Abstract
Activation of pregnane X receptor (PXR), a nuclear receptor that controls xenobiotic and endobiotic metabolism, is known to induce liver enlargement, but the molecular signals and the cell types responding to PXR-induced hepatomegaly remain unknown. In this study, the effect of PXR activation on liver enlargement and cell change was evaluated in several strains of genetically-modified mice and animal models. Lineage labelling using AAV-Tbg-Cre-treated Rosa26EYFP mice or Sox9-CreERT, Rosa26EYFP mice was performed and Pxr-null mice or AAV Yap shRNA-treated mice were used to confirm the role of PXR or YAP. Treatment with selective PXR activators induced liver enlargement and accelerated regeneration in wild-type and PXR-humanized mice but not in Pxr-null mice by increase of cell size, induction of a regenerative hybrid hepatocyte (HybHP) reprogramming, and promotion of hepatocyte and HybHP proliferation. Mechanistically, PXR interacted with yes-associated protein (YAP) and PXR activation induced nuclear translocation of YAP. Blockade of YAP abolished PXR-induced liver enlargement in mice. These findings revealed a novel function of PXR in enlarging liver size and changing liver cell fate via activation of the YAP signalling pathway. These results have implications for understanding the physiological functions of PXR and suggest the potential for manipulation of liver size and liver cell fate.
Keywords: pregnane X receptor, yes-associated protein, hybrid hepatocyte, hepatocyte proliferation, liver regeneration
Introduction
Given the many vital functions of the mammalian liver, especially metabolism and xenobiotic detoxification, the ability of the liver to maintain a constant size and mass is critical for organismal survival. The liver reaches a pre-programmed size during development, which is dynamically maintained during maturity and aging (1). Moreover, the liver modulates its size in response to physiological and pathological stimuli, for instance, hormonal fluctuations associated with pregnancy and lactation, acute-phase proteins involved in viral and bacterial infection and enzymes that metabolize xenobiotics (2). Furthermore, the liver has a tremendous capacity to regenerate after toxin-induced injury or surgical resection. However, the molecular mechanisms involved in the regulation of liver size and regeneration are highly complicated and remain unclear.
Yes-associated protein (YAP) is a critical regulator of liver size that rapidly switches hepatic growth on or off (3, 4). YAP hyperactivation can evoke proliferation and transdifferentiation of mature hepatocytes and part of the embryonic hepatic program (5). The expression and activity of YAP are critical for the process of liver regeneration (6), but the YAP activation context and its impact on liver enlargement and regeneration remain largely unclear.
Many cell types are changed within the liver in response to alterations in liver size and liver regeneration. During liver regeneration, differentiated hepatocytes re-enter the cell cycle, proliferate and replenish the lost tissue (7, 8). Bipotential liver progenitor cells (LPCs) were proposed as the main source of new hepatocytes and ductal cells under conditions in which normal hepatocyte proliferation was severely impaired (9, 10). In addition to cytokeratin 19 (CK19) and epithelial cell adhesion molecule (EpCAM), SRY (sex determining region Y)-box 9 (SOX9) was proposed as a novel marker for LPCs (5). Several studies have shown that LPCs only minimally contributed to restoring hepatocyte loss in most liver injury models (11, 12). However, a specialized type of cells bearing characteristics of both LPCs and mature hepatocytes were identified as hybrid hepatocytes (HybHPs). These cells express both the LPC marker SOX9 and the hepatocyte marker hepatocyte nuclear factor 4α (HNF4α). They are considered a pre-existing population of periportal hepatocytes located in the portal triads of healthy livers. HybHPs undergo extensive proliferation and replenish the liver mass after chronic hepatocyte-depleting injury (13). Thus, HybHPs may play critical roles in liver homeostasis and repair in response to injury.
Pregnane X receptor (PXR, NR1I2) is a ligand-activated transcription factor that belongs to the nuclear receptor superfamily. PXR is highly expressed in the liver and plays an integral role in the control of xenobiotic and endobiotic metabolism to maintain homeostasis of numerous endobiotics, such as glucose, lipids, steroids, bile acids, bilirubin, retinoic acid, and bone minerals (14, 15). Recent studies have explored the role of PXR in the regulation of disease risk and severity, including the development of cholestatic liver disease (16), inflammatory bowel disease (17), inflammatory liver disease (18), cholesterol gallstone disease (19), and non-alcoholic fatty liver disease (20). As a potential important therapeutic target, fully understanding the biological and physiological properties and functions of PXR would be of great value. Most recently, a mouse-specific PXR agonist pregnenolone-16α-carbonitrile (PCN) was found to induce significant liver enlargement. However, it is still unknown which cell types within the liver are responsible for the alterations in liver size, and the involved molecular signals remain poorly characterized. The risk of liver enlargement associated with PXR activators has not been extensively investigated and is important for the rational clinical use of therapeutic drugs that are PXR agonists.
The current study aimed to investigate the role of PXR in liver enlargement and to determine the mechanisms accounting for its effect. Here, a novel physiological role for PXR was uncovered showing that it controls liver size and changes cell fate by enlarging hepatocyte size, promoting hepatocyte proliferation, and inducing HybHPs programming. Notably, PXR was found to interact with the YAP signalling pathway that is essential for PXR-induced liver enlargement. These findings revealed a novel function for PXR in controlling liver size and changing liver cell fate via the YAP signalling pathway, which helps us better understand the PXR physiological functions and provides a clinically relevant argument for using PXR as a potential therapeutic target to promote hepatic repair.
Materials and Methods
MOUSE STRAINS
Eight- to nine-week-old male C57BL/6 mice weighing 20–22 g were provided by the centre of laboratory animal science of Guangdong (Guangzhou, China). Pxr-null mice (21) were obtained from Dr. Jeff L. Staudinger and Dr. Steven A. Kliewer. C57BL/6, Sox9-CreERT, and Rosa26EYFP reporter mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All animal studies were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University or by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health.
STATISTICAL ANALYSIS
The data are expressed as the means ± standard deviation (SD). One-way ANOVA followed by unpaired Student’s t-test or multiple t-test was used for statistical analysis of data using GraphPad Prism 5 (GraphPad Software Inc, San Diego, CA). P values less than 0.05 were considered significant.
Results
PXR ACTIVATION INDUCED LIVER ENLARGEMENT
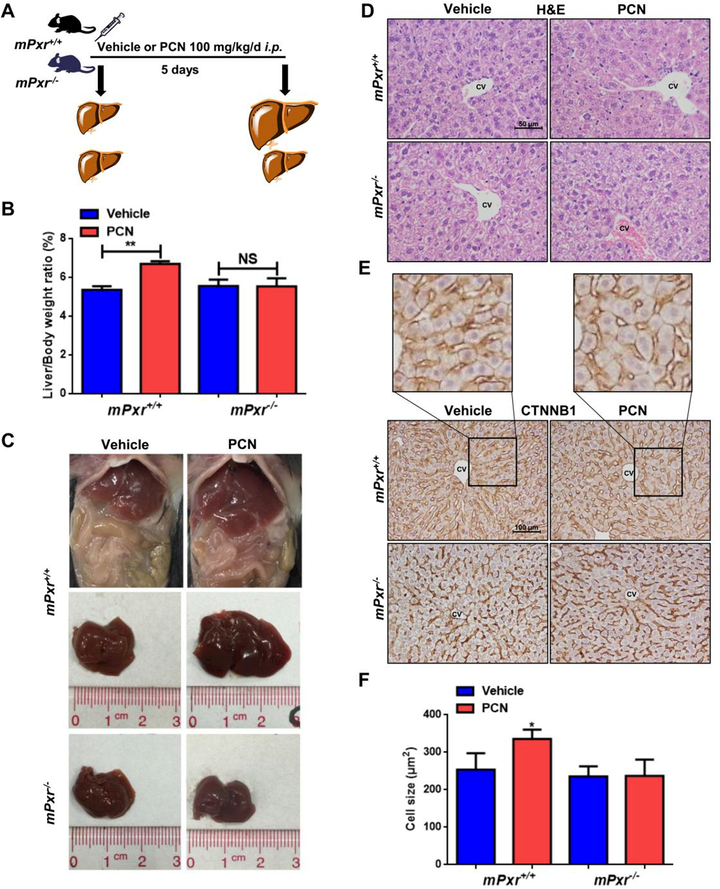
We first demonstrated PXR-induced liver enlargement using specific mouse or human PXR agonists in wild-type (WT) mice, Pxr-null mice or PXR-humanized mice (hPXR). After i.p. injection of 100 mg/kg/d PCN for 1 to 10 days, significant increases in liver-to-body-weight ratio were observed compared to the vehicle-treated group at the same time point (Supporting Fig. S1A-B). Recovery of the liver-to-body-weight ratio to the normal ratio took 15 days after 5 days of PCN treatment (Supporting Fig. S1C-D). WT and Pxr-null mice were then treated with vehicle or PCN for 5 days (Fig. 1A). Absence of PXR expression abolished PCN-induced liver enlargement (Fig. 1B-C). To examine the change of hepatic cell size after PCN treatment, hepatocyte size was measured by haematoxylin and eosin (H&E) and β-catenin (CTNNB1) staining in WT and Pxr-null mice. An increase in hepatocyte size around the central vein (CV) was observed in PCN-treated WT mice, which was abolished in Pxr-null mice (Fig. 1D-F).
Figure 1.
Effects of PXR activation on liver enlargement in wild-type (WT) and Pxr-null mice. (A) WT or Pxr-null mice were treated with vehicle (corn oil) or 100 mg/kg/d PCN for 5 days. (B) Liver-to-body-weight ratios (n = 6). (C) Representative gross pictures of WT or Pxr-null mice. (D) Histopathological analysis of representative mouse liver samples following H&E staining. (E) IHC staining of central vein areas for CTNNB1 (Membrane CTNNB1 staining was performed to describe the cell size). (F) Quantification of the cell size (n = 3). The data are expressed as the means ± SD. *P < 0.05 and **P < 0.01 compared with the vehicle group.
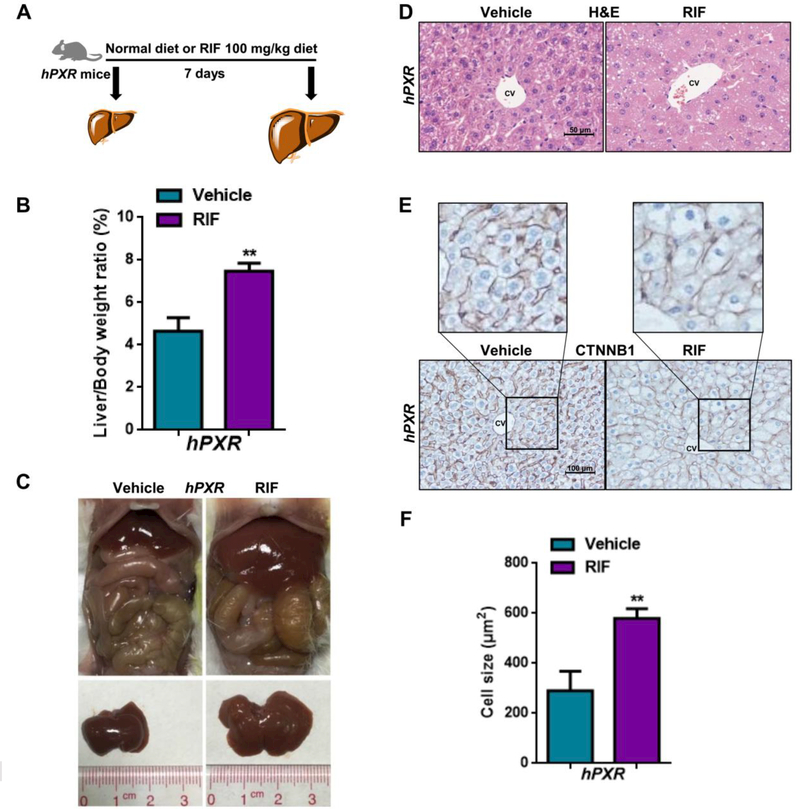
Human and mouse PXR have 95% homology in the DNA-binding domain but only 73% homology in the ligand-binding domain (22). To investigate whether liver overgrowth induced by PXR activation was species-dependent, hPXR mice were administered 100 mg/kg rifampicin (RIF), a human-specific PXR agonist, in the diet for 7 days (Fig. 2A). As expected, a significant increase in liver-to-body-weight ratio was also observed in RIF-treated mice (Fig. 2B-C). H&E and CTNNB1 staining revealed a significant increase in cell size in RIF-treated mice (Fig. 2D-F). Interestingly, both in WT mice and hPXR mice, significant cell size enlargement was mostly observed in CV instead of portal vein (PV) area (Supporting Fig. S2A-F). Since many therapeutic drugs were shown to affect PXR activity (23), the influence of some of these drugs on liver size was investigated. Treatment with mifepristone, PCN, schisandrol B, dexamethasone, nifedipine, or corticosterone for 5 days markedly induced liver enlargement (Supporting Fig. S2G-I).
Figure 2.
Effects of PXR activation on liver enlargement in PXR-humanized (hPXR) mice. (A) hPXR mice were fed a regular chow diet or the diet plus 100 mg/kg RIF for 7 days. (B) Liver-to-body-weight ratios (n = 4). (C) Representative gross pictures of hPXR mice. (D) Histopathological analysis of representative mouse liver samples following H&E staining. (E) IHC staining of central vein areas for CTNNB1. (F) Quantification of the cell size (n = 3). The data are expressed as the means ± SD. *P < 0.05 and **P < 0.01 compared with the vehicle group.
Increased liver size is known to be associated with inflammation and hepatotoxicity (24), and thus, several biochemical and inflammatory factors were measured to confirm whether the PXR-induced liver enlargement was due to inflammation. No structural degenerative or necrotic changes were observed between the vehicle-treated group and the PCN-treated group, as revealed by H&E staining, inflammatory factors, myeloperoxidase (MPO) staining and serum biochemical indexes (Supporting Fig. S3 A-E).
PXR ACTIVATION PROMOTED HEPATOCYTE PROLIFERATION AND INDUCED SOX9+ HybHPs.
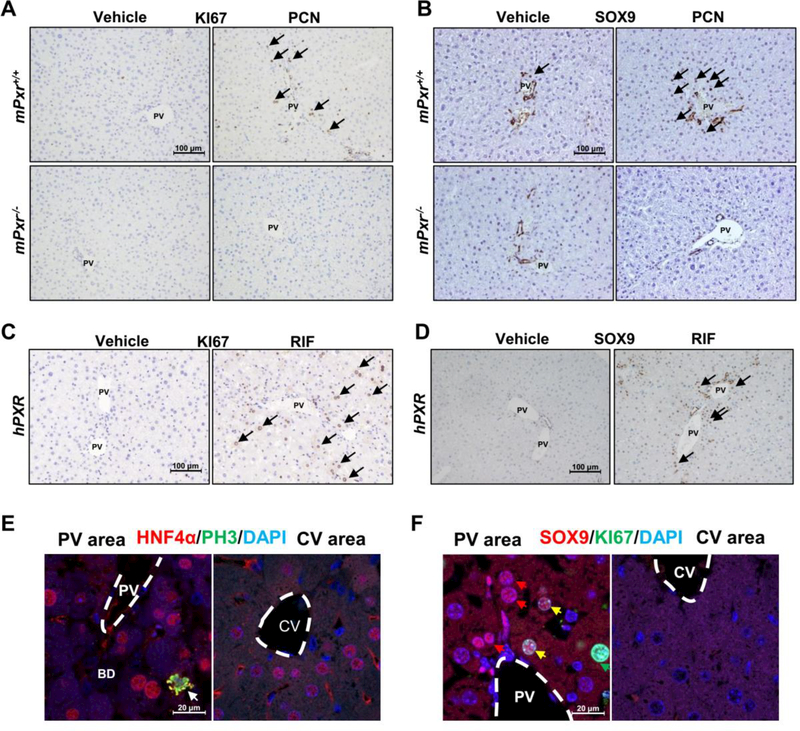
Next, the specific cell type responsible for PXR-induced liver enlargement was investigated. KI67 staining analysis demonstrated that PCN treatment significantly increased the number of KI67+ cells in WT mice; however, PCN failed to increase the number of KI67+ proliferating cells in Pxr-null mice (Fig. 3A, Supporting Fig. S4A). Interestingly, a robust increase in SOX9+ hepatocyte-like cells was observed around the PV area in the liver of PCN-treated mice (Fig. 3B, Supporting Fig. S4B). Similarly, an increase in the number of KI67+ hepatocytes and SOX9+ hepatocyte-like cells was also observed in 7-day RIF-treated hPXR mice (Fig. 3C-D, Supporting Fig. S4C-D). HNF4α/Phospho-Histone H3 (PH3) and HNF4α/KI67 double staining were performed, revealing that HNF4α+/PH3+ and HNF4α+/KI67+ proliferating hepatocytes were only observed in the PV area (Fig.3E, Supporting Fig. S4E). Furthermore, serial sections showed that most of the KI67+ proliferating cells were located in the PV area, especially the SOX9+ cell area, with very few KI67+ hepatocytes in the CV area (Supporting Fig. S4F). SOX9/KI67 double staining and quantified the percentage of KI67+ cells in SOX9+ hepatocytes were performed, and 25% of SOX9+ hepatocytes in PV area were also KI67+ (Fig. 3F, Supporting Fig. S4G).
Figure 3.
PXR activation results in hepatocyte proliferation and hybrid periportal hepatocyte (hepatic progenitor-like cell) reprograming. (A-B) IHC staining of portal vein areas for KI6 and SOX9 in WT or Pxr-null mice. (C-D) IHC staining of portal vein areas for KI67 and SOX9 in hPXR mice. (E) HNF4a and PH3 double staining of portal vein areas or central vein areas in PCN-treated mice. (F) SOX9 and KI67 double staining of portal vein areas or central vein areas in PCN-treated mice.
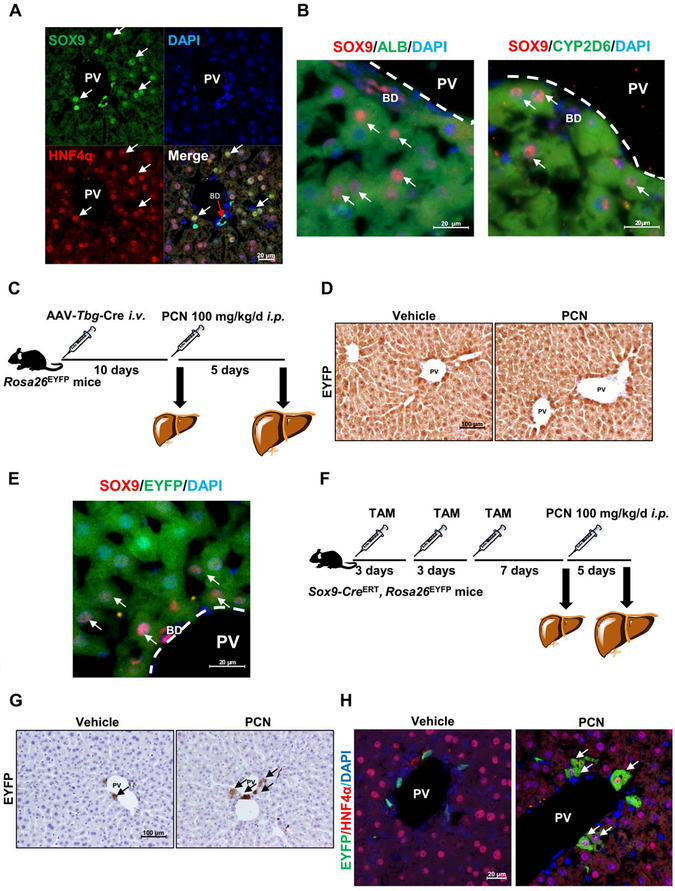
Since the location and SOX9+ expression pattern of these cells resembled those of HybHPs reported previously (13), SOX9 and HNF4α double staining was performed on livers from PCN-treated mice to clarify whether these SOX9+ hepatocyte-like cells were HybHPs. SOX9+ hepatocytes located in the PV area were also HNF4α+, suggesting that these SOX9+ hepatocyte-like cells are HybHPs (Fig. 4A). Moreover, to verify that the SOX9+ hepatocytes in PV area were HybHPs, SOX9/albumin (ALB) and SOX9/CYP2D6 staining in addition to HNF4α staining were performed. A population of SOX9+ hepatocytes were also positive for hepatocyte markers (Fig. 4B). To investigate the origin of these HybHPs after PXR activation, an adeno-associated virus (AAV) Tbg promoter-Cre recombinase vector (AAV-Tbg-Cre), in which Cre recombinase was only expressed in mature hepatocytes, was administered to Rosa26EYFP reporter mice (Fig. 4C). AAV-Tbg-Cre injection induced EYFP expression in more than 99% of hepatocytes but no other cells in the liver, such as bile duct cells, Kupffer cells and hepatic stellate cells (HSCs), as reported previously (25, 26). No hepatocytes were found without EYFP expression in the PV area after PCN treatment (Fig. 4D), and some of these cells showed positive SOX9/EYFP staining (Fig. 4E), suggesting that PCN-induced HybHPs were of hepatocyte origin. Sox9-CreERT;Rosa26EYFP transgenic mice were then treated with 50 mg/kg tamoxifen (TAM) to label the pre-existing SOX9+ cells, followed by vehicle or PCN administration (Fig. 4F). Very few hepatocytes were labelled with EYFP in the vehicle group, while EYFP+/HNF4α+ hepatocyte induction was observed extending along the periportal region in the PCN-treated group (Fig. 4G-H). These data supported the notion that PXR activation leads to HybHPs induction in the PV area.
Figure 4.
Clonal labelling confirmed cell type with PXR activation. (A) SOX9 and HNFα IF staining and DAPI staining in PCN-treated WT mice. (B) SOX9/ALB and SOX9/CYP2D6 double staining in PCN-treated mice. (C) Experimental design for TBG lineage tracing used Rosa26EYFPmice. Rosa26EYFP mice administered AAV-Tbg-Cre and treated with 100 mg/kg/d of PCN for 5 days. (D) Representative images showing EYFP staining. (E) SOX9 and EYFP double staining in PCN-treated AAV-Tbg-Cre, Rosa26EYFP ‘mice (n = 3). (F) Experimental design for SOX9 lineage tracing used Sox9-Cre, Rosa26EYFP mice administered TAM three times (once every three days), following treatment with 100 mg/kg/d of PCN for 5 days. (G) Representative slides of EYFP staining from Sox9-CreERT, Rosa26EYFPmice. (H) EYFP and HNF4a double staining in PCN-treated Sox9-CreERT, Rosa26EYFP mice (n = 3).
PXR ACTIVATION ACCELERATED LIVER REGENERATION
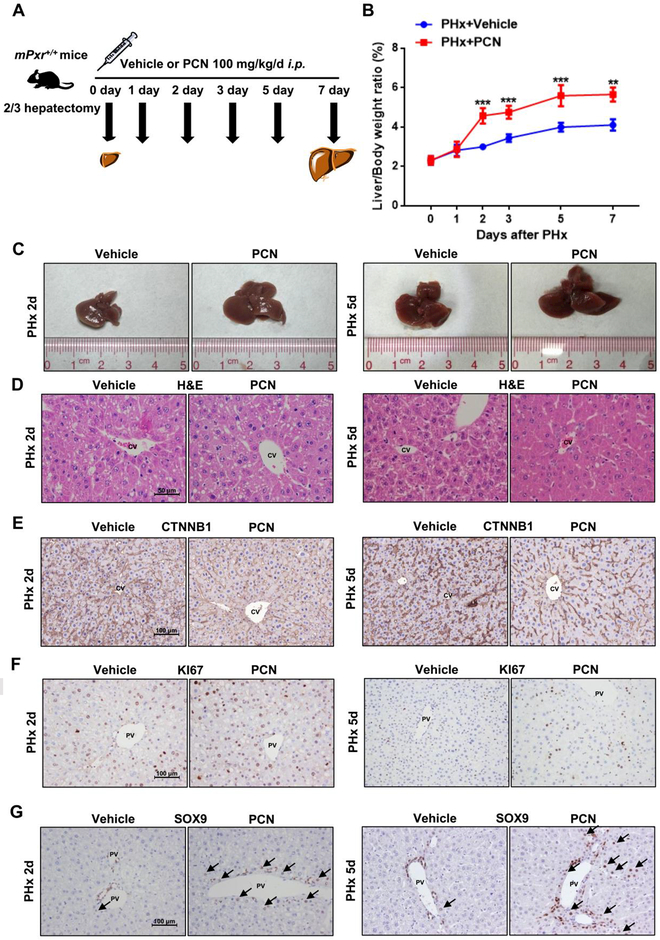
As noted above, PXR activation contributes to the regulation of liver size and hepatocyte proliferation in liver, in agreement with earlier studies that absence of PXR suppresses liver regeneration (27). Thus, by which PXR activation regulated liver regeneration after two-thirds partial hepatectomy (PHx) was examined (Fig. 5A). The liver-to-body-weight ratios of PCN-treated mice increased progressively and induced augmentative hepatomegaly after surgery (Fig. 5B-C). Enlarged hepatocytes in the PCN-treated group were also observed as revealed by H&E and CTNNB1 histological staining (Fig. 5D-E, Supporting Fig. S6A). Furthermore, the number of KI67+ hepatocytes in the PCN-treated group was higher than in the vehicle-treated group at day 2 and day 5 (Fig. 5F, Supporting Fig. S6B). Similarly, the number of SOX9+ HybHPs was substantially increased in the PV area of the PCN-treated mice (Fig. 5G Supporting Fig. S6C). The PXR-accelerated regeneration after PHx was further confirmed in hPXR mice treated with 50 mg/kg RIF after PHx (Supporting Fig. S5A). RIF also elevated liver-to-body-weight ratio after PHx (Supporting Figure S5B-C), and increased cell size (Supporting Fig. S5D-E, Supporting Fig. S6D). RIF-treated hPXR mice exhibited an increased number of KI67 positive hepatocytes and their postoperative SOX9+ hepatocytes were significantly increased compared with the vehicle-treated group (Supporting Fig. S5F-G Supporting Fig. S6E-F). Interestingly, during liver regeneration after PHx, no induction of SOX9 expression was observed in the vehicle-treated group, but an induction of SOX9+ hepatocytes was found in PCN/RIF-treated PHx mice. During tissue regrowth and liver regeneration, there is transient steatosis characterized by the accumulation of lipid used as substrates for energy production and membrane synthesis (28, 29), Pronounced hepatic triglyceride and cholesterol accumulation was observed in PCN-treated mice (Supporting Fig. S6G-J). Moreover, the level of serum albumin was not significantly changed in vehicle-treated mice and RIF/PCN-treated mice after PHx. However, PXR agonists inhibited the elevation of serum total bilirubin (TBIL) after PHx (Supporting Fig. S6K-N). These data suggested that PXR agonists had the potential to promote liver regeneration.
Figure 5.
Effects of PXR activation on liver regeneration after PHx in WT mice. (A) Mice were treated with PCN or vehicle following surgery and sacrificed at the indicated time points. (B) Liver-to-body-weight ratios. **P < 0.01 and ***P < 0.001 compared with the vehicle group at the same time point. The data are expressed as the means ± SD (n = 6). (C) Representative gross pictures of mice livers after PCN administration for 2 days and 5 days. (D-G) Histopathological and IHC staining of representative mouse liver samples following H&E (D), CTNNB1 (E), KI67 (F), and SOX9 (G) staining.
PXR INDUCED LIVER ENLARGEMENT IN A YAP-DEPENDENT MANNER
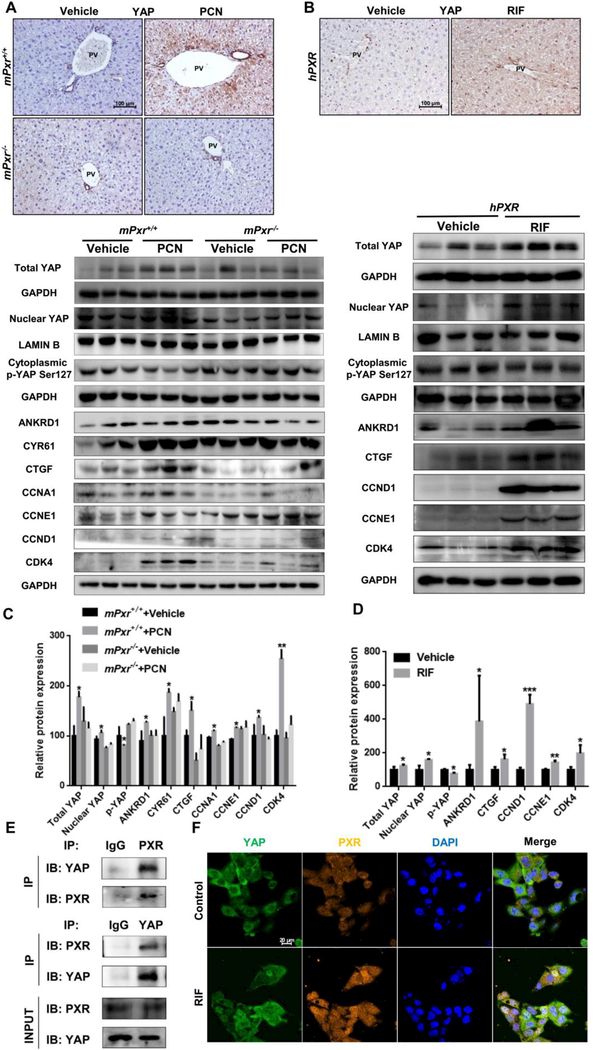
The YAP signalling pathway controls liver size (30) through the regulation of hepatocyte size and proliferation, and induction of ductal/progenitor cells (31). To determine whether PXR regulates liver growth through the YAP pathway, YAP expression was examined after PCN treatment in WT, Pxr-null, RIF treated hPXR mice. The Yap mRNA levels were measured and PXR activation induced significant increase in Yap mRNA (Supporting Fig. S7A-B). YAP is highly expressed in bile duct cells, where PXR agonist treatment induced YAP expression in all mouse lines except the Pxr-null mice (Fig. 6A-B). YAP phosphorylation increases its cytoplasmic retention and inhibits its function as a transcriptional co-activator (32). Levels of total YAP and nuclear YAP were significantly increased in PCN-treated WT mice and RIF-treated hPXR mice, while cytoplasmic phosphorylated YAP was reduced by both PCN and RIF, indicating that the YAP pathway is activated by PXR agonists (Fig. 6C-D). In support of this observation, expression of YAP downstream target proteins and proliferation-associated proteins, including the YAP target genes ankyrin repeat domain 1 (ANKRD1), cysteine rich angiogenic inducer 61 (CYR61) and connective tissue growth factor (CTGF) and the proliferation-associated proteins cyclin A1 (CCNA1), cyclin D1 (CCND1), cyclin E1 (CCNE1) and cyclin dependent kinase 4 (CDK4), were increased in PCN-treated WT mice and RIF-treated hPXR mice, but was not significantly changed in Pxr-null mice (Fig. 6C-D). However, the expression of upstream regulators, mammalian Ste20-like kinases 1/2 (MST1/2) p-MST1/2, large tumor suppressor 1/2 (LATS1/2) and p-LATS1/2, were not significantly changed (Supporting Fig. S7C-F), suggesting that PXR-induced YAP activation may not via YAP classical upstream.
Figure 6.
Effects of PXR activation on YAP signalling pathway. (A) IHC staining of YAP in WT and Pxr-null mice. (B) IHC staining of YAP in hPXR mice. (C) Western blotting and quantification of total YAP, nuclear YAP, cytoplasmic p-YAP, YAP downstream protein and proliferation protein expression in WT and Pxr-null mice. (D) Western blotting and quantification of total YAP, nuclear YAP and cytoplasmic p-YAP protein, YAP downstream protein and proliferation protein expression in hPXR mice. (E) Co-IP of PXR and YAP to assess their protein-protein interaction in primary hepatocyte extracts. (F) Confocal microscopy showing PXR and YAP distribution in HepG2 cells treated with 20 μΜ RIF for 48 hours. Nuclei were counterstained with DAPI. The data are expressed as the means ± SD. *P < 0.05, **P<0.01 and ***P<0.001 compared with the vehicle group (n = 3).
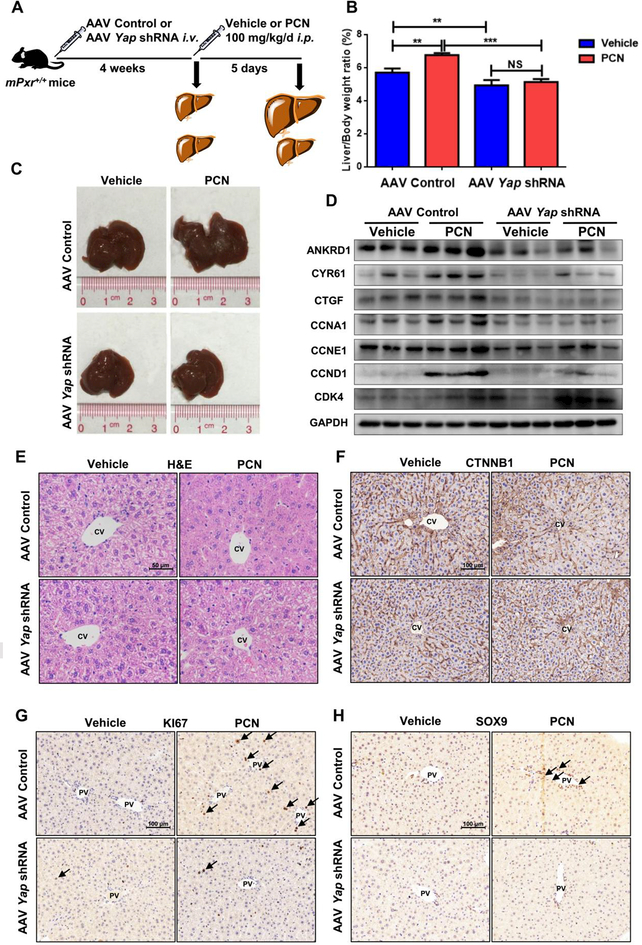
PXR and YAP are normally located in the cytoplasm and translocated into the nucleus after activation (33, 34). To explore the potential interaction of PXR and YAP, co-immunoprecipitation and co-localization analysis were performed. Endogenous PXR selectively bound to YAP in primary hepatocytes (Fig. 6E), and activation of PXR by RIF enhanced translocation of YAP to the nucleus in HepG2 cells (Fig. 6F). These data provided evidence that PXR interacts with YAP. Next, AAV Yap shRNA mice were used to investigate whether the PXR-induced liver enlargement depends on activation of the YAP pathway (Fig. 7A). Hepatic YAP was successfully reduced (Supporting Fig. S8A-B) without interfering PXR activation, as revealed by the protein expression of the PXR downstream genes CYP3A11, UGT1A1, and GSTM2 (Supporting Fig. S8C-D). The expression of YAP target genes revealed that by disabling Yap, PCN lost induction of ANKRD1, CYR61, CTGF, CCNA1, CCNE1 and CCND1 (Fig. 7D, Supporting Fig. S8E). Mice exposed to AAV control had a normal liver size, but AAV Yap shRNA-treated mice had a significant decrease in liver mass. PCN treatment induced liver enlargement and an increase in the number of KI67+ and SOX9+ hepatocytes in the presence of YAP, but failed to induce liver enlargement (Figure 7B-C), hepatocyte size (Fig. 7E-F, Supporting Fig. S8F) and elevation of the number of KI67+ and SOX9+ hepatocytes in the absence of YAP (Fig.7G-H, Supporting Fig. S8G-H). These results indicated that depletion of YAP prevented PCN-induced liver enlargement and liver cell fate change.
Figure 7.
Effects of PXR activation in AAV-Control and AAV Yap shRNA mice. (A) WT mice given AAV control or AAV Yap shRNA for 4 weeks and treated with 100 mg/kg/d PCN for 5 days. (B) Liver-to-body-weight ratios are shown. **P < 0.01 and ***P < 0.001. The data are expressed as the means ± SD (n = 5). (C) Representative gross pictures of mouse livers after vehicle or PCN administration. (D) Western blot was used to measure YAP downstream and proliferation protein expression in AAV-Control and AAV Yap shRNA mice. (E-H) Histopathological and IHC staining of representative mice liver samples following H&E (E), CTNNB1 (F), KI67 (G) and SOX9 (H) staining.
Discussion
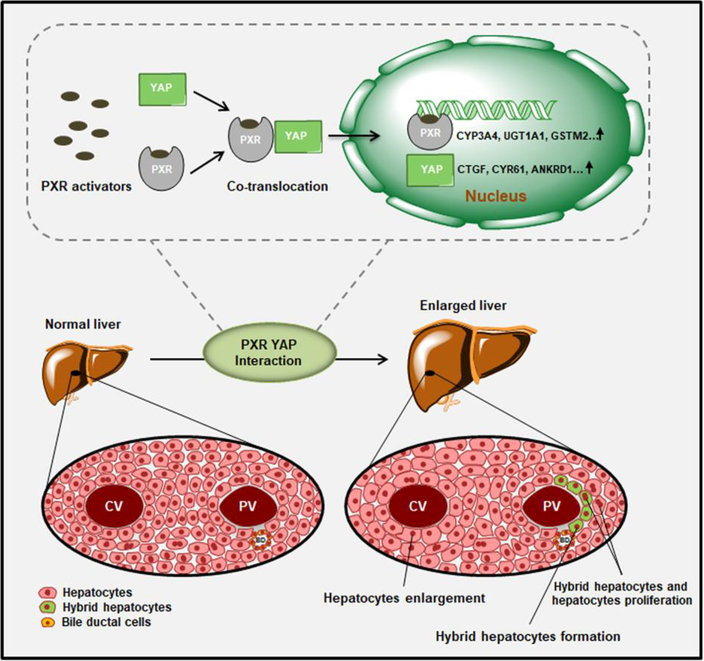
The liver plays a pivotal role in the metabolism and detoxification of xenobiotics, and hepatic size and mass must be strictly controlled. PXR activation was found to induce liver enlargement (35) and hepatocytes proliferation (36), but the related molecular signals and mechanisms have not been investigated. The cell types responding to PXR activation also remain unknown. The present study demonstrated that PXR activation induces liver enlargement by increasing hepatocyte size and proliferation (Fig. 8). Moreover, PXR activation induces hepatocytes to HybHPs reprogramming. Furthermore, activation of the YAP signalling pathway is necessary for PXR-induced liver enlargement. These findings reveal a novel function of PXR in changing liver size and reprogramming liver cell fate by potentially interacting with the YAP signalling pathway, thus revealing the mechanism by which PXR activation influences liver size and cell fate. The hepatic response to various stimuli may involve an increase in the liver size and functional capacity attributable to an increase in the size and/or number of hepatocytes.
Figure 8.
Proposed mechanisms of PXR regulated liver size and liver cell fate via YAP activation.
Liver size and mass are tightly controlled and many types of hepatic cells participate in liver size regulation. Among them, liver stem/progenitor cells play an important role in giving rise to both hepatocytes and cholangiocytes (37). During persistent and severe liver damage, part of hepatocytes undergo dedifferentiation into liver stem/progenitor cells for liver regeneration (38). HybHPs, a type of progenitor-like cell with lower SOX9 expression compared with cholangiocytes, and LPCs were identified as a pre-existing group of periportal hepatocytes with the highest regenerative and proliferative capacity in healthy livers (13, 39). Few SOX9+ hepatocytes exist under normal conditions, while PXR activation promoted an increase of SOX9+ hepatocytes in the PV area, which are also coincident with KI67+ proliferating cells. Of interest, PXR activation can induce KI67+ expression of mature hepatocytes around the PV area. PXR activation reprograms the cells to bear double biopotential of HybHP. The enzyme content and subcellular structures of hepatocytes from the periportal (afferent) and perivenous (efferent) zones of the liver parenchyma are varied. Since glycogen synthesis, glycolysis, lipogenesis, ketogenesis and detoxication are preferentially situated in the CV area (40), the enlargement of hepatocytes responding to PXR activation was primarily observed around the CV area. On the other hand, PXR induced-newly formed HybHPs were chiefly located near the PV area, which may due to the continuous hepatocytes supply from the SOX9-expressing precursor pool that located in the biliary duct. The newborn hepatocytes appeared to migrate very slowly from the PV region to the CV zone (5). Thus, PXR activation at the portal-central axis may induce a unique change in hepatic cell diversity that might correspond to different metabolic capacities.
Hepatic enzyme induction is generally an adaptive response associated with an increase in liver weight, induction of gene expression, and morphological changes in hepatocytes. Common xenobiotic enzyme inducers trigger pathways involving the constitutive androstane receptor (CAR), the peroxisome proliferator-activated receptor α (PPARα), and the aryl hydrocarbon receptor (AHR). In practice, it was found that these ligand-activated transcription factors can induce hepatocellular hypertrophy (41, 42). Since PXR broadly regulates xenobiotic-metabolizing metabolic enzymes, induction of one or more of these enzymes might contribute to PXR-induced hepatic growth. PXR might have a stage-specific effect on metabolic enzyme induction, which provides an energy source to support cell proliferation and tissue overgrowth.
The YAP signalling pathway, a critical regulator of organ and organism size, was shown to regulate hepatocyte proliferation and liver mass (43). It was also reported that over-activation of YAP leads to dedifferentiation of hepatocytes, which is associated with the appearance of progenitor cells (31, 44). In the current study, the PXR-induced liver enlargement was abolished after YAP suppression using AAV Yap shRNA, indicating that the YAP signalling pathway is essential for PXR-induced liver enlargement. Moreover, PXR regulated the expression of YAP downstream genes. As the major downstream effector of the Hippo pathway, unphosphorylated YAP translocates into the nucleus to induce target gene transcription, which is similar to PXR activation. Moreover, PXR is able to recruit members of the p160 family co-activators, such as peroxisome proliferator-activated receptor gamma co-activator lα and steroid receptor co-activator 1 (45, 46). Therefore, PXR and YAP might undergo nuclear co-translocation and co-activation. The present study also demonstrated a protein-protein interaction between PXR and YAP, which raises the possibility that PXR might regulate YAP activity via co-activator interactions that lead to nuclear translocation. However, the downstream signalling pathway of PXR-YAP in liver hypertrophy remains unclear. PXR is a direct activator of growth arrest and DNA damage-inducible 45β (GADD45B) (47), which was reported to be associated with CAR-induced liver hypertrophy (48). Whether GADD45B is involved in PXR-YAP-mediated liver enlargement still requires further study.
PXR was reported to play an important role in liver regeneration after PHx. However, the underlying mechanisms and cell types responding to PXR-related regeneration remain unknown. After PHx, all the existing mature cellular populations undergo cell division and proliferation to restore organ weight. A previous study reported that DNA synthesis in hepatocytes and bile duct cells was terminated 4–5 days after PHx (49). In the present study, PXR activation rapidly restored the liver mass by day 5 after PHx, yet some hepatocytes remained proliferative and dedifferentiated along the hepatic PV. These findings raise the possibility that both hepatocyte self-duplication and dedifferentiation into progenitor-like cells contribute to PXR-accelerated liver regeneration. Liver regeneration is a vital recovery process after liver damage, and thus, promoting regeneration though activating PXR might be an important strategy to promote liver regeneration associated with liver resection, liver cancer, and acute liver failure. The present study illustrated that many clinical drugs, such as nifedipine, mifepristone, and corticosterone, activate PXR and induce liver enlargement. Liver regeneration is the result of hepatocyte hyperplasia and hypertrophy (50), which could be modulated by PXR activation. Thus, the effect of these drugs to induce liver enlargement and promote liver regeneration should be considered prior to their potential clinical therapies.
PXR activation has broad biological and physiological implications. Thus, it is vital to fully understand the biological and physiological properties and functions of PXR. Overall, this study demonstrated that PXR activation induces liver enlargement and promotes liver regeneration by increasing cell size, inducing hepatocytes to HybHPs reprogramming, and promoting hepatocytes and HybHPs proliferation. However, the relative contributions of these three mechanisms to liver enlargement remain unclear and further studies are needed to clarify their relationship. Moreover, interaction with the YAP-signalling pathway is a key mechanism in PXR-induced liver enlargement and accelerated-regeneration. These findings provide a clinically relevant argument for using PXR as a potential therapeutic target for promoting hepatic development and liver repair.
Supplementary Material
Acknowledgments
Financial support
This work was supported by National Natural Science Foundation of China (Grants: 81522047, 81573489, 81730103, 81320108027), the 111 project (Grant: B16047), the National Key Research and Development Program (Grant: 2017YFE0109900, 2017YFC0909303), the Key Laboratory Foundation of Guangdong Province (Grant: 2011A060901014), and Natural Science Foundation of Guangdong (Grant: 1714050000188, 2015A030313124).
Abbreviations:
- PXR
pregnane X receptor
- HybHP
hybrid hepatocyte
- HNF4α
hepatocyte nuclear factor 4α
- YAP
yes-associated protein
- LPC
liver progenitor cell
- CK19
cytokeratin 19
- EpCAM
epithelial cell adhesion molecule
- SOX9
SRY (sex determining region Y)-box 9
- PCN
pregnenolone-16α-carbonitrile
- hPXR
PXR-humanized mice
- SD
standard deviation
- WT
wild-type
- H&E
haematoxylin and eosin
- CTNNB1
β-catenin
- CV
central vein
- RIF
rifampicin
- PV
portal vein
- MPO
myeloperoxidase
- PH3
Phospho-Histone H3
- ALB
albumin
- CYP2D6
cytochrome P450 2D6
- AAV
adeno-associated virus
- TAM
tamoxifen
- HSC
hepatic stellate cell
- PHx
partial hepatectomy
- TBIL
total bilirubin
- ANKRD1
ankyrin repeat domain 1
- CYR61
cysteine rich angiogenic inducer 61
- CTGF
connective tissue growth factor
- CCNA1
cyclin A1
- CCND1
cyclin D1
- CCNE1
cyclin E1
- CDK4
cyclin dependent kinase 4
- MST1/2
mammalian Ste20-like kinases 1/2
- LATS1/2
large tumor suppressor 1/2
- FoxA2
forkhead box A2
- CAR
constitutive androstane receptor
- PPAR
peroxisome proliferator-activated receptor
- AhR
aryl hydrocarbon receptor
- GADD45β
growth arrest
- DNA
damage-inducible 45β
Footnotes
Other experimental information is provided in the Supplementary Information.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 2016;13:473–485. [DOI] [PubMed] [Google Scholar]
- 2.Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, Singh B, et al. Hepatic enzyme induction: histopathology. Toxicol Pathol 2010;38:776–795. [DOI] [PubMed] [Google Scholar]
- 3.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007;130:1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SH, Camargo FD, Yimlamai D. Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis. Gastroenterology 2017;152:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011;43:34–41. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Xiao Y, Zhang S, Ji S, Wei L, Fan F, Geng J, et al. The Ets transcription factor GABP is a component of the hippo pathway essential for growth and antioxidant defense. Cell Rep 2013;3:1663–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taub R Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 2004;5:836–847. [DOI] [PubMed] [Google Scholar]
- 8.Yin S, Wang H, Park O, Wei W, Shen J, Gao B. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3. Am J Pathol 2011;178:1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 2013;14:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fausto N Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 2004;39:1477–1487. [DOI] [PubMed] [Google Scholar]
- 11.Tarlow BD, Finegold MJ, Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology 2014;60:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S, Upadhyay N, Greenbaum LE, Kaestner KH. Ablation of Foxl1-Cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology 2015;148:192–202 e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell 2015;162:766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998;92:73–82. [DOI] [PubMed] [Google Scholar]
- 15.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 2000;406:435–439. [DOI] [PubMed] [Google Scholar]
- 16.Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol 2015;62:S25–37. [DOI] [PubMed] [Google Scholar]
- 17.Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, Smyth CM, et al. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology 2006;130:341–348; quiz 592. [DOI] [PubMed] [Google Scholar]
- 18.Wallace K, Cowie DE, Konstantinou DK, Hill SJ, Tjelle TE, Axon A, Koruth M, et al. The PXR is a drug target for chronic inflammatory liver disease. J Steroid Biochem Mol Biol 2010;120:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Nishida S, Xu M, Makishima M, Xie W. PXR prevents cholesterol gallstone disease by regulating biosynthesis and transport of bile salts. Gastroenterology 2011;140:2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem 2007;18:184–195. [DOI] [PubMed] [Google Scholar]
- 21.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A 2001;98:3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie W, Evans RM. Pharmaceutical use of mouse models humanized for the xenobiotic receptor. Drug Discov Today 2002;7:509–515. [DOI] [PubMed] [Google Scholar]
- 23.Vignati LA, Bogni A, Grossi P, Monshouwer M. A human and mouse pregnane X receptor reporter gene assay in combination with cytotoxicity measurements as a tool to evaluate species-specific CYP3A induction. Toxicology 2004;199:23–33. [DOI] [PubMed] [Google Scholar]
- 24.Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, Kuttler K, et al. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes--conclusions from the 3rd International ESTP Expert Workshop. Toxicol Pathol 2012;40:971–994. [DOI] [PubMed] [Google Scholar]
- 25.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev 2013;27:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu X, Espanol-Suner R, Mederacke I, Affo S, Manco R, Sempoux C, Lemaigre FP, et al. Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment. J Clin Invest 2015;125:3891–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai G, He L, Bu P, Wan YJ. Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology 2008;47:1277–1287. [DOI] [PubMed] [Google Scholar]
- 28.Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 2004;40:1322–1332. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, Tebar F, et al. Caveolin-1 is essential for liver regeneration. Science 2006;313:1628–1632. [DOI] [PubMed] [Google Scholar]
- 30.Tordjmann T Hippo signalling: liver size regulation and beyond. Clin Res Hepatol Gastroenterol 2011;35:344–346. [DOI] [PubMed] [Google Scholar]
- 31.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, et al. Hippo pathway activity influences liver cell fate. Cell 2014;157:1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007;21:2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawana K, Ikuta T, Kobayashi Y, Gotoh O, Takeda K, Kawajiri K. Molecular mechanism of nuclear translocation of an orphan nuclear receptor, SXR. Mol Pharmacol 2003;63:524–531. [DOI] [PubMed] [Google Scholar]
- 34.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev 2013;27:355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos 2001;29:1467–1472. [PubMed] [Google Scholar]
- 36.Shizu R, Abe T, Benoki S, Takahashi M, Kodama S, Miayata M, Matsuzawa A, et al. PXR stimulates growth factor-mediated hepatocyte proliferation by cross-talk with the FOXO transcription factor. Biochem J 2016;473:257–266. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 2000;32:1230–1239. [DOI] [PubMed] [Google Scholar]
- 38.Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology 2014;59:1617–1626. [DOI] [PubMed] [Google Scholar]
- 39.Li D, Li W, Hui L. Hybrid hepatocyte: A newly identified player for regeneration in hepatic injuries. Hepatology 2016;64:2244–2246. [DOI] [PubMed] [Google Scholar]
- 40.Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev 1989;69:708–764. [DOI] [PubMed] [Google Scholar]
- 41.Crampton RF, Gray TJ, Grasso P, Parke DV Long-term studies on chemically induced liver enlargement in the rat. I. Sustained induction of microsomal enzymes with absence of liver damage on feeding phenobarbitone or butylated hydroxytoluene. Toxicology 1977;7:289–306. [DOI] [PubMed] [Google Scholar]
- 42.Crampton RF, Gray TJ, Grasso P, Parke DV Long-term studies on chemically induced liver enlargement in the rat. II. Transient induction of microsomal enzymes leading to liver damage and nodular hyperplasia produced by safrole and Ponceau MX. Toxicology 1977;7:307–326. [DOI] [PubMed] [Google Scholar]
- 43.Pan D Hippo signaling in organ size control. Genes Dev 2007;21:886–897. [DOI] [PubMed] [Google Scholar]
- 44.Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H, Park O, et al. Hippo signaling interactions with Wnt/beta-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest 2017;127:137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell 1995;83:841–850. [DOI] [PubMed] [Google Scholar]
- 46.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 1999;20:321–344. [DOI] [PubMed] [Google Scholar]
- 47.Kodama S, Negishi M. Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J Biol Chem 2011;286:3570–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Columbano A, Ledda-Columbano GM, Pibiri M, Cossu C, Menegazzi M, Moore DD, Huang W, et al. Gadd45beta is induced through a CAR-dependent, TNF-independent pathway in murine liver hyperplasia. Hepatology 2005;42:Π18–Π26. [DOI] [PubMed] [Google Scholar]
- 49.Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997;276:60–66. [DOI] [PubMed] [Google Scholar]
- 50.Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol 2012;22:1166–1175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.