Abstract
The objective of the present study is to investigate the role of α4, α5, α6 or β2 nAChR subunits in the antidepressant-like effect of bupropion. Adult male mice were treated with subcutaneous acute doses of bupropion (3 and 10 mg/kg) 30 min before the forced swim test (FST) in α4, α5, α6, or β2 nAChR subunit knockout (KO) and wild-type (WT) mice. In addition, the effects of β2* antagonist dihydro-β-erythroidine (DHβE, 3 mg/kg) on antidepressant-like effects of bupropion in C57BL/6J mice were assessed. Our results showed that baseline immobility and climbing time did not differ between KO and corresponding WT mice except for β2 KO. Bupropion significantly decreased immobility time and increased climbing time in the α4, α6 and β2 nAChR KO mice in comparison to WT littermates, indicating that lack of these nAChR subunits enhanced antidepressant effects of bupropion. On the contrary, the α5 nAChR subunit deletion did not alter the FST behavior in the bupropion-treated mice. Not only in the transgenic mice, bupropion also showed antidepressant-like effects in the WT mice. In addition, DHβE pretreatment before bupropion administration resulted in decreased immobility time and increased climbing time. Taken together, the present study provides evidence on the involvement of α4*, α6*, and β2* (* indicates possible presence of other subunits) nAChRs in the antidepressantlike effects of bupropion in the FST.
Keywords: bupropion, cholinergic, depression, forced swim test, nicotinic receptor
1. Introduction
Depression is a common, chronic, and potentially severe mental condition that adversely affects one's life characterized by sadness, feelings of guilt or low confidence; loss of interest, pleasure or concentration. Depression alone accounts for nearly 4% of the global burden of disease and affects up to about 300 million people worldwide [48]. About 7% of adults in the United States have been diagnosed with major depression in 2016 [41]. The current antidepressants primarily target monoamine transporters regulating the reuptake of dopamine, serotonin, and norepinephrine [5]. While most clinically effective therapeutic drugs including bupropion target monoamine transporters; these agents can also inhibit neuronal nicotinic acetylcholine receptors (nAChRs) [5, 43]. Emerging evidence suggests that nAChRs play an important role in the etiology of depression [21, 43].
The link between nicotine and depression is well established by clinical studies [31]. Numerous reports pointed out that smokers demonstrated more depressive symptoms when compared with nonsmokers and smokers diagnosed with depression were more dependent on smoking [9, 16, 22]. One of the alternative theories of depression is Cholinergic-adrenergic hypothesis proposed in the early 70s [21]. It suggests a hyperactivity of the cholinergic system over that of the adrenergic system in the brain. Apparently, high cholinergic transmission is associated with depression [21, 49] and inhibition of nAChRs may promote antidepressant effects [35, 43, 44]. Preclinical pharmacological studies demonstrated that central, not peripheral, nAChRs are involved in depression-like behaviors [1, 32]. Administration of nAChR antagonists and partial agonists also showed antidepressant-like effects [27, 32, 36]. In addition, nAChR antagonists were shown to potentiate the antidepressant effects of clinically effective antidepressants such as bupropion [29, 37].
Bupropion [(±)-2-(tert-Butylamino)-3’-0-chloropropiophenone], synthesized in 1969 [26], is effective as an antidepressant [3] and smoking cessation aid [19]. The mechanism of action of bupropion is not clear. Bupropion primarily acts as a norepinephrine-dopamine reuptake inhibitor [7]. It is also able to block activation of α3β2, α4β2, and α7 nAChRs [7]. It has been reported that bupropion antagonized in vivo nicotine’s antinociceptive, motor, hypothermic, and convulsive effects, after systemic administration in the mice [12, 44]. In addition, bupropion noncompetitively inhibited acetylcholine activation of rat α3β2- and α4β2-nAChRs with IC50 values of 1.3 and 8 μM, respectively, expressed in Xenopus oocytes [44]. Moreover, hydroxybupropions are major metabolites of bupropion and are believed to contribute to neurobiological effects of bupropion [10, 11]. The previous report from our laboratory showed that hydroxyl metabolites of bupropion are more potent blockers of α4β2 nAChRs [10]. Although evidence indicates that nAChRs play important role in the antidepressant action of bupropion [2], the involvement of α4β2* nAChR subunits (* indicates a variable subunit either α5 or α6) in bupropion’s therapeutic effect is largely unknown.
We hypothesized that these nAChR subunits associated with α4β2* nicotinic receptors may modulate the antidepressant-like effect of bupropion. For that, we used wild-type (WT) or α4, α5, α6, β2 nAChR knockout (KO) mice in the forced swim test (FST). The FST is a widely used screening tool to identify antidepressant potential of drugs. In addition, we determined the effects of a selective α4β2* nAChR antagonist (dihydro-β-erythroidine, DHβE) on antidepressant properties of bupropion in the FST.
2. Materials and Methods
2.1. Animals
Male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice null (KO) for the β2, α6 (Institut Pasteur, Paris, France) [8], α5 (Jackson Laboratories, Bar Harbor, ME) [39] and α4 subunits (provided by Dr. Henry Lester at the California Institute of Technology, with the permission of Dr. John Drago) [38] and their WT littermates were bred in an animal care facility at Virginia Commonwealth University. All the mice used in each experiment were backcrossed at least 10 to 12 generations. Mutant and wild types were obtained from crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Eight or more animals per group were used in most of our experiments. However, the number of animals varied as we were limited by the breeding outcome of littermate KO and WT mice. Mice were housed in a 21 °C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)-approved animal care facility. They were housed in groups of six and had free access to food and water under a 12-h light/dark cycle (lights on at 7:00 a.m.). Mice were 8–10 weeks of age and weighed approximately 20–30 g at the start of all the experiments. All experiments were performed during the normal light cycle (between 7:00 a.m. and 7:00 p.m.) and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Drugs
Bupropion HCl and dihydro-β-erythroidine (DHβE) were purchased from Sigma-RBI (St. Louis, MO, USA). The doses and route of administration of these drugs were based on our previous studies [10, 53]. These drugs were dissolved in 0.9% saline and injected subcutaneously (s.c.) at a volume of 10 ml/kg body weight. All doses are expressed as the free base of the drug.
2.3. Forced swimming test (FST) and procedure
The test was performed as described earlier [30]. Briefly, mice were gently placed individually into an open glass cylindrical container (diameter 10 cm, height 25 cm) containing 15 cm of water, maintained at 24°C that was filled with tap water, and left there for 6 min. The test was performed by the same well-trained experimenter, who was blinded to the treatment administered. Immobility was recorded during the last 4 min [50]. Durations of diving and swimming were not recorded. A mouse was considered to be immobile when it floated in an upright position, and made only small movements to keep its head above water but did not produce displacements. Notably, immobility is not a depression-like phenotype but a behavioral adaptation to the acute stressor [51,52]. The duration of immobility and climbing time were recorded and scored in sec. Immediately after the testing, mice were removed from the water, gently dried with paper towels, and placed inside a cage warmed by a heating pad.
To verify the effects of α4, α5, α6, and β2 nAChR subunits on the depression-like behavior, we first evaluated the behavioral responses for the untreated KO and WT mice in the forced swim test (FST). We tested the role of these subunits in the possible antidepressant effect of bupropion in a separate cohort of mice. For this set of experiment, α4, α5, α6, and β2 KO or WT mice were injected with s.c. bupropion (3 and 10 mg/kg) or vehicle (saline). After 30 min bupropion injection, animals were exposed to the FST.
In a separate cohort, effects of bupropion (3 or 10 mg/kg) in the FST were assessed in presence of β2 nAChR antagonist DHβE (3 mg/kg). For that, C57BL/6J mice were injected with DHβE (3 mg/kg, s.c.) or vehicle followed by bupropion (3 and 10 mg/kg, s.c.) 20 min later. Finally, the FST was performed after 30 min of the last injection.
2.4. Locomotor activity in mice
Mice were placed into individual Omnitech photocell activity cages (28 × 16.5 cm) and interruptions of the photocell beams (two banks of eight cells each) were then recorded for the next 10 min. We have selected the minimum duration of locomotor activity allowed by our instrument (10 min) to closely match the duration of the FST. Data were expressed as number of photocell interruptions.
For the locomotor activity measures, α4, α6, or β2 KO and WT mice were injected with bupropion (3 and 10 mg/kg, s.c.) or vehicle (saline). After 30 min bupropion injection, mice were tested in activity cages. In addition, C57BL/6J mice were injected with DHβE (3 mg/kg, s.c.) or saline 20 min before bupropion (3 and 10 mg/kg, s.c.) treatment. Thirty min after s.c. administration of either saline or bupropion, mice were assessed in the locomotor activity test.
2.5. Statistical analysis
The data obtained were analyzed using the GraphPad software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Data were tested using ordinary two-way analysis of variance (ANOVA), followed by the Sidak post hoc correction. Baseline value differences in immobility and climbing time of untreated WT and KO mice were tested by t test. The p values < 0.05 were considered significant.
Results
3.1. Forced swim test in untreated α4, α5, α6 or β2-nAChR subunit KO mice
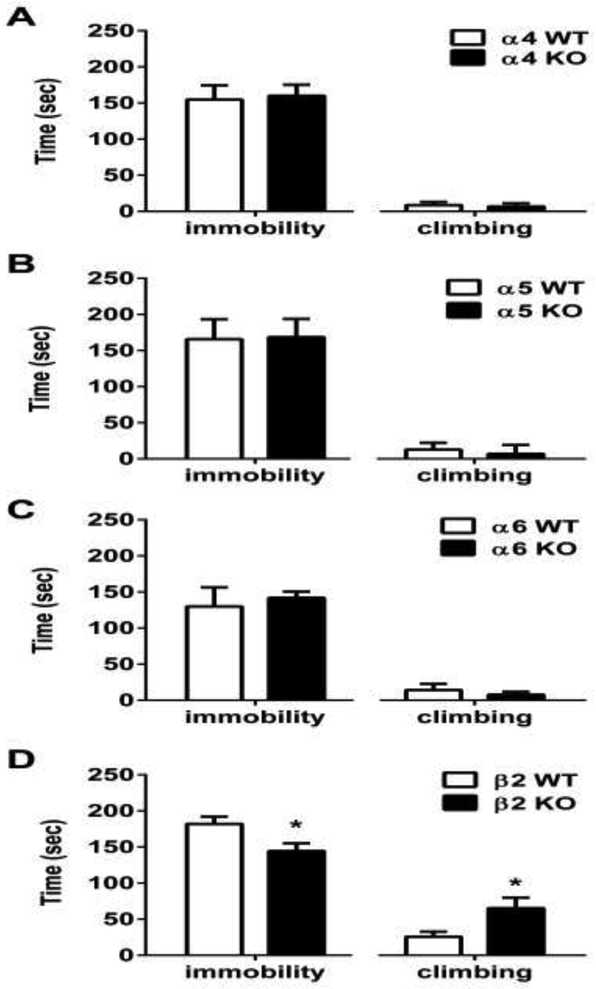
Two-way ANOVA was used to evaluate baseline immobility time and climbing time in KO mice and their WT littermates in the FST. There was no significant difference in immobility and climbing behavior between untreated α4 (timmobility=0.592, tclimbing=0.8831, df=14), α5 (timmobility=0.2024, tclimbing=0.9336, df=12), and α6 (timmobility=1.219, tclimbing=1.937, df=15) KO mice when compared to their WT littermates in the FST all p’s > 0.05, (Fig. 1 A-C). However, untreated β2 KO mice showed a lower duration of immobility and a longer climbing period (timmobility=2.578, tclimbing=2.433, df=14; p<0.05)
Figure 1. Time spent immobile and climbing by α4, α5, α6, and β2 nAChR KO mice in the FST.
Behavioral response in α4 (A), α5 (B) α6 (C), and β2 (D) KO mice and their WT littermates. Results are expressed as the mean ± S.E.M of 6-9 mice. α4 (n=9 WT and 7 for KO mice), α5 (n=8 WT and 6 for KO mice), α6 (n=9 WT and 8 for KO mice) and β2 (n=7 WT and 7 for KO mice).
3.2. Antidepressant-like effects of bupropion in the in α4, α5, α6, and β2 knockout mice in the FST
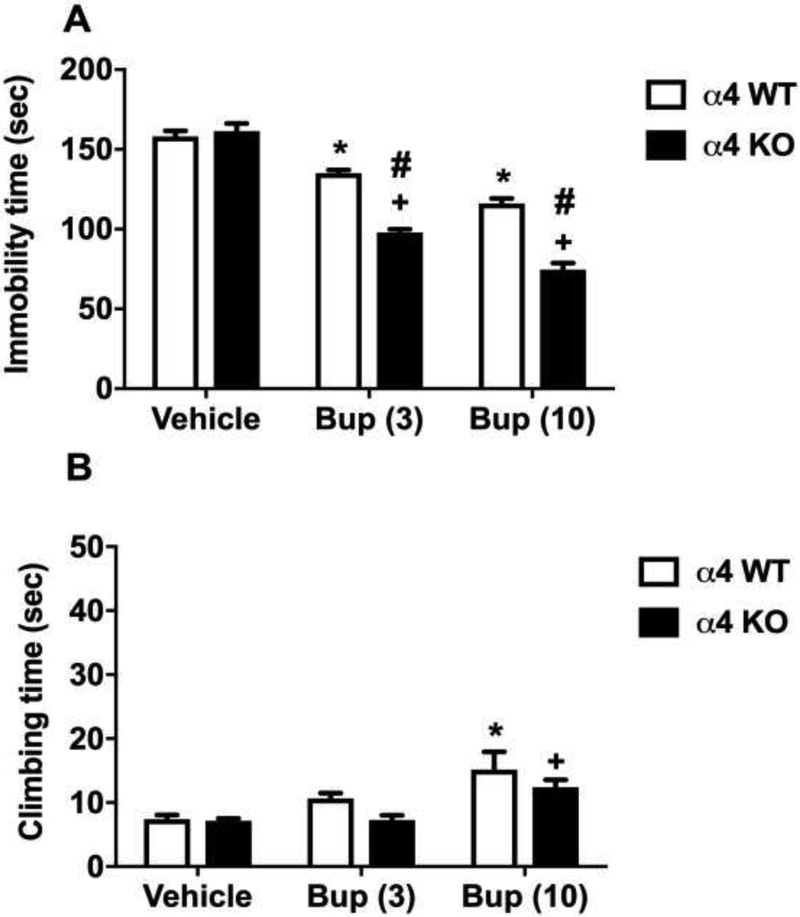
Two-way ANOVA performed in α4 KO and WT mice indicated significant effects of bupropion treatment on genotype, treatment, interaction of genotype and treatment for the immobility of FST [Fgenotype(1,42)=74.32, p<0.0001; Ftreatment(2,42)=169.9, p<0.0001 and Fgenotype × treatment interaction (2,42)=24.10, p<0.0001]. Bupropion (3 and 10 mg/kg) attenuated the immobility time in WT and KO mice when compared with vehicle treatment in the same genotype (Sidak post hoc, p<0.05) (Fig 2A). Moreover, immobility duration in bupropion treated mice was different between the KO and WT mice. While vehicle treatment did not show any difference between the KO and WT genotyped animals, bupropion (3 and 10 mg/kg) treatment significantly reduced immobility duration in KO mice (Sidak post hoc, p<0.05) (Fig 2A). Interestingly, the climbing time did not significantly differ between the bupropion-treated α4 WT and KO mice [Fgenotype(1,42)=3.65, p=0.06 and Fgenotype × treatment interaction (2,42)=0.73, p=0.48]; but high dose of bupropion (10 mg/kg) significantly increased the climbing time when compared with vehicle treatment [Ftreatment(2,42)=12.27, p<0.001] (Fig 2B).
Figure 2. The involvement of α4 nAChRs in the antidepressant-like effects of bupropion in the FST.
Effects of bupropion on immobility (A) and climbing (B) behavior in the FST in α4 KO mice and their WT littermates. After 30 min bupropion (3 and 10 mg/kg, s.c.) or vehicle (saline) injection, mice were exposed to the FST. Results are expressed as the mean ± S.E.M of 8 (WT) and 7 (KO) mice. * p < 0.05, significantly different from vehicle treated WT group; + p < 0.05, significantly different from vehicle treated KO group; # p < 0.05, significantly different from its corresponding control group (KO vs WT group). Bup: bupropion
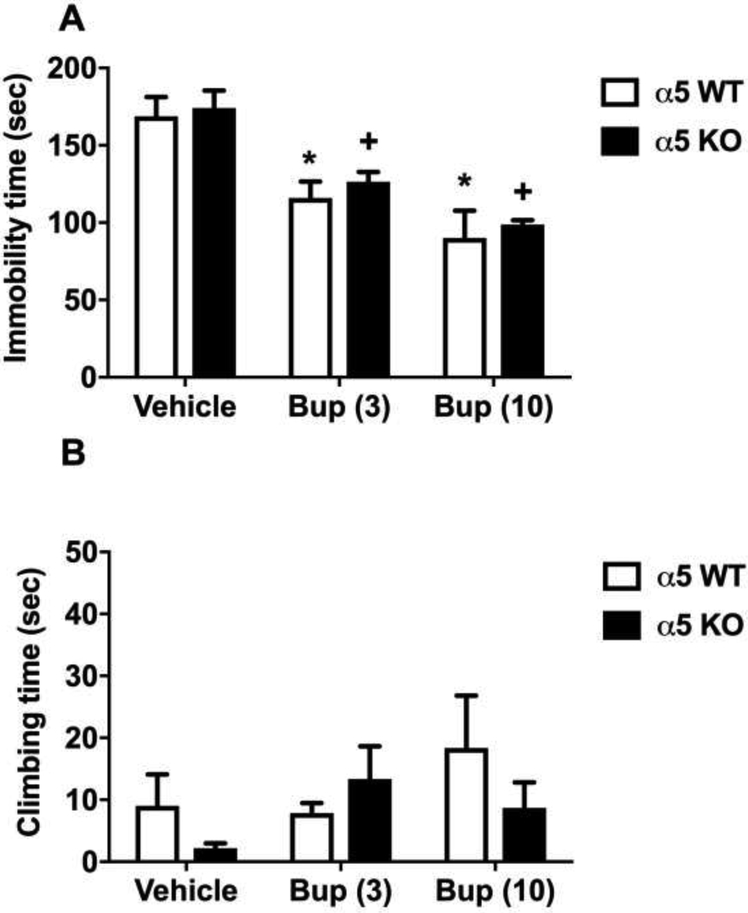
The antidepressant-like effects of bupropion in α5 nAChRs KO mice and their WT littermates are shown in Fig. 3. While there was a reduction in immobility time following the administration of bupropion in α5 nAChRs KO, it was not statistically significant in comparison to WT (Fig 3A). The effect of bupropion on climbing time was also not significantly different between KO and WT mice (Fig 3B).Bupropion (3 and 10 mg/kg) injection significantly decreased immobility time in both WT and KO mice [Ftreatment(2,44)=735.1, p<0.001]. Both doses of bupropion resulted in a significant reduction in immobility time in a dose-dependent manner (Sidak post hoc, p<0.05; denoted by an asterisk for WT and plus for KO). However, the immobility time of bupropion-treated mice was significantly shorter in the KO mice compared to WT littermates; hence the efficacy of bupropion was higher in the KO mice [Fgenotype(1,44)=602.3, p<0.001, denoted by hashtag].
Figure 3. The involvement of α5 nAChRs in the antidepressant-like effects of bupropion in the FST.
Effects of bupropion on immobility (A) and climbing (B) behavior in the FST in α5 KO mice and their WT littermates. After 30 min bupropion (3 and 10 mg/kg, s.c.) or vehicle (saline) injection, mice were exposed to the FST. Results are expressed as the mean ± S.E.M of 6 mice. * p < 0.05, significantly different from vehicle treated WT group; + p < 0.05, significantly different from vehicle treated KO group. Bup: bupropion
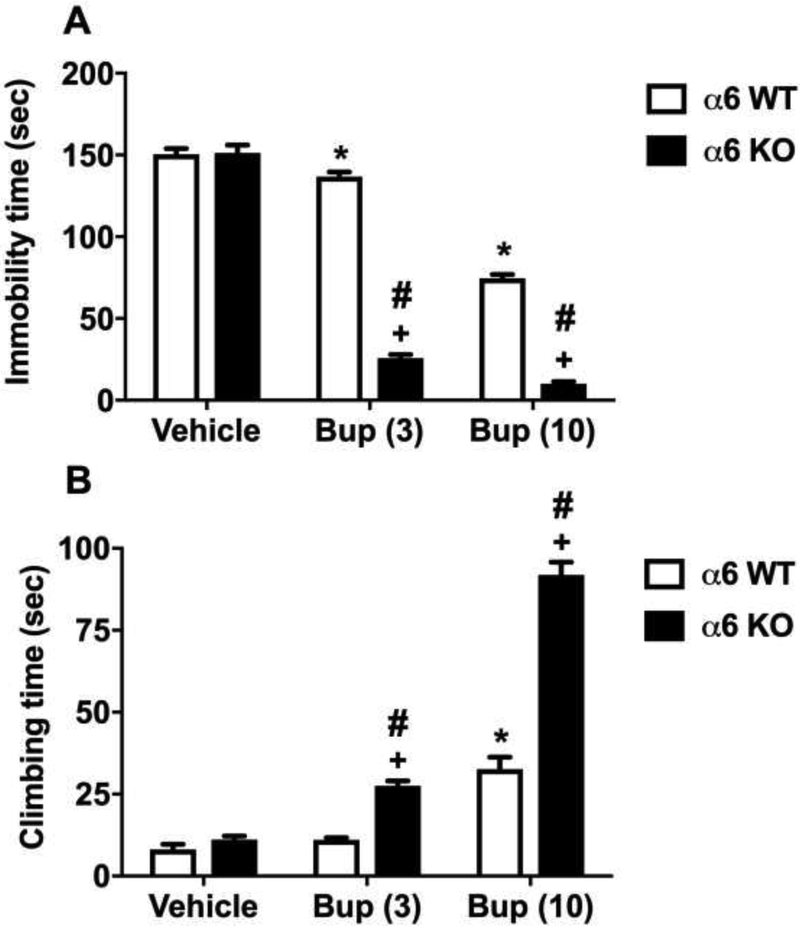
Two-way ANOVA revealed a significant interaction for the α6 nAChRs and bupropion treatments [Fgenotype × treatment interaction (2,44)=172.3, p<0.001] (Fig 4A). Similarly, Fig. 4B shows the time spent in climbing was significantly longer in bupropion treated mice in both KO an WT mice [Ftreatment(2,42)=199.7, p<0.001]. While higher dose of bupropion (10 mg/kg) enhanced the climbing time in WT mice, the climbing time was increased by low and high dose of bupropion (3 and 10 mg/kg) in KO mice (Sidak post hoc, p<0.05; denoted by asterisk for WT and plus for KO). Furthermore, α6 nAChR gene deletion in the mice resulted in a significant increase in climbing time compared to WT mice (Sidak post hoc, p<0.05; denoted by hashtag), a significant main effect of genotype [Fgenotype(1,42)=118.2, p<0.001], and a significant interaction [Fgenotype × treatment interaction (2,42)=54.56, p<0.001], (Fig 4B).
Figure 4. The involvement of α6 nAChRs in the antidepressant-like effects of bupropion in the FST.
Effects of bupropion on immobility (A) and climbing (B) behavior in the FST in α6 KO mice and their WT littermates. After 30 min bupropion (3 and 10 mg/kg, s.c.) or vehicle (saline) injection, mice were exposed to the FST. Results are expressed as the mean ± S.E.M of 11 (WT) and 9 (KO) mice. * p < 0.05, significantly different from vehicle treated WT group; + p < 0.05, significantly different from vehicle treated KO group; # p < 0.05, significantly different from its corresponding control group (KO vs WT group). Bup: bupropion
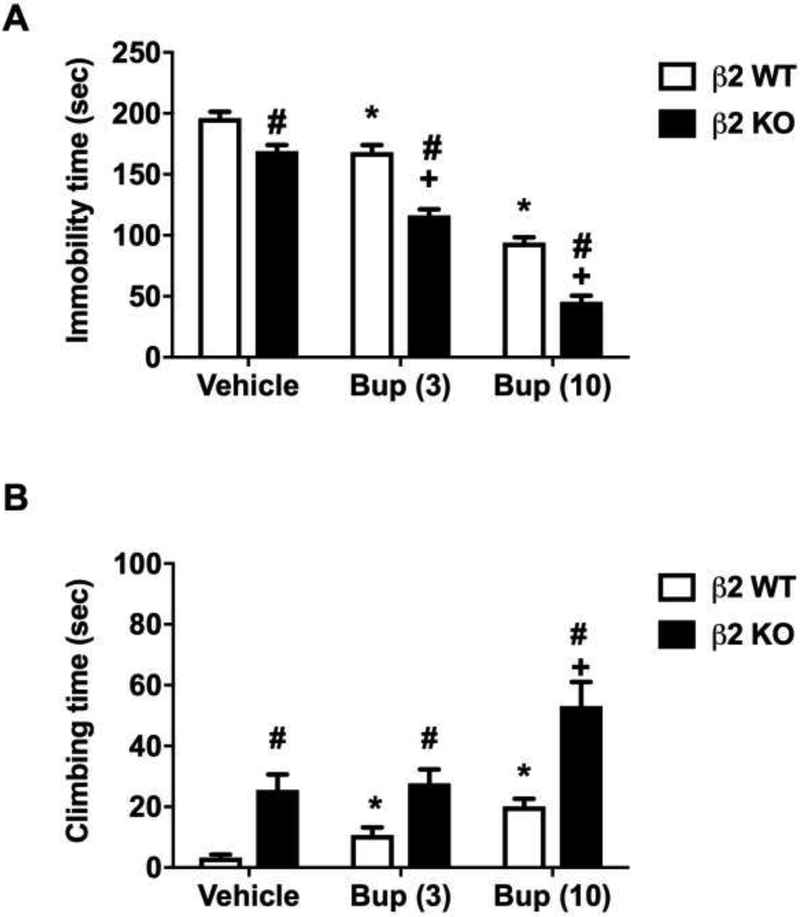
Figure 5 shows the effects of bupropion on β2 KO mice and their WT littermates in the FST. The β2 KO mice differed from the other KO in that they had reduced baseline immobility time compared to their WT littermates following administration of vehicle (Sidak post hoc, p<0.05). However, bupropion at a dose of 3 and 10 mg/kg was still able to significantly reduce the immobility time in WT and KO mice (Ftreatment (2, 36) = 250.9, p<0.001) in a dose-dependent manner (Sidak post hoc, p<0.05, denoted by asterisk for WT and plus for KO). There was also a significant reduction in immobility time between β2 KO and their WT littermates, indicating that bupropion was more effective in the KO mice [Fgenotype (1,36) = 104.3, p<0.001, denoted by hashtag]. There was also a significant interaction for the genotype and bupropion [Fgenotype × treatment interaction (2, 36) = 3.54, p<0.05] (Fig 5A). Similar to immobility time, β2 KO mice had significantly longer baseline climbing time compared to their WT littermates (Sidak post hoc, p<0.05, denoted by hashtag) (Fig 5B). However, only bupropion at a dose of 10 mg/kg was able to significantly increase climbing time in β2 KO mice [Ftreatment (2,36) = 13.42, p< 0.001, denoted by plus]. There was also a significant main effect of genotype [Fgenotype (1,36) = 42.77, p < 0.001].
Figure 5. The involvement of β2 nAChRs in the antidepressant-like effects of bupropion in the FST.
Effects of bupropion on immobility (A) and climbing (B) behavior in the FST in β2 KO mice and their WT littermates. After 30 min bupropion (3 and 10 mg/kg, s.c.) or vehicle (saline) injection, mice were exposed to the FST. Results are expressed as the mean ± S.E.M of 7 (WT) and 11 (KO) mice. * p < 0.05, significantly different from vehicle treated WT group; + p < 0.05, significantly different from vehicle treated KO group; # p < 0.05, significantly different from its corresponding control group (KO vs WT group). Bup: bupropion
3.3. Antidepressant-like effects of DHβE in the C57BL/6J mice in the FST
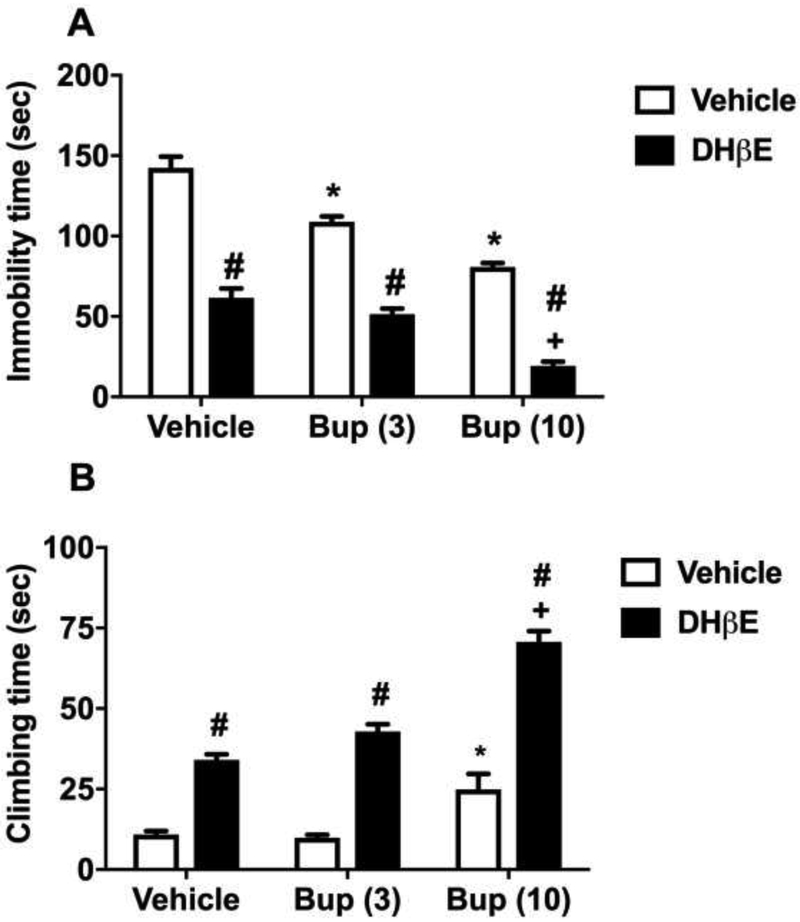
We then evaluated the effects of β2* nAChR selective antagonist, DHβE, on bupropion-induced behavioral responses in the FST. Fig. 6 displays the effect of combined treatment with DHβE and bupropion in the C57BL/6J male mice. There was a significant main effect of DHβE treatment [FDHβE(1,30)=329.2, p<0.001 ], a significant main effect of bupropion treatment [Ftreatment(2,30)=67.44, p<0.001] and a significant interaction of combination [Finteraction(2,30)=3.851, p<0.05] in the immobility time. Bupropion (3 and 10 mg/kg) dose-dependently attenuated the immobility time when compared with vehicle treatment in the C57BL/6J mice (Sidak post hoc, p<0.05) (Fig 6A). Moreover, DHβE alone diminished the immobility time when compared with vehicle-treated mice. The immobility time was also reduced in a dose-related manner in mice treated with both DhβE and bupropion (3 and 10 mg/kg) (Sidak post hoc, p<0.05). However, in the presence of DHβE, immobility time was also significantly decreased by bupropion (3 and 10 mg/kg) when compared with mice treated with either bupropion alone (Sidak post hoc, p<0.05; denoted by hashtag) or DHβE alone (Sidak post hoc, p<0.05; denoted by plus) (Fig 6A).
Figure 6. The involvement of α4β2 nAChRs in the antidepressant-like effects of bupropion in the FST.
Effects of bupropion on immobility (A) and climbing (B) behavior in the FST in dihydro-β-erythroidine (DHβE) pretreated C57BL/6J mice. For this reason, mice were injected s.c. DHβE (3 mg/kg) or vehicle (saline) with a 20 min pretreatment time. Continuously, same mice were administrated with bupropion (3 and 10 mg/kg, s.c.) or vehicle (saline). The FST was performed after 30 min of last drug administration. Results were expressed as the mean ± S.E.M of 6 mice. * p < 0.05, significantly different from vehicle group in vehicle pretreated group; + p < 0.05, significantly different from vehicle group in DHβE pretreated group; # p < 0.05, significantly different from its corresponding control group (DHβE pretreated bupropion group vs vehicle pretreated bupropion group). Bup: bupropion
Fig. 6B shows the effect of DHβE and bupropion treatment on the climbing time. ANOVA revealed a significant main effect of DHβE treatment [FDHβE(1,30)=225.1, p<0.001], a significant main effect of bupropion treatment [Ftreatment(2,30)=48.28, p<0.001] and a significant interaction of bupropion by DHβE [Finteraction(2,30)=8.385, p<0.05] in the time spent climbing. Bupropion (3 and 10 mg/kg) increased the climbing time in a dose-related manner when compared with vehicle treatment. Bupropion alone increased the time spent in climbing only at a dose of 10 mg/kg (Sidak post hoc, p<0.05). DHβE (3 mg/kg) administration significantly increased climbing time either injected alone or combined with bupropion (3 and 10 mg/kg) when compared with corresponding control mice (Sidak post hoc, p<0.05) (Fig 6B).
3.4. Effects of bupropion on locomotor activity in the α4, α6 knockout, and DHβE-treated mice
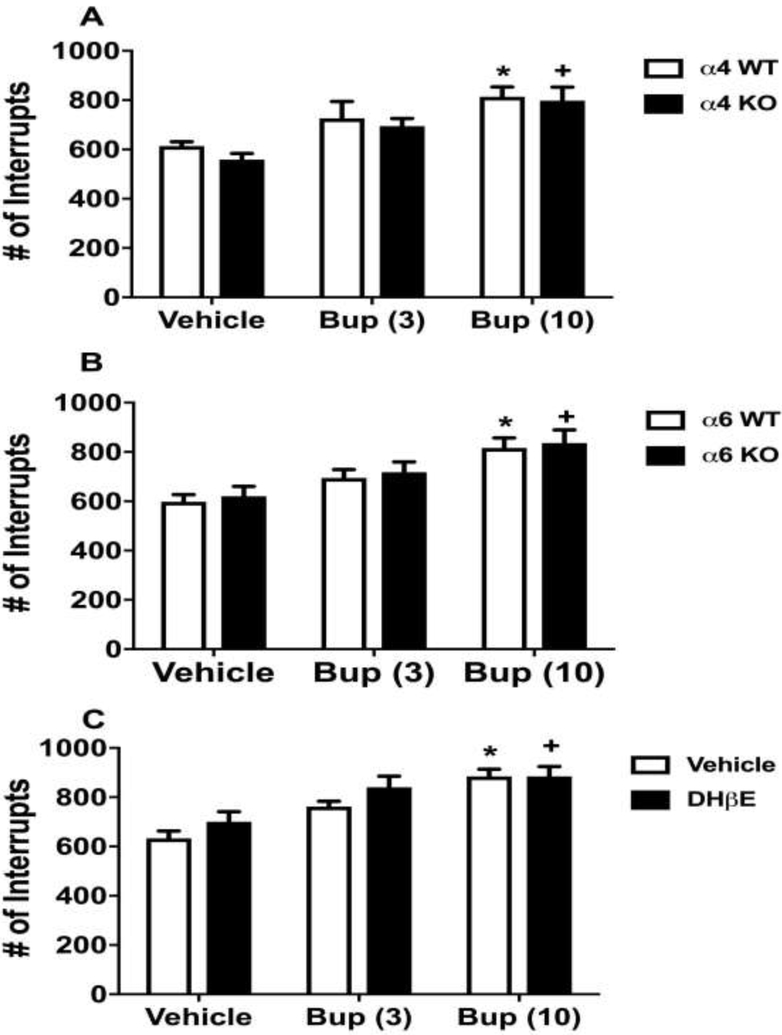
We finally investigated whether the changes observed above in the FST are due to alterations in the locomotor activity of mice. We assessed the effects of bupropion on the locomotor activity test using α4 KO and WT mice (Fig. 7A). Bupropion (3 and 10 mg/kg) dose relatedly increased the locomotor activity [Ftreatment (2,30) = 13.13, p<0.001] in α4 WT and KO mice when compared with vehicle treatment in the same genotype (Sidak post hoc, p<0.05). However, no significant difference was found in bupropion treatment in the locomotor activity between α4 WT and KO mice [Fgenotype(1,30)=0.9674, p=0.3332] (Fig 7A). In addition, ANOVA revealed that no significant interaction between α4 genotype and bupropion treatments for the spontaneous activity [Fgenotype × treatment interaction (2,30) = 0.1028, p=0.9026]. In addition, α6 WT and KO animals showed similar results. Bupropion (3 and 10 mg/kg) significantly increased the locomotor activity based on the high dose of bupropion without any relation to the α6 genotype. ANOVA revealed no significant main effect of α6 nAChRs [Fgenotype(1,38)=0.4266, p=0.5176], a significant main effect of bupropion treatment [Ftreatment(2,38)=10.68, p<0.001] and no significant interaction of bupropion by α6 nAChRs [Fgenotype × treatment interaction (2,38=0.004714, p=0.9953] in the locomotor activity (Fig 7B). Similar to α4 and α6 nAChR KO mice, there was no significant difference in locomotor activity between β2 nAChR KO and WT mice (number of interrupts, β2 KO: 622±42, WT: 659±50, ns).
Figure 7. The effect of bupropion on the locomotor activity in various nAChRs subtypes.
Mice were placed into photocell activity cages for 10 min after 30 min s.c. administration of bupropion (3 and 10 mg/kg) in α4 KO (A), α6 KO (B) and WT mice (C). The C57BL/6J mice (C) were injected s.c. DHβE (3 mg/kg) or vehicle (saline) 20 min before bupropion treatment. After 30 min bupropion (3 and 10 mg/kg, s.c.) or vehicle (saline) injection, mice were tested in activity cages. Results were expressed as the mean ± SEM as the number of photocell interruptions of α4 6 (WT) and 6 (KO); α6 8 (WT) and 8 (KO); . * p < 0.05, significantly different from vehicle group in WT animals or in vehicle pretreated group C57BL/6J mice; + p < 0.05, significantly different from vehicle group in KO animals or in DHβE pretreated group. Bup: bupropion
Fig. 7C shows the effect of combined treatment with DHβE and bupropion on the locomotor activity in the C57BL/6J mice. The two-way ANOVA revealed no significant main effect of DHβE treatment [FDHβE(1,35)=3.317, p=0.00771], a significant main effect of bupropion treatment [Ftreatment(2,35)=15.18, p<0.001] and no significant interaction of bupropion and DHβE [Finteraction(2,35)=0.526, p=0.5955]. Only the high dose of bupropion (10 mg/kg) significantly increased the locomotor activity either alone or in combination with DHβE (3 mg/kg) when compared to corresponding control-treated mice (Sidak post hoc, p<0.05). Bupropion (3mg/kg) did not significantly affect the locomotor activity (Sidak post hoc, p>0.05).
4. Discussion
One of the major findings of the present study was that lack of α4, α6, and β2 nAChR subunits enhanced the effects of bupropion in the FST. Bupropion (3 or 10 mg/kg) treatment reduced immobility time and increased climbing time in WT mice and transgenic mice, indicating antidepressant-like effects. However, the effects of bupropion were not enhanced in α5 nAChR KO mice in comparison to their WT littermates. We also found that the baseline depression-like behaviors in α4, α5, or α6 KO mice were similar to WT counterparts except in β2 KO mice. Consistent with previous findings [32], The mice lacking β2 nAChR subunit showed a reduction in immobility and elevation in climbing time compared to WT mice. In addition, the β2* nAChR antagonist DHβE (3 mg/kg) significantly reduced immobility time and increased climbing time in C57BL/6J mice. Moreover, DHβE enhanced the effects of bupropion in the FST. While the higher dose of bupropion (10 mg/kg) elevated locomotor activity, the effects were similar in both WT and transgenic mice used in this study. These findings support our initial hypothesis that nicotinic subunits associated with α4β2* nAChRs play an important role in the antidepressant effects of bupropion. The higher dose of bupropion (10 mg/kg) increased locomotor activity, indicating that part of reduction in immobility time could result from enhanced swimming. However, there was no significant difference in locomotor activity between WT and transgenic mice after bupropion treatment, whereas immobility time either differed or remained unchanged between WT and transgenic mice, indicating locomotor activity played a limited role in antidepressant-like effects in our study.
Previous studies suggest that antidepressant effects in mice are produced by inhibition of α4β2 nAChRs or activation of α7 nAChRs [1, 32]. While nicotinic agonists were inactive in the FST, non-selective antagonist mecamylamine, partial agonist cytisine, as well as the β2*-selective nAChR antagonist DHβE and α7 nAChR antagonist MLA showed antidepressant-like activities [1, 27]. Moreover, the antidepressant effect of mecamylamine was attenuated in β2 and α7 KO mice [32]. The present study is in line with previous reports that found inhibition of α4β2 nAChR activity induced antidepressant-like effects in mice.
The absence of the α4 or α6 subunit in the mouse prevents the formation of α4* or α6* containing nAChRs; such as α4β2, α4β4 or α6β2. However, the acute effects of bupropion are still noticeable in both α4 and α6 KO mice. Therefore, the deletion of those receptors does not abolish the effects of bupropion suggesting that α4 or α6 subunit-containing nAChRs are not the only ones responsible for those manifestations. Unlike mecamylamine, bupropion is a nAChR antagonist and a norepinephrine and dopamine transporter inhibitor [7]. We observed a significant reduction of immobility time and an elevation of climbing time by bupropion treatment in α4, β2, and α6 KO mice; indicating enhancement of the antidepressant effect of bupropion. According to Lucki et al. [23], the climbing behavior in the FST is primarily increased by induction of catecholamine neurotransmission. It is possible that increased climbing behavior in bupropion treated nAChR KO mice is associated with increased catecholamine transmission. In other words, the enhanced effect of bupropion in KO mice could be due to a complete genetic blockade of nAChR subunits which cannot be achieved by pharmacological blockade from systemic administration alone.
Interestingly, bupropion treatment did not affect depression-like behavior in α5 nAChR KO mice. The α5 subunit cannot yield functional receptors when expressed alone and it requires at least one other α subunit as well as a β2 and/or β4 subunit to form a functional nAChR [15, 18, 34]. The (α4β2)2α5 nAChRs are a prominent presynaptic subtype among α4β2* nAChRs, depending on the brain region. Approximately 90% of the heteromeric nAChRs are α4β2* subtype, and of these, about 10-37% contain α5 subtype in the cerebral cortex of adult male rats [25]. In addition, a class of nAChRs with a predominantly synaptic location on neurons contain receptors having at least three types of subunits encoded by the α3, β4, and α5 nAChR genes [46]. Incorporation of α5 subunit into α4β2*, α3β2*, and α3β4* nAChRs (where * denotes the possible inclusion of additional nAChR subunits) greatly influences a drug’s modulation of receptor function and pharmacological properties of these receptor subtypes in response to the drug in heterologous expression systems [4, 15, 18, 34, 45]. Our results showed that lack of α5 nAChR subunit does not alter the antidepressant effects of bupropion in mice, indicating that α5 nAChR subunits are not entirely related with those manifestations.
Interestingly, α6 KO mice were the most sensitive to the antidepressant effects of bupropion in the present study. The α6* subunit containing nAChRs, in particular, are highly expressed in mesolimbic pathways and mediate nicotine-related reward [6, 20, 28]. It has been reported that activation of α6β2* nAChRs is sufficient for dopamine-stimulating effects of nicotine [14]. The α6 subunit is greatly expressed in the ventral tegmental area and nucleus accumbens [8, 40, 47]. The α4, α6, β2, and β3 subunits exhibit a localization gradient across both medial habenula and interpeduncular nucleus [42]. Besides, heterologously expressed nAChRs containing α6 subunits can be defined in a straightforward fashion; such as α6β4 nAChRs if composed only of α6 and β4 subunits [24]. The α3, α4, β2, β3, and β4 subunits grouping into an extended family of α2/α4 and α3/α6 pairs [24]. On the other hand, α6* nAChRs are also co-expressed with the α4β2* nAChRs. Because α6* subunit containing nAChRs are selectively expressed in dopamine neurons and participate in cholinergic transmission, α6* specific agonists or antagonists have been suggested to provide a method for manipulating DA transmission in neural disorders by targeting endogenous cholinergic mechanisms in midbrain or striatum [14]. It is possible that in α6 KO mice, bupropion mediates its antidepressant effects primarily via other pathways such as noradrenergic neurotransmission rather than dopaminergic and cholinergic transmission. The present study cannot rule out the presence of compensatory mechanisms [13] due to overexpression of other non-α6 containing nAChRs. However, the role of α6 nAChRs in antidepressant effect of bupropion warrants further investigation.
The antidepressant effect of bupropion can also be modulated by other nAChR subunits which were not investigated in the present study. For example, it was reported that functional β4* containing nAChRs modulate acute and chronic antidepressant effects of bupropion [33].
Conclusions
Given the findings of the present study, we conclude that α4* and α6* nAChRs may play a modulatory role in the antidepressant-like effects of bupropion. Bupropion analogs which target these receptor subunits, could prove to be therapeutically useful for depression.
Highlights:
Role of nicotinic receptors was tested in the antidepressant effects of bupropion
α4, α5, α6, and β2 nicotinic receptor subunit WT&KO mice were studied using the FST
α4, α6, and β2 subunits modulate the antidepressant-like effects of bupropion
Acknowledgements.
The authors would like to thank Tie Shan-Han for his technical assistance. This work was supported by National Institutes of Health grants DA-019377 to MID.
Abbreviations:
- nAChR(s)
nicotinic acetylcholine receptor(s)
- s.c.
subcutaneous
- WT
wild-type
- KO
knock-out
- CNS
central nervous system
- (FST)
forced swim test
- DHβE
dihydro-β-erythroidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Andreasen JT, Olsen GM, Wiborg O, Redrobe JP, 2009. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J. Psychopharmacol 23, 797–804. doi: 10.1177/0269881108091587 [DOI] [PubMed] [Google Scholar]
- [2].Arias HR, 2009. Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? The international journal of biochemistry & cell biology, 41 (11), 2098–2108. [DOI] [PubMed] [Google Scholar]
- [3].Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, 1995. Bupropion: a review of its mechanism of antidepressant activity. J. Clin. Psychiatry 56, 395–401. [PubMed] [Google Scholar]
- [4].Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI, 2015. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem. Pharmacol 1–11. doi: 10.1016/j.bcp.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berton O, Nestler EJ, 2006. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci 7, 137–151. doi: 10.1038/nrn1846 [DOI] [PubMed] [Google Scholar]
- [6].Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM, 2010. Alpha-conotoxin Mii-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology 35, 665–673. doi: 10.1038/npp.2009.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carroll FI, Blough BE, Mascarella SW, Navarro HA, Lukas RJ, Damaj MI, 2014. Bupropion and bupropion analogs as treatments for CNS disorders, 1st ed, Advances in Pharmacology. Elsevier Inc. doi: 10.1016/B978-0-12-420118-7.00005-6 [DOI] [PubMed] [Google Scholar]
- [8].Champtiaux N, Han Z-Y, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux J-P, 2002. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J. Neurosci 22, 1208–1217. doi:22/4/1208 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Covey LS, Glassman AH, Stetner F, 1998. Cigarette smoking and major depression. J Addict Dis 17, 35–46. doi: 10.1300/J069v17n01_04 [DOI] [PubMed] [Google Scholar]
- [10].Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR, 2004. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol 66, 675–682. doi: 10.1124/mol.104.001313 [DOI] [PubMed] [Google Scholar]
- [11].Damaj MI, Grabus SD, Navarro HA, Vann RE, Warner JA, King LS, Wiley JL, Blough BE, Lukas RJ, Carroll FI, 2010. Effects of hydroxymetabolites of bupropion on nicotine dependence behavior in mice. J. Pharmacol. Exp. Ther 334, 1087–95. doi: 10.1124/jpet.110.166850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Damaj MI, Slemmer JE, Carroll FI, Martin BR, 1999. Pharmacological Characterization of Nicotine’s Interaction with Cocaine and Cocaine Analogs. Pharmacology 289, 1229–1236. [PubMed] [Google Scholar]
- [13].Drago J, McColl CD, Horne MK, Finkelstein DI, and Ross SA, 2003. Neuronal nicotinic receptors: insights gained from gene knockout and knockin mutant mice. Cellular and Molecular Life Sciences CMLS, 60(7), 1267–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, Lester HA, 2008. In Vivo Activation of Midbrain Dopamine Neurons via Sensitized, High-Affinity alpha 6 Nicotinic Acetylcholine Receptors. Neuron 60, 123–136. doi: 10.1016/j.neuron.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gerzanich V, Wang F, Kuryatov A, Lindstrom J, 1998. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J. Pharmacol. Exp. Ther 286, 311–20. [PubMed] [Google Scholar]
- [16].Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J, 1990. Smoking, Smoking Cessation, and Major Depression. JAMA 264, 1546–1549. doi: 10.1001/jama.1990.03450120058029 [DOI] [PubMed] [Google Scholar]
- [17].Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C, 2009. Rodent Habenulo-lnterpeduncular Pathway Expresses a Large Variety of Uncommon nAChR Subtypes, But Only the alpha 3 beta 4*and alpha 3 beta 3 beta 4*Subtypes Mediate Acetylcholine Release. J. Neurosci 29, 2272–2282. doi: 10.1523/jneurosci.5121-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoegberg BG, Lomazzo E, Lee NH, Perry DC, 2015. Regulation of α4β2α5 nicotinic acetylcholinergic receptors in rat cerebral cortex in early and late adolescence: Sex differences in response to chronic nicotine. Neuropharmacology 99, 347–355. doi: 10.1016/j.neuropharm.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hurt R, Sachs DPL, Glover E, Offord K, Johnston J, Dale L, Khayrallah M, Schroeder D, Glover P, Sullivan C, Croghan I, Sullivan P, 1997. Comparison of Sustained-Release Bupropion and Placebo for Smoking Cessation. N Engl J Med 337, 1195–202. doi: 10.1056/NEJM199710233371703 [DOI] [PubMed] [Google Scholar]
- [20].Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI, 2009. The Role of α6-Containing Nicotinic Acetylcholine Receptors in Nicotine Reward and Withdrawal. J. Pharmacol. Exp. Ther 331, 547–554. doi: 10.1124/jpet.109.155457.nAChRs [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ, 1972. A cholinergic-adrenergic hypothesis of mania and depression. Lancet (London, England) 2, 632–5. [DOI] [PubMed] [Google Scholar]
- [22].Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N, 1996. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict. Behav 21, 9–19. doi: 10.1016/0306-4603(95)00032-1 [DOI] [PubMed] [Google Scholar]
- [23].Lucki I, 1997. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behavioural pharmacology. 8,523–32. [DOI] [PubMed] [Google Scholar]
- [24].Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, et al. , 1999. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol. Rev 51, 397–401. [PubMed] [Google Scholar]
- [25].Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ, 2008. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem 104, 446–456. doi: 10.1111/j.1471-4159.2007.05011.x [DOI] [PubMed] [Google Scholar]
- [26].Mehta NB, 1983. The chemistry of bupropion. J. Clin. Psychiatry 44, 56–9. [PubMed] [Google Scholar]
- [27].Mineur YS, Somenzi O and Picciotto MR, 2007. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology, 52(5), 1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W, 2008. Crucial Role of 4 and 6 Nicotinic Acetylcholine Receptor Subunits from Ventral Tegmental Area in Systemic Nicotine Self-Administration. J. Neurosci 28, 12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Popik P, Kozela E, Krawczyk M, 2003. Nicotine and nicotinic receptor antagonists potentiate the antidepressant-like effects of imipramine and citalopram. Br. J. Pharmacol 139, 1196–1202. doi: 10.1038/sj.bjp.0705359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Porsolt RD, Le Pichon M and Jalfre ML, 1977. Depression: a new animal model sensitive to antidepressant treatments. Nature, 266(5604), 730. [DOI] [PubMed] [Google Scholar]
- [31].Quattrocki E, Baird A, Yurgelun-Todd D, 2000. Biological aspects of the link between smoking and depression. Harv. Rev. Psychiatry 8, 99–110. [PubMed] [Google Scholar]
- [32].Rabenstein RL, Caldarone BJ, Picciotto MR, 2006. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but notβ2- or α7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl). 189, 395–401. doi: 10.1007/s00213-006-0568-z [DOI] [PubMed] [Google Scholar]
- [33].Radhakrishnan R, Santamaria A, Escobar L, Arias HR, 2013. The β4 nicotinic receptor subunit modulates the chronic antidepressant effect mediated by bupropion. Neurosci. Lett 555, 68–72. doi: 10.1016/j.neulet.2013.08.009 [DOI] [PubMed] [Google Scholar]
- [34].Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L, 1996. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. doi: 10.1038/380347a0 [DOI] [PubMed] [Google Scholar]
- [35].Rana B, McMorn SO, Reeve HL, Wyatt CN, Vaughan PF, Peers C, 1993. Inhibition of neuronal nicotinic acetylcholine receptors by imipramine and desipramine. Eur J Pharmacol 250, 247–251. [DOI] [PubMed] [Google Scholar]
- [36].Roni MA and Rahman S, 2013. Antidepressant-like effects of lobeline in mice: behavioral, neurochemical, and neuroendocrine evidence. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 41, 44–51. [DOI] [PubMed] [Google Scholar]
- [37].Roni MA and Rahman S, 2015. Effects of lobeline and reboxetine, fluoxetine, or bupropion combination on depression-like behaviors in mice. Pharmacology Biochemistry and Behavior, 139, 1–6. [DOI] [PubMed] [Google Scholar]
- [38].Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF and Drago J, 2000. Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. Journal of Neuroscience, 20(17), 6431–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R and De Biasi M, 2003. The nicotinic acetylcholine receptor subunit α5 mediates short-term effects of nicotine in vivo. Molecular pharmacology, 63(5), 1059–1066. [DOI] [PubMed] [Google Scholar]
- [40].Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ, 2005. The subunit composition and pharmacology of α-Conotoxin Mii-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology 48, 696–705. doi: 10.1016/j.neuropharm.2004.12.011 [DOI] [PubMed] [Google Scholar]
- [41].SAMHSA. Behavioral health barometer: United States, volume 4: Indicators as measured through the 2015 National Survey on Drug Use and Health and National Survey of Substance Abuse Treatment Services. HHS publication no. SMA–17–BaroUS–16. Rockville: Substance Abuse and Mental Health Services Administration, 2017. [PubMed] [Google Scholar]
- [42].Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, Drenan RM, 2014. Differential Expression and Function of Nicotinic Acetylcholine Receptors in Subdivisions of Medial Habenula. J. Neurosci 34, 9789–9802. doi: 10.1523/JNEUROSCI.0476-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shytle RD, Silver a a, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR, 2002. Nicotinic acetylcholine receptors as targets for antidepressants. Mol. Psychiatry 7, 525–535. doi: 10.1038/sj.mp.4001035 [DOI] [PubMed] [Google Scholar]
- [44].Slemmer JE, Martin BR, Damaj MI, 2000. Bupropion is a nicotinic antagonist. J. Pharmacol. Exp. Ther 295, 321–7. [PubMed] [Google Scholar]
- [45].Tapia L, Kuryatov A, Lindstrom J, 2007. Ca2+ Permeability of the (α4)3(β2)2 Stoichiometry Greatly Exceeds That of (α4)2(β2)3 Human Acetylcholine Receptors. Mol. Pharmacol 71, 769–776. doi: 10.1124/mol.106.030445.units [DOI] [PubMed] [Google Scholar]
- [46].Vernallis AB, Conroy WG, Berg DK, 1993. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron 10, 451–464. doi: 10.1016/0896-6273(93)90333-M [DOI] [PubMed] [Google Scholar]
- [47].Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, Collins AC, Marks MJ, 2002. Involvement of the alpha3 subunit in central nicotinic binding populations. J. Neurosci 22, 2522–9. doi:20026184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].WHO, W.H.O., 2013. Mental Health Action Plan 2013-2020, WHO Press: Geneva, Switzerland. [Google Scholar]
- [49].Yu LF, Zhang HK, Caldarone BJ, Brek Eaton J, Lukas RJ, Kozikowski AP, 2014. Recent Developments in Novel Antidepressants Targeting α4β2-Nicotinic Acetylcholine Receptors, doi: 10.1021/jm401937a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zomkowski ADE, Rosa AO, Lin J, Santos AR, Calixto JB and Rodrigues ALS, 2004. Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test. Brain research, 1023(2), pp.253–263. [DOI] [PubMed] [Google Scholar]
- [51].Molendijk ML and de Kloet ER, 2015. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 62, 389–391. [DOI] [PubMed] [Google Scholar]
- [52].De Kloet ER and Molendijk ML, 2016. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Damaj MI, Welch SP, Martin BR, 1995. In vivo pharmacological effects of dihydro-beta-erythroidine, a nicotinic antagonist, in mice. Psychopharmacology (Berl). 117, 67–73. [DOI] [PubMed] [Google Scholar]