Abstract
Sensory gating, the ability to suppress sensory information of irrelevant stimuli, is affected in several neuropsychiatric diseases, notably schizophrenia and autism. It is currently unclear how these deficits interact with other hallmark symptoms of these disorders, such as social withdrawal and difficulty with interpersonal relationships. The highly affiliative prairie vole (Microtus ochrogaster) may be an ideal model organism to study the neurobiology underlying social behavior. In this study, we assessed unimodal acoustic sensory gating in male and female prairie voles using the prepulse inhibition (PPI) paradigm, whereby a lower amplitude sound (prepulse) decreases the startle response to a high amplitude sound (pulse) compared to the high amplitude sound alone. Prairie voles showed evidence of PPI at all prepulse levels compared to pulse alone, with both males and females showing similar levels of inhibition. However, unlike what has been reported in other rodent species, prairie voles did not show a within-session decrease in startle response to the pulse alone, nor did they show a decrease in startle response to the pulse over multiple days, highlighting their inability to habituate to startling stimuli (short- and long-term). When contrasted with a cohort of male wildtype C57Bl/6J mice that underwent a comparable PPI protocol, individual voles showed significantly higher trial-by-trial variability as well as longer latency to startle than mice. The benefits and caveats to using prairie voles in future sensory gating experiments are discussed.
Keywords: sensory gating, acoustic startle, habituation, comparative neuroscience, schizophrenia, autism
Introduction
The prairie vole (Microtus ochrogaster) has been used extensively in research on monogamy and pair-bonding due to its robust affiliative behaviors [1, 2] and the relationship of the neuropeptides oxytocin and vasopressin with such behaviors [3]. Despite this strong rationale for use as a model studying social behaviors, voles have been underutilized to model social deficits in neuropsychiatric illnesses, likely due to their high behavioral and genetic heterogeneity compared to other laboratory rodents [2]. However, as recent treatments for schizophrenia and autism spectrum disorder (ASD) have targeted oxytocin as a potential therapeutic target [4–7], the prairie vole may offer valuable insight into the neurochemistry of such mental illnesses [6, 8–10].
The ability of the nervous system to filter out irrelevant stimuli (e.g. sensory gating) is a fundamental feature of attention and is compromised in neuropsychiatric disorders [11–14]. Sensory gating can be seen throughout the animal kingdom, from simple invertebrates to humans [15]. The fundamentally conserved nature of sensory gating allows the systematic comparison of animal models to more complex human conditions. Prepulse inhibition (PPI), a measure of the ability to inhibit response to a startling stimulus if preceded by a weaker non-startling stimulus, is commonly used as a biomarker for disorders with dysfunctional sensory gating, such as schizophrenia, where patients show significantly less inhibition than controls [16].
In this paper, we characterize sensory gating behavior in prairie voles using the PPI procedure, a necessary first step towards modeling social and attentional deficits in ASD and schizophrenia. Habituation to the startle reflex, which is also compromised in schizophrenic patients, was assessed in the short-term (within-session) and long-term (over multiple sessions). We also compared male and female voles to determine if there were sex differences, as has been found in some strains of rodents and in humans [17, 18]. Finally, we compared voles with an inbred mouse line, C57BL/6J, to determine differences in individual variability in startle responsiveness.
Methods
Subjects
Prairie voles:
The prairie vole colony was established in 2005, derived from field-caught prairie voles in Illinois. Genetic diversity was maintained through generous outside donations (2007, 2008, 2014, 2015). Voles weighed between 25–40 grams and were postnatal day 39–40 (p40) at time of testing. Animals were group housed with same sex littermates (2–4/cage) in temperature and humidity controlled rooms with a 14:10 light/dark cycle (lights on at 8:00 AM). Female voles were housed in a different colony room than males. Voles had ad libitum access to food and water.
Mice:
For studies involving mice, C57Bl/6J wildtype male mice (Jackson Laboratory, Bar Harbor, ME, USA), ~79 days old and weighing 23–30 grams were used. Mice were single housed in a 12:12 light/dark cycle (lights on at 7:00 AM) with ad libitum access to food and water.
Experiment 1: Single session PPI -
Short-term habituation and within subject variability were measured in a single session of prepulse inhibition. Eighty-eight prairie voles (37 female, 51 male) were used in this study. This experiment was originally part of an experiment to disrupt early life sleep. All voles had been exposed to a novel object test (p34), as this experiment was just one of a series of cognitive, social, and sensory tests that were performed (data not shown). No difference in acoustic startle or PPI existed between early life sleep groups (P>0.15 for all comparisons), thus experimental groups were combined.
Experiment 2: Multiple session PPI -
A follow-up experiment was performed on a separate cohort of prairie voles (N=7, all female) to determine if long-term habituation over multiple days occurs to the startle pulse. PPI exposure was identical to that experienced by voles with a single PPI exposure, but continued for five consecutive days. All voles were naïve to previous testing.
Experiment 3: Single session PPI in Mice -
Single session PPI was run in mice to compare an established PPI model to results found in voles. Twenty-three mice were tested once for PPI and acoustic startle response.
All procedures and housing conditions were approved by the Institutional Animal Care and Use Committee and the Veterinary Medical Unit of the Veterans Administration in Portland, Oregon, an AAALAC accredited facility, and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
PPI Apparatus
PPI experiments took place in a room separate from the colony rooms. Tests were controlled by the Startle Response System (San Diego Instruments, 2005). Subjects were placed in a 2.54cm diameter hollow plastic tube, with plastic inserts that made the effective length of the tube approximately 8.9cm. This tube was in a sound-attenuating cubicle illuminated by an LED light (Sylvania “Dot-It”) at approximately 25–30 lux. Before the first test session of each day, sound was calibrated at three decibel levels (76, 90, 115dB) and checked with a sound level meter (Radio Shack). Data were collected via an accelerometer attached to each box, with readings in millivolts. All experiments made use of the same PPI equipment and calibration settings.
PPI Protocol
Voles:
Males were always tested first to minimize olfactory cues that might influence results (i.e. focus of anticipated mating) and each testing session included only one sex. A 65dB background noise was present for the duration of the test. After voles were placed in their chambers, a one-minute habituation period began. Following this, the PPI session began with a baseline measure of startle using a 120dB white noise stimulus for 40ms. Trials were interspersed with prepulse noise, 20ms in duration, of various set amplitudes (76, 80, 84, 90, or 96dB) with a 40ms pause before a 120dB stimulus, null trials where no sound was present except the 65dB background, as well as 90, 96, and 120dB noises without a prepulse. Recording of startle response began at presentation of the pulse and recorded for 60ms with a resolution of 1ms. Sessions were a total of 135 trials, with 10–20s (variable) between each trial. Chambers were wiped with water and paper towels between sessions. After all sessions had completed, the PPI chambers were cleaned with Nolvasan.
Mice:
Mice were tested for PPI in the same equipment and enclosure tubes with identical calibration as the voles. Differences in trials that involved mice included: 5 minute habituation period and 11 blocks consisting of each of the following trial types: null, 120dB pulse alone, 72dB PPI, 80dB PPI, and 84dB PPI (trial order within block pseudorandomized). All remaining session parameters were identical to sessions with voles.
Statistical Analysis
PPI -
As previously described by Valsamis & Schmid [19], PPI was assessed by normalizing each animal’s response to their respective average 120dB pulse-only startle response. A “% Inhibition” score was generated by subtracting each value by 100, therefore the average percent inhibition of 120dB pulse-only was set to 0, and a higher number indicates less startle response. Repeated measures ANOVA was run to test for sex effects with prepulse amplitude as the within subjects factor and sex as the between subjects factor. A one sample t-test was run between the % inhibition of each prepulse to a test value of 0 (normalized startle response to the pulse-only) to determine if startle inhibition was significant. To maintain a familywise error rate of 0.05, P values were only considered significant at an alpha level less than 0.01.
Short-term Habituation -
Short-term habituation was analyzed in male and female voles following the methods of Valsamis and Schmid (2011) by comparing the first two pulse-only startle responses to the last ten pulse-only startle responses. All pulse-only trials for an animal were normalized to that animal by taking it as a percentage of the average of the first two pulse-only trials. Therefore the first two pulse-only behavioral responses average to 100, and all other pulse-only trials are proportional to that. A repeated measures ANOVA was run comparing the first two pulse-only responses with the last 10, with sex as a between subjects factor.
Long-term Habituation -
Long-term habituation was analyzed by normalizing the overall average of the pulse-only startle responses of each vole per day to the average pulse-only startle response of the first day (first day = 100). We reasoned that normalizing the data for the overall average rather than looking at the first two pulse-only responses was sufficient to account for any short-term habituation that occurred during the session. A repeated-measures ANOVA was run comparing the normalized pulse-only responses.
Variability -
Individual variability of voles within trials of the same type was analyzed and compared to a cohort of mice that underwent a similar PPI procedure in the same equipment but on separate days. Scores from voles and mice were individually normalized to their average pulse-only responses for the first 10 pulse-only presentations. Mean-centered Levene’s test was run to compare equality of variance for the startle response between the two species. Only male subjects were used for comparisons.
Startle latency -
Average time to achieve a max startle response (Tmax) was averaged for all startle-only trials and was analyzed between mice and voles using Mann-Whitney U Test due to unequal variances between groups.
Results
Male and female prairie voles show robust acoustic prepulse inhibition
There were no differences in prepulse inhibition between males and females (repeated measures ANOVA, between group effect of sex F(1,86)=0.078, P=0.780) (Fig 1a). Given the lack of a sex difference, males (n=51) and females (n=37) were combined into one group for analysis. With males and females collapsed, each prepulse amplitude resulted in significant inhibition of the startle response compared to pulse alone (Table 1). There was a significant reduction in startle response (normalized startle response compared to 0) when the pulse was preceded by a 76dB, 80dB, 84dB, 90dB, and 94dB prepulse (one sample t-test, test value=0; all Ps<0.0001).
Figure 1: PPI in the prairie vole.
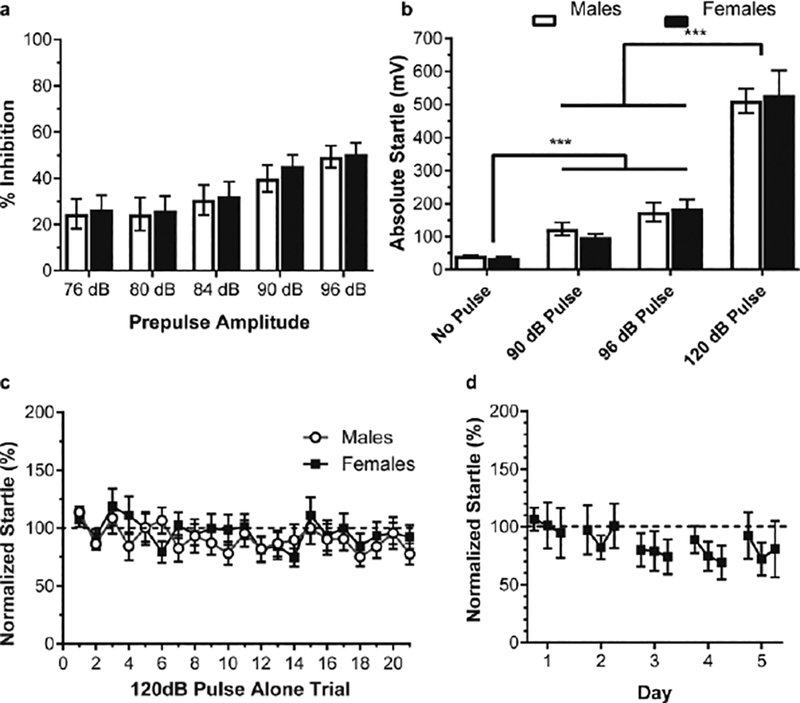
a, There was no effect of sex on prepulse inhibition. Startle was inhibited in both males (n=51) and females (n=37) when the pulse was preceded by a prepulse. Displayed as normalized percent inhibition in response versus 120 dB startle pulse alone, n=87. b) Absolute startle values to pulse alone of varying amplitudes (no prepulses) with males and females collapsed for analysis. Prairie voles startled significantly more to a 90dB pulse and a 94dB pulse compared to no sound presentation, but significantly less than the 120dB pulse alone and c) Lack of significant short-term habituation of behavioral response to a startling pulse. There was no effect of sex on startle response to pulses alone. The first two pulse-only responses were normalized and compared to the last ten, as described by Valsamis & Schmid, however, data points represented for each trial for visualization. Voles did not show habituation to the pulse within the PPI session. Dashed line is normalized value (100). d) Lack of significant long-term habituation of behavioral response to startling pulse. Data points are startle to pulse alone trials (each point is the average of 7 pulse alone trials for each day) normalized to day 1 and compared for five consecutive days.
Table 1 -. Prepulse inhibition in prairie voles.
Males and females were collapsed for analysis. Each prepulse amplitude resulted in significant inhibition of the startle response (normalized to pulse alone for each animal) after Bonferroni correction. Means are percent inhibition during PPI trials compared to pulse alone trials. One sample t-test, test value=0 (0=no change) (df=87)
| Prepulse Level | Mean | SD | T statistic | P value |
|---|---|---|---|---|
| 76 dB | 25.524 | 41.969 | 5.705 | 1.5754E–7 |
| 80 dB | 25.156 | 45.978 | 5.133 | 0.000002 |
| 84 dB | 31.354 | 42.995 | 6.841 | 1.0447E–9 |
| 90 dB | 42.232 | 36.788 | 10.769 | 1.1072E–17 |
| 96 dB | 49.912 | 32.045 | 14.611 | 3.8615E–25 |
To verify this effect was not due to startle in response to the prepulse itself, trials were interspersed with pulse-only trials at either 90 or 96dB, which were then compared to 120dB pulse-only trials (Figure 1b). There was a significant effect of dB level on acoustic startle response (repeated measures ANOVA, within subjects F(2,172)=116.054, P<0.001). Additionally, the absolute value of the average startle response to pulse alone trials was not influenced by sex (between subjects effect of sex: F(1,86)=0.002, P=0.965). Follow-up tests revealed that voles startled significantly less to both the 90dB and 96dB pulse–only compared to the absolute value of their average 120dB startle (paired t-tests: 90dB vs. 120dB t(87)=11.420, P<0.0001; 96dB vs. 120dB t(87)=11.263, P<0.0001). However, when compared to baseline trials without any sound other than background, voles startled significantly more to both the 90dB (paired t-test vs. null: t(87)=5.942, P<0.0001) and 96dB pulses (paired t-test vs. null t(87)=6.824, P<0.0001).
Short and long-term startle habituation are not observed in prairie voles
Short-term habituation was assessed by comparing the percent inhibition of the first two pulse-only behavioral responses (normalized, mean set to 100.0, SD: 0.0) and the last ten pulse-only responses (Females mean: 90.99, SD: 49.72; Males mean: 89.04, SD: 62.66) using a repeated measures ANOVA with sex as the between subjects factor. There was no short-term habituation (within subjects, F(1,86)=2.579, P=0.112) and no difference between sexes (between subjects, F(1,86)=0.025, P=0.876) (Figure 1c).
Long-term habituation was also assessed in a separate experiment using naïve voles, whereby voles (N = 7) were exposed to the same PPI session as the previous experiment over five consecutive days. Average response to pulse-only was normalized to the first day (Mean: 100, SD: 0.0). Voles did not show a significant difference in response (Mean: 89.3, SD: 22.9; Figure 1d), and a repeated measures ANOVA showed no significant change in startle over days (F(4,24)=0.53, P=0.72).
Male prairie voles display more variable startle responses than male mice
Given that previous studies have shown widely different prepulse inhibition of startle response depending on the mouse strain used [20], we investigated whether the outbred nature of prairie voles would have increased variability in response compared to a widely used inbred mouse strain, C57BL/6J. Male voles were compared with male mice on startle to the first 10 pulse alone trials with data normalized (to the average startle pulse, as above). Because the distribution of startle responses failed to meet a normality assumption (Shapiro-Wilk test; W = 0.87; P < 0.001), a mean-centered Levene’s test was run to compare the equality of variance for startle response between the two species. This test, which modeled startle variance against the interaction between species and individual animal to account for the repeated-measures design, indicated that voles have a greater variance in startle response than mice (Levene’s test; F = 2.11; P < 0.001)(Fig 2a). Startle amplitude and PPI values were not compared between species due to differences in the number and types of trials presented.
Figure 2: Acoustic startle response in male prairie voles and male C57Bl/6J mice.
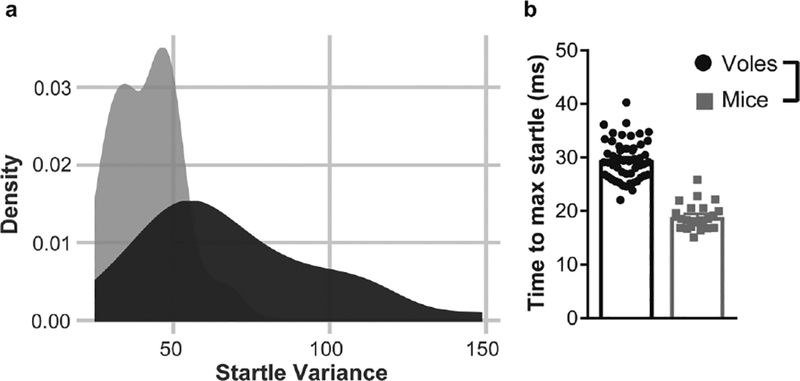
a) Increased variance of startle response in prairie voles compared to mice during first 10 pulse-only trials (120 dB). Male prairie voles (n=51) showed significantly greater variability in their startle response than male C57Bl/6J mice (n=23) (Levene’s F=2.11, P<0.001). Startle response was collected as Vmax for each 120dB pulse-only trial and the variance for each individual animal was calculated and plotted as density curves representing distribution of variance. b) Latency to startle (Tmax) in mice and voles averaged across all startle-only trials. Voles took significantly longer to reach their maximum startle amplitude than mice (P<0.001).
Startle response time
Latency to maximum startle was compared between mice and voles. Tmax values were averaged across all startle only trials for each animal. There were no sex differences in startle latency in the prairie voles (P>0.6) and there was no effect of weight on startle latency when entered as a covariate for analyses (One-way ANCOVA P>0.4). Male prairie voles took significantly longer than male mice to reach their maximum startle response to pulse-only trials (Mann-Whitney U Test, P<0.001, Figure 2b).
Discussion
Expanding upon a previous report in the literature using PPI in prairie voles [21], we found a robust inhibition of startle response to an acoustic pulse by an acoustic prepulse. Perkeybile et al. (2013) reported cross-modal potentiation (acoustic startle pulse and tactile prepulse) of startle in voles, but inhibition with a pulse and prepulse of the same modality (acoustic). We found intact PPI at all levels of prepulse tested, with PPI increasing with magnitude of prepulse. A concern for future experiments is the naturally occurring variability among individual prairie voles, which results in inconsistent responses from trial to trial. To counter this, we utilized a longer session duration and increased the total number of trials compared to what is typically utilized in inbred mouse studies, with voles exposed to each decibel prepulse a total of 18 times, and acoustic pulses alone 21 times, for an overall total of 135 trials. This gave us considerable power to detect prepulse inhibition despite the high variability in response. The enhancement of PPI beyond 90 dB is in agreement with the results reported by Perkeybile and provides further evidence that increased prepulse magnitude, compared to that used in typical mouse studies [20], may be necessary to see differences between experimental groups that utilize prairie voles. However, it should be noted that sounds above 90 dB resulted in a significant startle response in the prairie voles and the use of such decibel levels as a prepulse may confound interpretation of the startle response produced by a follow up pulse.
Observed differences in the literature as well as the current study could be due to varied developmental ages. In both rats [22] and humans [23] age has been shown to be an important factor in development of PPI, with PPI reaching maximum inhibition in middle age. This work sought to determine the methodological possibility of using prairie voles to explore PPI due to their aforementioned clinical relevance. Thus, P40 voles were used due to their similarity in size to adult mice, the rodent model most widely used in previous PPI work. Future work can explore developmental differences that may exist, and contribute to differing results.
Given that previous PPI studies have shown lower PPI in females compared to males in both human [24–26] and rat [18] subjects, we hypothesized that we would see a sex difference in voles. However, male and female voles showed no differences in inhibition to an acoustic startle following a prepulse after responses were normalized. It has been shown that menstrual/estrous phase can have a strong impact on whether sex differences in PPI and startle response are observed, with estrogen level significantly impacting the magnitude of startle response and the effect of prepulse on inhibition of facilitation [27], though in a dose-dependent manner that may be influenced by estrogen’s effect on dopamine levels in the brain [28]. As female voles are induced ovulators [29, 30], and the voles in this study were reproductively naïve, estrogen levels were likely low and stable. Future studies should investigate whether a sex difference develops following the spike in estradiol that occurs after exposure to a male or reproduction.
Unlike mice and rats, voles did not show short or long-term habituation to pulse alone using standard protocols applied in other laboratory rodents [19]. Although between species comparisons should be interpreted with caution due to a number of potentially confounding variables that go beyond species specific characteristics (e.g. diet, light cycle, body size), voles also took significantly longer to reach their maximum startle amplitude compared to laboratory mice. It is unclear why this is the case but does offer some distinct advantages. With intact PPI but a lack of long-term habituation and increased latency to startle, voles could be a useful model for testing the effectiveness of drugs that influence sensory gating, since they would tolerate repeated testing without habituation effects. Additionally, longer startle latencies in prairie voles resemble longer startle latencies found in humans with schizophrenia [31]. The well mapped oxytocin and vasopressin system in this species combined with the prepulse inhibition and startle behavioral data provided here could allow for rapid screening of drugs to treat neuropsychiatric conditions associated with dysfunction within these neuropeptide systems.
Acknowledgements
The authors would like to thank Dr. Ky Dehlinger and the animal care staff at the Portland VA Veterinary Medical Unit. The authors would also like to thank Dr. Robert Hitzemann for generously donating equipment for prepulse inhibition testing. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Funding
This work was supported by VA Biomedical Laboratory Research & Development (BLR&D) Career Development Award (CDA) # IK2 BX002712, Portland VA Research Foundation, Brain & Behavior Foundation NARSAD Award, Collins Medical Trust, and NIH EXITO Institutional Core, #UL1GM118964 to MML; NIH T32 5T32AA7468–29 and NIH T32 5T32HL083808–10 to CEJ; and NIH T32 5T32AA007468–32 to TMN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Carter CS, Devries AC, and Getz LL, Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience & Biobehavioral Reviews, 1995. 19(2): p. 303–314. [DOI] [PubMed] [Google Scholar]
- 2.McGraw LA and Young LJ, The prairie vole: an emerging model organism for understanding the social brain. Trends in neurosciences, 2010. 33(2): p. 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho MM, et al. , The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behavioral neuroscience, 1999. 113(5): p. 1071. [DOI] [PubMed] [Google Scholar]
- 4.Ebstein RP, et al. , Arginine vasopressin and oxytocin modulate human social behavior. Annals of the New York Academy of Sciences, 2009. 1167(1): p. 87–102. [DOI] [PubMed] [Google Scholar]
- 5.Levin R, et al. , Association between arginine vasopressin 1a receptor (AVPR1a) promoter region polymorphisms and prepulse inhibition. Psychoneuroendocrinology, 2009. 34(6): p. 901–908. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald K and Feifel D, Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta neuropsychiatrica, 2012. 24(3): p. 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer-Lindenberg A, et al. , Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience, 2011. 12(9): p. 524. [DOI] [PubMed] [Google Scholar]
- 8.Aydın O, et al. , Associations of oxytocin and vasopressin plasma levels with neurocognitive, social cognitive and meta cognitive function in schizophrenia. Psychiatry research, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Johnson ZV and Young LJ, Oxytocin and vasopressin neural networks: implications for social behavioral diversity and translational neuroscience. Neuroscience & Biobehavioral Reviews, 2017. 76: p. 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin LH, et al. , Peripheral oxytocin and vasopressin modulates regional brain activity differently in men and women with schizophrenia. Schizophrenia Research, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin AL and Brown RE, The lonely mouse: verification of a separation-induced model of depression in female mice. Behavioural brain research, 2010. 207(1): p. 196–207. [DOI] [PubMed] [Google Scholar]
- 12.Orefice LL, et al. , Peripheral mechanosensory neuron dysfunction underlies tactile and behavioral deficits in mouse models of ASDs. Cell, 2016. 166(2): p. 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orekhova EV, et al. , Sensory gating in young children with autism: relation to age, IQ, and EEG gamma oscillations. Neuroscience letters, 2008. 434(2): p. 218–223. [DOI] [PubMed] [Google Scholar]
- 14.Powell SB and Geyer MA, Overview of animal models of schizophrenia. Current protocols in neuroscience, 2007. 39(1): p. 9.24. 1–9.24. 20. [DOI] [PubMed] [Google Scholar]
- 15.Geyer MA and Swerdlow NR, Measurement of startle response, prepulse inhibition, and habituation. Current protocols in neuroscience, 1998. 3(1): p. 8.7. 1–8.7. 15. [DOI] [PubMed] [Google Scholar]
- 16.Clementz BA, Geyer MA, and Braff DL, P50 suppression among schizophrenia and normal comparison subjects: a methodological analysis. Biological psychiatry, 1997. 41(10): p. 1035–1044. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow NR, et al. , Men are more inhibited than women by weak prepulses. Biological psychiatry, 1993. 34(4): p. 253–260. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann J, Pryce CR, and Feldon J, Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behavioural brain research, 1999. 104(1–2): p. 113–117. [DOI] [PubMed] [Google Scholar]
- 19.Valsamis B and Schmid S, Habituation and prepulse inhibition of acoustic startle in rodents. Journal of visualized experiments: JoVE, 2011(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paylor R and Crawley JN, Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology, 1997. 132(2): p. 169–180. [DOI] [PubMed] [Google Scholar]
- 21.Perkeybile AM, Griffin L, and Bales KL, Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Frontiers in behavioral neuroscience, 2013. 7: p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acri JB, et al. , Strain and age differences in acoustic startle responses and effects of nicotine in rats. Pharmacology Biochemistry and Behavior, 1995. 50(2): p. 191–198. [DOI] [PubMed] [Google Scholar]
- 23.Ellwanger J, Geyer MA, and Braff DL, The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biological psychology, 2003. 62(3): p. 175–195. [DOI] [PubMed] [Google Scholar]
- 24.Aasen I, Kolli L, and Kumari V, Sex effects in prepulse inhibition and facilitation of the acoustic startle response: implications for pharmacological and treatment studies. Journal of Psychopharmacology, 2005. 19(1): p. 39–45. [DOI] [PubMed] [Google Scholar]
- 25.Jovanovic T, et al. , Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology, 2004. 41(3): p. 401–406. [DOI] [PubMed] [Google Scholar]
- 26.Kumari V, et al. , Sex differences in prepulse inhibition of the acoustic startle response. Personality and individual differences, 2003. 35(4): p. 733–742. [Google Scholar]
- 27.Koch M, Sensorimotor gating changes across the estrous cycle in female rats. Physiology & behavior, 1998. 64(5): p. 625–628. [DOI] [PubMed] [Google Scholar]
- 28.Van den Buuse M and Eikelis N, Estrogen increases prepulse inhibition of acoustic startle in rats. European journal of pharmacology, 2001. 425(1): p. 33–41. [DOI] [PubMed] [Google Scholar]
- 29.Carter C, et al. , Hormonal correlates of sexual behavior and ovulation in male-induced and postpartum estrus in female prairie voles. Physiology & behavior, 1989. 46(6): p. 941–948. [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Parsons M and Carter CS, Males increase serum estrogen and estrogen receptor binding in brain of female voles. Physiology & behavior, 1987. 39(3): p. 309–314. [DOI] [PubMed] [Google Scholar]
- 31.Hasenkamp W, et al. , Heritability of acoustic startle magnitude, prepulse inhibition, and startle latency in schizophrenia and control families. Psychiatry research, 2010. 178(2): p. 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]


