Abstract
Hepatocellular carcinoma (HCC) surveillance is associated with early tumor detection and improved survival in patients with cirrhosis; however, effectiveness is limited by underuse. We compared the effectiveness of mailed outreach and patient navigation strategies to increase HCC surveillance in a racially diverse cohort of patients with cirrhosis. We conducted a pragmatic randomized clinical trial comparing mailed outreach for screening ultrasound (n=600), mailed outreach plus patient navigation (n=600), or usual care with visit-based screening (n=600) among 1800 patients with cirrhosis at a large safety-net health system from December 2014 to March 2017. Patients who did not respond to outreach invitations within 2 weeks received reminder telephone calls. Patient navigation included an assessment of barriers to surveillance and encouragement of surveillance participation. The primary outcome was HCC surveillance (abdominal imaging every 6 months) over an 18-month period. All 1800 patients were included in intention-to-screen analyses. HCC surveillance was performed in 23.3% of outreach/navigation patients, 17.8% of outreach-alone patients, and 7.3% of usual care patients. HCC surveillance was 16.0% (95%CI 12.0–20.0%) and 10.5% (95%CI 6.8–14.2%) higher in outreach groups than usual care (p<0.001 for both) and 5.5% (95%CI 0.9–10.1%) higher for outreach/navigation than outreach-alone (p=0.02). Both interventions increased HCC surveillance across pre-defined patient subgroups. The proportion of HCC patients detected at an early stage did not differ between groups; however, a higher proportion of patients with screen-detected HCC across groups had early stage tumors than those with HCC detected incidentally or symptomatically (83.3% vs. 30.8%, p=0.003).
Conclusion
Mailed outreach invitations and navigation significantly increased HCC surveillance versus usual care in patients with cirrhosis.
Registration
ClinicalTrials.gov identifier: NCT02312817
Keywords: Liver cancer, early detection, navigation, screening
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 3rd leading cause of cancer-related death worldwide.1 HCC incidence in the U.S. and Europe has doubled over the past two decades, and HCC is the leading cause of death in patients with cirrhosis.2
Tumor stage is the strongest prognostic indicator in HCC patients, with curative treatments only available for patients with early stage HCC. Ultrasound-based surveillance is associated with improvements in early detection and overall survival in at-risk patients, including those with cirrhosis, and is recommended by professional societies including the National Comprehensive Cancer Network, European Association for the Study of the Liver, and American Association for Study of Liver Diseases (AASLD).3 A randomized clinical trial with >18,000 hepatitis B-infected persons demonstrated HCC surveillance significantly lowered mortality by 37%.4 Similarly, several cohort studies have shown cirrhosis patients receiving HCC surveillance have a higher odds of early detection and improved survival, after adjusting for lead-time bias, than those not receiving surveillance.5,6,7
As with breast and colorectal cancer screening, HCC surveillance is typically only offered opportunistically during face-to-face clinic encounters.8 Only a minority of cirrhosis patients undergo any HCC screening, and less than 5% undergo repeat semi-annual surveillance.9,10 Population outreach programs that systematically invite patients for screening and patient navigation interventions have effectively increased screening participation for other cancers including breast and colon cancer.11–14 For HCC, a mailed outreach strategy increased one-time screening participation in cirrhosis patients despite unique challenges surrounding patient identification, higher burden of medical illness, and patient access barriers to preventive care.15 However, there are few data evaluating patient navigation strategies in cirrhosis patients, and the effectiveness of either intervention strategy to promote HCC surveillance over longer periods of time remains unknown.
This study reports the primary outcome of completing HCC surveillance every 6 months over an 18-month period from a pragmatic, randomized clinical trial comparing a mailed outreach strategy, mailed outreach plus patient navigation, and usual care in patients with cirrhosis.
METHODS
Study Population
The trial was conducted at Parkland Health and Hospital System (Parkland) from December 2014 to March 2017. Parkland is a publically funded integrated safety-net health system that includes a 900-bed hospital, 12 community-based primary care clinics, specialty hepatology and oncology clinics, and radiology suites. Parkland offers a sliding fee scale program, which provides access to primary and subspecialty medical care, including HCC surveillance, at low cost for uninsured Dallas County residents.
The study was approved by UT Southwestern’s IRB. The UT Southwestern IRB determined a waiver of consent was ethical because: 1) the study posed minimal risk as HCC surveillance is standard of care and available for at-risk patients (including those with cirrhosis) through usual care, 2) waiver of consent would not adversely affect rights or welfare of participants, and 3) requiring consent would introduce volunteer bias threatening generalizability and validity as a population health strategy.
The trial protocol (ClinicalTrials.gov #NCT01710215) is available in Appendix. As previously described15, we used Parkland’s electronic medical record (EMR) to identify adult patients with documented or suspected cirrhosis and at least one outpatient clinic visit in the year preceding randomization. We included patients with suspected cirrhosis given many HCC patients fail to undergo screening due to unrecognized cirrhosis.16 “Documented cirrhosis” was defined using ICD-9 codes for cirrhosis or cirrhosis-related complications, which have high accuracy for identifying cirrhosis.17 “Suspected cirrhosis” was initially defined as AST to platelet ratio index (APRI) ≥1.0 in the presence of liver disease; however, the cut-off was increased to 1.5 in January 2015 to increase its positive predictive value for cirrhosis.18 Patients with HCC or significant comorbid conditions, including Child C cirrhosis, were excluded given limited benefit of HCC surveillance in those subgroups.19 We also excluded patients with no address or phone number on file or language other than English or Spanish.
Randomization and Blinding
Eligible persons were randomly assigned to receive usual care (Group 1), mailed outreach invitations for abdominal ultrasound (Group 2), or mailed outreach invitations plus patient navigation (Group 3), allocated in a 1:1:1 ratio using a computer-generated randomization sequence. Randomization was stratified by documented vs. suspected cirrhosis. Research staff conducted all mailings and reminder telephone calls; thus, participants and clinicians were blinded to the presence of other intervention groups. Although clinicians may have been aware of the trial, they did not have knowledge of group assignments.
HCC Screening Intervention Components
Usual Care (all groups) included visit-based HCC surveillance as ordered by clinicians during any outpatient visit. Parkland clinics do not have HCC surveillance reminders or provider-level audit and feedback for performance. The Radiology Department makes automated reminder calls to persons scheduled for ultrasounds 3 days preceding the appointment. Radiology uses an EMR alert to inform clinicians of findings suspicious for HCC; however, use of this system is at the discretion of the interpreting radiologist.20 Patients in the intervention groups were eligible for visit-based surveillance as recommended through usual care.
Mailed Outreach (Groups 2 and 3) was initiated for each 6-month period with one-page low-literacy letters in English and Spanish providing basic information about HCC risk and recommending surveillance. Trained bilingual research staff conducted telephone calls using standardized scripts for persons who did not respond to mailed invitations within two weeks. Telephone calls were stopped for persons with non-working phone numbers and those not reached after three attempts. Patients who did not complete screening were mailed a repeat letter recommending HCC surveillance six months later. Patients with normal imaging received a letter informing them of the results and inviting them for repeat surveillance during the next 6-month period. Patients with an abnormal result were contacted by research staff and referred for diagnostic contrast-enhanced CT or MRI.
Patient Navigation (Group 3) included standardized phone scripts for research staff to explore barriers and encourage participation for those who declined surveillance during telephone calls. Research staff also called patients 5–7 days prior to ultrasound appointments to remind them of the appointment, address any concerns, and reschedule the appointment if needed.
Primary and Secondary Outcomes
The primary outcome was receipt of HCC surveillance, defined as completion of abdominal imaging (ultrasound, CT, or MRI) during each 6-month interval over the 18-month study. Patient follow-up was censored at death or HCC diagnosis. For example, patients who completed surveillance every 6 months prior to death or HCC diagnosis were defined as meeting the primary outcome. For persons randomized to intervention groups, we included tests completed through outreach or usual care. To ascertain surveillance participation for all persons, research staff who did not deliver interventions and were blinded to intervention status queried the EMR for completed ultrasounds, contrast-enhanced CT, or contrast-enhanced MRI. Although professional societies do not recommend CT or MRI for screening, their completion satisfies the need for liver imaging and precludes the need for screening ultrasound. Alpha fetoprotein was not required because it was removed from AASLD guidelines during the study period. Subgroup analyses were planned a priori to examine effect modification by race/ethnicity, documented vs. suspected cirrhosis, Child Pugh class, and receipt of hepatology care preceding randomization.
A secondary outcome, defined a priori, was the proportion of patients with early stage HCC. HCC cases were adjudicated to confirm they met AASLD diagnostic criteria, i.e. presence of a typical vascular pattern on imaging (arterial enhancement and delayed washout) or histology.3 The Barcelona Clinic Liver Cancer (BCLC) system was used for tumor staging, with early stage defined as BCLC stage 0 or A.
Three post-hoc secondary analyses were performed using more liberal definitions of surveillance completion. First, we compared effectiveness of the interventions to promote HCC surveillance every 7 months over a 21-month period. Second, we compared receipt of one-time abdominal imaging during the 18-month study period between groups. Finally, we compared the proportion time covered (PTC) as the number of days patients were up-to-date with HCC surveillance, with each ultrasound providing 6 months (180 days) of time covered, divided by the number of days of follow-up (from randomization to date of HCC diagnosis, death, or study end).
Statistical Analysis
We used intent-to-screen principles to guide analyses. The Pearson Chi-Square test was used to compare primary and secondary outcomes between groups. Our primary comparisons of interest were 1) outreach-alone vs. usual care and 2) outreach plus navigation vs. outreach-alone. Participants without outpatient visits after randomization were considered as lost to follow-up but retained in analyses. Univariate logistic regression analyses were conducted to evaluate for effect modification across patient subgroups. We performed a per-protocol analysis excluding patients who died or were diagnosed with HCC after randomization but prior to cohort entry. Missing data were rare and reported as unknown.
Sample size calculations were determined a priori to compare HCC surveillance across groups. With 600 persons randomly assigned to each group, we had 90% power to detect a difference of at least 7.1% in surveillance completion between groups, assuming surveillance completion of 10% in usual care at a pre-specified two-sided significance level of 0.025 (=0.05/2 accounting for Bonferroni correction). Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Study Population
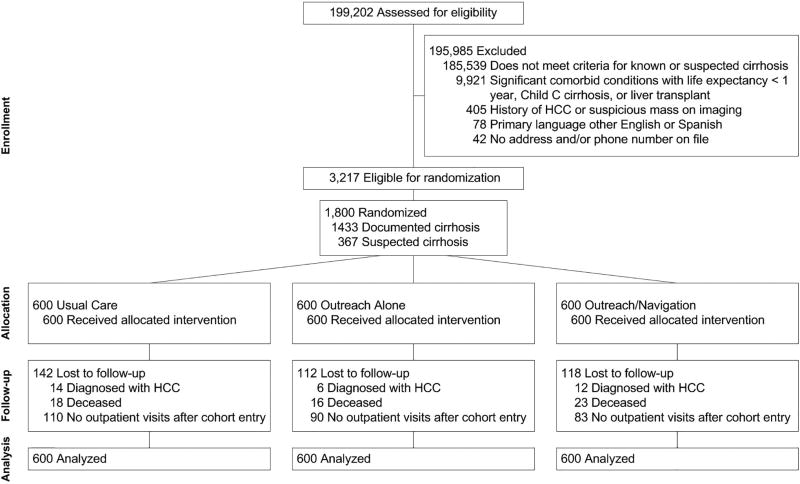
Of 1800 persons (mean age 55.3 years; 59.4% men), 600 were randomly assigned to mailed outreach and patient navigation, 600 to mailed outreach, and 600 to usual care (Figure 1, Table 1). Participants were racially/ethnically diverse with 37.8% Hispanic, 32.1% Black, and 28.3% White. Most (79.6%) patients had documented cirrhosis, and 20.4% had suspected cirrhosis. Most patients had compensated cirrhosis, with only 28.2% having ascites and 12.7% having hepatic encephalopathy. Although >90% of patients had ≥1 primary care visit in the year preceding randomization (median 4 visits), only 25.7% had ≥1 hepatology clinic visit. One-third (31.1%) of patients received abdominal imaging within 6 months preceding randomization.
Figure 1.
Study Consort Diagram
Table 1.
Characteristics of cirrhosis patients enrolled in a pragmatic randomized clinical trial promoting HCC surveillance, overall and by study group
| Usual Care n=600 |
Outreach Alone n=600 |
Outreach/ Navigation n=600 |
Total N=1,800 |
|
|---|---|---|---|---|
|
| ||||
| Age (years) | ||||
| 21–50 | 183 (30.5) | 174 (29.0) | 158 (26.3) | 515 (28.6) |
| 51–60 | 259 (43.2) | 272 (45.3) | 269 (44.8) | 800 (44.4) |
| 61–90 | 158 (26.3) | 154 (25.7) | 173 (28.8) | 485 (26.9) |
|
| ||||
| Male sex (%) | 350 (58.3) | 361 (60.2) | 358 (59.7) | 1,069 (59.4) |
|
| ||||
| Race/Ethnicity (%) | ||||
| Non-Hispanic White | 182 (30.3) | 165 (27.5) | 163 (27.2) | 510 (28.3) |
| Hispanic White | 217 (36.2) | 230 (38.3) | 234 (39.0) | 681 (37.8) |
| Non-Hispanic Black | 186 (31.0) | 197 (32.8) | 195 (32.5) | 578 (32.1) |
| Other/Unknown | 15 (2.5) | 8 (1.3) | 8 (1.3) | 31 (1.7) |
|
| ||||
| Etiology of Liver Disease (%) | ||||
| Hepatitis C | 320 (53.3) | 285 (47.5) | 313 (52.2) | 918 (51.0) |
| Alcohol-related | 98 (16.3) | 115 (19.2) | 104 (17.3) | 317 (17.6) |
| Nonalcoholic steatohepatitis | 104 (17.3) | 101 (16.8) | 94 (15.7) | 299 (16.6) |
| Hepatitis B | 21 (3.5) | 27 (4.5) | 14 (2.3) | 62 (3.4) |
| Other/unknown | 57 (9.5) | 72 (12.0) | 75 (12.5) | 204 (11.3) |
|
| ||||
| Presence of documented cirrhosis (%)1 | 472 (78.7) | 479 (79.8) | 482 (80.3) | 1,433 (79.6) |
|
| ||||
| Hepatic decompensation (%)2 | 181 (30.2) | 192 (32.0) | 201 (33.5) | 574 (31.9) |
|
| ||||
| Child Pugh Class (% Child A)3 | 432 (72.0) | 435 (72.5) | 424 (70.7) | 1,291 (71.7) |
|
| ||||
| Charlson Comorbidity Index (%)2 | ||||
| 0 | 76 (12.7) | 95 (15.8) | 79 (13.2) | 250 (13.9) |
| 1 | 140 (23.3) | 143 (23.8) | 149 (24.8) | 432 (24.0) |
| 2 | 103 (17.2) | 99 (16.5) | 84 (14.0) | 286 (15.9) |
| 3+ | 281 (46.8) | 263 (43.8) | 288 (48.0) | 832 (46.2) |
|
| ||||
| Number of primary care visits2 | 4 (IQR 2–7) | 3 (IQR 2–7) | 4 (IQR 2–7) | 4 (IQR 2–7) |
|
| ||||
| Receipt of hepatology care2 | 153 (25.5) | 153 (25.5) | 157 (26.2) | 463 (25.7) |
IQR – interquartile range
Defined using ICD-9 codes for cirrhosis or cirrhosis-related complications
During year prior to randomization
Defined using validated measure based on EMR data26
Primary Outcome: Surveillance Completion
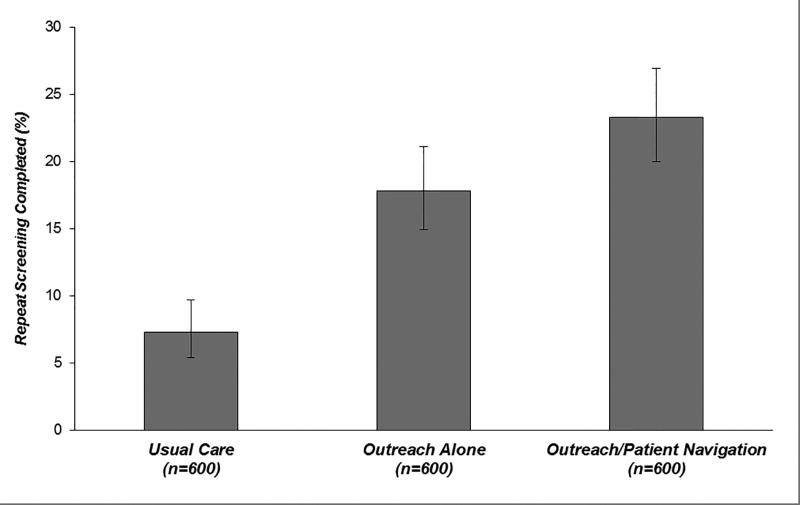
HCC surveillance was performed in 23.3% (95%CI 20.0 – 26.9%) of outreach/navigation patients, 17.8% (95%CI 14.9 – 21.1%) of outreach-alone patients, and 7.3% (95%CI 5.4 – 9.7%) of usual care patients (Table 2). Compared to usual care, surveillance completion was significantly higher in outreach-alone (+10.5%; 95%CI 6.8 – 14.2%) and outreach/navigation (+16.0%; 95%CI 12.0 – 20.0%) groups (p<0.001 for both). Adding navigation to outreach increased surveillance completion by 5.5% (95%CI 0.9 – 10.1%; p=0.02) (Figure 2). There was no appreciable change in direction or magnitude of results in a per-protocol analysis excluding persons who died (n=4) or were diagnosed with HCC (n=3) after randomization but prior to cohort entry. In this analysis, surveillance completion was significantly higher in both intervention groups compared to usual care (p<0.001 for both), and adding navigation increased surveillance completion compared to outreach alone (p=0.02).
Table 2.
HCC surveillance completion over 18-month study period
| Intervention Group | Completed HCC Surveillancea (n) |
Proportion Completed Surveillance (95% CI) |
Difference in Proportion Completing HCC Surveillance by Intervention Group (95% CI) |
|
|---|---|---|---|---|
|
| ||||
| vs. Usual Care | vs. Outreach Alone | |||
| Outreach/Navigation (n=600) | 140 | 23.3 (20.0– 26.9) | +16.0 (12.0– 20.0) | +5.5 (0.9– 10.1) |
| Outreach Alone (n=600) | 107 | 17.8 (14.9– 21.1) | +10.5 (6.8– 14.2) | --- |
| Usual Care (n=600) | 44 | 7.3 (5.4– 9.7) | --- | --- |
HCC surveillance was defined as receipt of abdominal imaging during each 6-month period after randomization.
Figure 2.
HCC surveillancea completion over 18-month study period by intervention group
aHCC surveillance was defined as receipt of abdominal imaging during each 6-month period after randomization.
Legend: Compared to usual care, HCC surveillance was significantly higher in the outreach-alone (+10.5%; 95%CI 6.8 – 14.2%) and outreach/navigation (+16.0%; 95%CI 12.0 – 20.0%) groups (p<0.001 for both). Adding navigation to outreach increased the surveillance proportion by 5.5% (95%CI 0.9 – 10.1%; p=0.02).
There was no evidence of effect modification for either outreach-alone or outreach/navigation interventions, compared to usual care, by race/ethnicity (white vs. non-white) or presence of documented cirrhosis (documented vs. suspected diagnosis); however, the magnitude of intervention effect varied by receipt of hepatology care in the year prior to randomization and Child Pugh class (Appendix Figure 1A and 1B). Although confidence intervals overlapped, the interventions (outreach alone and outreach/ navigation vs. usual care) had a stronger effect among patients with Child B cirrhosis and those who did not have hepatology care in the year preceding randomization. Among all pre-defined subgroups, patient navigation increased surveillance completion compared to outreach alone (Appendix Figure 1C).
Secondary Outcomes
Secondary outcomes did not significantly differ between intervention groups. HCC was diagnosed in 1.8% (95%CI 0.9 – 3.3%) of outreach/navigation patients, 1.0% (95%CI 0.4 – 2.2%) of outreach-alone patients, and 2.3% (95%CI 1.3 – 3.9%) of usual care patients (Table 3). Similarly, the proportion of HCC patients detected at an early stage did not differ between study groups (p=1.0), with 63.6% (95%CI 30.8 – 89.1%) of outreach/navigation HCC patients, 66.7% (95%CI 22.3 – 95.7%) of outreach-alone HCC patients, and 57.1% (95%CI 28.9 – 82.3%) of usual care HCC patients diagnosed at an early stage. Cholangiocarcinoma was diagnosed in one patient in the outreach/navigation group. Of note, 8 (57.1%) HCC patients in the usual care group were screen-detected; conversely, 7 (41.2%) HCC patients in the intervention groups presented incidentally or symptomatically. Overall, screen-detected patients had a higher proportion of early stage tumors than those detected incidentally or symptomatically (83.3% vs. 30.8%, p=0.003) (Appendix Table 1).
Table 3.
Hepatocellular carcinoma surveillance outcomes, by study group
| Intervention Group | Any Stage HCC Diagnosis | Early Stage HCC Diagnosisa | ||
|---|---|---|---|---|
|
| ||||
| n |
% of study group, (95% CI) |
n |
% of all HCC diagnoses in study group (95% CI) |
|
| Outreach/Navigation (n=600) | 11 | 1.8 (0.9 – 3.3) | 7 of 11 | 63.6 (30.8 – 89.1) |
| Outreach Alone (n=600) | 6 | 1.0 (0.4 – 2.2) | 4 of 6 | 66.7 (22.3 – 95.7) |
| Usual Care (n=600) | 14 | 2.3 (1.3 – 3.9) | 8 of 14 | 57.1 (28.9 – 82.3) |
Early HCC was defined as Barcelona Clinic Liver Cancer (BCLC) stage 0 or stage A.
In a post-hoc analysis evaluating a more liberal definition of HCC surveillance every 7 months over a 21-month period, 28.2% (95%CI 24.6 – 31.8%) of outreach/navigation patients, 20.8% (95%CI 17.6 – 24.1%) of outreach-alone patients, and 9.5% (95%CI 5.4 – 9.7%) of usual care patients completed surveillance. HCC surveillance was 18.7% (95%CI 14.4 – 23.0%) and 11.3% (95%CI 7.3 – 15.3%) higher in outreach groups than usual care (p<0.001 for both) and 7.4% (95%CI 2.5 – 12.2%) higher for outreach/navigation than outreach-alone (p=0.003) (Appendix Table 2). Similarly, receipt of any abdominal imaging was 22.4% (95%CI 16.9 – 27.8%) and 19.7% (95%CI 14.2 – 25.2%) higher in outreach/navigation and outreach-alone groups than usual care (p<0.001 for both), respectively (difference between outreach groups: 2.7% (95%CI 0 – 8.0%; p=0.3) (Appendix Table 3). Finally, PTC was 25.3% (95%CI 22.9 – 27.6%) in the usual care group, 40.9% (95% CI 38.3 – 43.6%) in the outreach-alone group, and 44.0% (95% CI 41.2% – 46.8%) in the outreach/navigation group. Both the outreach-alone and outreach/navigation groups had significantly higher PTC compared to usual care (p<.0001 for both); however there was no significant difference between the two outreach groups (p=0.11).
DISCUSSION
In this pragmatic, randomized clinical trial among a large cohort of cirrhosis patients, a mailed outreach intervention significantly increased HCC surveillance every 6 months compared with usual care. Adding patient navigation further increased HCC surveillance compared to outreach alone. Both interventions were effective, independent of patient sex, race/ethnicity, receipt of hepatology care, or presence of documented vs. suspected cirrhosis, although the magnitude of benefit appeared stronger in patients with Child B cirrhosis and those not engaged in hepatology care. Despite improvements in the primary outcome, HCC surveillance in both intervention groups remained below 30%, highlighting a need for more intensive interventions.
Despite literature demonstrating HCC surveillance underuse in cirrhosis patients, few studies have evaluated interventions to increase surveillance. Two small studies suggested a benefit of nursing protocols and automated reminders, but both were conducted among selected patients followed by hepatologists.21 In clinical practice, primary care providers are often responsible for liver-related care of cirrhosis patients, particularly in rural areas where access to subspecialty care is limited.22 Primary care providers report several barriers to HCC surveillance, including inadequate knowledge and clinic time constraints, so it is unclear if these interventions would be equally effective among these patients.23 Another study suggested EMR clinical reminders may increase HCC surveillance among cirrhosis patients followed by primary care providers, but this study relied on visit-based care and only included patients with documented cirrhosis.24 Our study suggests mailed outreach invitations can be an effective population health strategy to increase HCC surveillance among at-risk patients. Compared to other interventions, our mailed outreach strategy can increase surveillance participation among patients not regularly engaged in clinical care and those with suspected cirrhosis but without documented ICD-10 codes, who represent over one-third of patients failing to receive HCC surveillance.16
Prior intervention studies only examined one-time or intermittent HCC surveillance completion, with none evaluating semi-annual surveillance, as recommended by AASLD guidelines. Our study extends this literature by demonstrating mailed outreach strategies and patient navigation can increase HCC surveillance over longer periods of time. This is important because cirrhosis patients have a 2–4% annual risk of developing HCC. HCC surveillance at regular intervals is critical to identify incident cancer at an early stage.25,26 Our primary outcome of HCC surveillance moves a step closer to evaluating screening process completion, which includes initial screening, repeat screening among patients with normal screen results, and diagnostic evaluation among those with abnormal screen results. This distinction is important, as studies have demonstrated failures at each step in the HCC screening process.9,27–29 Although semi-annual HCC surveillance is a more rigorous outcome than prior studies, this still represents an imperfect surrogate for clinical outcomes including early HCC detection and improved survival. Although we found no significant differences in early HCC detection between groups, our study was not powered to detect differences in tumor stage or survival. The lack of statistical power in our study was exacerbated by a lower than anticipated HCC incidence rate. It is unclear if this was related to a lower incidence given increased dissemination of hepatitis C anti-viral therapy and shift to non-viral cirrhosis, pragmatic trial design and imperfect specificity of ICD-9 codes for presence of cirrhosis, or ascertainment bias over the relatively short 18-month duration of the trial. An ongoing multi-center study is evaluating the effect of outreach strategies to improve outcomes such as screening process completion and early HCC detection, although this trial is years away from reporting.
We found patient navigation increased HCC surveillance every 6 months compared to outreach alone, with the benefit more pronounced in a secondary analysis evaluating surveillance every 7 months. Prior studies have suggested high patient acceptance of HCC surveillance although patient barriers, including challenges with scheduling and transportation, are associated with lower surveillance participation.30,31 Patient navigation is an effective strategy to address screening barriers for other cancer screening programs but has not been previously evaluated for HCC surveillance.12,32 Despite demonstrated effectiveness, less than one-third of patients receiving outreach and navigation in this study underwent HCC surveillance, highlighting a need for more intensive interventions. Patient navigation in our study consisted of only barrier assessment, motivational education, and assistance with ultrasound scheduling. More extensive navigation to overcome barriers, such as transportation assistance or evening/weekend ultrasound appointments so patients do not miss work, may be effective and warrant evaluation. Alternatively, prior studies have shown receipt of subspecialty care is significantly associated with higher surveillance rates, so efforts to increase referrals may be effective for systems with sufficient Hepatology capacity.
This study had the following limitations. First, our study was conducted in a safety-net health system and results may not generalize to other health systems. However, racially diverse, socioeconomically disadvantaged patients represent a difficult-to-reach population and are important to study given lower HCC surveillance receipt and higher HCC mortality rates.33,34 Second, patients may have received abdominal imaging at outside institutions; however, this is unlikely because many patients did not have insurance for care outside the safety-net health system. Further, we would not expect receipt of surveillance imaging at outside institutions to differ between study groups given the randomized nature of the study. Third, low surveillance completion may be partly explained by patients no longer following at Parkland or having contraindications to screening, e.g. increased comorbidity or liver function deterioration, which may be under-recognized given the trial’s pragmatic design. Fourth, we could not differentiate imaging indication (diagnostic versus screening purposes); however, imaging for either purpose is sufficient for surveillance completion.
This study has several strengths including its large sample size with various cirrhosis etiologies, racially and socioeconomically diverse patient population, and innovation comparing mailed outreach with and without patient navigation to promote HCC surveillance over 18 months. Our trial’s pragmatic design also avoided volunteer bias, included cirrhosis patients with minimal exclusion criteria, and used processes (ultrasound scheduling, outcome ascertainment, and results notification) that could easily be adopted into clinical care.35
In summary, mailed outreach invitations are effective for increasing HCC surveillance among cirrhosis patients. Adding patient navigation to the outreach strategy further increased HCC surveillance. Given the pervasive nature of HCC screening underuse among cirrhosis patients, mailed outreach with or without patient navigation can be an effective strategy for improving HCC screening delivery.
Supplementary Material
Acknowledgments
The authors would like to thank Blue Faery: The Adrienne Wilson Liver Cancer Association for their help and support of this project.
Financial Source: This study was conducted as part of the Center for Patient-Centered Outcomes Research with support from AHRQ Grant R24 HS022418, NIH RO1 CA212008, Cancer Prevention Research Institute of Texas (CPRIT) RP150587, and NIH/NCI Cancer Center Support Grant P30 CA142543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AHRQ. The funding agency had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Abbreviations
- HCC
hepatocellular carcinoma
- AASLD
American Association for the Study of Liver Diseases
- CRC
colorectal cancer
- EMR
electronic medical record
- APRI
AST to platelet ratio index
- BCLC
Barcelona Clinic Liver Cancer
Footnotes
Author contributions: Dr. Singal had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design (Singal, Tiro, and Halm); Acquisition, analysis and interpretation of the data (Singal, Tiro, Murphy, Marrero, McCallister, Fullington, Mejias, Waljee, Bishop, Santini, and Halm); Drafting of the manuscript (Singal); Critical revision of the manuscript for important intellectual content (Singal, Tiro, Murphy, Marrero, McCallister, Fullington, Mejias, Waljee, Bishop, Santini, and Halm); Obtained funding (Singal, Halm); Administrative, technical, and material support (Singal and Halm); and Study supervision (Singal)
Conflicts of Interest: None of the authors have any relevant conflicts of interest to declare.
References
- 1.Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AG, Pillai A, Tiro J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014;11(4):e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival Among Patients with Cirrhosis in the US. Am J Med. 2017;130(9):1099–1106. e1091. doi: 10.1016/j.amjmed.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 7.van Meer S, de Man RA, Coenraad MJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J Hepatol. 2015;63(5):1156–1163. doi: 10.1016/j.jhep.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Breen N, Meissner HI. Toward a system of cancer screening in the United States: trends and opportunities. Annu Rev Public Health. 2005;26:561–582. doi: 10.1146/annurev.publhealth.26.021304.144703. [DOI] [PubMed] [Google Scholar]
- 9.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal AG, Tiro J, Li X, Adams-Huet B, Chubak J. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-based Integrated Health Care Delivery System. J Clin Gastroenterol. 2017;51(7):650–655. doi: 10.1097/MCG.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genoff MC, Zaballa A, Gany F, et al. Navigating Language Barriers: A Systematic Review of Patient Navigators' Impact on Cancer Screening for Limited English Proficient Patients. J Gen Intern Med. 2016;31(4):426–434. doi: 10.1007/s11606-015-3572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krok-Schoen JL, Oliveri JM, Paskett ED. Cancer Care Delivery and Women's Health: The Role of Patient Navigation. Front Oncol. 2016;6:2. doi: 10.3389/fonc.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muliira JK, D'Souza MS. Effectiveness of patient navigator interventions on uptake of colorectal cancer screening in primary care settings. Jpn J Nurs Sci. 2016;13(2):205–219. doi: 10.1111/jjns.12102. [DOI] [PubMed] [Google Scholar]
- 14.Singal AG, Gupta S, Skinner CS, et al. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial. JAMA. 2017;318(9):806–815. doi: 10.1001/jama.2017.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singal AG, Tiro JA, Marrero JA, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology. 2017;152(3):608–615. e604. doi: 10.1053/j.gastro.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AG, Yopp AC, Gupta S, et al. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev Res (Phila) 2012;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47(5):e50–54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abd El Rihim AY, Omar RF, Fathalah W, El Attar I, Hafez HA, Ibrahim W. Role of fibroscan and APRI in detection of liver fibrosis: a systematic review and meta-analysis. Arab J Gastroenterol. 2013;14(2):44–50. doi: 10.1016/j.ajg.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Trevisani F, De NS, Rapaccini G, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience) Am J Gastroenterol. 2002;97(3):734–744. doi: 10.1111/j.1572-0241.2002.05557.x. [DOI] [PubMed] [Google Scholar]
- 20.Mokdad A, Browning T, Mansour JC, Zhu H, Singal AG, Yopp AC. Implementation of a Voice Messaging System is Associated With Improved Time-to-Treatment and Overall Survival in Patients With Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2016;14(1):38–46. doi: 10.6004/jnccn.2016.0005. [DOI] [PubMed] [Google Scholar]
- 21.Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11(7):850–858. e851–854. doi: 10.1016/j.cgh.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Singal AG, X L, Tiro J, et al. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice Patterns and Attitudes of Primary Care Providers and Barriers to Surveillance of Hepatocellular Carcinoma in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved Surveillance for Hepatocellular Carcinoma with a Primary Care-Oriented Clinical Reminder. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136(1):138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 27.Singal AG, Tiro JA, Gupta S. Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastroenterol Hepatol. 2013;11(5):472–477. doi: 10.1016/j.cgh.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel N, Yopp AC, Singal AG. Diagnostic Delays are Common Among Patients wtih Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2014 doi: 10.6004/jnccn.2015.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singal AG, Waljee AK, Patel N, et al. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2013;11(9):1101–1108. doi: 10.6004/jnccn.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singal A, Volk M, Rakoski M, et al. Patient Involvement is Correlated with Higher HCC Surveillance in Patients with Cirrhosis. J Clin Gastroenterol. 2011;45(8):727–732. doi: 10.1097/MCG.0b013e31820989d3. [DOI] [PubMed] [Google Scholar]
- 31.Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65(3):875–884. doi: 10.1002/hep.28770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Percac-Lima S, Ashburner JM, Zai AH, et al. Patient Navigation for Comprehensive Cancer Screening in High-Risk Patients Using a Population-Based Health Information Technology System: A Randomized Clinical Trial. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.0841. [DOI] [PubMed] [Google Scholar]
- 33.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116(5):1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 34.Ha J, Yan M, Aguilar M, et al. Race/Ethnicity-specific Disparities in Hepatocellular Carcinoma Stage at Diagnosis and its Impact on Receipt of Curative Therapies. J Clin Gastroenterol. 2016;50(5):423–430. doi: 10.1097/MCG.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 35.Ford I, Norrie J. Pragmatic Trials. New England Journal of Medicine. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.