Abstract
Background
Adult body size is related to ovarian cancer risks, but size in childhood may also influence risks. We investigated if childhood body mass index (BMI; kg/m2), height, and growth patterns were associated with ovarian cancer overall and by histologic subtypes, including effects of birthweight.
Methods
A cohort of 155,958 girls from the Copenhagen School Health Records Register, born 1930–1989 with measured weights and heights from 7–13 years were included. During follow-up, 1,041 ovarian cancers were recorded. Overweight was defined using International Obesity Task Force criteria. Cox regressions were performed.
Results
Compared with non-overweight girls, at most ages girls with overweight had increased risks of ovarian cancer overall (hazard ratio (HR) range: 1.24–1.34), mucinous, endometrioid and clear cell ovarian cancers, but not serous and other ovarian cancers. Childhood height had positive and significant associations with ovarian cancer overall (HR range: 1.07–1.10 per z-score) and the endometrioid subtype but not with the other sub-types. Adjusting for birthweight minimally altered the associations with childhood body size. In growth analyses, girls with overweight or who were tall at 7 and 13 years had increased risks of ovarian cancer overall compared with average-sized girls at both ages.
Conclusions
Ovarian carcinogenesis is linked to childhood overweight, tallness and growth, with variations across histological subtypes, suggesting that early life play a role in the origins of this disease.
Impact
These findings emphasize that healthy body size and growth during childhood are important as they may contribute to reducing ovarian cancer risks.
Keywords (MeSH): body weights and measures, child, growth and development, histology, obesity, ovarian neoplasms
Introduction
Ovarian cancer is the deadliest gynecological malignancy and it ranks as the seventh most common cancer among women worldwide and the tenth most common cancer in Denmark (1, 2). Currently there are no effective ovarian cancer screening approaches and as clinical symptoms are uncommon at early stages, it is often diagnosed at advanced stages (3, 4). The etiology of ovarian cancer is complex and heterogeneous with respect to genomic pathways, histological presentation, and prognosis (5, 6).
Few risk factors for ovarian cancer are established, and these include older age, family history, reproductive factors (nulliparity, history of infertility, early age at menarche, and late age at menopause) and hormone replacement therapy (3, 7). There are also indications that excess body fatness in adult life increases risks for ovarian malignancies (3, 8, 9), however, evidence is more convincing for taller adult height (3, 8). Excessive weight gain in adult life may also be associated with increased risks of developing ovarian cancer (10). Although not investigated in the majority of previous studies on adult women, risk factors, including body size, most likely differ by tumor sub-type (11–13).
The timing of the development and the duration of exposure to excess body fatness may be important for risks of ovarian cancer. Two studies that examined links between childhood or adolescent adiposity and ovarian cancer overall yielded inconsistent results; child size did not matter whereas adolescent size did, and neither study investigated effects of weight patterns during childhood (14, 15). Although it is plausible that childhood height and growth matter as well, previous studies have not reported on this nor have they examined the associations with ovarian cancer sub-types or the influence of birthweight - each of which may be important factors for the risks of this disease.
We examined if childhood overweight and height at each age from 7 to 13 years are associated with risks of ovarian cancer overall and its different histological subtypes. Additionally we investigated if associations between childhood body size and ovarian cancer overall were influenced by birthweight and whether childhood overweight and growth patterns were associated with risks of ovarian cancer overall.
Materials and Methods
The Copenhagen School Health Records Register (CSHRR) contains information from mandatory school health examinations on 184,276 girls born 1930–1989. Weight and height at ages 7–13 years were measured by trained school personnel. Parents or guardians reported their child’s birthweight from the birth year 1936 onwards(16).
Women were followed for information in national health registers based on record linkages using a unique government-issued personal identification number assigned to all Danish residents alive in 1968 or born thereafter (17). The information was systematically recorded on individual health cards along with the girl’s name, sex and date of birth (16). Identification numbers for girls who were in school in 1968 or later were recorded on the health cards and for girls leaving school prior to this time identification numbers were retrieved (16). Incident ovarian cancers were identified in the comprehensive and valid Danish Cancer Registry (18). Using International Classification of Disease (ICD)-10 codes, ovarian tumors were classified as C56.0, C56.2-3 and C56.9. Using ICD-O-3 morphology codes, the epithelial ovarian cancers were defined and sub-classified as serous, mucinous, endometrioid, clear cell and other epithelial ovarian cancers (Supplementary Table S1). We excluded non-epithelial ovarian cancers, very rare and unspecific ovarian cancers, cancers with uncertain primary origin and cancers without morphology information from all analyses. Information on vital status was obtained from the Danish Civil Registration System (17) and information on bilateral oophorectomy or salpingo-oophorectomy from the Danish National Patient Register, which contains information on all hospital discharge diagnoses since 1977 (19).
Among the total population of girls in the CSHRR, we excluded women without an identification number (N=21,717), who emigrated, died or were lost to follow-up prior to age 18 or January 1, 1978 (N=3,784), who had an oophorectomy or salpingo-oophorectomy prior to age 18 (N=2) or 1978 (N=50), an ovarian cancer diagnosis prior to 1978 (N=49), lacked a date for the ovarian cancer diagnosis (N=2), were missing height and/or weight measurements at all childhood ages (N=2,710) or with outlying height or BMI z-scores at all ages (z-score <−4.5 or >4.5) (N=4), resulting in 155,958 women available for the analyses (Supplementary Figure S1). Women were followed from 18 years or from her age in 1978, whichever came later. Follow-up ended on the date of a diagnosis of ovarian cancer, oophorectomy/salpingo-oophorectomy, death, emigration, loss to follow-up, or December 31, 2014, whichever came first.
Statistical analyses
BMI (kg/m2) values were calculated. BMI and height were transformed into z-scores using an internal reference and interpolated or extrapolated to exact ages. For BMI, the values were subsequently back-transformed so that girls could be classified using International Obesity Task Force into non-overweight or overweight (including obesity). At age 7 years, the cut-off corresponds to 17.69 kg/m2 and cut-offs for other ages are in Supplementary Table S2 (20). We conducted Cox proportional hazards analyses stratified by 5-year birth cohorts using age at risk as the underlying time scale to investigate the associations between childhood body size and ovarian cancer risks. In the sub-sample of women born 1936–1989 with information available on birthweight, we repeated these analyses on the outcome of ovarian cancer overall and tested for effect modification by birthweight. As this was not found (all p-values ≥ 0.09) we conducted these analyses only adjusting for birthweight.
Non-linearity was assessed by linear splines, the likelihood ratio test and visual inspections of graphs. We detected non-linearity in associations between childhood BMI and ovarian cancer, and these were further explored by categorical analyses (5 categories using a priori cut-points corresponding to the 10, 25, 75 and 90th percentiles (Supplementary Table S3)). Categorical analyses using IOTF cut-offs were conducted. Non-linearity was not identified for associations with childhood height.
Growth in relation to ovarian cancer overall was assessed in girls with body size available at 7 and 13 years. In analyses on changes in weight status, girls who were not overweight at both ages were the reference group. Growth in height was examined in a model with height at age 7 years and change in height from age 7 to 13 years.
We examined the proportional hazards assumptions underlying the Cox models by testing if associations between childhood body size and ovarian cancer risks differed by categories of age at risk using likelihood ratio tests. Interactions of birth cohort with the associations between childhood body size and ovarian cancer risks were similarly investigated using likelihood ratio tests. No violations of the proportional hazards assumption or birth cohort effects were detected. Potential heterogeneity in the associations with childhood body size across subtypes was evaluated using likelihood ratio tests by including an interaction term between childhood BMI and height, respectively, and ovarian cancer sub-types.
This study was approved by the Danish Data Protection Agency. According to Danish law, ethical approval is not required for register-based studies.
Results
During 37 years and 4.61 million woman-years of follow-up of 155,958 women, 1,041 were diagnosed with epithelial ovarian cancer. Among these cases, 570 (54.8%) were serous, 110 (10.6%) mucinous, 104 (10.0%) endometrioid, 39 (3.7%) clear cell and 218 (20.9%) were other epithelial ovarian cancers (Supplementary Table S2). The median age at diagnosis was 58 years (range: 20–82) for ovarian cancers overall and it varied slightly across subtypes (Table 1). As expected, median values for BMI and height increased with childhood age (Table 1).
Table 1.
Age at diagnosis among cases and BMI (kg/m2) and height (cm) values for 155,958 girls included in this study from the Copenhagen School Health Records Register
| N | Median (range) | |
|---|---|---|
| Age at diagnosis (years) | ||
| Overall | 1,041 | 58 (20–82) |
| Serous | 570 | 58 (20–82) |
| Mucinous | 110 | 55 (29–76) |
| Endometrioid | 104 | 53 (21–81) |
| Clear cell | 39 | 56 (35–76) |
| Other epithelial | 218 | 60 (20–81) |
|
| ||
| N | Median (5–95 percentiles) | |
|
| ||
| BMI (kg/m2) | ||
| 7 | 145,821 | 15.3 (13.5–18.0) |
| 8 | 147,519 | 15.6 (13.7–18.7) |
| 9 | 142,518 | 16.0 (13.9–19.6) |
| 10 | 139,003 | 16.4 (14.2–20.4) |
| 11 | 138,103 | 16.8 (14.4–21.3) |
| 12 | 137,041 | 17.5 (14.8–22.2) |
| 13 | 135,13 | 18.3 (15.3–23.2) |
| Height (cm) | ||
| 7 | 145,821 | 121.7 (113.0–130.4) |
| 8 | 147,519 | 126.9 (117.9–136.0) |
| 9 | 142,518 | 132.1 (122.7–142.0) |
| 10 | 139,003 | 137.3 (127.3–148.0) |
| 11 | 138,103 | 142.9 (132.0–154.8) |
| 12 | 137,041 | 149.3 (137.1–161.7) |
| 13 | 135,130 | 155.5 (143.0–167.0) |
BMI, body mass index.
Overweight and ovarian cancer overall and by sub-type
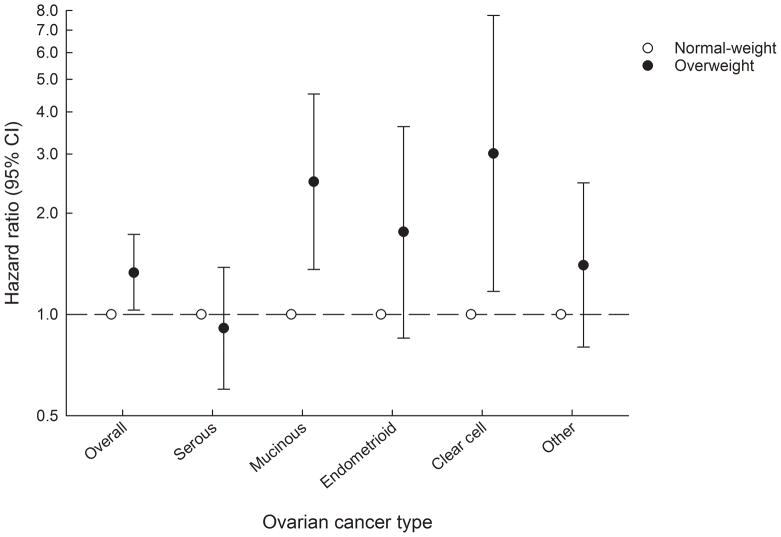
Girls with overweight had increased risks of ovarian cancer overall compared with girls who were not overweight as illustrated by the results for weight status at age 7 years (Figure 1). The associations were similar at other ages (hazard ratio (HR) range: 1.24–1.33), although the estimates did not reach statistical significance at a few ages (Supplementary Table S2). For the subtypes, childhood overweight at age 7 years was positively associated with mucinous, endometrioid and clear cell ovarian cancers, although the association did not reach statistical significance for the endometrioid sub-type (Figure 1). In contrast, there were no associations with serous or other epithelial ovarian cancers (Figure 1). Findings for the other childhood ages were similar with those for age 7 years, although the associations with the endometrioid sub-type were significant for most childhood ages (Supplementary Table S2). Despite indications of differences in the associations between childhood overweight and the different subtypes, the tests for heterogeneity were not statistically significant (P-values range: 0.35–0.75).
Figure 1.
Childhood weight status at age 7 years and the risks of ovarian cancer overall and by histological subtype a.The figure shows the risks estimates (hazard ratios including 95% confidence intervals) for ovarian cancer risk for girls with overweight compared with girls who were non-overweight.
CI, confidence intervals.
aCox proportional hazards regression models stratified by 5-year birth cohorts.
Height and ovarian cancer overall and by sub-type
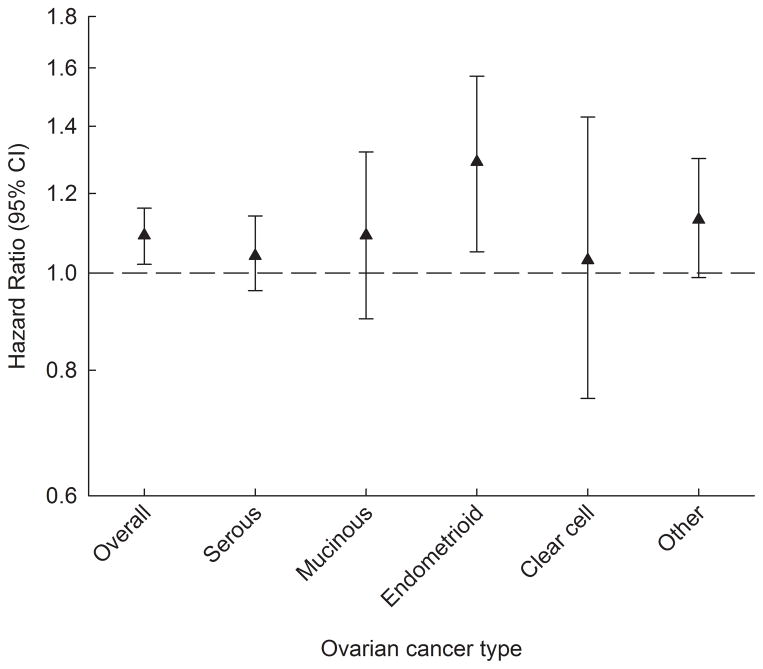
Childhood height was positively and significantly associated with ovarian cancer overall and associations were similar across all ages (HR range: 1.07–1.10 per z-score, corresponding approximately to 5.2 cm at age 7 years) (Figure 2, Supplementary Table S4). For the endometrioid subtype, the associations were similarly positive (HR range: 1.19–1.34 per z-score), whereas associations with serous, mucinous, clear cell and other epithelial ovarian cancers were generally not statistically significant (Figure 2, Supplementary Table S4). The tests for heterogeneity among sub-types were not statistically significant (P-value range: 0.21–0.63).
Figure 2.
Childhood height at age 7 years and the risks of ovarian cancer overall and by histological subtypea. The figure shows the risks estimates (hazard ratios including 95% confidence intervals) for ovarian cancer risk per z-score increase in height.
CI, confidence intervals.
aCox proportional hazards regression models stratified by 5-year birth cohorts.
Adjustment for birthweight
In the sub-sample of 118,229 women with information available on birthweight there were 663 cases of ovarian cancer. In this sub-sample, the associations between childhood overweight and height, respectively, and ovarian cancer overall were minimally affected by adjustment for birthweight (Supplementary Tables S5–6).
Growth and ovarian cancer overall
Among 126,700 girls with measurements of body size at ages 7 and 13 years there were 920 diagnoses of ovarian cancer. In these analyses we found that girls with overweight at both ages had an increased risks of ovarian cancer overall compared with non-overweight girls. Girls who became overweight or who changed from being classified as overweight to non-overweight during childhood did not have increased risks of ovarian cancer overall (Table 2). Girls who were tall throughout childhood (represented by the change estimate in the growth model) had significantly increased risks of ovarian cancer overall compared with average-height girls (HR=1.04 [95% confidence interval (CI): 1.01–1.08] per 0.5 z-score increase). In contrast, girls who grew tall (represented by the age 7 estimate in the growth model) during childhood did not have increased risks of ovarian cancer overall compared with girls who had average growth (HR=0.95 [95% CI: 0.89–1.01] per 0.5 z-score increase, corresponding approximately to 20.6 cm of growth).
Table 2.
Weight status patterns and the risks of ovarian cancer overall including women born 1930–1989a
| Overweight statusb | N | Cases | HR (95% CI) |
|---|---|---|---|
| Non-overweight at 7 and 13 years | 114,229 | 839 | 1.00 (ref.) |
| Non-overweight at 7 years, overweight at 13 years | 4,828 | 32 | 1.09 (0.77–1.55) |
| Overweight at 7 years, non-overweight at 13 years | 3,435 | 21 | 0.98 (0.64–1.51) |
| Overweight at 7 and 13 years | 4,208 | 36 | 1.67 (1.19–2.33) |
BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Cox proportional hazards regression models stratified by 5-year birth cohorts.
Overweight defined using International Obesity Task Force criteria
Discussion
In this large population-based study, with an extensive follow-up period of 37 years, girls with overweight had greater risks of ovarian cancer overall than girls who were not classified as overweight. Similar patterns were identified for the histological subtypes of mucinous, endometrioid and clear cell ovarian cancers, but not with serous and other ovarian cancers. Taller girls had increased risks of ovarian cancer overall and the endometrioid subtype, but not the other subtypes. Adjusting for birthweight minimally affected the associations with childhood body size. Finally, girls with overweight or who were tall at ages 7 and 13 years had elevated risks of ovarian cancer overall compared with girls who were not overweight or not tall at both ages.
Our findings on childhood overweight, although not directly comparable due to age differences, are in accord with those from a Norwegian study of adolescents (14–19 years) that also used measured values of height and weight (15). Our results differ from an American study that combined data from the Nurses’ Health Study and Nurses’ Health Study II and did not find strong indications of associations between body size at 5 to 10 years and risks of ovarian cancer. As the previous study used recalled and self-reported somatotypes from childhood, differences with our study findings may be explained by differences in the ascertainment of childhood body size (14).
Although ovarian epithelial cancers are recognized to differ histologically, the majority of epidemiological studies are unable to explore associations by sub-type due to low numbers of cases and a lack of available histological information. A growing body of evidence suggests that many ovarian cancer risk factors are differentially associated with histological sub-types, but findings are generally inconsistent, and this also applies to adult BMI and height (11–13). Risk factors are well-established only for the rare non-serous sub-type. In analyses on associations with ovarian cancer sub-types, we generally found positive associations between childhood overweight and mucinous, endometrioid and clear cell ovarian cancers as well as positive associations between childhood height and the endometrioid subtype. These sub-type analyses offer an insight into how childhood overweight and height relate to these forms, and consistent with previous studies, we do not find indications of associations with the common and fatal serous ovarian cancers.
We and others have previously shown u-shaped associations between birth weight and ovarian cancer risks (21, 22). However, adjusting for birthweight minimally changed our findings for childhood body size, suggesting that size at birth, as an indicator of fetal growth, does not affect these associations with ovarian cancer risks.
Our novel analyses on growth found that girls with overweight at 7 and 13 years had higher risks of ovarian cancer overall than girls who were not overweight at both ages. Becoming overweight or reducing size to being classified as non-overweight, however, was surprisingly not associated with ovarian cancer as compared with girls who were not overweight at both ages. One other study on growth and ovarian cancer risks examined BMI trajectories from childhood to midlife, but as it was limited by few cases it did not detect associations with ovarian cancer (23). We also found that girls who were consistently tall, compared with girls who were not, had significantly increased risks of ovarian cancer overall. As we did not find that girls who became overweight or grew tall during these ages were at increased risks of ovarian cancer, these findings suggest that the biological processes underlying these associations may be initiated earlier in childhood.
Biological mechanisms underlying the association between childhood body size and ovarian cancer risks are likely to vary across histological sub-types, and they remain largely unknown. Adult adiposity may be linked to ovarian carcinogenesis through influences on the bioavailability or synthesis of endogenous sex hormones (24, 25). Similarly, excess childhood body fatness is speculated to contribute to a continuous accumulation of risk throughout the life-course or alternatively, the timing of developing excess body fatness may be critically important. As child and adult BMI are only moderately correlated at ages when ovarian cancers emerge (r values of 0.26–0.43) (26), which is typically from the 5th decade of life, body size tracking is unlikely to entirely explain these findings. Height is likely a marker for underlying etiologic factors such as genetic predispositions, environmental and nutritional factors, exposure to insulin, and sex steroid and growth hormones (27, 28). Additionally, taller height and excess body fatness may be indicators of earlier puberty (29, 30) and contribute to an increased lifetime number of ovulatory cycles (31) that may be critical for ovarian cancer risks.
Our study included a large population of school-aged girls with measured heights and weights from mandatory school-based examinations enhancing the validity as compared with self-reported data. Girls in this study were largely of Western European origin, thus our results likely apply to significant portions of populations outside of Denmark. These girls were followed through national health registers for an extensive period with minimal loss to follow-up. A distinct strength includes the detailed pathology information on morphologically-verified tumors from the high-quality Danish Cancer Registry (18), which allowed us to divide ovarian cancers into subtypes based on histological traits. Information on tumor grade is not electronically available in Danish registers, thus, we could not evaluate if associations with serous ovarian malignancies depend on the degree of differentiation as suggested for adult body size (32). Even though information on birthweight was available in this study, we lack information on body size from the period after birth to age 7 years making us unable to evaluate these ages in childhood, where associations with body size may be established. Moreover, we do not have information available on potential confounding factors in childhood. Finally, apart from adjusting for birth weight, other covariates of potential interest, such as adult body size, menopausal hormone therapy, and reproductive factors, were not taken into account. These factors are unlikely to confound the associations but could potentially act as mediators. Nonetheless, it was beyond the scope of the current study to examine the potential mediating roles of these factors.
In conclusion, our study suggests that childhood overweight and tallness increase the risks of ovarian cancer overall and that girls who had growth patterns of overweight or tallness at ages 7 and 13 had increased risks of ovarian cancer overall. Interestingly, there were indications that the associations may vary by histological sub-type. We did not find any associations with serous ovarian cancer, which is the most common type of ovarian cancer, and future studies therefore need to identify other risk factors for this subtype. Whether the associations are independent of adult body size remains to be explored. Nonetheless, the identification of factors early in life associated with later ovarian cancer risks, although they may only be indicators of risks, adds to the currently limited understanding of ovarian cancer etiology.
Supplementary Material
Acknowledgments
Financial information
This work was supported by the European Research Council under the European Union’s Seventh Framework Programme [(FP/2007–2013)/ERC, 281419 to JLB], the Danish Council for Independent Research [(DFF)|FSS 1331-00218 to JLB], the European Union’s Horizon 2020 research and innovation programme [DynaHEALTH, 633595 to JLB] and Dr. Sofus Carl Emil Friis and wife Olga Doris’ Fund to JLB. Additionally, this work was supported from the Johannes Clemmesen’s Fund, Bispebjerg and Frederiksberg Hospital, and Else and Mogens Wedell-Wedellsborg’s Fund to JAA. The intramural research program of the US National Cancer Institute funded BT and NW. The funding sources had no involvement in the study design, collection, analysis and interpretation of data, writing the article and decision to submit.
The CSHRR was established by the former Institute of Preventive Medicine (now the Center for Clinical Research and Prevention). It was built in collaboration with the Copenhagen City Archives in Denmark.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Globocan. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. International Agency on Cancer (IARC). World Health Organization (WHO); [Google Scholar]
- 2.NORDCAN, Association of the Nordic Cancer Registries. Cancer Statistics [Google Scholar]
- 3.World Cancer Research Fund / American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Ovarian Cancer. 2014 [Google Scholar]
- 4.Wentzensen N. Large ovarian cancer screening trial shows modest mortality reduction, but does not justify population-based ovarian cancer screening. Evid Based Med. 2016;21:159. doi: 10.1136/ebmed-2016-110411. [DOI] [PubMed] [Google Scholar]
- 5.Soslow RA. Histologic subtypes of ovarian carcinoma: an overview. Int J Gynecol Pathol. 2008;27:161–74. doi: 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- 6.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43:420–32. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 7.Brinton LA, Sahasrabuddhe VV, Trabert B. Epidemiology of Gynecologic Cancers. In: Barakat R, Berchuck A, Markman M, Randall ME, editors. Principles and Practice of Gynecologic Oncology. 6. Philadelphia: Wolters Kluwer Health; 2013. pp. 1–29. [Google Scholar]
- 8.Aune D, Navarro Rosenblatt DA, Chan DS, Abar L, Vingeliene S, Vieira AR, et al. Anthropometric factors and ovarian cancer risk: a systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J Cancer. 2015;136:1888–98. doi: 10.1002/ijc.29207. [DOI] [PubMed] [Google Scholar]
- 9.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv088. [DOI] [PubMed] [Google Scholar]
- 11.Yang HP, Trabert B, Murphy MA, Sherman ME, Sampson JN, Brinton LA, et al. Ovarian cancer risk factors by histologic subtypes in the NIH-AARP Diet and Health Study. Int J Cancer. 2012;131:938–48. doi: 10.1002/ijc.26469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34:2888–98. doi: 10.1200/JCO.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol. 2010;171:45–53. doi: 10.1093/aje/kwp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baer HJ, Hankinson SE, Tworoger SS. Body size in early life and risk of epithelial ovarian cancer: results from the Nurses’ Health Studies. BrJCancer. 2008;99:1916–22. doi: 10.1038/sj.bjc.6604742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. JNatlCancer Inst. 2003;95:1244–8. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- 16.Baker JL, Olsen LW, Andersen I, Pearson S, Hansen B, Sørensen TIA. Cohort profile: the Copenhagen School Health Records Register. IntJEpidemiol. 2009;38:656–62. doi: 10.1093/ije/dyn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen CB. The Danish Civil Registration System. ScandJPublic Health. 2011;39:22–5. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 18.Gjerstorff ML. The Danish Cancer Registry. ScandJPublic Health. 2011;39:42–5. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 19.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. ScandJPublic Health. 2011;39:30–3. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 20.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. PediatrObes. 2012;7:284–94. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 21.Trabert B, Aarestrup J, Ulrich LG, Wentzensen N, Sorensen TIA, Baker JL. Birth weight and the risk of histological subtypes of ovarian and endometrial cancers: Results from the Copenhagen School Health Records Register. Gynecol Oncol. 2018;148:547–52. doi: 10.1016/j.ygyno.2017.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang TO, Reeves GK, Green J, Beral V, Cairns BJ. Birth weight and adult cancer incidence: large prospective study and meta-analysis. AnnOncol. 2014;25:1836–43. doi: 10.1093/annonc/mdu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138:2383–95. doi: 10.1002/ijc.29981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. NatRevCancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 25.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 26.Aarestrup J, Bjerregaard LG, Gamborg M, Ängquist L, Tjønneland A, Overvad K, et al. Tracking of body mass index from 7 to 69 years of age. Int J Obes (Lond) 2016;40:1376–83. doi: 10.1038/ijo.2016.88. [DOI] [PubMed] [Google Scholar]
- 27.Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. EpidemiolRev. 2001;23:313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 28.Rogol AD. Sex steroids, growth hormone, leptin and the pubertal growth spurt. Endocr Dev. 2010;17:77–85. doi: 10.1159/000262530. [DOI] [PubMed] [Google Scholar]
- 29.Aksglaede L, Juul A, Olsen LW, Sørensen TIA. Age at puberty and the emerging obesity epidemic. PLoSOne. 2009;4:e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SH, Tzeng SJ, Cheng JY, Chie WC. Height and weight change across menarche of schoolgirls with early menarche. Arch Pediatr Adolesc Med. 2000;154:880–4. doi: 10.1001/archpedi.154.9.880. [DOI] [PubMed] [Google Scholar]
- 31.Yang HP, Murphy KR, Pfeiffer RM, George N, Garcia-Closas M, Lissowska J, et al. Lifetime Number of Ovulatory Cycles and Risks of Ovarian and Endometrial Cancer Among Postmenopausal Women. Am J Epidemiol. 2016;183:800–14. doi: 10.1093/aje/kwv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20:251–62. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.