Abstract
Autism spectrum disorder (ASD) is consistently associated with alterations in brain connectivity, but there are conflicting results as to where and when individuals with ASD display increased or reduced functional connectivity. Such inconsistent findings may be driven by atypical neurodevelopmental trajectories in ASD during adolescence, but no longitudinal studies to date have investigated this hypothesis. We thus examined the functional connectivity of three neurocognitive resting-state networks – the default mode network (DMN), salience network, and central executive network (CEN) – in a longitudinal sample of youth with ASD (n=16) and without ASD (n=22) studied during early/mid- and late adolescence. Functional connectivity between the CEN and the DMN displayed significantly altered developmental trajectories in ASD: typically developing (TD) controls – but not youth with ASD – exhibited an increase in negative functional connectivity between these two networks with age. This significant interaction was due to the ASD group displaying less negative functional connectivity than the TD group during late adolescence only, with no significant group differences in early/mid-adolescence. These preliminary findings suggest a localized age-dependency of functional connectivity alterations in ASD and underscore the importance of considering age when examining brain connectivity.
Keywords: adolescence, autism spectrum disorder, functional magnetic resonance imaging, brain development, functional connectivity
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder that is diagnosed on the basis of impairments in social communication and the presence of repetitive behaviors and restricted interests (American Psychiatric Association, 2013). ASD is also associated with impairments in cognitive domains such as executive function (see Lai et al., 2017 for review), which significantly predicts later academic achievement and adaptive functioning (Pugliese et al., 2015; Pugliese et al., 2016; St John et al., 2017). Converging lines of evidence – including genetics, postmortem, and in vivo neuroimaging studies – suggest that ASD is characterized by atypical brain connectivity (Zikopoulos & Barbas, 2013; Bourgeron, 2015; Hernandez et al., 2015; Kana et al., 2014; Picci et al., 2016). Instrinsic functional connectivity, derived from resting-state functional magnetic resonance imaging (fMRI) data, is one in vivo method of measuring brain connectivity in humans (Smith et al., 2013). Numerous studies have found functional connectivity alterations in ASD relative to typically developing (TD) controls, with some studies demonstrating that such differences are correlated with ASD symptomatology and can be used to predict an ASD diagnosis at accuracies significantly above chance (e.g., Uddin et al., 2013a; Plitt et al., 2015a; Yerys et al., 2015). However, studies investigating resting-state network (RSN) functional connectivity have yielded conflicting results regarding the directionality of alterations in ASD, with some studies reporting hyperconnectivity among participants with ASD and others reporting hypoconnectivity (see Hull et al., 2016; Picci et al., 2016 for review).
One recent hypothesis argues that such discrepancies in the functional connectivity literature are due to atypical RSN trajectories in ASD, as hyperconnectivitiy is more likely to be reported by papers focusing on children and hypoconnectivity by papers focusing on adolescents or adults (Uddin et al., 2013b). Cross-sectional studies directly investigating the age-dependence of functional connectivity differences in ASD have supported the existence of atypical development between childhood and adulthood (Anderson et al., 2011; Di Martino et al., 2011; Wiggins et al., 2011; Wiggins et al., 2012; Padmanabhan et al., 2013; Bos et al., 2014; Washington et al., 2014; Alaerts et al., 2015; Doyle-Thomas et al., 2015; Jiang et al., 2015; Nomi & Uddin, 2015; Burrows et al., 2016; Dajani & Uddin, 2016; Farrant & Uddin, 2016; Long et al., 2016; Lee et al., 2017; Woodward et al., 2017; but see Shih et al., 2011), a pattern which has also been found when examining the impact of autistic traits on functional connectivity development across a combined sample of individuals with and without ASD (Neufeld et al. 2017). However, the exact trajectories of resting-state functional connectivity differences in ASD is still unclear and may also be regionally dependent (Padmanabhan et al., 2013; Alaerts et al., 2015; Burrows et al., 2016; Dajani & Uddin, 2016; Farrant & Uddin, 2016; Lee et al., 2017). When focusing on higher-order cortico-cortical functional connectivity in cross-sectional samples spanning childhood through adulthood, there are still inconsistent findings: some results are suggestive of developmental normalization (Alaerts et al., 2015; Nomi & Uddin, 2015; Burrows et al., 2016; Dajani & Uddin, 2016; Farrant & Uddin, 2016; Lee et al., 2017), whereas other findings suggest continued atypical developmental trajectories in ASD (Wiggins et al., 2011; Wiggins et al., 2012; Bos et al., 2014; Doyle-Thomas et al., 2015; Jiang et al., 2015; Burrows et al., 2016; Dajani & Uddin, 2016; Farrant & Uddin, 2016; Lee et al., 2017).
Notably, all studies investigating the developmental trajectories of functional connectivity in ASD thus far have used cross-sectional samples. However, longitudinal studies are crucial for better understanding how functional connectivity alterations in ASD may relate to age. Longitudinal samples allow for greater sensitivity when mapping trajectories and comparing age groups, as well as greater confidence that age-dependent effects are not due to inherent differences between the sample characteristics of different age cohorts. We therefore examined resting-state functional connectivity in early/mid- and late adolescence using what is, to the best of our knowledge, the first longitudinal sample of resting-state fMRI data from individuals with ASD and matched TD controls.
Amongst the many RSNs and regions of interest that have been investigated in prior studies, three core neurocognitive RSNs are of particular interest in ASD: the default mode network (DMN), cingulo-opercular salience network (SN), and fronto-parietal central executive network (CEN). The DMN, SN, and CEN are interrelated, in that the DMN and the CEN are negatively connected in neurotypical adults and the SN is thought to modulate activity in these two networks as appropriate for the task at hand (Fox et al., 2005; Menon, 2011; Chai et al., 2014; Uddin, 2015). Importantly, the functional connectivity within and between these three networks not only exhibits significant differences in ASD, but can also be used to predict changes in autistic traits and adaptive behaviors over time (Nomi & Uddin, 2015; Plitt et al., 2015b; Abbott et al., 2016). We thus chose to focus on measures of functional connectivity within and between the DMN, SN, and CEN. Our results, although preliminary given our relatively small sample size, indicate that the developmental trajectories of functional connectivity between the CEN and the DMN is atypical among adolescents with ASD, underscoring the importance of considering age in our understanding of brain connectivity in ASD.
Methods
Participants
Participants were recruited from the UCLA Center for Autism Research and Treatment and the greater Los Angeles area for another study that served as the first time point for the current set of analyses; for this study, recruitment was completed with the goal of matching groups on gender. All subjects who were early/mid-adolescents during this initial study were subsequently re-contacted for participation in a second study. Data for the second time point was collected from interested participants who were available approximately 3 years following their first scan and who had no metal implants (including braces) at this time. Inclusion criteria for the ASD group consisted of a confirmed ASD diagnosis using the Autism Diagnostic Observation Schedule – Generic (ADOS) (Lord et al., 2000), the Autism Diagnostic Interview – Revised (ADI-R) (Lord et al., 1994), and best clinical judgment. Exclusionary criteria for both groups included full-scale IQ < 70 as assessed using the 4th edition of the Wechsler Intelligence Scale for Children (WISC) (Wechlser, 2003), or the 1st or 2nd edition of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechlser, 1999, 2011). Additional exclusionary criteria for the ASD group included a history of neurological, psychiatric or other developmental disorders prior to joining the study, with the exception of anxiety, depression, and attention-deficit/hyperactivity disorder (ADHD) due to their frequent comorbidity with ASD (Lai et al., 2014). However, ASD was required to be the primary diagnosis for study inclusion. Additional exclusionary criteria for the TD group consisted of any psychiatric or neurological disorder as ascertained prior to study start, the existence of any first- or second-degree relative with ASD, and a total t-score > 65 on the parent-report version of the Social Responsiveness Scale – 2nd Edition (SRS-2) (Constantino & Gruber, 2012), a measure of social functioning. A total of 41 subjects provided data at both time points, and a subset of these longitudinal data are now publicly available on the Autism Brain Imaging Data Exchange II (Di Martino et al., 2017). For the current study, 1 TD and 1 ASD subject were excluded from our current sample due to excessive head motion during MRI scanning (maximum absolute motion > 5 mm) and 1 TD participant was excluded due to an elevated SRS-2 t-score at the second time point. No additional subjects were excluded to match groups, and our final total sample size was 16 participants with ASD and 22 TD participants.
Descriptive statistics and P-values for all measures are presented in Table 1, with the reported group comparisons completed using t-tests, chi-squared tests, or their non-parametric equivalent as appropriate. The first time point (“Time 1” or “T1”) was collected in early to mid-adolescence, with a mean age of 12.7 years old (range: 11.5 – 14.3 years old). Participants returned a mean of 3.1 years later (range: 2.6 - 3.6 years), and the second time point (“Time 2” or “T2”) was collected in late adolescence, with a mean age of 15.8 years old (range: 14.4 – 17.5 years old). The ASD and TD groups were matched on full-scale IQ at T1 (P > 0.2), in addition to being matched on the following at both time points: age, gender, handedness, the number of single-subject independent components labeled as motion or noise during fMRI data preprocessing, and mean relative motion (all Ps > 0.1), with the exception of age at T2 where there was a trend for the ASD group to be younger (P = 0.097). Importantly, however, there was no significant difference in time between scans when comparing the ASD and TD groups (P > 0.5). When comparing the difference in motion-related measures between early/mid- and late adolescence, both groups moved less as they got older, measured by both the number of components labeled as motion (both P < 0.05) and mean relative motion (ASD: P = 0.098; TD: P = 0.03). Notably, however, there were no significant differences between the ASD and TD groups when examining how these motion-related measures changed between T1 and T2 (both P > 0.6).
Table 1.
Mean and Standard Deviation of Sample Descriptives
| ASD
|
TD
|
ASD vs TD P-Values
|
T1 vs T2 P-Values
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | Time 1 | Time 2 | ASD | TD | ASD vs TD | |
| Male/Female Subjects | 15/1 | 15/1 | 22/0 | 22/0 | 0.42 | - | - | - | - |
| Age (years) | 12.5 ± 0.8 | 15.5 ± 0.8 | 12.9 ± 0.9 | 16.0 ± 0.9 | 0.13 | 0.097 | <0.001 | <0.001 | 0.59 |
| Handedness (R/L) | 15/1 | 15/1 | 21/1 | 21/1 | 1.00 | - | - | - | - |
| Full-Scale IQ | 101.3 ± 17.7 | - | 107.8 ± 13.5 | - | 0.21 | - | - | - | - |
| Mean Relative Motion (mm) | 0.13 ± 0.11 | 0.08 ± 0.05 | 0.10 ± 0.06 | 0.07 ± 0.04 | 0.44 | 0.49 | 0.098 | 0.03 | 0.85 |
| # of Motion/Noise Components | 21.8 ± 6.0 | 17.7 ± 3.7 | 18.9 ± 5.5 | 15.8 ± 5.3 | 0.13 | 0.23 | 0.02 | 0.04 | 0.64 |
| Total SRS-2 (t-score) | 74.0 ± 10.4 | 69.4 ± 8.9 | 44.1 ± 4.5† | 45.6 ± 6.4 | <.001 | <.001 | 0.07 | 0.71 | 0.09 |
| ADOS Social Affect | 10.7 ± 2.1 | - | - | - | - | - | - | - | - |
| ADOS Repetitive & Restricted | 2.9 ± 1.5 | - | - | - | - | - | - | - | - |
| ADOS Comparison Score | 8.1 ± 1.2 | - | - | - | - | - | - | - | - |
n=20
ADOS: Autism Diagnostic Observation Schedule – Generic; SRS: Social Responsiveness Scale – 2nd Edition; L: Left; R: Right.
Medication information for our sample is as follows. In early/mid-adolescence, no participants in the TD group and 8 participants in the ASD group were on one or more medications: 2 on aripiprazole, 1 on atomexetine, 1 on citalopram, 1 on fluoxetine, 3 on guanfacine, 1 on lisdexamfetamine, 2 on methylphenidate, and 1 on sertraline. In late-adolescence, 1 TD participant was on medication (escitalopram), and 10 participants with ASD were on one or more medications as follows: 1 on amphetamine/dextroamphetamine, 2 on aripiprazole, 1 on citalopram, 2 on dexmethylphenidate, 1 on dexmethylphenidate hydrochloride, 3 on guanfacine, 2 on lisdexamfetamine, and 3 on sertraline. Six participants with ASD changed medication types between the two time points.
MRI Data Acquisition
Scans were acquired on two Siemens 3T Trio scanners (identical hardware, software, and sequences) at UCLA using a 12-channel head coil, with groups and time points matched on scanner (all Ps > 0.2). During the 6-min resting-state fMRI scan, subjects were told to relax and look at a black fixation cross in the center of a white background, presented through MR-compatible goggles (TR=3000ms, TE=28ms, field of view [FOV]=192mm, 34 slices, slice thickness=4mm, in-plane voxel size=3×3mm). For registration purposes, we additionally collected a high-resolution echo planar scan that was co-planar to the fMRI scan to ensure identical distortion characteristics (TR=5000ms, TE=34ms, FOV=192mm, 36 slices, slice thickness=4mm, in-plane voxel size of 1.5×1.5mm).
fMRI Data Preprocessing
Resting-state fMRI data for each time point underwent standard preprocessing using FMRIB’s Software Library (FSL) (Smith et al., 2004) and Analysis of Functional NeuroImages (AFNI) (Cox, 1996), including skull stripping, motion correction, smoothing with a 6 mm full width at half maximum (FWHM) Gaussian kernel, and affine registration with 6 degrees of freedom to each subject’s high-resolution matched bandwidth coplanar image from the same time point using FMRIB’s Linear Image Registration Tool (FLIRT) (Jenkinson & Smith, 2001; Jenkinson et al., 2002), followed by affine registration with 12 degrees of freedom to the standard MNI template of 152 averaged brains (2mm isotropic resolution) using FLIRT. Scans from both time points were directly registered to the same standard template to avoid the bias inherent in registering all images to the baseline time point (Reuter & Fisch, 2011; Thompson & Holland, 2011); each registration was visually inspected as part of quality control procedures. To remove potential motion confounds, the automatic independent component classifier ICA-AROMA (Pruim et al., 2015a; Pruim et al., 2015b) was used to regress out components labeled as motion or noise at the single-subject level. This approach was used instead of deleting individual motion-contaminated volumes (Power et al., 2012; Power et al., 2014) to effectively control for motion while retaining data from the full scan. To further reduce noise and other confounds, data were bandpass filtered (0.01 Hz < t < 0.1 Hz) and the following measures were included as nuisance regressors at the single-subject level: mean white matter time series, mean cerebrospinal fluid time series and mean global time series, as well as the temporal derivatives of these regressors (Power et al., 2014).
fMRI Data Analysis
To examine resting-state functional connectivity of the DMN, SN, and CEN, average residual time series were extracted from 5mm radius seeds located in the precuneus for the DMN (MNI coordinates: −6, −52, 43; Fox et al., 2005), the orbital frontoinsula for the SN (MNI coordinates: 38, 26, −10; Seeley et al., 2007), and the dorsolateral prefrontal cortex for the CEN (MNI coordinates: 44, 36, 30; Seeley et al., 2007). The time series from each seed was subsequently correlated with that of every other voxel in the brain, and the resulting correlation map for each network was then converted into a z-statistic map using Fisher’s r-to-z transform.
All whole-brain group-level contrasts were conducted in FSL using FMRIB’s Local Analyses of Mixed Effects (FLAME 1+2), with variance estimated separately for the ASD and TD groups. For each network, we examined the effect of group at each time point, the effect of time point within each group, and the interaction between group and time point. All analyses covaried for demeaned age at the second time point due to the marginally trending difference in age between groups at T2. To control for multiple comparisons across the brain, Gaussian random-field theory was used with a voxelwise threshold of Z > 2.3 and a corrected cluster threshold of P < 0.001; we elected to use this comparatively stringent threshold to minimize concerns of false positives. As we were primarily interested in functional connectivity within and between the DMN, SN, and CEN, all group analyses were limited to voxels which belonged to one of these networks at a threshold of Z > 2.3, P < 0.001 within the ASD or TD group at either time point. That is, all group analyses were prethreshold masked using a combined mask of the DMN, SN, and CEN across both of our groups and both of our time points.
To aid with results interpretation, connectivity z-statistics were extracted from all significant clusters using FSL and then analyzed with SPSS Statistics version 24.0.0.0 (IBM Corporation, Armonk, NY, USA). A general linear model that included demeaned age at T2 was used to test if each cluster that exhibited a significant effect of group or time point displayed positive or negative functional connectivity.
To ascertain whether each significant cluster represented within- or between-network functional connectivity, we defined the network membership of each cluster using an approach which is similar to that used by Sherman et al. (2014) and is in keeping with prior developmental studies that have used the more mature time point for such labeling purposes (e.g., Fair et al., 2009; Marek et al., 2015). Specifically, for each resting-state network we created a combined mask across our ASD and TD groups using their data at T2. For instance, the combined DMN mask was the summation of our ASD group’s DMN at T2 and our TD group’s DMN at T2. We then used AFNI to calculate the overlap between each of the three network masks (DMN, SN, CEN) and each significant cluster. Each cluster was subsequently classified as belonging to the network with which it displayed the greatest percentage overlap. As an example, if the DMN precuneus seed exhibited significant functional connectivity changes between groups and/or time points with a cluster in the frontal lobe, the extent to which this frontal cluster overlapped with the DMN, SN, and CEN masks would then be calculated. If this significant cluster displayed the greatest percent overlap with the DMN mask, it would be labeled as within-network DMN functional connectivity. However, if this cluster displayed the greatest percent overlap with the SN (or CEN) mask, it would instead be labeled as between-network functional connectivity between the DMN and the SN (or CEN, respectively).
To verify that our whole-brain functional connectivity results were not driven by medication status (Linke et al., 2017), residual motion (Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012), the use of parametric statistical tests to correct for multiple comparisons (Eklund et al., 2016), or the effect of global signal regression (Murphy & Fox, 2017), four sets of additional analyses were conducted. First, we used a general linear model controlling for demeaned T2 age to investigate the impact of medication status among participants with ASD on the extracted connectivity z-statistics. Second, all group-level contrasts were completed with the addition of mean relative motion as a group-level covariate, where relative motion was calculated using FSL’s MCFLIRT tool (Jenkinson et al., 2002). Third, all comparisons were repeated using FSL’s randomise with threshold-free cluster enhancement to conduct permutation testing at a corrected threshold of P < 0.05 (Winkler et al., 2014). Fourth, all group-level analyses were completed using a single-subject preprocessing stream which did not include global signal or its derivative as nuisance regressors.
Results
Within-Group Resting-State Network Maps
The DMN maps included the precuneus/posterior cingulate cortex, medial prefrontal cortex (mPFC)/ventromedial prefrontal cortex (vmPFC), angular gyrus, and superior frontal gyrus/middle frontal gyrus for both groups at T1 and T2 (Fig. S1, Table S1). TD participants additionally displayed functional connectivity with the middle temporal gyrus (MTG) and thalamus at both time points, while participants with ASD displayed significant functional connectivity with these structures only during late adolescence. Further regions that were part of the DMN for only one group and time point (i.e., cerebellum, caudate, parahippocampal gyrus) are shown in Fig. S1.
The SN maps of both groups included the anterior insula, anterior cingulate cortex, dorsomedial prefrontal cortex (dmPFC), frontal pole, ventrolateral prefrontal cortex (vlPFC), and subcortical structures at each time point (Fig. S2, Table S2). During both T1 and T2, TD participants furthermore exhibited functional connectivity between the SN seed and the dorsolateral prefrontal cortex (dlPFC), as well as functional connectivity with the mPFC at T1 only and functional connectivity with the supramarginal gyrus and MTG at T2 only. Participants with ASD displayed significant functional connectivity with the supramarginal gyrus, superior temporal gyrus, and MTG at both time points, in addition to dlPFC functional connectivity during the late adolescence.
Lastly, the CEN maps for both groups contained the intraparietal sulcus and dlPFC/vlPFC at both time points (Fig. S3, Table S3). The TD group also displayed significant functional connectivity with the dmPFC, insula, temporal lobe, and cerebellum at both time points, as well as significant functional connectivity with the thalamus during the late adolescent time point only. Of these additional regions which were part of the CEN in the TD group, the ASD group exhibited significant functional connectivity only with the insula and temporal lobe, and this functional connectivity was visible solely during early/mid-adolescence. Further structures that exhibited significant functional connectivity with the CEN for one diagnostic group and time point (caudate, pallidum) are shown in Fig. S3.
Effects of Group within Time Point
When comparing the ASD and TD groups at T1, no significant differences were noted in the DMN, SN, or CEN. Similarly, when examining DMN and SN functional connectivity at T2, no significant between-group differences emerged. During late adolescence, however, the CEN demonstrated significant functional connectivity differences with the mPFC/vmPFC (MNI peak coordinates: −12, 44, −8; max Z: 4.20) when contrasting the ASD and TD groups. This mPFC/vmPFC area is part of the DMN (Table S4), and extracted z-statistics revealed that the TD group displayed significant negative functional connectivity between the CEN seed and this area (P < 0.001), whereas the ASD did not show significant functional connectivity between these regions (P > 0.1) (Fig. 1).
Figure 1.
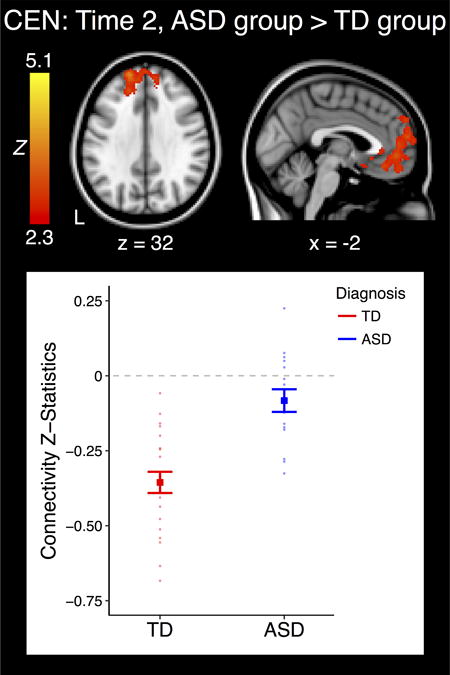
Cluster which displayed a significant effect of group within time point. The plot represents the connectivity values extracted from the significant cluster at left. Bold indicates group means, and each individual point represents the connectivity value from one subject. Error bars are standard error of the mean (+/− 1). ASD: Autism Spectrum Disorder; TD: Typically Developing; CEN: Central Executive Network; L: Left.
Effects of Time Point within Group
The ASD group displayed no significant changes in DMN, SN, or CEN functional connectivity between early/mid- and late adolescence. In contrast, the TD group displayed significant changes over time in all three networks.
With regards to the DMN, the TD group displayed reduced functional connectivity with increasing age with the right angular gyrus/MTG (MNI peak coordinates: 42, −56, 28; max Z: 5.01), a region within the DMN (Table S4). Connectivity z-statistics from this cluster showed that there was significant positive functional connectivity with this region during both time points among TD participants (both P < .01), although there was greater positive functional connectivity during T1 than T2 (Fig. 2A).
Figure 2.
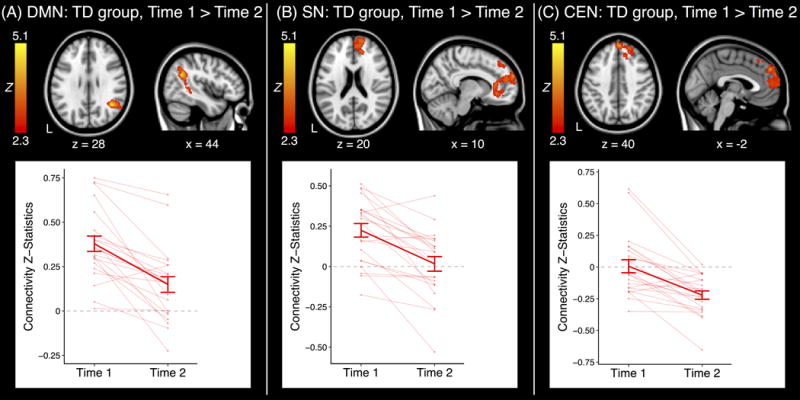
Clusters which displayed a significant effect of time point within group. Each plot represents the connectivity values extracted from the significant cluster at left. Error bars are standard error of the mean (+/− 1). ASD: Autism Spectrum Disorder; TD: Typically Developing; CEN: Central Executive Network; DMN: Default Mode Network; SN: Salience Network; L: Left.
When investigating the SN, the TD group displayed decreasing functional connectivity with the right mPFC (MNI peak coordinates: 12, 62, 24; max Z: 4.18) with increasing age. The mPFC is part of the DMN (Table S4), and extracted z-statistics revealed that the SN was positively connected with this region in early/mid-adolescence (P < 0.001) but not significantly connected with this area in late adolescence (P > 0.7) (Fig. 2B).
For the CEN, TD participants displayed an increase in negative functional connectivity with the mPFC/dmPFC from T1 to T2 (MNI peak coordinates: 2, 52, 36; max Z: 5.09), an area which again falls within the DMN (Table S4). The TD group did not display significant functional connectivity with this cluster in early/mid-adolescence (P > 0.6) but did display significant negative functional connectivity with this region during late adolescence (P < 0.001) (Fig. 2C).
Interactions between Group and Time Point
There was no significant interaction between group and time point for the DMN or the SN. However, a significant group × time point interaction was identified for the CEN with the mPFC/dmPFC (Fig. 3; MNI peak coordinates: 10, 42, 28; max Z: 4.00). This mPFC/dmPFC cluster is located within the DMN (Table S4) and notably overlaps with the region where the ASD and TD groups significantly differed in CEN functional connectivity during late adolescence (Fig. 1), as well as the region in which the TD group displayed a significant change in CEN functional connectivity over time (Fig. 2C).
Figure 3.
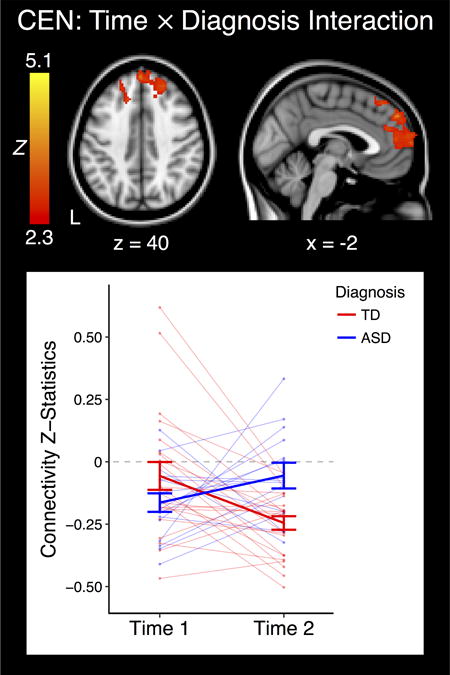
Cluster which displayed a significant interaction between group and time point. The plot represents the connectivity values extracted from the significant cluster at left. Bold indicates group means, and each individual point or line represents the connectivity value or values from one subject. Error bars are standard error of the mean (+/− 1). ASD: Autism Spectrum Disorder; TD: Typically Developing; CEN: Central Executive Network; L: Left.
To qualify this group × time point interaction, we extracted and analyzed connectivity z-statistics from this significant mPFC/dmPFC cluster. During early/mid-adolescence, the ASD group displayed significant negative functional connectivity between the CEN seed and this mPFC/dmPFC area (P < 0.001), whereas the TD group exhibited no significant functional connectivity between these regions (P > 0.4), although as described above there was no significant difference between the ASD and TD groups at the whole-brain level at this time point. By late adolescence the ASD group displayed no significant functional connectivity between the CEN seed and this cluster (P > 0.5), while the TD group exhibited negative functional connectivity (P < 0.001).
Additional Analyses
Medication status did not affect our results. Specifically, when examining connectivity z-statistics extracted from clusters showing significant effects at T1 (Fig. 3) or T2 (Fig. 1, Fig. 3), no significant differences were observed between unmedicated and medicated participants with ASD (all Ps > 0.3). Similarly, the change in connectivity z-statistics between Time 1 and Time 2 (Fig. 3) did not differ between those subjects with ASD who switched medication and those who did not (P > 0.5). When repeating our whole-brain analyses with mean relative motion as a group-level covariate, all whole-brain analyses yielded similar results (Table S5). Similarly, when using permutation testing all functional connectivity findings remained significant; there was additionally a significant change over time in CEN functional connectivity with the thalamus among TD participants (Table S5). When conducting analyses without global signal regression, the interaction between group and time point remained significant; the difference in T2 CEN functional connectivity between the ASD and TD groups, as well as the developmental change in CEN functional connectivity among TD participants, were also statistically significant albeit at a somewhat less stringent statistical threshold of (Z > 2.3, P < .01; Table S6).
Discussion
We found that adolescents with ASD exhibited age-dependent alterations relative to TD controls in functional connectivity between the CEN and the DMN. Previous studies have found that individuals with ASD generally display atypical functional connectivity, but conflicting results have emerged as to whether ASD is associated with increased or decreased functional connectivity (Rudie & Dapretto, 2013; Hernandez et al., 2015; Hull et al., 2016; Picci et al., 2016; Vasa et al., 2016). Such inconsistent findings may have been driven by differences in age between studies, coupled with atypical developmental trajectories in ASD between childhood an adulthood (Uddin et al., 2013b). As no prior longitudinal studies have investigated whether ASD does indeed display an altered pattern of brain development during this window, our results are the first longitudinal data to support the presence of atypical development of functional connectivity in adolescents with ASD. We focused on the DMN, SN, and CEN based on their previously-established relevance to ASD and their known relationships with each other: the DMN and CEN are anticorrelated in neurotypical adults, while the SN is theorized to influence activity within the DMN and CEN (Fox et al., 2005; Menon, 2011; Barber et al., 2013; Chai et al., 2014; Plitt et al., 2015b; Uddin, 2015; Abbott et al., 2016). More generally, the DMN typically deactivates during externally-driven tasks and has been associated with social cognition, whereas the CEN generally activates during cognitively demanding tasks and has previously been related to executive functioning and decision making (Menon, 2011). The SN is thought to play a role in detecting and coordinating a response to salient interoceptive and exteroceptive stimuli, including modulating the DMN and CEN as necessary (Menon & Uddin, 2010).
When investigating differences between the ASD and TD groups within each time point, the CEN exhibited significantly less negative functional connectivity with the mPFC hub of the DMN among late adolescents with ASD than their TD counterparts, such that the CEN and the DMN were anticorrelated in typical development but not so in ASD. A prior study examining a mixed sample of children and adolescents similarly found less negative functional connectivity between the CEN and the DMN in ASD when using a preprocessing pipeline similar to ours (Abbott et al., 2016). Functional connectivity alterations between the CEN and DMN have also previously been demonstrated in other neurodevelopmental disorders (e.g., Hoekzema et al., 2014; Manoliu et al., 2014), and functional connectivity patterns between the CEN and the DMN in ASD can significantly predict changes in adaptive behavior among late adolescents with ASD (Plitt et al., 2015b). While prior research has also found alterations in within-network functional connectivity among individuals with ASD (see Hull et al., 2016 for review), we did not find any differences in within-network functional connectivity when contrasting the ASD and TD groups. This may be due to our strict statistical threshold, chosen to stringently control for false positives.
When examining longitudinal trajectories of the DMN, SN, and CEN within each group, the TD group displayed significant development in all three of these networks. The DMN displayed a reduction in short-range within-network functional connectivity, whereas the SN had decreased between-network functional connectivity with the mPFC hub of the DMN. The TD group additionally displayed an increase in negative functional connectivity between the CEN and the mPFC hub of the DMN, such that the CEN and DMN were significantly anticorrelated during late but not mid-adolescence. These developmental changes in our TD sample are in line with prior studies demonstrating that typical development between childhood and adulthood is characterized by a weakening of short-range connections, an increase in segregation between networks, and the development of anticorrelations between the CEN and the DMN (see Ernst et al., 2015; Stevens, 2016 for review). In contrast, our ASD sample displayed no significant longitudinal changes in the DMN, SN, or CEN from early/mid- to late adolescence. Some prior cross-sectional studies similarly found that ASD is associated with less change in functional connectivity over development (Padmanabhan et al., 2013; Washington et al., 2014), although other cross-sectional studies have not found such a relationship (Wiggins et al., 2011; Wiggins et al., 2012; Bos et al., 2014; Doyle-Thomas et al., 2015). As previous studies have suggested that typical development displays synaptic reorganization during adolescence (Blakemore, 2012), the lack of significant developmental changes among our participants with ASD may reflect atypical synaptic processes (Zikopoulos & Barbas, 2013), possibly driven by ASD-associated genetic variants which have been predicted to influence the structure and function of synapses (Bourgeron, 2015).
When examining the interaction between group and time point, the developmental trajectories of CEN functional connectivity with the mPFC hub of the DMN significantly differed between the ASD and TD groups. This finding was driven by the whole-brain results described above: the TD but not the ASD group displayed an increase in negative functional connectivity between the CEN and the DMN with age, which resulted in the TD but not the ASD group having significant anticorrelations between the CEN and the DMN during late adolescence. Our results suggest that TD individuals display developmental trajectories that allow them to attain mature patterns of negative functional connectivity between the CEN and DMN by late adolescence, whereas individuals with ASD do not. Prior studies have noted that the emergence of anticorrelations between the CEN and the DMN in typical development occurs during a similar developmental window as normative improvements in executive function (e.g., Luna et al., 2004; Chai et al., 2014), and stronger anticorrelations between the CEN and DMN are associated with better performance on lab-based measures of executive function and IQ in neurotypical individuals (Kelly et al., 2008; Hampson et al., 2010; Sherman et al., 2014). Our findings thus suggest the possibility that atypical developmental trajectories between the CEN and DMN in ASD, which prevent them from attaining typical mature patterns of functional connectivity, may be related to the persistent executive function deficits observed in ASD (e.g., Chen et al., 2016; Davids et al., 2016; Wang et al., 2017).
More broadly, our results support a localized age-dependency of functional connectivity alterations in ASD. This is the first longitudinal study to support the notion that variable functional connectivity findings in ASD may be driven by age differences (Uddin et al., 2013b). However, our results also suggest that the developmental trajectories for different functional networks are not equally impacted in ASD, underscoring the value of considering other factors which may be driving conflicting results in the ASD literature. For instance, functional connectivity estimates in ASD may potentially be impacted by genetic (Rudie et al., 2012; Bourgeron, 2015; Hernandez et al., 2017) and behavioral heterogeneity between different ASD cohorts, including variation in previously completed interventions, the presence of psychiatric comorbidities, and variability in intellectual functioning and autistic symptoms (Hazlett et al., 2006; Di Martino et al., 2013; Uddin et al., 2013a; Venkataraman et al., 2016); methodological differences, such as whether analyses were conducted on task-based or resting-state measures of functional connectivity, may similarly affect functional connectivity findings in ASD across studies (Muller et al., 2011; Nair et al., 2014; Hernandez et al., 2015). Our pattern of results additionally indicate that between-network functional connectivity may be a more sensitive metric for detecting neural alterations in ASD than focusing on within-network functional connectivity, as our participants displayed functional connectivity differences in the former but not the latter.
Based on our findings, future investigations which plan to use connectivity-based measures for pre-clinical purposes, such as predicting treatment response or creating biologically-based ASD subgroups, should consider including age as a variable of interest to improve the accuracy and meaningfulness of results. However, our sample was relatively small and future investigations with much larger longitudinal sample sizes, achievable through data-sharing, are needed to replicate the preliminary findings reported here and allow for a better characterization of brain-behavior relationships (Di Martino et al., 2017). Additionally, longitudinal studies with a wider age range should be used to more comprehensively delineate changes in functional connectivity among individuals with ASD over development. Such future longitudinal studies should also collect lab-based measures of executive function to further investigate the relationship between RSN development and executive function in ASD. Lastly, future investigations should additionally examine whether boys and girls with ASD display similar alterations in developmental trajectories, as prior studies suggest the existence of behavioral and genetic differences in ASD as a function of gender (Werling & Geschwind, 2013; Hull et al., 2017).
In conclusion, our results demonstrate that the developmental trajectories of the CEN and the DMN – as assessed in a longitudinal sample, covarying for motion, and using robust nonparametric statistics – are significantly altered in adolescents with ASD. These findings thus highlight the need to carefully consider participant age when examining functional connectivity in ASD.
Supplementary Material
Lay Summary.
Brain connectivity may develop differently during adolescence in youth with autism spectrum disorder (ASD). We looked at changes in brain connectivity over time within individuals and found that, for some brain regions, adolescents with ASD did not show the same changes in brain connectivity that typically developing adolescents did. This suggests it is important to consider age when studying brain connectivity in ASD.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (award number P50HD055784 to S.Y.B.), the National Institute of Mental Health (award number F31MH110140 to K.E.L. and T32MH073526 to L.M.H.), the National Institute of Neurological Disorders and Stroke (award number T32NS048004 to K.E.L. and F99NS105206 to L.M.H.), and the ARCS Foundation. We are also grateful for the generous support from the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Capital Group Companies Charitable Foundation, William M. and Linda R. Dietel Philanthropic Fund, and Northstar Fund. Research reported in this publication was also partially supported by the National Center for Research Resources and by the Office of the Director of the National Institutes of Health under award numbers C06RR012169, C06RR015431 and S10OD011939. Our appreciation additionally extends to Hilary Bowman, Rosemary McCarron, and Devora Beck-Pancer for data collection and coordination. The contents of this paper are solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Human subjects oversight and approval was provided by UCLA’s Institutional Review Board, and the authors declare no conflict of interest.
Contributor Information
Katherine E. Lawrence, Ahmanson-Lovelace Brain Mapping Center, University of California Los Angeles, Los Angeles, CA, USA Interdepartmental Neuroscience Program, University of California Los Angeles, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA.
Leanna M. Hernandez, Ahmanson-Lovelace Brain Mapping Center, University of California Los Angeles, Los Angeles, CA, USA Interdepartmental Neuroscience Program, University of California Los Angeles, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA.
Susan Y. Bookheimer, Center for Cognitive Neuroscience Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA.
Mirella Dapretto, Ahmanson-Lovelace Brain Mapping Center, University of California Los Angeles, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA.
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, et al. Patterns of Atypical Functional Connectivity and Behavioral Links in Autism Differ Between Default, Salience, and Executive Networks. Cereb Cortex. 2016;26(10):4034–4045. doi: 10.1093/cercor/bhv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K, Nayar K, Kelly C, Raithel J, Milham MP, Di Martino A. Age-related changes in intrinsic function of the superior temporal sulcus in autism spectrum disorders. Soc Cogn Affect Neurosci. 2015;10(10):1413–1423. doi: 10.1093/scan/nsv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington DC: 2013. [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011;21(5):1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51(1):156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61(2):397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Bos DJ, van Raalten TR, Oranje B, Smits AR, Kobussen NA, Belle J, et al. Developmental differences in higher-order resting-state networks in Autism Spectrum Disorder. Neuroimage Clin. 2014;4:820–827. doi: 10.1016/j.nicl.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16(9):551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- Burrows CA, Laird AR, Uddin LQ. Functional connectivity of brain regions for self- and other-evaluation in children, adolescents and adults with autism. Dev Sci. 2016;19(4):564–580. doi: 10.1111/desc.12400. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci. 2014;26(3):501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SF, Chien YL, Wu CT, Shang CY, Wu YY, Gau SS. Deficits in executive functions among youths with autism spectrum disorders: an age-stratified analysis. Psychol Med. 2016;46(8):1625–1638. doi: 10.1017/S0033291715002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale–Second Edition (SRS-2) Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dajani DR, Uddin LQ. Local brain connectivity across development in autism spectrum disorder: A cross-sectional investigation. Autism Res. 2016;9(1):43–54. doi: 10.1002/aur.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids RC, Groen Y, Berg IJ, Tucha OM, van Balkom ID. Executive Functions in Older Adults With Autism Spectrum Disorder: Objective Performance and Subjective Complaints. J Autism Dev Disord. 2016;46(9):2859–2873. doi: 10.1007/s10803-016-2831-4. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, O’Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, et al. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data. 2017;4:170010. doi: 10.1038/sdata.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle-Thomas KA, Lee W, Foster NE, Tryfon A, Ouimet T, Hyde KL, et al. Atypical functional brain connectivity during rest in autism spectrum disorders. Ann Neurol. 2015;77(5):866–876. doi: 10.1002/ana.24391. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA. fMRI functional connectivity applied to adolescent neurodevelopment. Annu Rev Clin Psychol. 2015;11:361–377. doi: 10.1146/annurev-clinpsy-032814-112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ. Atypical developmental of dorsal and ventral attention networks in autism. Dev Sci. 2016;19(4):550–563. doi: 10.1111/desc.12359. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28(8):1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol Psychiatry. 2006;59(1):1–6. doi: 10.1016/j.biopsych.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Hernandez LM, Krasileva K, Green SA, Sherman LE, Ponting C, McCarron R, et al. Additive effects of oxytocin receptor gene polymorphisms on reward circuitry in youth with autism. Mol Psychiatry. 2017;22(8):1134–1139. doi: 10.1038/mp.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Rudie JD, Green SA, Bookheimer S, Dapretto M. Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology. 2015;40(1):171–189. doi: 10.1038/npp.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernandez V, Bosch R, Soliva JC, et al. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum Brain Mapp. 2014;35(4):1261–1272. doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JV, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. Resting-State Functional Connectivity in Autism Spectrum Disorders: A Review. Front Psychiatry. 2016;7:205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull L, Mandy W, Petrides KV. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism. 2017;21(6):706–727. doi: 10.1177/1362361316669087. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jiang L, Hou XH, Yang N, Yang Z, Zuo XN. Examination of Local Functional Homogeneity in Autism. Biomed Res Int. 2015;2015:174371. doi: 10.1155/2015/174371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Uddin LQ, Kenet T, Chugani D, Muller RA. Brain connectivity in autism. Front Hum Neurosci. 2014;8:349. doi: 10.3389/fnhum.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Lai CLE, Lau Z, Lui SSY, Lok E, Tam V, Chan Q, et al. Meta-analysis of neuropsychological measures of executive functioning in children and adolescents with high-functioning autism spectrum disorder. Autism Res. 2017;10(5):911–939. doi: 10.1002/aur.1723. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Baron-Cohen S. Autism. The Lancet. 2014;383(9920):896–910. doi: 10.1016/s0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- Lee Y, Park BY, James O, Kim SG, Park H. Autism Spectrum Disorder Related Functional Connectivity Changes in the Language Network in Children, Adolescents and Adults. Front Hum Neurosci. 2017;11:418. doi: 10.3389/fnhum.2017.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke AC, Olson L, Gao Y, Fishman I, Muller RA. Psychotropic medication use in autism spectrum disorders may affect functional brain connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(6):518–527. doi: 10.1016/j.bpsc.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Duan X, Mantini D, Chen H. Alteration of functional connectivity in autism spectrum disorder: effect of age and anatomical distance. Sci Rep. 2016;6:26527. doi: 10.1038/srep26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40(2):428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Keown CL, Datko M, Shih P, Keehn B, Muller RA. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Hum Brain Mapp. 2014;35(8):4035–4048. doi: 10.1002/hbm.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld J, Kuja-Halkola R, Mevel K, Cauvet E, Fransson P, Bolte S. Alterations in resting state connectivity along the autism trait continuum: a twin study. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.160. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci. 2013;7:814. doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picci G, Gotts SJ, Scherf KS. A theoretical rut: revisiting and critically evaluating the generalized under/over-connectivity hypothesis of autism. Dev Sci. 2016;19(4):524–549. doi: 10.1111/desc.12467. [DOI] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Martin A. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. Neuroimage Clin. 2015a;7:359–366. doi: 10.1016/j.nicl.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Wallace GL, Kenworthy L, Martin A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc Natl Acad Sci U S A. 2015b;112(48):E6699–6706. doi: 10.1073/pnas.1510098112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015a;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015b;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Pugliese CE, Anthony L, Strang JF, Dudley K, Wallace GL, Kenworthy L. Increasing adaptive behavior skill deficits from childhood to adolescence in autism spectrum disorder: role of executive function. J Autism Dev Disord. 2015;45(6):1579–1587. doi: 10.1007/s10803-014-2309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese CE, Anthony LG, Strang JF, Dudley K, Wallace GL, Naiman DQ, et al. Longitudinal Examination of Adaptive Behavior in Autism Spectrum Disorders: Influence of Executive Function. J Autism Dev Disord. 2016;46(2):467–477. doi: 10.1007/s10803-015-2584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Dapretto M. Convergent evidence of brain overconnectivity in children with autism? Cell Rep. 2013;5(3):565–566. doi: 10.1016/j.celrep.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012;75(5):904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Muller RA. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, et al. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17(12):666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T, Dawson G, Estes A. Brief Report: Executive Function as a Predictor of Academic Achievement in School-Aged Children with ASD. J Autism Dev Disord. 2017 doi: 10.1007/s10803-017-3296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neurosci Biobehav Rev. 2016;70:13–32. doi: 10.1016/j.neubiorev.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Thompson WK, Holland D, Alzheimer’s Disease Neuroimaging, I. Bias in tensor based morphometry Stat-ROI measures may result in unrealistic power estimates. Neuroimage. 2011;57(1):1–4. doi: 10.1016/j.neuroimage.2010.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013a;70(8):869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013b;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Mostofsky SH, Ewen JB. The Disrupted Connectivity Hypothesis of Autism Spectrum Disorders: Time for the Next Phase in Research. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(3):245–252. doi: 10.1016/j.bpsc.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman A, Yang DY, Dvornek N, Staib LH, Duncan JS, Pelphrey KA, et al. Pivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorder. Neuroreport. 2016;27(14):1081–1085. doi: 10.1097/WNR.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang YB, Liu LL, Cui JF, Wang J, Shum DH, et al. A Meta-Analysis of Working Memory Impairments in Autism Spectrum Disorders. Neuropsychol Rev. 2017;27(1):46–61. doi: 10.1007/s11065-016-9336-y. [DOI] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT, Wolfe A, et al. Dysmaturation of the default mode network in autism. Hum Brain Mapp. 2014;35(4):1284–1296. doi: 10.1002/hbm.22252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechlser D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation; 1999. [Google Scholar]
- Wechlser D. Wechlser Intelligence Scale for Children–Fourth Edition (WISC-IV) San Antonio, TX: NCS Pearson; 2003. [Google Scholar]
- Wechlser D. Wechlser Abbreviated Scale of Intelligence–Second Edition (WASI-II) San Antonio, TX: NCS Pearson; 2011. [Google Scholar]
- Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26(2):146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Ashinoff S, Weng SJ, Carrasco M, Welsh RC, et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res. 2011;1380:187–197. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Bedoyan JK, Carrasco M, Welsh RC, Martin DM, et al. The impact of serotonin transporter genotype on default network connectivity in children and adolescents with autism spectrum disorders. Neuroimage Clin. 2012;2:17–24. doi: 10.1016/j.nicl.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Giraldo-Chica M, Rogers B, Cascio CJ. Thalamocortical dysconnectivity in autism spectrum disorder: An analysis of the Autism Brain Imaging Data Exchange. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(1):76–84. doi: 10.1016/j.bpsc.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, et al. Default mode network segregation and social deficits in autism spectrum disorder: Evidence from non-medicated children. Neuroimage Clin. 2015;9:223–232. doi: 10.1016/j.nicl.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci. 2013;7:609. doi: 10.3389/fnhum.2013.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


