Despite recent advances and ongoing intensive research, no antifibrotic treatment has been approved for liver fibrosis. Elucidation of the key steps in fibrogenesis may pave the road towards effective drugs. However, pathophysiological mechanisms are complex and severity of fibrosis may affect the efficacy of antifibrotic compounds. Earlier treatment may be more effective, (1) although reasons for the stage-dependent effectiveness of antifibrotic treatment are not entirely clear. For more than a decade, angiogenesis has been considered a contributor to liver fibrosis and inhibition of angiogenesis served as a model for antifibrotic treatment. However, success of this approach was variable. (2)
Increased stiffness occurs in the context of advanced liver fibrosis and measurement of stiffness by MR- or ultrasound-based elastography has been increasingly applied in clinical practice. However, recent advances in the field of mechanobiology have revealed that stiffness is more than just a bystander product of liver fibrosis; increased stiffness itself can promote inflammation and fibrosis through a process called mechanotransduction. (3) Different cell types can sense stiffness of the extracellular matrix (ECM) through various transmembranous receptors (such as integrins), which activate an intracellular program affecting cell migration and gene expression. (4) Stiffness can also direct angiogenesis, since ECM serves as a scaffold for endothelial cell anchorage and migration; this cell-ECM interaction can in turn further increase stiffness in an amplifying loop. (5–7) However, not much has been known so far about the link between stiffness, angiogenesis and liver fibrosis progression.
A recent publication in Nature Materials by Liu and colleagues investigated the role of mechanical tension generated during angiogenesis and its influence on the progression of liver fibrosis. (8) For the purpose of this study, the authors developed a novel in vitro tool to study the interaction between liver sinusoidal endothelial cells (LSEC) and hepatic stellate cells (HSC). This three-dimensional microfibrotic niche consisted of LSEC cultured on an underlying two-dimensional substrate with different degrees of stiffness, and an overlaid three-dimensional collagen type I hydrogel, which was embedded with HSC. Thereby, Liu et al. were able to analyze 1) spatial organization of LSEC and HSC, 2) dynamic interactions between LSEC and HSC in response to increased stiffness, 3) ECM remodeling during fibrosis progression, and 4) stage-dependent effects of anti-angiogenic drugs.
First, the authors showed that sinusoidal angiogenesis is markedly increased during early-stage fibrosis, but not at later stages in human patients and murine fibrosis models. In addition, Liu and colleagues found that LSEC behavior depends on the stiffness of their environment. While on low stiffness substrates, LSEC exhibited typical pro-angiogenic properties (such as tip cell migration, deposition of a basal membrane and increased formation of focal adhesion complexes), on high stiffness substrates LSEC showed random migration, sparsely distributed focal adhesion complexes and leaky sinusoids. Putting these data together, the authors hypothesized that a microfibrotic niche with a low stiffness represents an in vitro model for early-stage fibrosis in contrast to a high stiffness model, which mirrors late-stage fibrosis. To prove this concept, the authors analyzed the mRNA profiles of LSEC at an early and late-stage of fibrosis. Indeed, mechanobiologically modulated LSEC in microfibrotic niches largely recapitulated the featured mRNA changes of LSEC during fibrosis progression in vivo. (8) On a functional level, the authors observed that HSC activation was increased in early-stage compared to late-stage fibrosis. Of note, HSC activation was not mediated through paracrine factors, nor direct physical contact between the cells. Rather, HSC were directly activated by mechanical force conferred by stiffness.
Lastly, the authors found that anti-angiogenic drugs are beneficial in early-stage, but not late-stage fibrosis. Treatment with sorafenib reduced early-stage in vitro remodeling and attenuated early-stage fibrosis in vivo. In contrast, sorafenib did not attenuate collagen crosslinking in the late-stage in vitro model and even worsened late-stage-fibrosis in vivo despite its ongoing anti-angiogenic effect on endothelial cells. In later stage fibrosis, however, a small molecule inhibitor of the collagen crosslinking enzyme, lysyl oxidase (LOX) was able to reduce fibrosis in vitro and in vivo.
The study by Liu et al. enlightens the stage-dependent role of angiogenesis and angiogenesis-induced activation of HSC from a mechanobiological perspective. (8) Using a novel in vitro tool (microfibrotic niche), the authors reveal mechanotransduction as an important pathway in liver fibrosis progression. This has therapeutic implications: All steps involved in the process of mechanosensing- and transduction might serve as therapeutic targets, which go beyond classical growth factor and anti-angiogenic strategies. Using models for early and late-stage fibrosis, the authors were further able to investigate angiogenesis in a stage-dependent manner. The previously reported, controversial findings regarding angiogenesis in liver fibrosis can now be put together into a complex puzzle, where angiogenesis promotes fibrosis at an early disease stage, but not at a later stage. (2) Moreover, the study outlines an explanation for the stage-dependent effects of antifibrotic treatments: while in early stages, anti-angiogenic compounds may be useful, they might cause harm at later stages where angiogenesis is required for fibrosis resolution. In contrast, agents inhibiting collagen crosslinking may have a role in advanced disease although this conjecture remains to be fully proven. Despite these novel and enlightening findings, there are several limitations of the study: 1) the exact mechanisms how LSEC (and increased angiogenesis) affect HSC activation (and fibrosis) remain unanswered, 2) how LSEC sense increased stiffness to trigger angiogenesis and fibrosis was not elucidated, and 3) studies were conducted under stiffness values which may be considerably higher than those detected in liver cirrhosis.
In conclusion, the study highlights that time-point of treatment initiation matters in liver fibrosis depending on the chosen regimen. The authors give a rationale why anti-angiogenic treatment might have different effects in early versus late-stage fibrosis (Figure 1). However, it is too early to translate these findings into clinical practice. But considering the possible stage-dependent effects of anti-angiogenic and anti-fibrotic drugs will be critical in clinical trial design. Better three hours too soon than a minute too late (William Shakespeare, The Merry Wives of Windsor, 1602) – this might be true, at least when it comes to anti-angiogenesis therapy in liver fibrosis.
Figure 1.
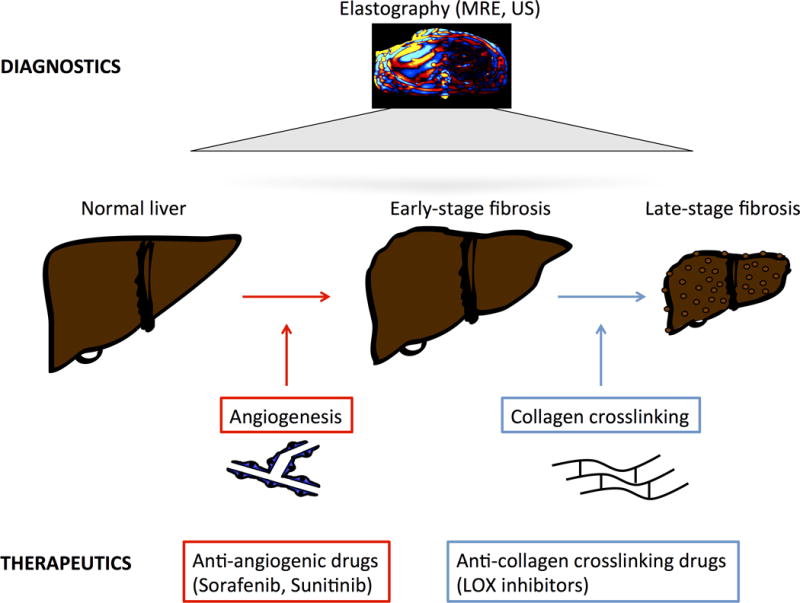
Stage-dependent model of liver fibrosis progression and anti-fibrotic treatment. Fibrosis progression and liver stiffness can be assessed and measured using MR- or ultrasound-based elastography (top). In early stages, angiogenesis contributes to liver fibrosis, suggesting possible benefit from anti-angiogenic drugs such as sorafenib or sunitinib. In advanced fibrosis, anti-angiogenic drugs may have detrimental effects. Drugs blocking collagen crosslinking, such as LOX inhibitors, could be beneficial at late stages (bottom).
Acknowledgments
Grant support: This work is supported in part by the Swiss National Science Foundation grant P2ZHP3_168561 (T.G.), an unrestricted Novartis Foundation grant (T.G.) and by the National Institutes of Health grants AA21788 and DK059615 (V.H.S)
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists
References
- 1.Leclercq IA, Sempoux C, Stärkel P, Horsmans Y. Limited therapeutic efficacy of pioglitazone on progression of hepatic fibrosis in rats. Gut. 2006;55:1020–1029. doi: 10.1136/gut.2005.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, Hu M, et al. Mechanotransduction and fibrosis. J Biomech. 2014;47:1997–2005. doi: 10.1016/j.jbiomech.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112(Pt 19):3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 6.Bishop PN. The role of extracellular matrix in retinal vascular development and preretinal neovascularization. Exp Eye Res. 2015;133:30–36. doi: 10.1016/j.exer.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, You Z, Yu H, Zhou L, Zhao H, Yan X, Li D, et al. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nat Mater. 2017;16:1252–1261. doi: 10.1038/nmat5024. [DOI] [PubMed] [Google Scholar]


