Abstract
Recruitment of liver sinusoidal endothelial cell progenitor cells, so-called sprocs, from the bone marrow by VEGF-sdf1 signaling promotes recovery from injury and drives liver regeneration. Matrix metalloproteinases (MMPs) can proteolytically cleave VEGF, which might inhibit progenitor cell recruitment, but systemic matrix metalloproteinase inhibition might prevent efflux of progenitors from the bone marrow. The hypothesis for this study was that liver-selective MMP-9 inhibition would protect the hepatic VEGF-sdf-1 signaling pathway, enhance bone marrow sproc recruitment, and thereby ameliorate liver injury and accelerate liver regeneration, whereas systemic MMP inhibition would impair bone marrow sproc mobilization and therefore have less benefit or would be detrimental. Results: Liver-selective MMP-9 inhibition accelerated liver regeneration after partial hepatectomy by 40%, whereas systemic MMP inhibition impaired liver regeneration. Liver-selective MMP-9 inhibition largely abolished warm ischemia-reperfusion injury. In the extended hepatectomy model, liver-selective MMP-9 inhibition restored liver sinusoidal endothelial cell integrity, enhanced liver regeneration, and reduced ascites. Liver-selective MMP-9 inhibition markedly increased recruitment and engraftment of bone marrow sprocs, whereas systemic MMP inhibition impaired mobilization of bone marrow sprocs and their hepatic engraftment. Hepatic MMP-9 proteolytically cleaved VEGF after partial hepatectomy. Liver-selective MMP-9 inhibition prevented VEGF cleavage and doubled protein expression of VEGF and its downstream signaling partner sdf-1. In contrast, systemic MMP inhibition enhanced recruitment and engraftment of infused allogeneic progenitors. Conclusion: Liver-selective MMP inhibition prevents proteolytic cleavage of hepatic VEGF, which enhances recruitment and engraftment of bone marrow sprocs after liver injury. This ameliorates injury and accelerates liver regeneration. Liver-selective MMP-9 inhibition may be a therapeutic tool for liver injury that damages the vasculature, whereas systemic matrix metalloproteinase inhibition can enhance the benefit of stem cell therapy with endothelial progenitor cells.
Keywords: endothelial progenitor cell, partial hepatectomy, liver sinusoidal endothelial cell, small-for-size graft injury, extended hepatectomy
After various forms of liver injury, hepatic VEGF is the central mediator that signals through stromal cell derived factor-1 (sdf1 or CXCL12) to induce proliferation in the bone marrow, mobilization to the circulation, and engraftment in the liver of CXCR7+ liver sinusoidal endothelial cell progenitor cells (sprocs) and signals through the nitric oxide pathway to induce the differentiation of engrafted sprocs to fenestrated liver sinusoidal endothelial cells (LSECs) (1–3). Although there is ample evidence that LSECs drive liver regeneration (4–6), our group has shown that this function is fulfilled by engrafted BM sprocs amongst the LSECs. Bone marrow suppression impairs liver regeneration after partial hepatectomy and the effect of bone marrow suppression is fully offset by infusion of whole bone marrow or of sprocs (1–3). Only the CXCR7+ subset of circulating sprocs engraft in the liver (1) and knockout of CXCR7+ endothelial cells in the body impairs liver regeneration (7). In addition to driving liver regeneration, recruitment of BM sprocs promotes recovery from toxic injury (2, 8).
There are two reasons to assume sprocs are a subset of endothelial progenitor cells. First, after partial hepatectomy there is a two-fold increase in circulating sprocs that persists for 72 hours (3). At 6 hours after partial hepatectomy, upwards of 90% of these cells are the CXCR7+ fraction that engrafts in the liver (1), but from 24 to 72 hours the circulating endothelial progenitor cells are almost all CXCR7-. Second LSECs are CD31+ (a classic endothelial marker) and CD45+ (8, 9) and we therefore define sprocs as progenitor cells with both these markers. Endothelial progenitor cells are often defined as the CD45- fraction of circulating progenitor cells. This further supports that LSEC progenitor cells or sprocs are a sub-set of endothelial progenitor cells.
Disparate types of injury that target LSECs increase matrix metalloproteinase (MMP) activity, including cold preservation injury (10), sinusoidal obstruction syndrome (11), acetaminophen toxicity (12), ischemia-reperfusion injury (13, 14), and small-for-size syndrome (15). The literature has conflicting reports on whether MMPs enhance [to cite just a few (16–18)] or reduce (19) VEGF effects. An explanation for MMP-mediated reduction of VEGF activity is the finding that MMPs, including MMP-9, can proteolytically cleave VEGF164 (19), generating VEGF with a dysfunctional angiogenic response. This suggests that inhibition of hepatic MMP activity after liver injury might enhance VEGF-sdf1 recruitment and repair by BM sprocs. Conversely, proteolytic cleavage by MMPs, including MMP-9, of extracellular matrix and cytokines that retain stem cells in their niche is necessary for mobilization of BM stem cells (20, 21). Thus inhibition of hepatic MMP activity might improve BM sproc recruitment and engraftment in the liver, whereas inhibition of BM MMP should impair BM sproc mobilization and diminish repair of liver injury.
Based on the above, we formulated the following two hypotheses. 1. Liver-selective inhibition of MMP with sparing of BM MMP should reduce liver injury and promote liver regeneration, whereas systemic MMP inhibition should be less beneficial or even detrimental. 2. Engraftment of infused sprocs for stem cell therapy should benefit from systemic MMP inhibition by preventing proteolytic cleavage of hepatic VEGF, and by reducing release of sprocs from the BM and thereby decreasing competition for engraftment.
The therapeutic benefit of liver-selective MMP-9 inhibition is shown in models of two-thirds partial hepatectomy (PH) (Fig 2), ischemia-reperfusion injury (Fig 3), and 90% hepatectomy as a model of small-for-size syndrome (Fig 4). Examination of the mechanisms by which liver-selective MMP-9 inhibition protects against injury and promotes liver regeneration are shown in figures 5 and 6. The use of systemic MMP inhibition to promote engraftment of infused sprocs, as a model for stem cell therapy, is shown in figure 7. Figure 8 is a diagram that describes the molecular pathways underlying the mechanisms.
Figure 2.
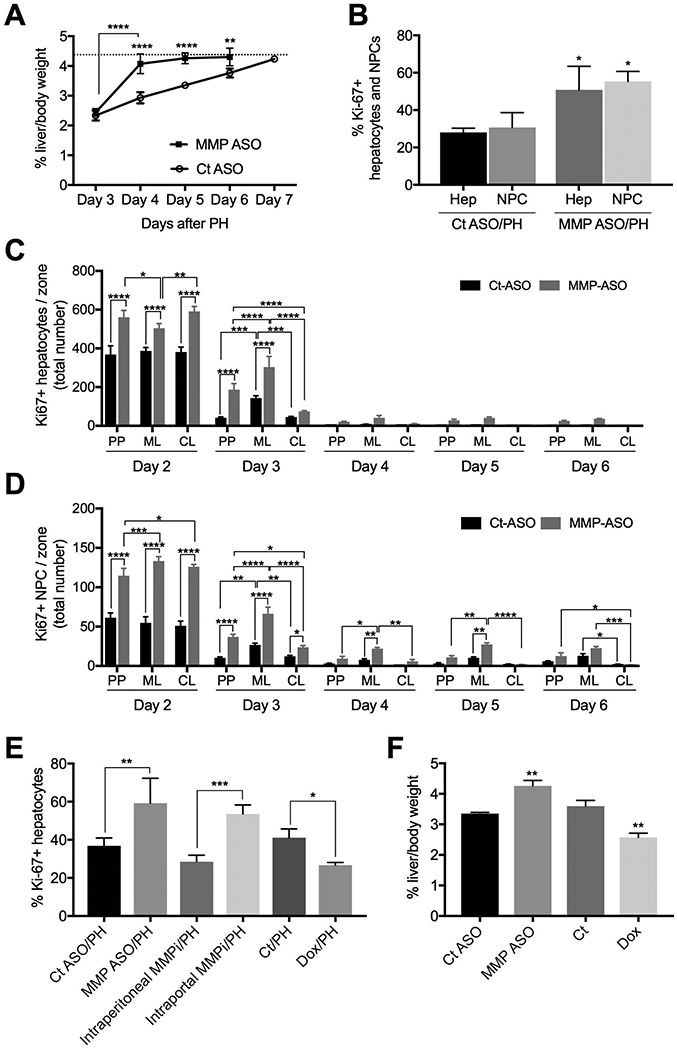
Liver-selective MMP-9 inhibition accelerates liver regeneration after two-thirds partial hepatectomy (PH). (A) Time-course from day 3-day 7: pretreatment with MMP-9 ASO accelerates liver regeneration after PH compared to scrambled control ASO (n=3). The dotted line is the average liver-to-body weight ratio of untreated control littermates. (B) Percentage of proliferating hepatocytes and non-parenchymal cells in the sinusoids on day 2 (ki-67) (n=3). (C) The number of hepatocytes proliferating in each zone from day 2-day 6 is shown for control ASO and MMP-9 ASO pretreated prior to PH (n=3 for each day). (D) The number of non-parenchymal cells proliferating in each zone from day 2-day 6 is shown for control ASO and MMP-9 ASO pretreated prior to PH (n=3 for each day). (E) Hepatocyte proliferation (ki-67) on day 2 after PH is enhanced by liver-selective MMP inhibition with MMP-9 ASO or by intraportal MMPi compared to intraperitoneal MMPi, whereas systemic inhibition with doxycycline reduces hepatocyte proliferation (n=3). (F) MMP-9 ASO increases liver/body weight ratio by 27% on day 5 after PH, whereas doxycycline reduces liver/body weight ratio by 29% (n=3). Abbreviations: CT, control; Dox, doxycycline; MMPi 2/9, inhibitor of MMP 2 and 9; PH, partial hepatectomy; PP, periportal, ML, midlobular, CL, centrilobular; / indicates two treatments, e.g. MMP ASO/PH is MMP ASO plus partial hepatectomy. Analysis by ANOVA was statistically significant and analyzed post-hoc by Fisher’s least significant difference. Levels of statistical significance are * p< 0.05, ** p< 0.01, *** p< 0.001, and **** p < 0.0001. Unless otherwise indicated, significance is based on comparison with the appropriate control. Unprocessed original scans of blots are shown in Supporting Figure S2B and 2C.
Figure 3.
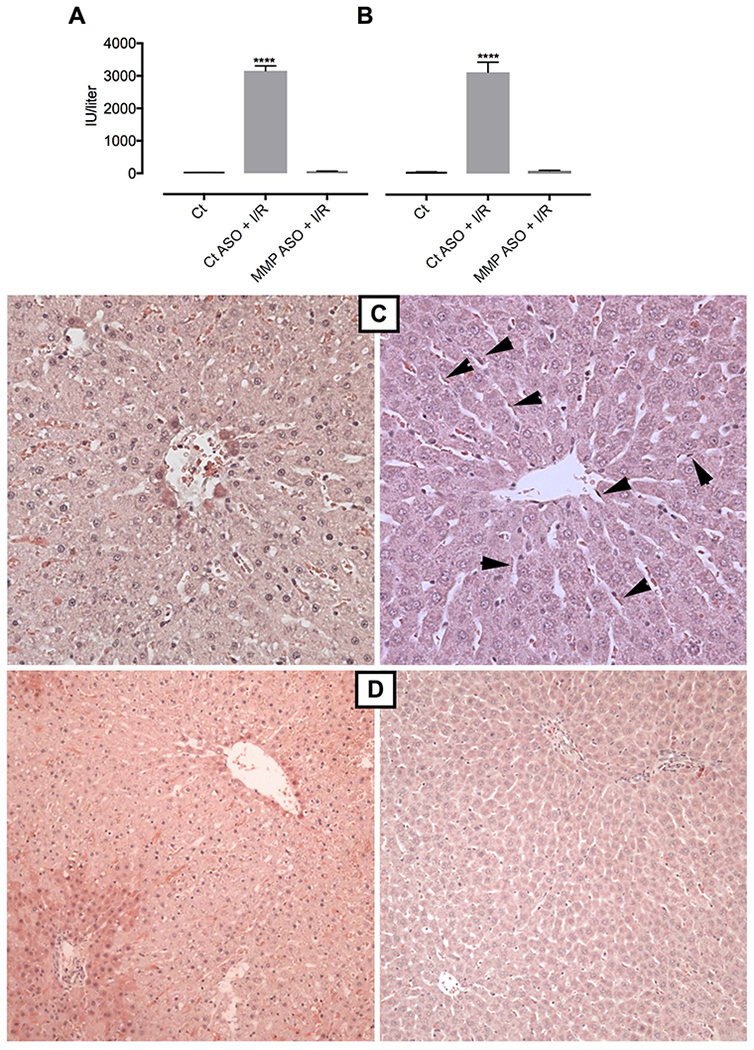
Liver-selective MMP-9 inhibition prevents ischemia-reperfusion (I/R) injury. (A) ALT (n=3) and (B) AST in littermate controls, and after pretreatment with control ASO or MMP-9 ASO followed by I/R injury, assessed by colorimetric assay (n=3). (C) high power hematoxylin-eosin (H & E) stain of pericentral lobule: control ASO/I/R injury (left panel) shows early hepatocyte injury with clearing of cytoplasm, lobular disarray, and lack of LSECs compared to normal appearing liver without hepatocyte injury or loss of LSECs (arrowheads) in the MMP-ASO/I/R group (right panel). (D) low power H & E stain demonstrates widespread hepatocyte changes with sparing around portal tract (vessel bottom left quadrant) and of terminal hepatocytes near the central vein in control ASO/I/R injury (left panel) versus no visible injury in MMP-9 ASO/I/R injury (right panel). Analysis by ANOVA was statistically significant and analyzed post-hoc by Fisher’s least significant difference. **** p < 0.0001 compared to MMP-9 ASO/I/R.
Figure 4.
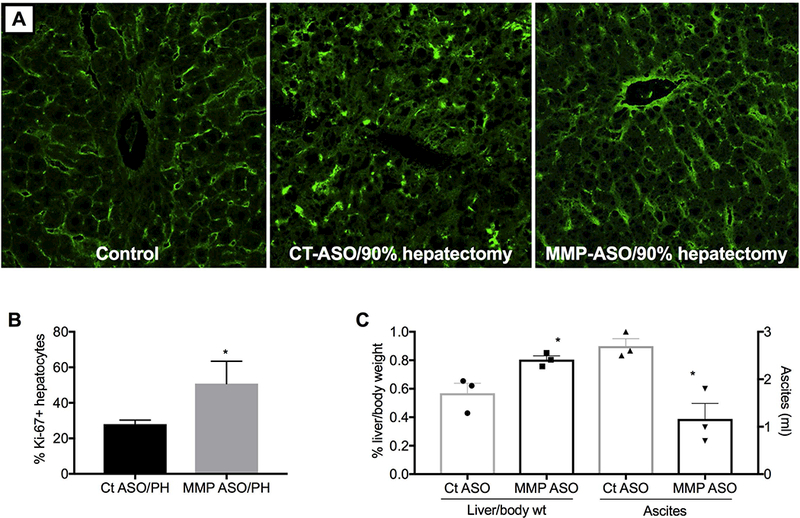
Liver-selective MMP-9 inhibition restores endothelial integrity, accelerates liver regeneration, and reduces ascites on day 2 after extended (90%) hepatectomy. (A) CD31 staining of normal liver (left panel), and on day 2 after extended hepatectomy in control ASO pretreated (middle panel) or MMP-9 ASO pretreated rats (right panel); images are centered on the portal tract. (B) Hepatocyte proliferation and (C) liver weight and ascites (n=3). *p<0.05 compared to control ASO by unpaired t-test.
Figure 5.
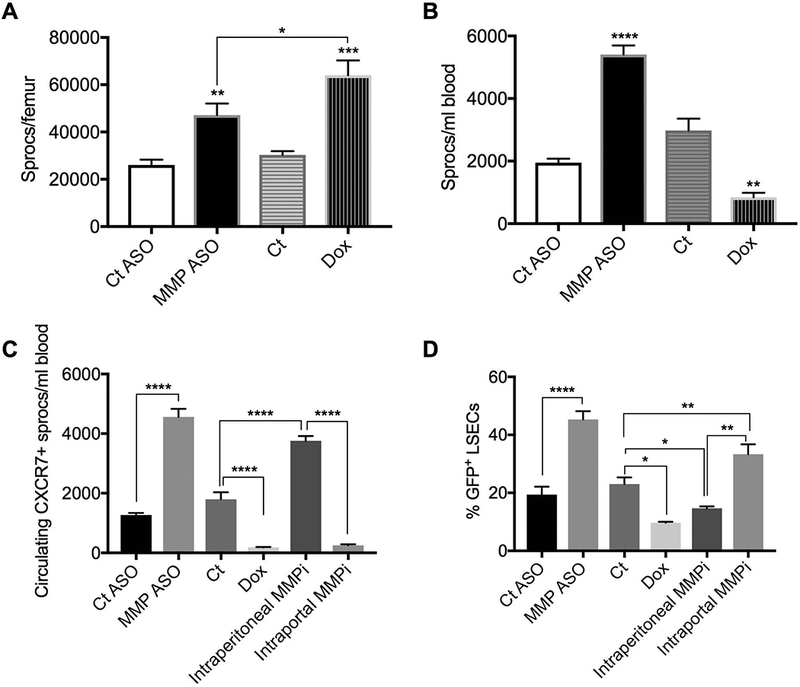
Recruitment of BM sprocs after PH is enhanced by liver-selective MMP inhibition and reduced by systemic MMP inhibition. (A) MMP-9-ASO (liver selective) and doxycycline (systemic) inhibition of MMP increase the number of sprocs in the BM (n=3). (B) MMP-9 ASO increases and systemic doxycycline decreases BM sproc mobilization to the circulation (n=3). (C) The number of circulating CXCR7+ sprocs are increased by liver-selective MMP inhibition (MMP-9 ASO or intraportal infusion of an MMP2/9 inhibitor), but reduced by systemic MMP inhibition (doxycycline or intraperitoneal MMP2/9 inhibitor) (n=3). (D) Hepatic engraftment after PH is enhanced by liver-selective MMP inhibition (MMP-9 ASO or intraportal MMP2/9 inhibitor), but reduced by systemic MMP inhibition (doxycycline or intraperitoneal MMP2/9 inhibitor) (n=3). Fig A-C are 6 hour time points; engraftment in Fig D was determined at 24 hours. Analysis by ANOVA was statistically significant and analyzed post-hoc by Fisher’s least significant difference. Levels of statistical significance are * p< 0.05, ** p< 0.01, *** p< 0.001, and **** p < 0.0001. Unless otherwise indicated, significance is based on comparison with the appropriate control.
Figure 6.
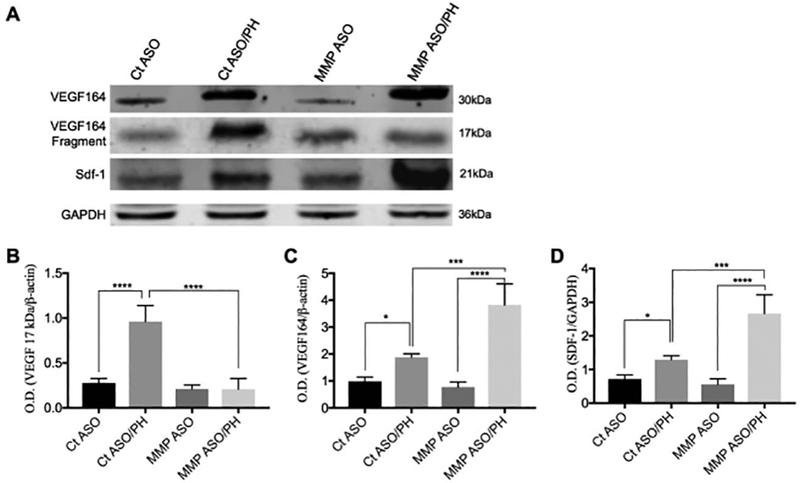
MMP-9 cleaves VEGF164 with attenuation of sdf-1 expression. Increased MMP-9 after PH causes proteolytic cleavage of VEGF164, resulting in formation of a 17 kDa fragment, assessed 6 hours after PH. Pretreatment with MMP-9 ASO prevents the formation of the 17 kDa product and thereby increases expression of VEGF164 and the downstream signaling partner of VEGF, sdf-1. (A) Immunoblot of VEGF164, VEGF164 cleavage fragment, and sdf-1 (n=3). Quantitation of the immunoblots showing (B) the 17 kDa cleavage product of VEGF164, with an increase after PH that is blocked by MMP-9 ASO pretreatment; (C, D) VEGF164 and sdf-1, with an increase after PH that is further increased after MMP-9 ASO pretreatment. Analysis by ANOVA was statistically significant and analyzed post-hoc by Fisher’s least significant difference. Levels of statistical significance are * p< 0.05, ** p< 0.01, *** p< 0.001, and **** p < 0.0001. Unprocessed original scans of blots are shown in Supporting Figure S6.
Figure 7.
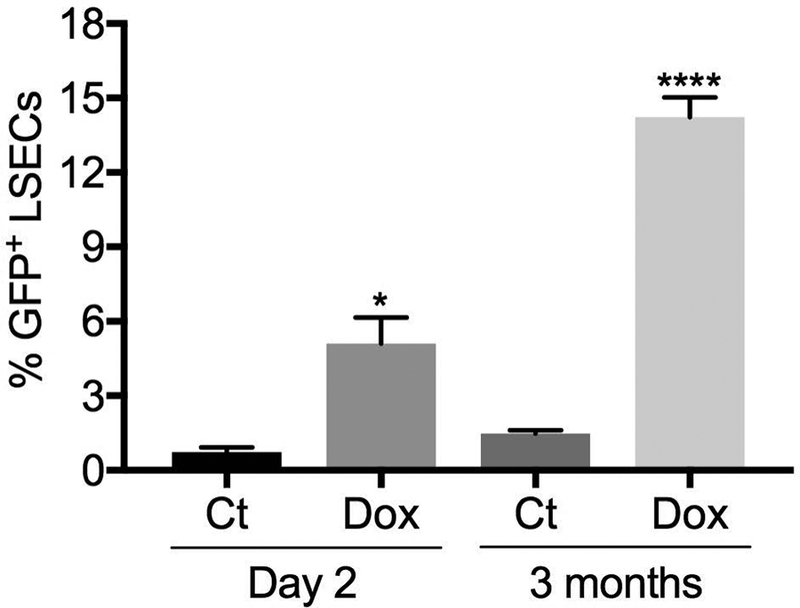
Model of stem cell therapy. Systemic MMP inhibition with doxycycline followed by PH and infusion of GFP+ allogeneic sprocs. Doxycycline enhances engraftment of infused sprocs, expressed as the percentage of GFP+ LSECs two days and three months later (n=3). **** p<0.0001 and * P<0.05 compared to control by unpaired t-test.
Figure 8.
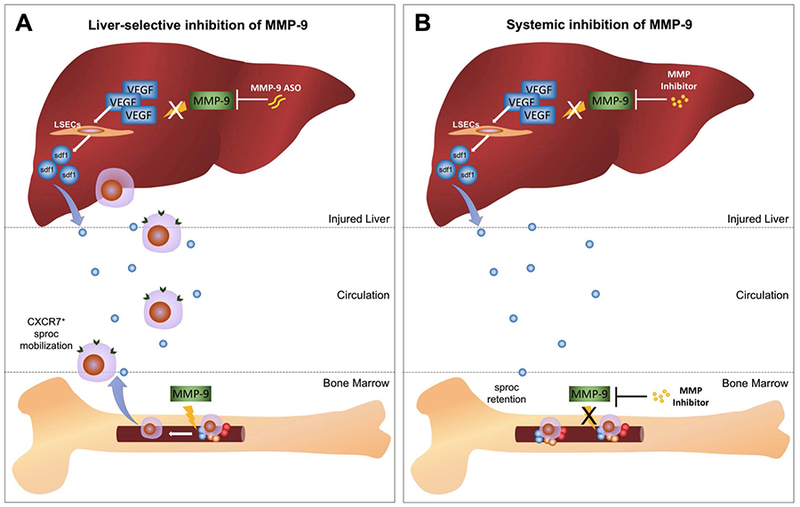
Molecular pathway diagram contrasting the effect of liver-selective and systemic MMP-9 inhibition. (A) Liver-selective MMP-9 inhibition prevents hepatic MMP-9 from proteolytically digesting hepatic VEGF after liver injury or partial hepatectomy. This permits the hepatic VEGF-sdf1 pathway to recruit CXCR7+ sprocs from the bone marrow, but does not inhibit MMP-9 activity in the bone marrow needed to mobilize sprocs. (B) Systemic inhibition of MMP prevents proteolytic cleavage of VEGF by hepatic MMP-9, thus preserving the VEGF-sdf1 pathway, but prevents sprocs from leaving the bone marrow by inhibiting the bone marrow MMP activity needed for release of the sprocs. The net effect is to reduce circulating sprocs and impair recruitment of sprocs to the liver.
MATERIALS AND METHODS
Reagents.
Chemicals were obtained from Sigma-Aldrich unless stated otherwise. All antibodies and dilutions used are listed in Supporting Table S1.
Animal studies.
Lewis rats were obtained from Harlan (Placentia, CA). Breeding pairs of Lew-Tg(CAG-EGFP)ys rats were obtained from the National Institutes of Health Rat Resource and Research Center at the University of Missouri. Male rats were used for the experiments. Rats were kept in conventional housing for rats, consisting of Allentown polycarbonate rat cages with filter tops and Sani-Chips bedding (wood product), in a 12:12-h light-dark cycle (lights on from 6 AM to 6 PM) at room temperature (21–23°C), with 5-μm-filtered water delivered to cages via an Edstrom automatic watering valve. Purina Lab Diet 5001 and water were provided ad libitum. Rats that showed distress postoperatively, based on activity level, behavior, appearance, reduced intake of food or water, or respiratory distress, were euthanized.
All protocols were reviewed and approved by the Animal Care and Use Committee at the University of Southern California to ensure ethical and humane treatment of the animals. This study followed the guidelines outlined in the Office of Laboratory Animal Welfare “Public Health Service Policy on Humane Care and Use of Laboratory Animals” (2015).
Partial (70%) hepatectomy was performed under general anesthesia with ketamine-xylazine (80–90 mg/kg ip). For 70% partial hepatectomy, the median and left lateral lobes were removed. Buprenorphine SR (1 mg/kg) was used postoperatively for analgesia.
Extended hepatectomy.
To achieve 90% hepatectomy, the medial and left lateral, and the superior and inferior portion of the right lobe were resected with preservation only of the caudate lobe. To prevent post-operative hypoglycemia, animals were gavaged once with 0.5 ml 20% glucose and for the first 16 hours 20% glucose was used as oral hydration that was provided through a liquid diet feeding tube (Bioserv cat 9010) that was positioned so rats could drink without significant exertion, followed by free access to water. Rats received standard laboratory chow ad libitum. All rats survived until they were euthanized on day 2.
Ischemia–reperfusion model.
Ischemia was induced by clamping the vessels in the hilum that perfuse the medial and left lateral lobes with a nontraumatic microvascular clip. After 1 hour, the microvascular clip was released. The abdomen was closed and the rats were left to recover with free access to standard chow diet and water ad libitum. Preliminary studies established that ALT and AST peaked at 6 hours.
Bone marrow transplantation.
BM cells were obtained from one tibia and femur from the donor. Recipients underwent 1,000 cGy total body irradiation and were injected via tail vein with 50 million BM cells. Rats received oxytetracycline (200 mg/ml) diluted 1:1,000 in the drinking water starting two days before irradiation and continuing until one week after irradiation. BM was allowed to engraft for 2 months before use. BM from Lew-Tg(CAG-EGFP)ys Lewis rats was transplanted into wild-type Lewis rats and cells were tracked by GFP expression.
Liver-selective MMP-9 inhibition:
Knockdown of MMP-9 was achieved by injection of one of two MMP-9 antisense oligonucleotides that were a kind gift from Ionis Pharmaceuticals (Ionis No 283953 and 283973, Carlsbad, CA; Supporting Table S2), 20 mg/kg intraperitoneally twice weekly for 4 weeks.
Hepatic MMP-2/9 inhibition in other studies was performed by infusing 2-[(4-biphenylsulfonyl)amino]-3-phenyl-propionic acid (Abcam, Cambridge, MA), 100 μg/h, into the portal circulation by an Alzet mini-osmotic pump (model 2ML1; Alza Corporation, Palo Alto, CA) via a cannula inserted into the inferior mesenteric vein starting 2 days before the relevant study.
Systemic MMP inhibition:
Doxycycline (Sigma, St. Louis), 15 mg/kg i.g., was given twice daily starting 2 days before the relevant study. Control studies were performed with the same dose of a chemically modified tetracycline, isochlorotetracycline, which is only a weak MMP inhibitor. In other experiments, MMP-2/9 was inhibited by infusing 2-[(4-biphenylsulfonyl)amino]-3-phenyl-propionic acid, 100 μg/kg/h, intraperitoneally with an Alzet mini-osmotic pump (model 2001, Durect Corporation, Cupertino CA).
LSEC isolation.
LSECs were isolated by collagenase perfusion, iodixanol density gradient centrifugation, and centrifugal elutriation, as previously described (22, 23). Yields averaged 86 × 106 cells per normal rat liver (range 69–95 × 106) with average viability of 94% (range 91–96%). Purity of the cells was 99%, as determined by uptake of formaldehyde-treated serum albumin (kind gift from Bård Smedsrod, University of Trømso, Trømso, Norway), a function specific to LSECs. Cells isolated by this protocol have an appropriate range of fenestrae organized in sieve plates.
Sproc isolation.
BM and circulating sprocs were isolated by immunomagnetic selection for CD133 using a CD133 Cell Isolation Kit (Miltenyi Biotec, Auburn, CA) and separation with an autoMACS Pro (Miltenyi Biotec, Auburn, CA), followed by FACS sorting for CD45 and CD31 (Supporting Fig S1).
To isolate resident sprocs, LSEC were isolated as described above and the CD133+ fraction was obtained by immunomagnetic selection (3).
To determine the number of sprocs in the circulation and BM from Lewis rats 6 hours after partial hepatectomy, CD133+ cells were isolated by immunomagnetic selection for CD133 from peripheral blood or BM. Cell suspensions were preblocked with FcR blocking reagent and incubated for 60 minutes with antibodies to rat CD45 and CD31. CD133+45+31+ cells were quantified by flow cytometry. In some experiments cells were also stained with antibody to rat CXCR7, to identify CD133+45+31+CXCR7+ cells in the circulation.
Flow cytometry.
Flow cytometry was performed using a FACSCalibur (BD Biosciences). Isotype control antibodies were used to determine appropriate gates, voltages, and compensation required for multivariate flow cytometry. Cell Quest Pro software was used for analysis
Immunohistochemistry for Ki-67
Deparaffinized 5 μm sections of liver were treated with Bond ER-2 antigen retrieval (Leica Biosystems, Buffalo Grove, IL) for 20 min. After preblocking for 10 min with Rodent Block R (Cat#RBR962G, Biocare, Pacheco, CA), sections were incubated with antibody against Ki-67 for 60 min. Immunohistochemistry was performed by incubating sections with rabbit-on-rodent HRP-Polymer (cat # RMR622G, Biocare) for 30 min. Slides were rinsed in Bond diaminobenzidine (Leica Biosystems) for 10min. After counterstaining with Bond hematoxylin, slides were dehydrated and covered using a coverslip with resinous mounting medium (Leica Biosystems).
Ki-67 positive cells were determined using photographs taken with a 20×-objective in 15 lobules per rat, n=3. To assess the percentage of proliferating hepatocytes and non-parenchymal cells, slides were coded and the percentage Ki-67 positive cells was determined blindly by X.W. To determine zonation of proliferating cells, each lobule was divided into three even fields and the total number of ki-67 positive cells/field was counted.
Measurements of serum ALT and AST were performed by IDTOX Alanine Transaminase (ALT) Color Endpoint Assay kit (cat # sup 6001-c, Empire genomics, Buffalo, NY) and IDTOX Aspartate Transaminase (AST) Color Endpoint Assay kit (cat # sup 6002-c, Empire genomics).
CD31 staining.
Frozen sections of liver were fixed in cold acetone and stained with a mouse monoclonal anti-CD31 and m-IgG BP-FITC anti-mouse IgG. Slides were examined using a Nikon PCM-2000 confocal microscope with a 488-nm laser excitation wavelength.
Immunoblotting.
Samples from freshly isolated liver were harvested using triple lysis buffer (TLB: 50mM Tris base, 150mM NaCl, 3mM sodium azide, 12mM sodium deoxycholate, 0.1% SDS, 1% Nonidet-P40) supplemented with 2mM phenylmethylsulphonyl fluoride (PMSF), 1mM sodium orthovanadate and 1% protease inhibitors cocktail (Santa Cruz sc-24948). Proteins were purified by centrifugation at 15,000g at 4°C for 10 min and quantified using DC™ Protein Assay Kit (BioRad). The samples obtained from bone marrow extracellular fluid were preserved in PBS. For MMP-9 analysis, 100μg of total protein was used from each sample per lane and resolved on NuPAGE™ Novex™ 10% Bis-Tris Protein Gels using MOPS running buffer. Proteins were transferred onto 0.45μm nitrocellulose membranes via electroblotting using XCell II™ Blot Module (Invitrogen), for 1h, 30V. For SDF-1 and VEGF analysis, 75μg of total protein was used from each sample per lane and resolved on NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels using MES running buffer. Proteins were transferred onto 0.2μm nitrocellulose membranes via electroblotting using Trans-blot SD Semi-Dry Transfer Cell™ (Bio-rad), for 20min, 25V. Membranes were blocked using NAP-BLOCKER™. Blots were probed with primary antibody overnight followed by IRDye secondary antibodies. Membrane digital images were acquired with the Odyssey Infrared Imaging System (LI-COR) and analyzed using the LI-COR Image Studio™. Analyses were performed in triplicate and normalized by GAPDH or ß-actin values.
Statistical analysis.
Unless stated otherwise, statistical analysis was performed by ANOVA and, if the ANOVA was statistically significant, analyzed post-hoc by Fisher’s least significant difference using Graphpad Prism. Levels of statistical significance are * p< 0.05, ** p< 0.01, *** p< 0.001, and **** p < 0.0001. Unless otherwise indicated, statistical significance is compared to the appropriate control. All experiments were performed with n=3 unless stated otherwise.
RESULTS
Acceleration of liver regeneration
Pro-MMP-9 is stored in granules in cells and its proteolytic activity occurs extracellularly after degranulation and cleavage to MMP-9. In normal liver, LSECs are a major source of MMP-9 (Supporting Fig S2A) which is consistent with the LSEC as the liver cell with the highest MMP-9 activity (11). PH increased MMP-9 expression in the liver and MMP-9 antisense oligonucleotides (ASO) abrogated the increase (Fig 1A and B, Supporting Fig S2B). Liver-selectivity of the ASO was confirmed by measuring MMP-9 activity in the extracellular fluid of the BM. BM MMP-9 activity was increased after PH, but MMP-9 ASO pretreatment did not alter BM MMP-9 activity (Fig 1C and D, Supporting Fig S2C).
Figure 1.
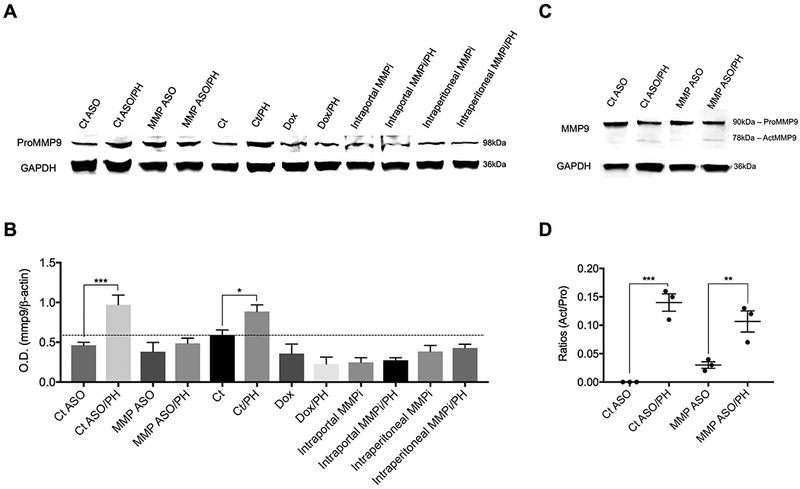
Hepatic and bone marrow MMP-9 after PH (A) Immunoblots of hepatic pro-MMP-9 protein expression assessed 6 hours after PH. (B) Quantification of panel A, shows increased hepatic MMP-9 protein expression after PH and this increase is abrogated by MMP-9 ASO, doxycycline, and intraportal or intraperitioneal administration of an MMP-2/9 inhibitor (n=3). (C) Immunoblot of pro-MMP-9 and MMP-9 in the BM extracellular fluid. (D) Quantification of activation of BM MMP-9, expressed as the ratio of MMP-9/pro-MMP-9 in BM extracellular fluid, increases after PH (n=3). There is no significant difference in the increase after PH with pretreatment by control ASO vs MMP-9 ASO.
Figure 2A is a key panel. MMP-9 ASO accelerated liver regeneration by 40%; to the best of our knowledge, this degree of acceleration is unprecedented. A time-course of liver-to-body-weight from day 3 to day 7 after PH demonstrates that the liver-to-body weight ratio reaches that of control littermates by day 4 after MMP-9 ASO pretreatment compared to day 7 in the control-ASO pretreated group. MMP-9 ASO pre-treatment increased proliferation of hepatocytes and non-parenchymal cells by 80% on day 2 (Fig 2B), Hepatocyte proliferation in the MMP-9 ASO group is higher in all 3 zones of the liver on day 2 and higher in the periportal and midlobular region on day 3 (Fig 2C). For non-parenchymal cells from rats treated with MMP-9 ASO, proliferation is higher in all 3 zones on day 2 and day 3 and proliferation is higher in the midlobular region on days 4 and 5 (Fig 2D). Liver-selective MMP-9 inhibition with either MMP-9 ASO or intraportal MMP-2/9 inhibitor enhances hepatocyte proliferation on day 2 compared to their respective controls, whereas systemic MMP inhibition with doxycycline, which inhibits several MMPs including MMP-9 (24, 25), reduces hepatocyte proliferation compared to its solvent control (Fig 2E). Of note, neither doxycycline nor intraportal MMP-2/9 inhibitor given to controls was hepatotoxic (Supporting Fig S3A).
Comparison of liver-selective versus systemic MMP inhibition demonstrates that liver-to-body weight ratio on day 5 is increased in the MMP-9 ASO pretreatment group by 27% compared to control ASO pretreatment (Fig 2F). Systemic MMP inhibition with doxycycline reduces liver-to-body weight ratio on day 5 by 29% compared to its solvent control (Fig 2F). To rule out the possibility that liver-selective MMP inhibition recruited a stem cell for one of the other liver cell types, livers were digested on day 2 after PH and examined by flow cytometry. At least 94% of CD133+ cells were CD31+ cells. i.e. endothelial cells (Supporting Fig S3B).
Prevention of ischemia/reperfusion (I/R) injury
Induction of ischemia for 1 hour, followed by 6 hours of reperfusion lead to extremely high elevations of ALT and AST (Fig 3A and3B). Pre-treatment with MMP-9 ASO largely abolished injury as indicated by ALT (58 IU/l ± 10) and AST (95 IU/l ± 12) in the near-normal or modestly elevated range, respectively (Fig 3A and3B). Histology (Fig 3C and3D) showed clearing of hepatocyte cytoplasm, lobular disarray, and absence of LSECs in the control-ASO/I/R injury group (left panels), whereas the MMP-9 ASO/I/R injury group showed preservation of hepatocyte integrity and presence of LSECs (right panels). The findings suggest that the predominant injury at 6 hours was to LSECs rather than to hepatocytes: LSECs were largely absent in the control ASO pretreated group (Fig 3C), but there was only clearing of cytoplasm and no frank necrosis of hepatocytes. Thus pretreatment by MMP-9 ASO prevented subsequent hepatocyte necrosis by promoting repair of the LSEC lining.
Attenuation of small-for-size syndrome
In living-donor related transplantation or after extensive liver resection for metastases, the recipient’s existing portal vein flow does not adapt to the small liver. Damage to the liver graft or remnant is thought to be due to hyper-perfusion and the injury is known as small-for-size syndrome. Extended hepatectomy, removal of 90% of the liver, is a model for small-for-size syndrome. On day 2 after extended hepatectomy, CD31 staining demonstrated a marked absence of LSECs lining the sinusoids (Fig 4A, middle panel), which is consistent with collapse of sinusoids seen during the first 72 hours after PH (26). In contrast, the sinusoids of rats pre-treated with MMP-9 ASO followed by extended hepatectomy (Figure 4A, right panel) had LSEC lining that appears comparable to the untreated control livers (Fig 4A, left panel). The 3 panels of Fig 4A are centered on the pericentral lobule for the sake of comparison, but the respective changes in LSEC lining in the pericentral lobule were representative of the whole liver (data not shown). Pretreatment with MMP-9 ASO also reduced ascites on day 2 by 57% compared to control ASO pretreatment (Fig 4C), likely due to patent sinusoids that improved liver hemodynamics.
Increased recruitment and engraftment of BM sprocs
To examine the mechanism of the therapeutic benefit of liver-selective MMP inhibition, the respective effects of liver-selective and systemic MMP inhibition on BM sproc recruitment and engraftment were examined in the PH model. MMP-9-ASO/PH increased the number of sprocs in the BM compared to control ASO/PH (Fig 5A). Systemic MMP inhibition in the doxycycline/PH group increased the number of sprocs in the BM significantly more than MMP-ASO/PH, consistent with impairment of sproc release by MMP inhibition of the BM.
Mobilization: MMP-9-ASO/PH increased BM sproc mobilization to the circulation compared to ASO control/PH by 180%, whereas doxycycline/PH reduced mobilization of BM sprocs by 72% (Fig 5B). This is consistent with the hypothesis that liver-selective MMP-9 inhibition protects the chemoattractant signaling to BM sprocs and promotes recruitment of sprocs from the BM, whereas systemic MMP inhibition prevents release of sprocs from the BM. BM sprocs that engraft in the liver are CXCR7+ (1), so addition of this marker is a closer approximation of actual sprocs, i.e. LSEC-specific endothelial progenitors cells in the circulation. Liver-selective MMP inhibition with either MMP-9-ASO or with slow infusion into the portal circulation (intraportal infusion) of an MMP-2/9 inhibitor, (2R)-2-[(4-Biphenylylsulfonyl)amino]-3-phenylpropionic acid, increased CXCR7+ sprocs in the circulation by 260 and 110%, respectively, after PH, whereas systemic MMP inhibition with either doxycline or intraperitoneal injection of the MMP-2/9 inhibitor reduced the number of CXCR7+ sprocs mobilized to the circulation by 90 and 86%, respectively (Fig 5C). Pharmacological inhibition with the MMP-2/9 inhibitor abrogated the increase in hepatic MMP-9 expression when given either liver-selectively by intraportal infusion or intraperitoneally (Fig 1A).
Engraftment: To track BM sproc engraftment in the liver, wild type rats underwent BM transplantation with BM from transgenic EGFP+ rats. Liver-selective MMP inhibition with either MMP-9 ASO/PH or intraportal MMP-2/9 inhibitor/PH increased hepatic engraftment of GFP+ BM sprocs by 134% and 45%, respectively, whereas systemic inhibition with either doxycycline/PH or intraperitioneal MMP-2/9 inhibition/PH reduced hepatic engraftment of BM sprocs by 58% and 36%, respectively, compared to control/PH (Fig 5D). As a control, the effect of a different MMP-9 ASO on engraftment was examined and this had near-identical effect on engraftment to the MMP-ASO described above (Supporting Fig S4). To confirm that the effect of doxycycline was due to MMP inhibition rather than an antibiotic effect, isochlorotetracycline was administered prior to PH. Isochlorotetracycline had no effect on engraftment (Supporting Fig S5).
Mechanism of liver-selective MMP inhibition
Upregulation of MMP-9 expression after PH (Fig 1A and B) (27) was accompanied by increased expression of a 17 kDa VEGF cleavage fragment (Fig 6A and B), which is consistent with previous reports of MMP-9 producing a VEGF164 cleavage product that has dysfunctional angiogenic properties (19, 28). Pretreatment with MMP-9 ASO completely prevented increased expression of the 17 kDa cleavage product after PH and lead to significantly higher hepatic VEGF164 and sdf1 in the MMP-9 ASO/PH group compared to the ASO Ct/PH group (Fig 6 A–D, Supporting Fig S6). MMPs, including MMP-9, can also cleave sdf-1, leading to decreased chemoattraction. We observed faint expression of a 13 kDa band of sdf-1 that increased after PH, but MMP-9 ASO pretreatment did not diminish the size of the 13 kDa band (data not shown).
Increased engraftment of infused stem cells
Stem cell therapy with infused cells requires engraftment. However progenitor cells that are infused after injury compete with BM progenitors. Indeed infusion of EGFP+ sprocs 6 hours after PH resulted in only 0.7% of LSECs derived from the infused sprocs on day 2 and 1.5% by 3 months (Fig 7). Systemic inhibition of MMP with doxycycline, which reduces mobilization of BM sprocs (see above), increased engraftment of infused sprocs seven-fold by day 2 after infusion and almost ten-fold by 3 months (Fig 7).
DISCUSSION
Liver-selective MMP inhibition accelerated liver regeneration after two-thirds PH by 40%, largely abolished I/R injury, and accelerated liver regeneration and reduced ascites formation after extended hepatectomy. The mechanism is through inhibition of proteolytic cleavage by MMP-9 of VEGF164, which resulted in increased expression of VEGF164 and the downstream chemokine sdf-1. The VEGF-sdf1 pathway recruits BM endothelial progenitor cells of the LSECs, so-called BM sprocs, that are essential for liver regeneration (1, 2). In contrast, systemic MMP inhibition impaired recruitment of BM sprocs to the liver and reduced liver regeneration after PH by preventing mobilization of BM progenitor cells to the circulation. Consistent with this, systemic pharmacological inhibition of MMPs has been studied in liver injury models, but often with modest benefit (12, 13, 29, 30). Figure 8 summarizes the findings described above.
Previous studies have demonstrated MMP-9 activity in LSECs but undetectable activity in hepatocytes, Kupffer cells, or hepatic stellate cells (11). Although antisense oligonucleotides have been designed that are more hepatocyte specific, the 2’-methoxyethyl ASOs used in the current study target both hepatocytes and non-parenchymal cells. Thus LSECs are the likely target of the MMP ASO used in the current study.
The findings reported here have translational implications for the liver. MMP inhibitors failed clinical trials to treat cancer, but good candidate drugs reportedly have no toxicity with short-term administration. Treatment of a cadaveric organ donor with systemic MMP inhibition might protect multiple organs from ischemia-reperfusion injury without inhibiting MMP in the recipients’ BM. Serendipitous application of liver-selective MMP-2/9 inhibition completely prevented severe toxin-induced sinusoidal obstruction syndrome (11). The extended hepatectomy studies presented here suggest that liver-specific MMP inhibition would attenuate small-for-size syndrome. For living-related liver donor transplantation and for extended hepatectomy for liver tumors, MMP-9 inhibition would need to be strictly liver-specific to prevent MMP inhibition of the BM.
The basic mechanistic processes described here in liver injury mimic that found in other organs. MMPs increase after injury in several organs, including heart (31, 32), brain (33, 34), lung (35, 36), kidney (33, 37), and pancreas (38), and vascular wall (39). VEGF and sdf1 signaling recruit endothelial progenitor cells to the heart (40, 41), brain and spine (42–44), lung (45), kidney (46), peripheral nerves (47), bone (48, 49), and limbs (50). Thus the benefit of end-organ specific MMP inhibition is likely applicable to other organs and should apply to recovery from insults that injure the vasculature. The challenge will be to develop end-organ specific delivery of an MMP inhibitor, given the observation that systemic inhibition that inhibits BM MMP is detrimental. Slow infusion of MMP inhibitor into a specific organ may be an option if (near)-complete uptake of the MMP inhibitor by the organ can be achieved.
Stem cell therapy with infused cells requires engraftment and the current study demonstrates that engraftment of infused endothelial progenitor cells after injury is markedly increased by systemic MMP inhibition. One could envision that one application of this would be enhanced engraftment of endothelial progenitor cells that are gene-edited to be anticoagulant to reduce the risk of recurrent thrombosis or vascular occlusion in a compromised vascular bed.
Supplementary Material
Acknowledgements.
The authors thank Gary Kanel, USC Dept of Pathology, for review of the pathology slides. Both MMP-9 antisense oligonucleotides and the control antisense oligonucleotides were a kind gift provided by Ionis Pharmaceuticals (provided under an MTA). Competing financial interest: the University of Southern California and Laurie DeLeve have filed a provisional patent based on the findings of this study.
Financial support:
This work was supported by NIH grant DK46357 (L.D.D.), by a New Investigator Transition Award from the Research Center for Liver Diseases (A.M.M.) and by the Research Center for Liver Diseases (NIH grant P30DK048522) Cell Separation and Culture Core, Cell and Tissue Imaging Core, Liver Histology Core, and the Proteomics Subcore. The authors thank Gary Kanel, USC Dept of Pathology, for review of the pathology slides.
Abbreviations:
- sdf-1
stromal-derived factor
- BM
bone marrow
- sproc
liver sinusoidal endothelial cell progenitor cell
- LSEC
liver sinusoidal endothelial cell
- MMP
matrix metalloproteinase
- PH
partial hepatectomy
- ASO
antisense oligonucleotides
- I/R
ischemia-reperfusion
References
- 1.DeLeve LD, Wang X, Wang L. VEGF-sdf1 recruitment of CXCR7+ bone marrow progenitors of liver sinusoidal endothelial cells promotes rat liver regeneration. American Journal of Physiology-Gastrointestinal and Liver Physiology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Wang X, Wang L, Chui JD, van de Ven G, Gaarde WA, DeLeve LD. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology 2012;143:1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver Sinusoidal Endothelial Cell Progenitor Cells Promote Liver Regeneration in Rats. Journal of Clinical Investigation 2012;122:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding B-S, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010;468:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver: Upregulation in sinusoidal endothelial cells after carbon tetrachloride. Journal of Clinical Investigation 1993;91:2244–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science 2003;299:890–893. [DOI] [PubMed] [Google Scholar]
- 7.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 2014;505:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harb R, Xie G, Lutzko C, Guo Y, Wang X, Hill C, Kanel G, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology 2009;137:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie G, W L, W X, W L, DeLeve LD. Isolation of periportal, mid-lobular and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am J Physiol-Gastrointest Liver Physiol 2010;299:G1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upadhya GA, Harvey RP, Howard TK, Lowell JA, Shenoy S, Strasberg SM. Evidence of a role for matrix metalloproteinases in cold preservation injury of the liver in humans and in the rat. Hepatology 1997;26:922–928. [DOI] [PubMed] [Google Scholar]
- 11.DeLeve LD, Wang X, Tsai J, Kanel GC, Strasberg SM, Tokes ZA. Prevention of sinusoidal obstruction syndrome (hepatic venoocclusive disease) in the rat by matrix metalloproteinase inhibitors. Gastroenterology 2003;125:882–890. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Abril ER, Bethea NW, McCuskey RS. Inhibition of matrix metalloproteinases minimizes hepatic microvascular injury in response to acetaminophen in mice. Toxicological Sciences 2005;83:190–196. [DOI] [PubMed] [Google Scholar]
- 13.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2008;47:186–198. [DOI] [PubMed] [Google Scholar]
- 14.Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. Fibronectin-alpha4beta1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. The American journal of pathology 2007;170:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori T, Uemoto S, Chen F, Ann-Baine MT, Gardner LB, Hata T, Kuribayashi K, et al. Effect of cold ischemia/reperfusion injury and/or shear stress with portal hypertension on the expression of matrix metalloproteinase-9. Ann Gastroenterol 2012;25:345–351. [PMC free article] [PubMed] [Google Scholar]
- 16.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med 2005;9:267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai H, Ma Y, Jiang L, Mu Z, Jiang Z, Chen X, Wang Y, et al. Hypoxia Response Element-Regulated MMP-9 Promotes Neurological Recovery via Glial Scar Degradation and Angiogenesis in Delayed Stroke. Mol Ther 2017;25:1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, Lendermon JB, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017;17:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. The Journal of Cell Biology 2005;169:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein G, Schmal O, Aicher WK. Matrix metalloproteinases in stem cell mobilization. Matrix Biology 2015;44–46:175–183. [DOI] [PubMed] [Google Scholar]
- 21.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. The Journal of clinical investigation 2003;111:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLeve LD, Wang X, McCuskey MK, McCuskey RS. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic sorting lack fenestrae and sieve plates. American Journal of Physiology-Gastrointestinal and Liver Physiology 2006;291:G1187–1189. [DOI] [PubMed] [Google Scholar]
- 23.Steffan AM, Gendrault JL, McCuskey RS, McCuskey PA, Kirn A. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology 1986;6:830–836. [DOI] [PubMed] [Google Scholar]
- 24.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med 1991;2:297–321. [DOI] [PubMed] [Google Scholar]
- 25.Greenwald RA, Moak SA, Ramamurthy NS, Golub LM. Tetracyclines suppress matrix metalloproteinase activity in adjuvant arthritis and in combination with flurbiprofen, ameliorate bone damage. J Rheumatol 1992;19:927–938. [PubMed] [Google Scholar]
- 26.Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology 2001;33:363–378. [DOI] [PubMed] [Google Scholar]
- 27.Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology 2000;31:75–82. [DOI] [PubMed] [Google Scholar]
- 28.Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, Ferrara N. The Carboxyl-terminal Domain(111165) of Vascular Endothelial Growth Factor Is Critical for Its Mitogenic Potency. Journal of Biological Chemistry 1996;271:7788–7795. [DOI] [PubMed] [Google Scholar]
- 29.Cursio R, Mari B, Louis K, Rostagno P, Saint-Paul MC, Giudicelli J, Bottero V, et al. Rat liver injury after normothermic ischemia is prevented by a phosphinic matrix metalloproteinase inhibitor. FASEB Journal 2002;16:93–95. [DOI] [PubMed] [Google Scholar]
- 30.Ma ZY, Qian JM, Rui XH, Wang FR, Wang QW, Cui YY, Peng ZH. Inhibition of Matrix Metalloproteinase-9 Attenuates Acute Small-for-Size Liver Graft Injury in Rats. American Journal of Transplantation 2010;10:784–795. [DOI] [PubMed] [Google Scholar]
- 31.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 2000;101:1833–1839. [DOI] [PubMed] [Google Scholar]
- 32.Falk V, Soccal PM, Grunenfelder J, Hoyt G, Walther T, Robbins RC. Regulation of matrix metalloproteinases and effect of MMP-inhibition in heart transplant related reperfusion injury. Eur J Cardiothorac Surg 2002;22:53–58. [DOI] [PubMed] [Google Scholar]
- 33.Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, Lei J, et al. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol 2009;216:35–46. [DOI] [PubMed] [Google Scholar]
- 34.Guan W, Kozak A, El-Remessy AB, Johnson MH, Pillai BA, Fagan SC. Acute treatment with candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl Stroke Res 2011;2:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J 2009;33:77–84. [DOI] [PubMed] [Google Scholar]
- 36.Willems S, Verleden SE, Vanaudenaerde BM, Wynants M, Dooms C, Yserbyt J, Somers J, et al. Multiplex protein profiling of bronchoalveolar lavage in idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Ann Thorac Med 2013;8:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turunen AJ, Lindgren L, Salmela KT, Kyllonen LE, Andersson S, Pesonen E. Matrix Metalloproteinase-9 and Graft Preservation Injury in Clinical Renal Transplantation. Transplant Proc 2015;47:2831–2835. [DOI] [PubMed] [Google Scholar]
- 38.Gukovsky I, Lugea A, Shahsahebi M, Cheng JH, Hong PP, Jung YJ, Deng QG, et al. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2008;294:G68–79. [DOI] [PubMed] [Google Scholar]
- 39.George SJ, Zaltsman AB, Newby AC. Surgical preparative injury and neointima formation increase MMP-9 expression and MMP-2 activation in human saphenous vein. Cardiovasc Res 1997;33:447–459. [DOI] [PubMed] [Google Scholar]
- 40.Tu TC, Nagano M, Yamashita T, Hamada H, Ohneda K, Kimura K, Ohneda O. A Chemokine Receptor, CXCR4, Which Is Regulated by Hypoxia-Inducible Factor 2alpha, Is Crucial for Functional Endothelial Progenitor Cells Migration to Ischemic Tissue and Wound Repair. Stem Cells Dev 2016;25:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H, Kim S, Baek SH, Kwon SM. Pivotal Cytoprotective Mediators and Promising Therapeutic Strategies for Endothelial Progenitor Cell-Based Cardiovascular Regeneration. Stem Cells Int 2016;2016:8340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobrino T, Perez-Mato M, Brea D, Rodriguez-Yanez M, Blanco M, Castillo J. Temporal profile of molecular signatures associated with circulating endothelial progenitor cells in human ischemic stroke. J Neurosci Res 2012;90:1788–1793. [DOI] [PubMed] [Google Scholar]
- 43.Paczkowska E, Golab-Janowska M, Bajer-Czajkowska A, Machalinska A, Ustianowski P, Rybicka M, Klos P, et al. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: the role of endothelin-1. J Neurol Sci 2013;325:90–99. [DOI] [PubMed] [Google Scholar]
- 44.Paczkowska E, Roginska D, Pius-Sadowska E, Jurewicz A, Piecyk K, Safranow K, Dziedziejko V, et al. Evidence for proangiogenic cellular and humoral systemic response in patients with acute onset of spinal cord injury. J Spinal Cord Med 2015;38:729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi Y, Qian L, Sun B, Liu L, Wu P, Sun L. Inhaled NO contributes to lung repair in piglets with acute respiratory distress syndrome via increasing circulating endothelial progenitor cells. PLoS One 2012;7:e33859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bo CJ, Chen B, Jia RP, Zhu JG, Cao P, Liu H, Wu R, et al. Effects of ischemic preconditioning in the late phase on homing of endothelial progenitor cells in renal ischemia/reperfusion injury. Transplant Proc 2013;45:511–516. [DOI] [PubMed] [Google Scholar]
- 47.Kim BJ, Lee JK, Schuchman EH, Jin HK, Bae JS. Synergistic vasculogenic effects of AMD3100 and stromal-cell-derived factor-1alpha in vasa nervorum of the sciatic nerve of mice with diabetic peripheral neuropathy. Cell Tissue Res 2013;354:395–407. [DOI] [PubMed] [Google Scholar]
- 48.Kawakami Y, Ii M, Matsumoto T, Kuroda R, Kuroda T, Kwon SM, Kawamoto A, et al. SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial progenitor cells contributes to bone fracture healing. J Bone Miner Res 2015;30:95–105. [DOI] [PubMed] [Google Scholar]
- 49.Herrmann M, Verrier S, Alini M. Strategies to Stimulate Mobilization and Homing of Endogenous Stem and Progenitor Cells for Bone Tissue Repair. Front Bioeng Biotechnol 2015;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiang KH, Cheng WL, Shih CM, Lin YW, Tsao NW, Kao YT, Lin CT, et al. Statins, HMG-CoA Reductase Inhibitors, Improve Neovascularization by Increasing the Expression Density of CXCR4 in Endothelial Progenitor Cells. PLoS One 2015;10:e0136405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


