Abstract
Background:
Previously, we observed a strong relationship between circulating serum inflammation proteins in relation to lung cancer diagnosis and risk, both in case-control and prospective cohorts. Low dose CT (LDCT) screening has a high prevalence of false positive nodules, thus companion non-invasive biomarkers that can distinguish between benign and malignant nodules could have clinical utility and positive impact on patient outcomes.
Methods:
We conducted a nested case-control study within the National Lung Screening Trial (NLST). Concentrations of 30 inflammation proteins were measured on plasma samples of 262 cases and 528 controls using a highly sensitive and analytically validated electrochemiluminescence V-PLEX immunoassay.
Results:
Comparing the 4th quartile with 1st quartile, we found increased IFN-γ and IL-12/IL-23p40 associated with increased odds of a lung cancer diagnosis (OR 1.89, 95% C.I. 1.16–3.09; OR 2.49, 95% C.I. 1.46–4.23, respectively). Confirming our previous observations, we also detected a relationship between increased IL-6, IL-8 and CRP with lung cancer diagnosis. These relationships were significant after adjustment for age, gender, race, smoking, BMI, family history of lung cancer and previous diagnoses of inflammatory conditions. However, none of these proteins could distinguish between a benign and malignant lung nodule (IL-6: OR 1.25, 95% C.I. 0.59–2.64; IL-8: OR 1.40, 95% C.I. 0.70–2.81; CRP: OR 0.98, 95% C.I. 0.45–2.12).
Conclusions:
We have discovered new associations for IFN-γ and IL-12/IL-23p40 with lung cancer but have no evidence that these proteins can distinguish between benign and malignant lung nodules.
Impact:
Circulating inflammation proteins are unlikely to have utility as companion LDCT biomarkers.
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States and worldwide (1). For all stages of lung cancer combined, the 5-year survival rates are still approximately 15%. One of the major contributing factors to this dismal survival rate is the stage at which the majority of lung cancers are diagnosed. Unfortunately, greater than 50% of lung cancer is diagnosed at stage 3 or 4, a time when both local and systemic treatments are unlikely to be curative. The results of the National Lung Screening Trial mark a new gold standard for lung cancer screening based on low-dose computed tomography (LDCT) imaging (2,3). However, the enhanced sensitivity of LDCT is tempered by its low specificity and the simultaneous detection of benign indolent nodules. Up to 97% of suspicious lesions detected with LDCT are benign at time of surgery, and the number of false positives diagnosed by LDCT significantly increases the cost of screening and patient morbidity (3,4). Biomarkers that can differentiate between benign and malignant tumors could reduce the number of surgeries and invasive procedures for patients and ultimately improve lung cancer outcomes.
The role of circulating immune proteins as cancer biomarkers is of interest given the documented relationship between smoking and inflammation with this disease (5–9). A chronic inflammatory state can cause a high rate of cell turnover and an increase in oxidative and nitrosative stress (10). These reactive oxygen species can then bind to DNA, leading to damage and mutations, possibly also exacerbating the DNA damage caused by tobacco smoke (5,11–14). Several chronic inflammatory and infectious conditions are associated with an increased risk of lung cancer (8,15). Furthermore, cytokine concentrations are altered when inhaled smoke particulates and chemical irritants induce an immune response (16–18), and research has shown that microenvironmental, lung cancer and premalignant epithelial cells can also secrete cytokines (19–21).
Modulation of the inflammatory milieu within the tissue microenvironment can be evident systemically (21–23). Indeed, we and others have previously demonstrated an elevation of certain inflammation proteins at the time of lung cancer diagnosis (22,24–26). In addition, some inflammation proteins were also associated with lung cancer risk (22,25,27) suggesting that they may be useful for risk stratification. While molecular epidemiology studies do not directly imply causation, they do suggest that inflammation is related to the etiology of lung cancer. This suggestion is supported by epidemiological studies that show a relationship between inflammatory conditions and risk of developing lung cancer (8,28), and mouse models that define a causal relationship between inflammation and lung cancer (11).
Considering the relationship between inflammation with lung cancer risk and diagnosis, and the inflammatory signals that egress from malignant tumors, we sought to investigate if circulating inflammation proteins could be a companion diagnostic tool for lung cancer screening and potentially discriminate between malignant and benign nodules.
Methods
Study Population
This study utilized data and biospecimens from the NLST, a randomized screening trial that recruited subjects at high risk for lung cancer (2,3). The NLST compared the use of low-dose CT with chest radiography. It was a collaborative effort of the Lung Screening Study (LSS), administered by the NCI Division of Cancer Prevention, and the American College of Radiology Imaging Network (ACRIN), sponsored by the NCI Division of Cancer Treatment and Diagnosis, Cancer Imaging Program. The NLST population selection and eligibility criteria, methods and results have been previously described (2,3). Briefly, all subjects were aged between 55 and 74 years old with at least a 30 pack-year history of cigarette smoking. Former smokers were defined as those who had quit smoking less than 15 years before entry into the study. Participants were randomly assigned into two study arms. Participants in the first arm were randomized to receive three annual low-dose CT assessments, with the second arm scheduled to receive three annual single-view posterior-anterior chest radiographs. Both arms were then followed for 5 years.
Positive low-dose CT scans were defined as one or more non-calcified nodules at least 4 mm in the longest diameter (2,3). Subjects within the low-dose CT arm with a positive scan were further evaluated for lung cancer diagnoses by follow-up with their physician. Participants were given a questionnaire upon entry into the study that included questions related to their past medical history, smoking habits and family history of cancer. In total, 53,454 persons at high-risk for lung cancer were enrolled in the NLST. There were 649 lung cancers, for which diagnostic information was complete, diagnosed in the LDCT-arm.
Study Design
Biospecimens were collected from a subset of ACRIN centers (n=15). Of the 649 lung cancers diagnosed with LDCT, plasma was collected and available for 265. Of the 265, analysis was successful on 262 (note, plasma for three individuals was not included due to insufficient volume). Of these 262 cases, 61 were diagnosed at T0, 62 were diagnosed at T1 and 139 were diagnosed at T2. Further, of these 262 cases, 134 were diagnosed at the time of their screen, 122 had a sample drawn before diagnosis and 6 were drawn after diagnosis. A breakdown of these collections, stratified by time of cancer diagnosis, is presented in Supplementary Table 1. Controls were then selected based on incidence-density matching of each case to two random controls. The samples were 2 to 1 incidence density matched based on timepoint sample taken (T0, T1, T2), number of screens, age at diagnosis (± 5 years), smoking status (current/former), gender (male/female), cigarettes per day (± 1) and duration of smoking (± 3 years). These criteria were possible for 187 cases. The cigarettes/day criterion was relaxed to +/−5 for 42 individuals. There was matching based only on time on study, number of screens, age, and gender for 73 individuals, matching based on time on study and number of screens for 12 individuals and matching only by time on study for 100 individuals. We performed two nested case control studies. The first sought to validate and confirm our previous observations within the NLST samples using a traditional case-control subset. The second sought to compare inflammation profiles between benign and malignant nodules. Matching was not conducted with consideration for the positive or negative status of the scan. A summary of the participant characteristics is outlined in Table 1.
Table 1:
Characteristics of the lung cancer cases and controls
| Cases | Controls | P1 | P2 | ||
|---|---|---|---|---|---|
| Demographics | (n=262) | Negative Scan N=418 | Positive Scan N=110 | ||
| Age | |||||
| Median (IQR) in years | 64 (60–68) | 64 (59–68) | 64.5 (61–68) | 0.50 | 0.57 |
| Gender | |||||
| Male | 159 | 263 | 59 | 0.56 | 0.21 |
| Female | 103 | 155 | 51 | ||
| Race | |||||
| White | 488 | 384 | 104 | ||
| Black or African-American | 22 | 19 | 3 | ||
| Asian | 6 | 6 | 0 | 0.36 | 0.27 |
| American Indian or Alaskan Native | 1 | 1 | 0 | ||
| Native Hawaiian or other Pacific Islander | 3 | 3 | |||
| More than one race | 5 | 4 | 1 | ||
| Missing | 3 | 1 | 2 | ||
| Smoking Status at Randomization | |||||
| Former | 124 | 182 | 49 | 0.33 | 0.62 |
| Current | 138 | 236 | 61 | ||
| Pack-years of Smoking | |||||
| Median (IQR) in years | 57 (43–76.5) | 51 (43–76) | 50 (42.4–69) | 0.22 | 0.05 |
| BMI | |||||
| Median (IQR) in years | 26.4 (24.0–29.2) | 26.6 (24.4–30.1) | 26.2 (22.9–30.13) | 0.06 | 0.77 |
| Family History of Lung Cancer | |||||
| No | 192 | 304 | 82 | 0.71 | 0.61 |
| Yes | 57 | 84 | 21 | ||
| Tumor Stage | |||||
| IA | 160 | ||||
| IB | 25 | ||||
| IIA | 5 | ||||
| IIIA | 15 | ||||
| IIIB | 23 | ||||
| IV | 10 | ||||
| Unable to assess | 7 | ||||
| Tumor Histology | |||||
| Large cell carcinoma, NOS | 6 | ||||
| Large cell neuroendocrine carcinoma | 2 | ||||
| Tumorlet, NOS | 14 | ||||
| Oat cell carcinoma | 1 | ||||
| Small cell carcinoma | 3 | ||||
| Non-small cell carcinoma | 21 | ||||
| Squamous cell carcinoma | 52 | ||||
| Squamous cell carcinoma, keratinizing, NOS | 6 | ||||
| Squamous cell carcinoma, large cell, non-keratinizing | 1 | ||||
| Adenocarcinoma | 87 | ||||
| Carcinoid | 2 | ||||
| Bronchiolo-alveolar adenocarcinoma | 40 | ||||
| Bronchiolo-alveolar adenocarcinoma, non-mucinous | 3 | ||||
| Bronchiolo-alveolar adenocarcinoma, mixed | 5 | ||||
| Adenocarcinoma with mixed subtypes | 3 | ||||
| Papillary adenoma | 6 | ||||
| Signet ring cell carcinoma | 5 | ||||
| Acinar cell adenoma | 3 | ||||
| Mixed squamous cell and glandular papilloma | 4 | ||||
| Adenocarcinoma with squamous metaplasia | 1 | ||||
P denotes differences between controls with a negative LDCT scan and lung cancer patients
P denotes differences between controls with a positive LDCT scan and lung cancer patients
Inflammation Protein Measurement
Concentrations of 30 inflammation proteins were measured on serum samples of 265 cases and 528 controls using a highly sensitive and analytically validated electrochemiluminescence V-PLEX immunoassay (MSD® Rockville, MD), following the manufacturer’s instructions. Plasma samples from all participants were randomly distributed across the plates. Control samples to assess inter-plate variability, and analyte-specific standards to generate standard curves, were also included with each plate. Briefly, 25μl of patient sera were assayed for circulating levels of Chemokine Panel 1 Kit (Eotaxin, Eotaxin-3, IL-8, IP-10, MCP-1, MCP-4, MDC, MIP-1α, MIP-1β, TARC), Proinflammatory Panel 1 Kit (IFN-γ, IL-10, IL-12p70, IL-13, IL-1β, IL-2, IL-4, IL-6, TNF-α), Cytokine Panel 1 Kit (GM-CSF, IL-1α, IL-5, IL-7, IL-12/IL-23p40, IL-15, IL-16, IL-17A, TNF-β, and VEGF-A) and CRP. All signal results were extrapolated into concentrations (pg/ml) from the standard curves. Five control samples (random plasma samples from healthy volunteers) were also included on each plate to assess intraplate variance (see Supplementary Table 2 for coefficients of variance). To ensure quality data for further analyses and interpretation, detection level criteria were applied to the measurements obtained for each protein (Supplementary Tables 3 and 4) based on fit curve ranges defined by the plate-specific-standard curves generated for each analyte using standard dilutions (computations were conducted using Workbench 4.0 (MSD® Rockville, MD). If an analyte had more than 50% of samples that fell outside of the detection range, the protein was excluded from further analysis (this criterion applied to high-sensitivity IL-8, MIP-1α, GM-CSF, IL-1α, IL-5, IL-1β, IL-12p70, IL-13, and IL-4). Median and quartile levels are chosen based on the respective control populations (i.e., negative scan or positive scan).
Data Processing and Normalization
Discovery Workbench (MesoScale Diagnostics LLC, Rockville, MD, USA) was used to fit analyte- and plate-specific signal vs concentration standard curves based on seven calibrator dilutions. Fitted curves were applied to convert measured signals to concentrations on biological samples. In the process of calibration, samples with signal intensities outside the standard curve range were assigned out-of-range (OOR) concentrations. Concentrations were log2-transformed. The subsequent analysis was separately carried out for unknown and control samples. For each analyte, the fraction of samples with concentrations labeled as OOR was calculated; analytes with OOR concentrations on >15% of the samples were removed from the subsequent analysis. Analytes that passed this sensitivity-based quality control filter were grouped by plex (CRP, Chemokine, Cytokine, and Proinflammatory) (29).
Each of these groups was separately normalized across plates using the R package MDimNormn, which minimizes plate effects based on a multidimensional MA (multi- MA) mean centering normalization approach (27). For each analyte, 1-way ANOVA analysis was performed to verify that any significant plate effects (F-value<0.05) were indeed removed after multi-MA normalization. For each protein, the difference between each sample and the median across samples in units of mean absolute deviation (MAD) was used as a robust statistic of deviation. Outlier samples were defined as samples that had absolute deviation >3 for >15% of analytes (and the same sign in all of the deviates above the absolute deviation threshold).
Statistical Analysis
Demographic and smoking status variables were described for cases and controls. To evaluate differences between cases and controls, chi-squared tests were used for categorical variables and Student’s t-tests for continuous variables. Tumor characteristics were further described for cases. Univariable and multivariable logistic regression was utilized to estimate the magnitude of association between inflammation protein expression and case/control status. Multivariable analyses were used to control for possible confounding variables: age, gender, race, smoking status at randomization, and pack-years of smoking. Final adjusted models included the above variables with BMI, family history of cancer and for previous diagnoses of inflammatory conditions including COPD, emphysema, bronchiectasis, sarcoidosis, tuberculosis or chronic bronchitis. Odds ratios (ORs) and 95% confidence intervals (CIs) were derived. A Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs for time from randomization to status at last follow up among cases. Models were adjusted for age, gender, smoking status, pack-years of smoking, tumor stage, histological subtype, and treatment status. A comparison of ROC and AUC statistics was conducted using the roccomp command. All statistical analyses were performed using STATA (version 14) and two-sided significance levels were set at P<0.05.
There were 40 tumors diagnosed as BAC (bronchioalveolar carcinoma) in this dataset. Since the completion of the NLST, BAC has undergone a reclassification as adenocarcinoma in situ, minimally invasive adenocarcinoma (MIA), lepidic predominant adenocarcinoma (LPA), predominantly invasive adenocarcinoma with some nonmucinous lepidic component and invasive mucinous adenocarcinoma. The previous classification included a heterogenous spectrum of subtypes that usually has a better prognosis (in some cases 5-year survival was 100% at 5 years) (30), therefore for the survival analysis of this paper we also conducted anested analysis with removal of the BAC subtype.
Results
Study Population Characteristics
Descriptive characteristics of controls and lung cancer cases are presented in Table 1. The population was well matched, with no differences by age, gender, BMI, race or smoking status (Table 1). As this was an NLST population, the pack-year distribution was high for both cases and controls. As expected, the majority of the cases were diagnosed with stage I disease (n=160 stage IA and n=25 stage IB, combined stage 1=71%). Although the histological subtype distribution was broad, adenocarcinoma was the most prevalent (33%), followed by squamous cell carcinoma (20%) and bronchiolo-alveolar adenocarcinoma (19%).
Association Between Plasma Inflammation Proteins and Lung Cancer Diagnosis
As shown in Supplementary Table 5, most inflammation proteins had a similar distribution between cases and controls. However, several proteins, including IL-8, CRP and IFN-γ, were significantly higher among cases.
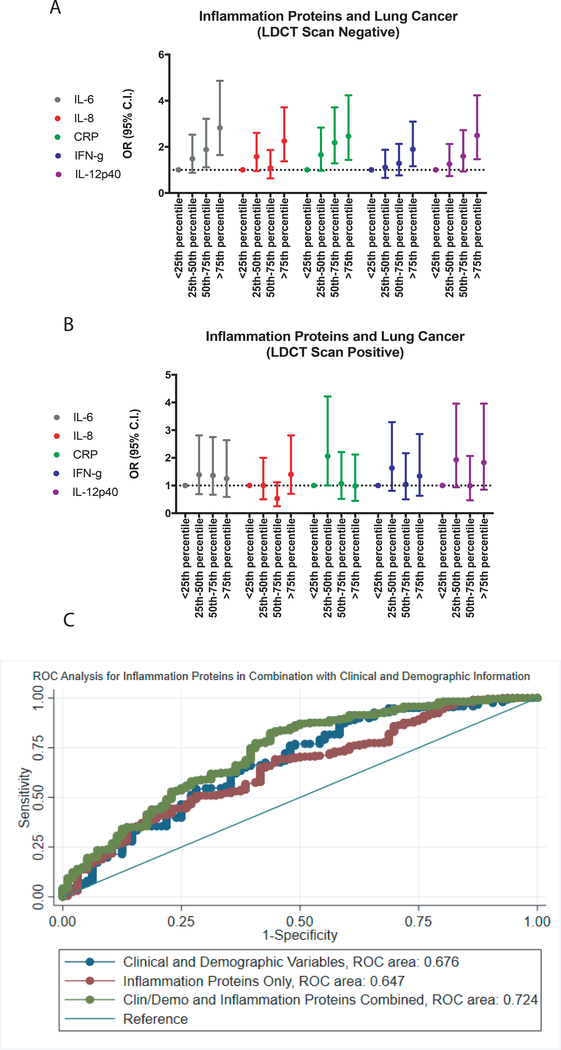
We initially attempted to confirm our previous observations with regard to CRP, IL-6 and IL-8 in the NLST population and conducted a nested case-control study based on cases with a positive screen and controls with a negative screen. Comparing quartile 4 with quartile 1, IL-6, IL-8 and CRP were associated with increased odds of a lung cancer diagnosis (OR 2.82, 95% C.I. 1.64–4.86; OR 2.25, 95% C.I. 1.37–3.71; OR 2.46, 95% C.I. 1.43–4.23, respectively). These observations confirmed our previous findings (22). We also found increased IFN-γ and IL-12/IL-23p40 associated with increased odds of a lung cancer diagnosis (OR 1.89, 95% C.I. 1.16–3.09; OR 2.49, 95% C.I. 1.46–4.23, respectively) (Table 2) (Figure 1A). These relationships were significant after adjustment for age, gender, race, smoking status, pack-years of smoking, BMI, family history of lung cancer and previous diagnoses of inflammatory conditions. The results for all inflammation proteins included in this study are shown in Supplementary Table 6.
Table 2:
Relationship between inflammation proteins with lung cancer diagnosis among individuals with a negative LDCT screen
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Control/Case | Participants with a negative LDCT scan | Participants with a negative LDCT scan | |||
| OR (95% C.I.) | P | OR (95% C.I.) | P* | ||
| IL-6 | |||||
| <25th Percentile | 103/42 | Reference | Reference | ||
| 25th – 50th Percentile | 103/58 | 1.38 (0.85–2.24) | 0.19 | 1.48 (0.87–2.53) | 0.15 |
| 50th – 75th Percentile | 103/73 | 1.74 (1.09–2.77) | 0.02 | 1.88 (1.11–3.21) | 0.02 |
| >75th Percentile | 103/83 | 2.00 (1.26–3.16) | 0.003 | 2.82 (1.64–4.86) | <0.001 |
| Trend | 1.40 (1.18–1.66) | 0.002 | 1.40 (1.18–1.66) | <0.001 | |
| <Median | 206/100 | Reference | Reference | ||
| >Median | 205/156 | 1.57 (1.14–2.15) | 0.005 | 1.84 (1.26–2.68) | 0.001 |
| IL-8 | |||||
| <25th Percentile | 105/50 | Reference | Reference | ||
| 25th – 50th Percentile | 104/69 | 1.40 (0.88–2.19) | 0.15 | 1.57 (0.95–2.60) | 0.08 |
| 50th – 75th Percentile | 105/52 | 1.04 (0.65–1.67) | 0.87 | 1.08 (0.63–1.86) | 0.77 |
| >75th Percentile | 104/91 | 1.84 (1.18–2.85) | 0.007 | 2.25 (1.37–3.71) | 0.001 |
| Trend | 1.18 (1.02–1.35) | 0.02 | 1.25 (1.06–1.46) | 0.006 | |
| <Median | 209/119 | Reference | Reference | ||
| >Median | 209/143 | 1.20 (0.88–1.63) | 0.25 | 1.30 (0.90–1.86) | 0.16 |
| CRP | |||||
| <25th Percentile | 105/50 | Reference | Reference | ||
| 25th – 50th Percentile | 104/69 | 1.57 (0.98–2.52) | 0.06 | 1.65 (0.96–2.83) | 0.07 |
| 50th – 75th Percentile | 105/52 | 1.81 (1.14–2.87) | 0.01 | 2.18 (1.28–3.71) | 0.004 |
| >75th Percentile | 104/91 | 1.76 (1.11–2.80) | 0.02 | 2.46 (1.43–4.23) | 0.001 |
| Trend | 1.19 (1.03–1.37) | 0.02 | 1.33 (1.13–1.58) | 0.001 | |
| <Median | 209/119 | Reference | Reference | ||
| >Median | 209/143 | 1.39 (1.02–1.90) | 0.04 | 1.73 (1.20–2.49) | 0.003 |
| IFN-g | |||||
| <25th Percentile | 104/50 | Reference | Reference | ||
| 25th – 50th Percentile | 103/57 | 1.15 (0.72–1.84) | 0.56 | 1.11 (0.65–1.87) | 0.72 |
| 50th – 75th Percentile | 104/64 | 1.28 (0.81–2.03) | 0.29 | 1.28 (0.76–2.13) | 0.35 |
| >75th Percentile | 103/91 | 1.84 (1.18–2.85) | 0.007 | 1.89 (1.16–3.09) | 0.01 |
| Trend | 1.22 (1.06–1.40) | 0.005 | 1.24 (1.06–1.45) | 0.007 | |
| <Median | 207/107 | Reference | Reference | ||
| >Median | 207/155 | 1.45 (1.06–1.98) | 0.02 | 1.50 (1.06–2.14) | 0.02 |
| IL-12/IL-23p40 | |||||
| <25th Percentile | 105/50 | Reference | Reference | ||
| 25th – 50th Percentile | 104/65 | 1.31 (0.83–2.07) | 0.24 | 1.25 (0.73–2.12) | 0.42 |
| 50th – 75th Percentile | 105/62 | 1.24 (0.78–1.97) | 0.36 | 1.59 (0.93–2.72) | 0.09 |
| >75th Percentile | 104/85 | 1.72 (1.10–2.67) | 0.02 | 2.49 (1.46–4.23) | 0.001 |
| Trend | 1.17 (1.02–1.35) | 0.03 | 1.36 (1.15–1.61) | 0.001 | |
| <Median | 209/115 | Reference | Reference | ||
| >Median | 209/147 | 1.27 (0.94–1.74) | 0.12 | 1.77 (1.22–2.57) | 0.002 |
P adjusted for age, gender, smoking status, pack-years of smoking, race, BMI, history of COPD, childhood asthma, adulthood asthma, asbestosis, chronic bronchitis, bronchiectasis, emphysema, fibrosis of the lung, pneumonia, sarcoidosis, silicosis, tuberculosis, diabetes, family history of lung cancer
Figure 1.
A graphical representation of odds ratios for inflammation proteins associated with lung cancer diagnosis among A, screen negative controls and B, screen positive controls. C, a ROC analysis for clinical and demographic variables and inflammation proteins among lung cancer patients and controls with positive LDCT scans.
One hundred and twenty-two patients had their blood taken before the lung cancer diagnosis. As the period between the first and final screen was 2 years, it isn’t possible to compare the relationship between these proteins with long-term lung cancer risk. However, we conducted a sensitivity analysis to filter out the samples taken at the time of a positive screen/lung cancer diagnosis (Supplementary Table 7). We then compared the levels of each inflammation protein in these cases to control participants with a negative LDCT scan (n=418). Increased levels of IL-6 (OR: 1.25, 95% C.I. 1.00–1.57), IFN-γ (OR 1.27, 95% C.I. 1.04–1.56) and IL-12/IL-23p40 (OR: 1.32, 95% C.I. 1.06–1.65) were each associated with increased likelihood of a lung cancer diagnosis in samples taken 1–2 years before a positive scan (Supplementary Table 7).
Given our repeated observations of elevated inflammation proteins at the time of lung cancer diagnosis among lung cancer patients, compared with population controls, we then conducted a second nested case-control study looking at levels of circulating proteins in malignant compared with benign nodules detected by LDCT. As shown in Table 3 and Figure 1B, none of the circulating inflammatory proteins measured in this study could distinguish a benign from malignant lung nodule (IL6: OR 1.25, 95% C.I. 0.59–2.64) (IL-8: OR 1.40, 95% C.I. 0.70–2.81) (CRP: OR 0.98, 95% C.I. 0.45–2.12) (IFN-γ: OR 1.34, 95% C.I. 0.63–2.86) (IL-12/IL23p40: OR 1.83, 95% C.I. 0.85–3.96). We also conducted an ROC-analysis looking at co-variables alone (AUC=0.676), the five inflammation proteins alone (AUC=0.647) and co-variables and inflammation proteins together. The combined AUC value was 0.724 (Figure 1C).
Table 3:
Relationship between inflammation proteins with lung cancer diagnosis among individuals with a positive LDCT scan
| Control/Case | Participants with a positive LDCT scan | ||
|---|---|---|---|
| OR (95% C.I.) | P* | ||
| IL-6 | |||
| <25th Percentile | Reference | ||
| 25th – 50th Percentile | 28/57 | 1.39 (0.69–2.81) | 0.35 |
| 50th – 75th Percentile | 27/66 | 1.36 (0.67–2.75) | 0.40 |
| >75th Percentile | 28/78 | 1.25 (0.59–2.64) | 0.56 |
| Trend | 27/55 | 1.07 (0.84–1.36) | 0.57 |
| <Median | 88/123 | Reference | |
| >Median | 55/133 | 1.10 (0.66–1.86) | 0.71 |
| IL-8 | |||
| <25th Percentile | Reference | ||
| 25th – 50th Percentile | 28/69 | 1.00 (0.50–2.00) | 0.99 |
| 50th – 75th Percentile | 27/63 | 0.53 (0.25–1.12) | 0.10 |
| >75th Percentile | 28/39 | 1.40 (0.70–2.81) | 0.34 |
| Trend | 27/91 | 1.06 (0.86–1.32) | 0.57 |
| <Median | 55/132 | Reference | |
| >Median | 55/130 | 0.96 (0.57–1.61) | 0.87 |
| CRP | |||
| <25th Percentile | Reference | ||
| 25th – 50th Percentile | 28/50 | 2.06 (1.00–4.22) | 0.05 |
| 50th – 75th Percentile | 27/104 | 1.07 (0.52–2.21) | 0.86 |
| >75th Percentile | 28/62 | 0.98 (0.45–2.12) | 0.96 |
| Trend | 28/46 | 0.91 (0.71–1.17) | 0.47 |
| <Median | 55/154 | Reference | |
| >Median | 55/108 | 0.68 (0.41–1.13) | 0.14 |
| IFN-g | |||
| <25th Percentile | Reference | ||
| 25th – 50th Percentile | 27/56 | 1.63 (0.81–3.29) | 0.17 |
| 50th – 75th Percentile | 27/85 | 1.04 (0.50–2.17) | 0.92 |
| >75th Percentile | 27/56 | 1.34 (0.63–2.86) | 0.45 |
| Trend | 27/65 | 1.04 (0.82–1.32) | 0.75 |
| <Median | 54/141 | Reference | |
| >Median | 54/121 | 0.89 (0.53–1.51) | 0.68 |
| IL-12/IL-23p40 | |||
| <25th Percentile | Reference | ||
| 25th – 50th Percentile | 28/66 | 1.93 (0.94–3.96) | 0.07 |
| 50th – 75th Percentile | 27/89 | 0.99 (0.47–2.07) | 0.98 |
| >75th Percentile | 28/51 | 1.83 (0.85–3.96) | 0.12 |
| Trend | 27/66 | 1.12 (0.88–1.43) | 0.37 |
| <Median | 55/145 | Reference | |
| >Median | 55/117 | 0.93 (0.55–1.59) | 0.80 |
P adjusted for age, gender, smoking status, pack-years of smoking, race, BMI, history of COPD, childhood asthma, adulthood asthma, asbestosis, chronic bronchitis, bronchiectasis, emphysema, fibrosis of the lung, pneumonia, sarcoidosis, silicosis, tuberculosis, diabetes, family history of lung cancer
As the case-control analysis included disease ranging from stage I-stage IV, it was possible that our case-control results reflected an elevation of inflammation proteins in late stage disease, which would explain the inability of the inflammation proteins to detect early stage disease. To test this possibility, we also conducted an analysis restricted to stage I disease (Supplementary Table 8). As shown, each of the 5 proteins were strongly associated with stage 1 disease.
Relationship Between Circulating Inflammation Proteins and Lung Cancer Survival in a Screened Population
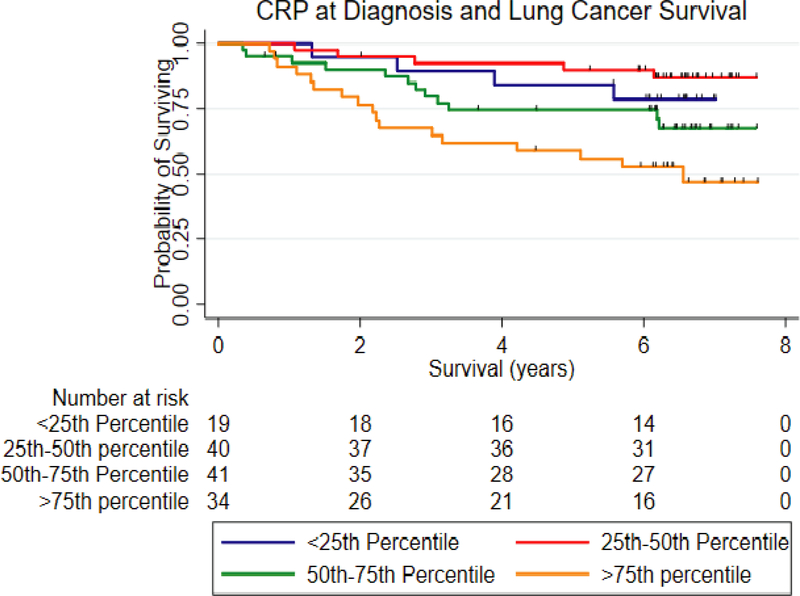
We previously found evidence that IL-6, IL-8, CRP and IL-17A were associated with lung cancer survival (31,32). These studies included lung cancer cases diagnosed based on clinical symptoms. We therefore asked whether any of the circulating proteins measured in this study were associated with survival in the NLST patient cohort. As shown in Figure 2, when considering levels of CRP only among cases where the samples was taken at the time of diagnosis, increased levels of CRP were associated with poor outcome (Table 4) (HR: 2.28, 95% C.I. 1.02–5.07, P=0.04).
Figure 2.
A Kaplan-Meier curve depicting the relationship between circulating levels of CRP with survival among lung cancer patients diagnosed with LDCT in the NLST.
Table 4:
Relationship between CRP with lung cancer survival in the NLST1
| N | HR (95% C.I.) | P* | N | HR (95% C.I.) | P^ | |
|---|---|---|---|---|---|---|
| CRP | ||||||
| <25th Percentile | 19 | Reference | 12 | Reference | ||
| 25th – 50th Percentile | 40 | 0.65 (0.26–1.62) | 0.35 | 32 | 0.87 (0.32–2.36) | 0.78 |
| 50th – 75th Percentile | 41 | 1.37 (0.60–3.12) | 0.45 | 36 | 1.62 (0.64–4.09) | 0.31 |
| >75th Percentile | 34 | 2.28 (1.02–5.07) | 0.04 | 33 | 2.77 (1.10–6.96) | 0.03 |
| Trend | 1.52 (1.18–1.96) | 0.001 | 1.57 (1.18–2.07) | 0.002 | ||
P adjusted for age, gender, smoking status, race, pack-years of smoking, treatment status, tumor stage and tumor histology.
P adjusted for age, gender, smoking status, race, pack-years of smoking, treatment status, tumor stage and tumor histology (with BAC removed).
denotes samples with a blood draw at the time of the screen/cancer diagnosis (n=134).
Discussion
We and others previously demonstrated that circulating proteins are altered among lung cancer cases compared with controls in both a case-control and prospective study setting (22,24–26). Herein, we replicated these previous observations for IL-6, CRP and IL-8. Our previous studies focused on a limited number of inflammation proteins (n=10), therefore we increased that number to 30 in this study and identified two additional proteins associated with lung cancer diagnosis, i.e., IFN-γ and IL-12/IL-23p40. To our knowledge, this is the first time that circulating levels of these two proteins have been linked with lung cancer diagnosis and/or risk. The participants in this study were high-risk, heavy smokers, with a smoking profile that was higher that other studies. This is a potential reason why our study was the first to report these observations. Of note, our findings were observed in plasma, while previous studies focused on serum (22,25–27), which could also explain why we found an association. Our validation of previous findings for IL-6, CRP and IL-8 in a different type of biospecimen further strengthens those observations.
A secondary aim of this study was to assess whether the utility of circulating inflammation proteins could be extended to use as companion biomarkers in the setting of LDCT screening for lung cancer. Previously, levels of CRP, IL-6, IL-8 proteins were found to be elevated in clinically-detected lung cancer patients compared with population controls. Further, in this analysis when only considering screen negative patients, levels were also elevated, including for IFN-γ and IL-12/IL-23p40. Surprisingly, however, these proteins were not elevated in lung cancer cases with a positive screen, compared with individuals with a suspicious lesion but no confirmed cancer diagnosis. The power in this analysis was lower, but the results appeared to be clear. We conducted an ROC analysis looking at whether the inflammation proteins added information to a discrimination model. Although the AUC value increased when these proteins were added, the value itself, 0.724, was still low, suggesting again that these proteins have little discrimination ability in this setting.
As nodules detected by LDCT screening are small, we tested whether the relationship between inflammation proteins and lung cancer diagnosis in the case control analysis was influenced by the detection of large, late-stage tumors—the study cohort included tumors from stage I-stage IV. Therefore, we conducted a sensitivity analysis restricted to stage I tumors only. However, our data show that the inflammation proteins were associated with diagnosis of stage I disease. This suggests that these proteins are elevated among patients with tumors 30 mm or less (size cut-off for stage I disease). It is possible that these inflammation proteins are not useful for detection of smaller lesions, i.e., <30 mm, but our findings also suggest that even inflammation proteins associated with lung cancer risk, and lung cancer diagnosis, may not be useful biomarkers for benign and malignant lung nodule discrimination. One reason for this could be that patients with detected nodules or positive LDCT scans could have additional inflammatory conditions that elevate baseline levels. Senescent cells within the nodule microenvironment could acquire a senescent-associated secretory phenotype (SASP) or a pre-malignant non-progressing lesion that secretes cytokines (20,23). Although a null relationship was observed both before and after adjustment for many inflammatory conditions in our study, we cannot rule out this possibility. The lack of an association is also unlikely to be due to the specific smoking and age profile of the patients in the NLST, as our first analysis, which was case-control and where a significant relationship was observed, had a similar smoking profile to the nodule discrimination analysis.
Our data suggest that alternative strategies are needed to find biomarkers for nodule discrimination. Indeed, recent work by several groups have shown progress. For example, results of the PANOPTIC trial measuring LG3BP (33) and C163A, and the MILD trial, measuring microRNAs (34), suggest that some circulating biomarkers will have sufficient discriminatory power to be clinically useful. Other strategies, such as that of Spira and colleagues, harness the field effect theory of lung carcinogenesis measuring gene expression signatures in bronchial washings and nasal epithelia (35), while the field of radiomics is also rapidly evolving and poised to impact the discrimination of nodules (36–39).
The third aim of this study was to assess whether proteins previously associated with lung cancer survival among symptomatic patients would also be related to survival in a screen-detected population. Herein, we validated our observations for CRP. Since the NLST was completed, BAC has been reclassified. As BAC tends to have a better prognosis overall, we also conducted a sensitivity analysis with BAC tumors removed, however it did not change our findings.
Our study has several strengths and limitations. A key strength of the study was assessment of samples from the national lung screening trial and the ability to pull from a large population of controls. Further, simultaneously assessing these proteins both in the context of a case control study and a screening study enabled us to extrapolate case control findings directly to the question of benign and malignant nodules within the same population. Another strength of the study was our ability to control for a broad range of inflammatory conditions (these data were extensively collected in the NLST), as these are potential confounders for the association between circulating inflammation proteins and lung cancer. One of the study’s limitations was the inclusion of a smaller population of screen positive controls, but in this case the study was limited to what was available.
In summary, our data show that while inflammation is involved in the etiology of lung cancer, the detection of circulating inflammation proteins (at least those measured in this study) is unlikely to have clinical use in distinguishing between a benign and malignant lung nodule. The majority of this population were white and therefore this conclusion still needs to be made for other racial and ethnic groups. Although samples from screening studies are precious in nature, our data also highlight the need to possibly avoid extrapolation from case-control to prospective to screening studies for the purposes of identifying molecules that will discriminate between benign and malignant nodules. Additionally, studying the intended use population, possibly via agnostic means, could be the best approach, at least in the search for these specific kinds of biomarkers. This approach has been validated by Spira and others (34,35). It is also worth noting that the issue of risk stratification for high-risk individuals is still a concern and biomarker work continues in this area as well (40). For example, while the general uptake of LDCT screening is still low (41), as its use broadens, there is isn’t sufficient medical capacity to screen all eligible individuals, estimated at over 7 million (41,42). Given previous data linking inflammation proteins to lung cancer risk (22,25,26), and the data from this study showing that IL-6, IFN-γ and IL-12/IL-23p40 were also elevated among lung cancer patients 1–2 years prior to a lung cancer diagnosis, it remains possible that there may be clinical utility for inflammation proteins in that setting.
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program of the National Cancer Institute. J.C. was supported by the Intramural Research Program of multiple NIH Institutes through the Trans-NIH Center for Human Immunology (CHI), NIAID, NIH.
Funding Statement: This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI. J.C. was supported by the Intramural Research Program of multiple NIH Institutes through the Trans-NIH Center for Human Immunology (CHI), NIAID, NIH.
Footnotes
Conflict of Interest Statement:
The authors do not have any conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patz EF, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer 2003;5:46–62 [DOI] [PubMed] [Google Scholar]
- 6.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276–85 [DOI] [PubMed] [Google Scholar]
- 7.Hijikata A, Kitamura H, Kimura Y, Yokoyama R, Aiba Y, Bao Y, et al. Construction of an open-access database that integrates cross-reference information from the transcriptome and proteome of immune cells. Bioinformatics 2007;23:2934–41 [DOI] [PubMed] [Google Scholar]
- 8.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther 2008;8:605–15 [DOI] [PubMed] [Google Scholar]
- 9.Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015;36:1085–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res 2007;85:462–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006;6:24–37 [DOI] [PubMed] [Google Scholar]
- 13.Peek RM, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a national cancer institute-sponsored meeting. Cancer Res 2005;65:8583–6 [DOI] [PubMed] [Google Scholar]
- 14.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. JClin Invest 2007;117:1175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blot WJ, Harrington JM, Toledo A, Hoover R, Heath CW Jr., Fraumeni JF Jr. Lung cancer after employment in shipyards during World War II. N Engl J Med 1978;299:620–4 [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015;160:62–73 [DOI] [PubMed] [Google Scholar]
- 17.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest 2007;131:1557–66 [DOI] [PubMed] [Google Scholar]
- 18.Kuschner WG, D’Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J 1996;9:1989–94 [DOI] [PubMed] [Google Scholar]
- 19.Fukuyama T, Ichiki Y, Yamada S, Shigematsu Y, Baba T, Nagata Y, et al. Cytokine production of lung cancer cell lines: Correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci 2007;98:1048–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 2010;29:273–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seike M, Yanaihara N, Bowman ED, Zanetti KA, Budhu A, Kumamoto K, et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst 2007;99:1257–69 [DOI] [PubMed] [Google Scholar]
- 22.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst 2011;103:1112–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson SD, De Costa AM, Young MR. Effect of the premalignant and tumor microenvironment on immune cell cytokine production in head and neck cancer. Cancers (Basel) 2014;6:756–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pine SR, Mechanic LE, Enewold L, Bowman ED, Ryan BM, Cote ML, et al. Differential Serum Cytokine Levels and Risk of Lung Cancer Between African and European Americans. Cancer Epidemiol Biomarkers Prev 2016;25:488–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiels MS, Katki HA, Hildesheim A, Pfeiffer RM, Engels EA, Williams M, et al. Circulating Inflammation Markers, Risk of Lung Cancer, and Utility for Risk Stratification. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013;105:1871–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner DR, Fanidi A, Grankvist K, Muller DC, Brennan P, Manjer J, et al. Inflammatory Cytokines and Lung Cancer Risk in 3 Prospective Studies. Am J Epidemiol 2017;185:86–95 [DOI] [PubMed] [Google Scholar]
- 28.Brenner DR, Boffetta P, Duell EJ, Bickeboller H, Rosenberger A, McCormack V, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol 2012;176:573–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong MG, Lee W, Nilsson P, Pawitan Y, Schwenk JM. Multidimensional Normalization to Minimize Plate Effects of Suspension Bead Array Data. J Proteome Res 2016;15:3473–80 [DOI] [PubMed] [Google Scholar]
- 30.Zell JA, Ou SH, Ziogas A, Anton-Culver H. Long-term survival differences for bronchiolo-alveolar carcinoma patients with ipsilateral intrapulmonary metastasis at diagnosis. Ann Oncol 2006;17:1255–62 [DOI] [PubMed] [Google Scholar]
- 31.Meaney CL, Zingone A, Brown D, Yu Y, Cao L, Ryan BM. Identification of serum inflammatory markers as classifiers of lung cancer mortality for stage I adenocarcinoma. Oncotarget 2017;8:40946–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan BM, Pine SR, Chaturvedi AK, Caporaso N, Harris CC. A Combined Prognostic Serum Interleukin-8 and Interleukin-6 Classifier for Stage 1 Lung Cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Thorac Oncol 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvestri GA, Tanner NT, Kearney P, Vachani A, Massion PP, Porter A, et al. Assessment of Plasma Proteomics Biomarker’s Ability to Distinguish Benign From Malignant Lung Nodules: Results of the PANOPTIC (Pulmonary Nodule Plasma Proteomic Classifier) Trial. Chest 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32:768–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, et al. A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer. N Engl J Med 2015;373:243–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Kim J, Balagurunathan Y, Hawkins S, Stringfield O, Schabath MB, et al. Prediction of pathological nodal involvement by CT-based Radiomic features of the primary tumor in patients with clinically node-negative peripheral lung adenocarcinomas. Med Phys 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Balagurunathan Y, Liu Y, Qi J, Schabath MB, Ye Z, et al. Comparison Between Radiological Semantic Features and Lung-RADS in Predicting Malignancy of Screen-Detected Lung Nodules in the National Lung Screening Trial. Clin Lung Cancer 2018;19:148–56 e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Wang H, Li Q, McGettigan MJ, Balagurunathan Y, Garcia AL, et al. Radiologic Features of Small Pulmonary Nodules and Lung Cancer Risk in the National Lung Screening Trial: A Nested Case-Control Study. Radiology 2018;286:298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tunali I, Stringfield O, Guvenis A, Wang H, Liu Y, Balagurunathan Y, et al. Radial gradient and radial deviation radiomic features from pre-surgical CT scans are associated with survival among lung adenocarcinoma patients. Oncotarget 2017;8:96013–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Integrative Analysis of Lung Cancer E, Risk Consortium for Early Detection of Lung C, Guida F, Sun N, Bantis LE, Muller DC, et al. Assessment of Lung Cancer Risk on the Basis of a Biomarker Panel of Circulating Proteins. JAMA Oncol 2018:e182078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer 2013;119:1381–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.