Abstract
Tobacco addiction each year causes millions of deaths worldwide. Brain nicotinic acetylcholine receptors have been shown to be central to tobacco addiction. Nicotine replacement therapy aids tobacco cessation, but the success rate is still far too low. This may in part be due to the fact that neurons with nicotinic receptors are not the only neural systems involved in tobacco addiction. Interacting neural systems also play important roles in tobacco addiction. Nicotine increases the release of a variety of neurotransmitters, including dopamine and serotonin. Dopamine, in particular dopamine D1 receptors, have been shown to be involved in the reinforcing action of nicotine. Serotonin through its actions on 5-HT2C receptors has been shown to play a key role in modulating the reinforcement of addictive drugs, including nicotine and alcohol. Combination of treatments could provide greater treatment efficacy. These studies were conducted to evaluate combination therapies utilizing nicotine replacement therapy in conjunction with either a dopamine D1 receptor antagonist SCH-23390 or a serotonin 5-HT2C receptor agonist, lorcaserin. Female Sprague-Dawley rats were given access to self-administer nicotine via IV infusions. Osmotic pumps were implanted to reproduce the kinetic of chronic nicotine patch therapy. SCH-23390 (0.02 mg/kg) or lorcaserin (0.6 mg/kg) were administered prior to nicotine self-administration sessions. Reproducing earlier findings SCH-23390, lorcaserin and nicotine replacement therapy were effective at reducing IV nicotine self-administration. 5HT2C agonist treatment had additive effects with chronic nicotine infusion for significantly lowering nicotine self-administration. This study demonstrates the feasibility of combination of chronic nicotine with therapies targeting non-nicotinic receptors as treatment options for tobacco addiction.
Keywords: Nicotine, Self-administration, SCH-23390, Lorcaserin, Dopamine D1 receptors, Serotonin 5HT2C receptors, Treatment, Addiction
Introduction
Tobacco use remains a leading cause of preventable death, claiming nearly 6 million lives world-wide each year, (Centers for Disease Control and Prevention, 2014). In the United States alone, the economic cost of smoking is estimated at over 300 billion dollars each year, including the loss of productivity and the medical costs associated with smoking (Centers for Disease Control and Prevention, 2014). Despite tobacco’s known health impairing effects, and increased efforts to educate young people of its dangers, people continue to smoke. As of 2013, nearly one in five adults in the United States were current cigarette smokers (Centers for Disease Control and Prevention, 2014), almost 70% of whom stated they want to quit smoking (Surgeon General, 2014). Despite this, most attempts to stop smoking are unsuccessful.
Nicotine replacement therapy (NRT) is frequently used as a component to aid in smoking cessation attempts, by supplying the body with nicotine from a source other than tobacco. NRT reduces the physiological withdrawal symptoms that result from tobacco dependence, and increases the likelihood of continued abstinence (Gourlay and McNeil, 1990). Additionally, NRTs generally provide lower concentrations of nicotine than would be available from smoking, with the added benefit that they do not provide the carcinogens or gasses associated with smoking cigarettes. The most common NRTs are the transdermal patch and gum, although other delivery mechanisms exist (Frishman, 2007). An examination of NRTs has revealed that although replacement therapies significantly increase smoking cessation by 50-70% over placebo, given the very low rate with placebo, cessation success with nicotine replacement remains at a low level (Stead et al.,2012).
There are few non-nicotinic pharmacologic therapies available to combat nicotine dependence. Currently, there are two non-nicotine pharmacologic therapies approved by the US FDA for treating tobacco addiction. Bupropion (Zyban®), initially approved by the FDA for depression, was approved to aid smoking cessation in 1997. The second drug, approved by the FDA in 2006, is varenicline (Chantix®). Varenicline acts as a nicotinic partial agonist for α4β2*, α6β2* and α3β4 receptor subtypes (Rollema et al., 2007), and a full agonist for α7 nicotinic receptors (Mihalak et al., 2006). Because of varenicline’s partial agonist property, it acts as an antagonist to α4β2* receptors in the presence of nicotine, and activates nicotinic receptors in nicotine’s absence (Gonzales et al., 2006). Importantly, only about one in five smokers are able to maintain prolonged abstinence, with any of the available pharmacotherapies. Thus, there is a great need for more effective pharmacotherapies to help these people combat tobacco smoking addiction. There is evidence for possible efficacy of treatments for tobacco smoking cessation mediated via other neurotransmitter systems. Brain nicotinic receptors interact with numerous neural circuits, many of which are integral to reinforcement and reward pathways. Nicotine has been shown to affect the release of various neurotransmitters other than acetylcholine, including the biogenic amines: serotonin (5HT), dopamine (DA), and histamine (H) (Corrigall et al., 1992; Wonnacott et al., 1990). Elevations in DA release have been noted as a major contributor to the reinforcing properties of nicotine (Di Chiara, 2000). The release of monoamines likely underlies the reinforcement of tobacco. DA systems in the brain are key to nicotine reinforcement. SCH-23390 is a potent D1 antagonist (Bourne, 2001). Subcutaneous injections of SCH-23390 reduce nicotine self-administration in rats (Corrigall and Coen, 1991; Hall et al., 2015b). Furthermore, studies demonstrated the effectiveness of SCH-23390 in reducing nicotine self-administration in rats with local infusion into the nucleus accumbens, insular cortex and posterior parietal association cortex (Hall et al., 2015a; Kutlu et al., 2013). These findings suggest that SCH-23390 can be an effective agent to help treat tobacco abuse. Over-expression of D1 receptors in the prelimbic frontal cortex of adult male rats increases high-risk behaviors, including increased reactivity to drug-related cues for nicotine (Sonntag et al., 2014). This implicates DA D1 family receptors as attractive drug targets to help with smoking cessation. SCH-23390 has also demonstrated off-target effects as a potent agonist of serotonin 5-HT2C receptors, (Millan et al., 2001), which may also play a role in its ability to decrease the reinforcing effects of nicotine.
In addition to DA, the serotonergic system in the brain also plays a significant role in addiction. One serotonergic receptor implicated in addiction is the 5-HT2C subtype (Liu et al., 2007; Stanford et al., 2005). Previous studies have shown that activation of 5-HT2C receptors in turn inhibits the release of mesolimbic dopamine (Di Giovanni et al., 2000; Di Matteo et al., 1999), Increased activation of 5-HT2C receptors then, provides a possible mechanism to mediate the increased release of dopamine by exogenous substances (i.e. nicotine). As a potent agonist of 5-HT2C, lorcaserin, a drug approved by the FDA for the treatment of obesity, meets the characteristics necessary for a drug with the potential to decrease the reinforcing effects of nicotine. Functional assays revealed that lorcaserin is over 100 times more selective for 5-HT2C in comparison to 5-HT2B, and over 20 times more selective in comparison to 5-HT2A (Smith et al., 2005). Additionally, lorcaserin has demonstrated the ability to decrease nicotine selfadministration in rats (Higgins et al., 2012; Levin et al., 2011b) as well as alcohol intake in alcohol preferring rats (Rezvani and Levin, 2014). These characteristics of lorcaserin implicate its possible utility in a combination therapy with NRT.
The purpose of these studies was to better understand the relationship between brain nicotinic acetylcholine receptors, DA D1 receptors, and 5-HT2C receptors in nicotine reinforcement, and to examine if a combination of treatment methods targeting both receptor systems can potentiate their individual effects. This study examined this by determining the effectiveness of a combination therapy of nicotine replacement and dopamine D1 antagonism with SCH-23390 and 5HT2C agonism with lorcaserin. We acknowledge the difficulty of exploring dose-response in studies of combination therapy and drug interactions. It is hypothesized that the combined treatments will provide greater and more long-lasting reduction in nicotine self-administration.
Methods
Subjects
Young adult female Sprague-Dawley rats were used in this study. Animals were individually housed in accordance with standard laboratory conditions. Housing was located in a temperature- and humidity-controlled room adjacent to the testing room to minimize the stress that can be induced by transportation. Animals were singly housed to prevent damage to catheters by cage mates. All animals were kept on a reverse 12:12 day/night cycle (lights on from 6 PM to 6 AM) at room temperature of 23±2 degrees °C with 50±10% humidity, with behavioral testing occurring during the dark, “active” phase of their diurnal cycle for this nocturnal species. All animals were given water ad libitum outside of their experimental sessions. Initially fed ad libitum, all animals were kept on an approved and modestly restricted diet of standard rat chow, given approximately 30 min after the completion of experimental session, once behavioral training commenced. The rats were fed daily, increasing feeding from 8-16 g/day as the rats grew. This kept them at a healthy lean weight 85% of age-adjusted ad lib weight for female Sprague-Dawley rats. The age of the female rats at the beginning of the drug study was 66-72 days with an average weight of 210 g). The rats progressively gained weight throughout the study. These studies were conducted under protocols approved by the Institutional Animal Care and Use Committee of Duke University and meets the requirements of state, federal and international regulatory bodies.
Drug Preparation
Drug solutions were prepared bi-weekly in sterilized isotonic solutions. Doses of nicotine ditartrate solutions were calculated as a function of the nicotine base weight. SCH-23390 and lorcaserin were calculated as of the salt weight and were dissolved in saline. The pH of solutions was adjusted using NaOH to 7.0. Solutions were then passed through a 0.22 μ Nalgene filter (Nalgene Nunc International, Rochester, NY, USA) to ensure sterilization. All nicotine solutions were kept covered to prevent photodegradation and all solutions were refrigerated between experiments. All doses of drugs were selected based on previous studies (Levin et al. 2011a, Rezvani et al., 2014). Selected doses for SCH-23390 and lorcaserin were 0.02 mg/kg and 0.6 mg/kg, respectively. The volume of SC injection for both drugs and the saline vehicle was 1 ml/kg given 15 min before nicotine IV self-administration sessions and during the week of enforced abstience.
Study Design
Two studies were conducted. Study 1 tested the interactions of chronic nicotine infusions via an osmotic minipump with repeated acute SC injections of the dopamine D1 antagonist SCH-23390 and Study 2 tested the interactions of chronic nicotine infusions via an osmotic minipump with repeated acute SC injections of the serotonin 5HT2c agonist lorcaserin. The experimental sequence is shown in Table 1. The doses chosen for chronic nicotine infusion as well as repeated SCH-23390 and lorcaserin injections were selected as those that we had found in previous studies to cause modest but significant decreases in nicotine self-administration (Hall et al., 2015b; Levin et al., 2011a; Levin et al., 2016). In both studies the nicotine treatment was the same: Under general anesthesia, osmotic minipumps (Alzet Model 2ML4, Durect Inc., Cupertino, CA, USA), filled with nicotine (2.5 mg/kg/day) were placed in subdermal s.c. pockets. The rats were given the osmotic minipump on a day with no test sessions. The resumed testing the day after starting on the minipump. The osmotic pumps continuously and with the zero-order kinetics delivered nicotine for four consecutive weeks. This delivery modeled the zero-order diffusion of nicotine to individuals by nicotine patches. Following the recovery from the minipump surgery, the effects of lorcaserin and SCH-23390 as well as the control vehicle on IV nicotine self-administration was tested for two consecutive weeks. The drug treatments continued for four consecutive weeks with an additional week of testing after the end of drug treatment. Rats were randomly assigned to treatment groups. After the first week on nicotine, the rats began either SCH-23390 or lorcaserin (or control) injections.
Table 1:
The sequence of experimental treatments, training and measurements.
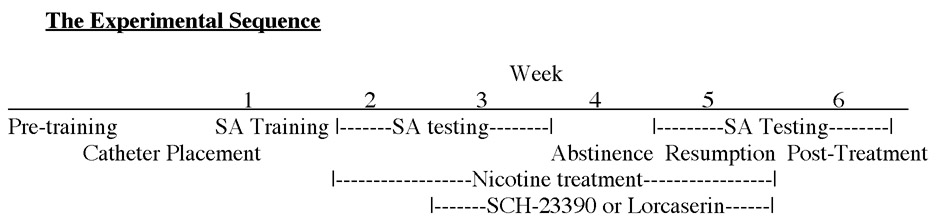 |
A period of forced abstinence from self-administration trials was implemented to model the self-enforced abstinence of people trying to quit smoking. After two weeks of treatments with nicotine self-administration access there was a week of enforced abstinence during which the rats were not given access to nicotine for self-administration but continue to receive acute injections of SCH-23390 (0.02 mg/kg) or lorcaserin (0.6 mg/kg) similar to how an individual would continue to use treatments for a period following cessation of smoking. Following the hiatus from nicotine reinforcement, rats were reintroduced access to nicotine self-administration again without any treatment. The last part of the experiment evaluated the drug-seeking behavior of rats once the treatments have ceased. This can be demonstrated by decreased reward-driven behavior towards the nicotine reinforcement, even after treatments have been withdrawn.
Behavioral Training
Before minipump surgery and nicotine self-administration trials began, all rats were trained to lever press in a standard dual-lever experimental chamber. Each chamber was equipped with two levers (one active, the other inactive), two cue lights, one located above each lever, a house light, and a tone generator. A computer programmed with MED-PC software managed experimental events and data collection. Initially, rats were trained to respond to a lever to receive a 45 mg food pellet reward, under a fixed ratio (FR) 1 schedule of reinforcement. Half the animals were trained to respond to the right lever, and the other half was trained to respond to the left lever. The cue light over the correct lever was illuminated, while the cue light over the inactive lever remained dark. Food restricted rats were put in operant box for one hour/day for 3 consecutive days and number of lever presses were recorded for food pellet. Once rats met a criterion of ≥50 correct lever responses for food in three consecutive one-hour training sessions, they had jugular catheter surgeries to allow them to receive IV nicotine infusions and began nicotine self-administration trials. Nicotine self-administration sessions were conducted in the same dual-lever experimental chambers used for pellet training. Following catheterization, animals began self-administration sessions with nicotine (0.03 mg/kg/infusion) as a reinforcer. During each session, an illuminated light over the active lever indicated the correct lever to press. Responding to the active lever resulted in a 0.5 s tone generation, and a 50 μL infusion of nicotine (0.03 mg/kg/infusion) over less than one second. Each infusion was followed by a one-min timeout phase, where the cue light was exterminated and responses were recorded, but no reinforcement was given for responses. Inactive lever presses were recorded, but resulted in no infusion deliveries, serving as a control. All self-administration sessions lasted one hour. Five baseline sessions were administered to each rat before test sessions with SCH-23390, lorcaserin or vehicle injections.
Surgeries
Animals went under two surgeries. First catheter surgery and a week later the minipump surgery. Following completion of training with food reinforcements, animals were anesthetized using 60 mg/kg of ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA) and 0.15 mg/kg of dexdomitor (Pfizer, New York, NY, USA) given via i.p. injections, and a catheter (Strategic Application Inc., Libertyville, IL, USA) was implanted into the jugular vein using aseptic techniques, to facilitate nicotine infusions. After anesthetization, a small incision slightly lateral to the frontal midline was made, and the jugular vein was exposed via blunt dissection. The area of jugular vein distal to the desired region was tied off to prevent bleeding, and an incision was made in the vein for catheter insertion. The catheter was inserted until the tip was close to the heart. Once in place, the catheter was sutured to both the vein and deep muscle using silk. The remaining portion of catheter was routed subcutaneously around the back, through a skin pocket created by blunt instrument, and threaded through a small incision made between the scapulae. The catheter was then attached to an infusion harness (SAI Infusion Technologies, Libertyville, IL, USA), which could be tethered to the infusion pump during experimental sessions. Surgical wounds were sutured using polypropylene thread. Harnesses were also tethered to each animal using polypropylene thread. All catheters were flushed daily prior to self-administration sessions with a 0.3 mL solution containing 100 U/mL heparinized saline (Baxter Health Corporation, Deerfield, IL, USA). Post-sessions, the nicotine solution remaining in each animal’s harness was removed and replaced with a 0.3 mL sterile lock containing heparinized saline 500 U/mL with 8 mg/mL gentamicin (American Pharmaceutical Partners, Schaumberg, IL, USA). Upon completion of self-administration sessions, animals were tested for jugular-catheter patency using phenobarbital before sacrifice. Data was only included from animals with verified patent catheters. A total of 72 animals went under surgery and 48 of them remained patent to the end of the study. Only the data from the rats with patent catheter were used for analysis.
After five sessions of nicotine self-administration training was complete and the drug treatment studies began, rats underwent another surgery to implant osmotic pumps (Alzet Model 2ML4, Durect Corporation, Palo Alto, CA, USA) to infuse either saline or nicotine (2.5 mg/kg/day) chronically for four weeks. Nicotine dose was calculated as a function of nicotine base weight. Pumps were implanted subcutaneously into a pocket on the back, created by a blunt instrument through an incision. The incision was closed once the pump was implanted using a combination of polypropylene thread and surgical clips, and a topical antibiotic was applied. Each animal was administered ketoprofen (5 mg/kg, s.c.) for post-operative pain, and a topical anesthetic, bupivacaine (Levin et al., 2011a; Rezvani et al. 2014).
Chronic Nicotine Infusion
After 5 baseline sessions, rats began chronic treatments with saline or 2.5 mg/kg/day of nicotine for 28 consecutive days via osmotic pump. The nicotine dose was calculated as a function of the nicotine base weight at the time of the surgery. The dose was chosen to simulate plasma levels of nicotine observed in chronic heavy smokers (Murrin et al., 1987) and has been shown to alter cholinergic receptor expression in adult rats (Trauth et al., 1999). Previously, we have found that this dose of chronic nicotine causes significant, reduction of nicotine self-administration in rats (Levin et al., 2016). After their pump surgeries, all animals continued with nicotine self-administration sessions.
SCH-23390 and Lorcaserin Injections
Starting after five sessions of baseline training for nicotine self-administration as described above, therapeutic drug tests began. The rats began repeated injections s.c. with either saline, SCH-23390 (Study 1), or lorcaserin (Study 2) during weeks 2–4 of chronic nicotine infusion via osmotic pump. Rats were injected with vehicle, SCH-23390 (0.02 mg/kg, s.c.), or lorcaserin (0.6 mg/kg, s.c.). These are doses of SCH-23390 and lorcaserin, which we have previously shown to significantly reduce nicotine self-administration in rats with this SA procedure (Hall et al., 2015b; Levin et al., 2011a). Each solution was injected subcutaneously in a volume of 1 ml/kg body weight approximately 20 min before the self-administration session begins. Thus, each animal received injections while chronically treated with nicotine or vehicle infusions, including before IV nicotine self-administration and during the week of enforced abstinence.
After two weeks (5 sessions/week) of receiving chronic pump treatment and one week of receiving injections of SCH-23390 or lorcaserin with nicotine self-administration trials, rats were put on one-week hiatus from self-administration trials. While on hiatus, rats received the same doses of SCH-23390, lorcaserin or vehicle injections, according to their assigned group (i.e.five injections over the course of the week) as well as having continued nicotine infusions via the osmotic pump.
Post-hiatus, rats were reintroduced to nicotine self-administration trials. Rats also resumed saline, SCH-23390 or lorcaserin s.c. injections 20-min prior to beginning each session, according to each rat’s group placement. Resumption of the nicotine self-administration trials ran for five consecutive days.
Once the resumption week was completed, the osmotic pumps were removed under anesthesia using ketamine and dexdormitor as described above. Once the pump was removed, the incision was sutured shut using polypropylene thread, topical antibiotics were applied, and ketoprofen was administered for post-operative pain. Rats were given at least 24 hours to recover from this surgery. Rats then returned to complete five additional nicotine self-administration sessions, over the course of a week, without receiving injections and also without their nicotine pumps. After completion of the last session, each rat’s catheter was tested for patency using phenobarbital before sacrifice. Data was only included from animals with verified patent catheters.
Data Analysis
Analysis of variance was used to determine statistical significance of the collected data (nicotine infusions per session). There were three factors in each study: chronic nicotine, chronic SCH-23390 or lorcaserin (depending on study), and week. The two drug factors were between subjects factors and the time factor was a repeated measure. Planned comparisons were made between treatment groups and controls. The repeated measure was the week-by-week self-administration performance (infusions/session) during the two weeks of drug administration, the week of resumption after enforced abstinence and the week after the end of drug treatment. As recommended by Snedecor and Cochran interactions of p<0.05 (one-tailed) were followed-up tests of the simple main effects (Snedecor and Cochran, 1967). They make the point that by their nature interactions are one-tailed tests. All of the final analyses had p<0.05 (two-tailed) as the threshold for significance. An alpha level of p<0.05 (two-tailed) was used as a cutoff for statistical significance. Dunnett’s tests were used to correct for multiple comparisons to determine which treatments caused significant decreases in nicotine self-administration relative to control.
Results
Study 1: SCH-23390 Interactions with Chronic Nicotine
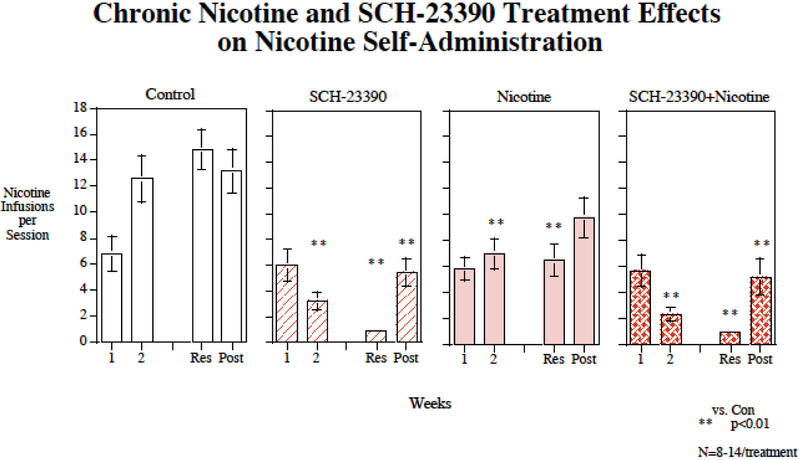
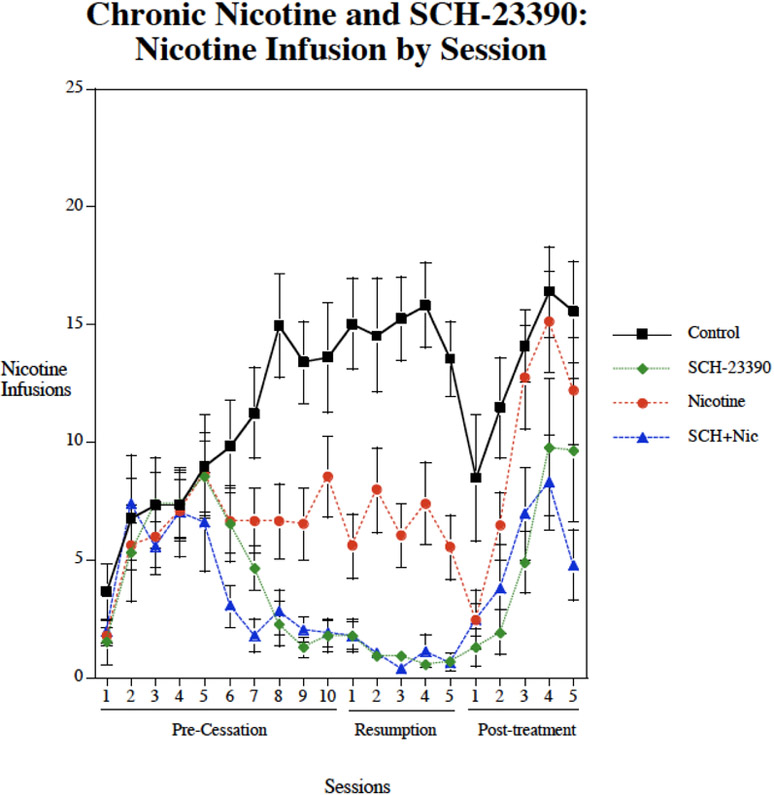
Chronic subcutaneous (s.c.) infusions of nicotine via osmotic pump (2.5 mg/kg/day) significantly decreased nicotine self-administration (F(1,44)=5.47, p<0.025) (Fig. 1). There was also a significant main effect of SCH-23390 (F(1,44)=49.93, p<0.0005). There was no significant nicotine × SCH-23390 interaction. There were interactions of SCH-23390 × week (F(3,132)=14.24, p<0.0005) and SCH-23390 × Nicotine × week (F(3,132)=2.50, p<0.07) that called for analyses of drug effects during each week of treatment. As shown in figure 1, no significant effect was seen during the first week of treatment. The controls were still self-administering relatively low nicotine levels during the first week of treatment given the limited number of pretraining sessions. During continued testing during the second week and beyond the controls continued to rise in their levels of nicotine self-administration as has been seen previously, whereas the treated groups did not show such a rise. During the second week, both nicotine (p<0.01) and SCH-23390 (p<0.01) given by themselves significantly lowered nicotine self-administration relative to control. The combination of nicotine plus SCH-23390 injections also significantly (p<0.01) reduced nicotine self-administration relative to control during the second week. This combination also significantly reduced nicotine self-administration relative to nicotine alone (p<0.025), but was not to SCH-23390 alone. During the week of resumed access after the enforced abstinence, nicotine alone (p<0.01), SCH-23390 alone (p<0.01) and the combination (p<0.01) all significantly reduced nicotine self-administration relative to control. Continuing the effectiveness of the combined treatment, the combination of nicotine plus SCH-23390 significantly lowered nicotine self-administration relative to nicotine alone (p<0.005), but not to SCH-23390 alone. After cessation of treatment the groups given nicotine alone rose and was no longer different from control. In contrast, the groups, which had received SCH-23390 alone or nicotine plus SCH-23390 treatment showed increases in nicotine self-administration but remained significantly (p<0.01) lower than control. During the post-treatment testing phase, the combination treatment was significantly (p<0.05) lower than nicotine alone. Figure 2 shows the session-by-session data. All groups showed a temporary decrease in nicotine self-administration on the day after the minipump was removed. The controls and nicotine alone group showed increases in nicotine self-administration after withdrawal of the treatment such that they were not significantly different from controls. The groups that received either SCH-23390 alone both nicotine + SCH-23390 showed an increase from a lower ongoing level. There were significant decreases relative to control (18.4±5.8) in inactive lever pressing with the SCH-23390 given alone (5.0±1.9, p<0.05), nicotine given alone (5.0, p<0.05) and the combination (1.5±0.3, p<0.01). During the post-treatment phase there were no longer significant effects of drug treatment on inactive lever presses.
Figure 1:
Repeated SCH-23390 injections interacting with chronic nicotine infusion, affect IV nicotine self-administration The weeks listed are weeks of drug treatment with four weeks of chronic nicotine infusion (Week 1 through resumption) and three weeks of lorcaserin injections (Week 2 through nicotine access resumption). Average infusions per session refer to the average number of nicotine infusions delivered per 60-min self-administration session with each treatment condition. Results from nicotine self-administration sessions, averaged over week-long periods of the experiment (weeks 1 and 2 of continued nicotine access (1 and 2), resumed access (Res) and post-treatment (Post), grouped by treatment condition. Chronic continuous nicotine infusions (4 weeks at 2.5 mg/kg/day) and chronic s.c. SCH-23390 (4 weeks at 0, or 0.02 mg/kg) injection effects on IV nicotine self-administration, weekly average response for the entirety of the experiment. Data represent mean ± sem, N=9-14/treatment
Figure 2:
Individual nicotine self-administration sessions during SCH-23390 + Chronic Nicotine treatment study (mean±sem).
Study 2: Lorcaserin Interactions with Chronic Nicotine
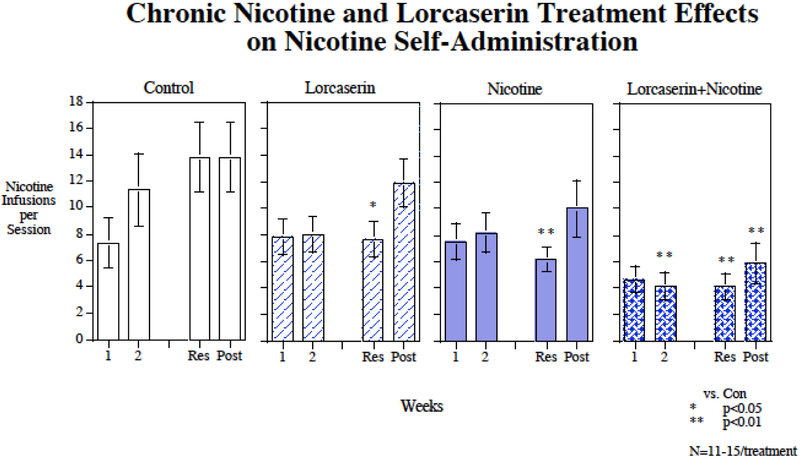
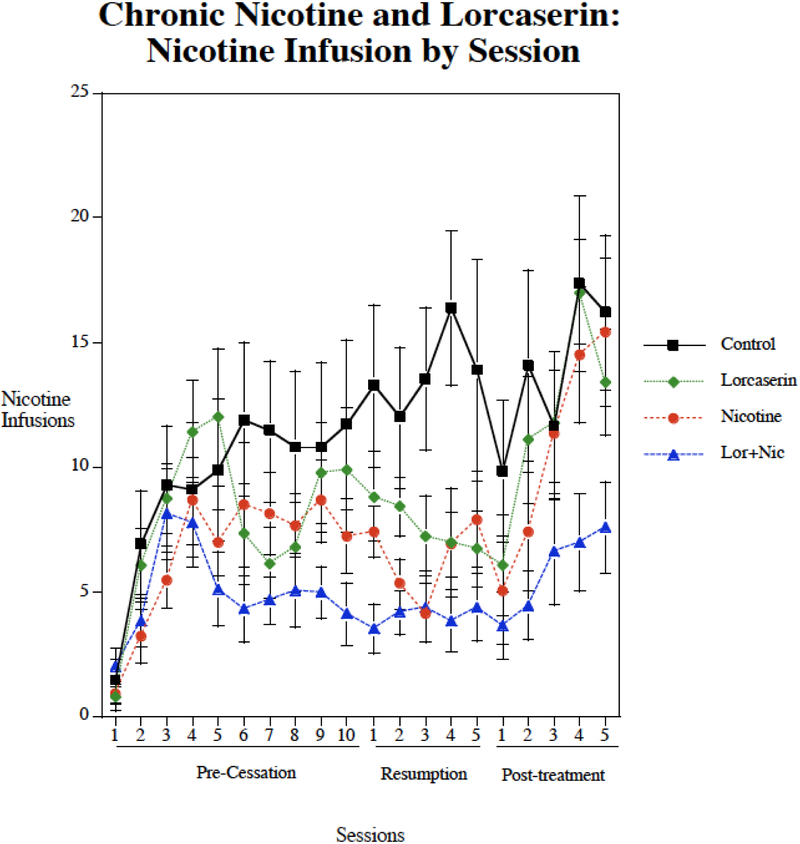
Chronic nicotine infusions via osmotic pump (2.5 mg/kg/day) significantly decreased nicotine self-administration (F(1,46)=7.85, p<0.01). There was not a significant main effect of lorcaserin (p=0.07). There was no significant nicotine × lorcaserin interaction. There was a significant interaction of lorcaserin × week (F(3,138)=4.64, p<0.005) that prompted tests of the drug effects during each week of treatment. As shown in figure 3, no significant effect was seen during the Week 1 of treatment. As seen previously in Study 1, the controls were still self-administering relatively low levels during the first week of treatment given the limited number of pretraining sessions. During continued testing with week two and beyond the controls continued to rise in their levels of nicotine self-administration, whereas the treated groups did not show such a rise. During the second week of treatment only the combination of nicotine plus lorcaserin significantly (p<0.01) reduced nicotine self-administration relative to control. During the week of resumed access after the enforced abstinence, nicotine alone (p<0.01), lorcaserin alone (p<0.05) and the combination (p<0.01) all significantly reduced nicotine self-administration relative to control. After cessation of treatment with lorcaserin and nicotine, the groups previously given nicotine alone or lorcaserin alone rose substantially and were no longer different from the control group. In contrast, the group withdrawn from nicotine plus lorcaserin treatment showed only a small increase in nicotine self-administration that remained significantly (p<0.01) lower than control. During the post-treatment testing phase, the combination treatment was significantly lower than the group formerly given lorcaserin alone (p<0.05). but not the group formerly given nicotine alone. Figure 4 shows the session-by-session data for the lorcaserin + nicotine study. With this more fine-grain look, it becomes clearer that after the end to treatment the nicotine self-administration in the lorcaserin or chronic alone groups quickly return to control levels while that of the group that had received lorcaserin+chronic nicotine continued to show lower levels. No significant drug treatment effects were seen with inactive lever pressing, suggesting no appreciable drug effects on general activity in this study.
Figure 3:
Repeated lorcaserin injections interacting with chronic nicotine infusion, affect rates of nicotine self-administration. The weeks listed are weeks of drug treatment with four weeks of chronic nicotine infusion (Week 1 through resumption) and three weeks of lorcaserin injections (Week 2 through resumption). Average infusions per session refer to the average number of nicotine infusions delivered per 60-min self-administration session with each treatment condition. Results from nicotine self-administration sessions, averaged over week-long periods of the experiment (weeks 1 and 2 of continued nicotine access (1 and 2), resumed access (Res) and post-treatment (Post), grouped by treatment condition. Chronic continuous nicotine infusions (4 weeks at 0 or 2.5 mg/kg/day) and chronic s.c. lorcaserin (4 weeks at 0 or 0.6 mg/kg) injection effects on IV nicotine self-administration, weekly average response for the entirety of the experiment. Data represent mean ± sem, N=11-15/treatment
Figure 4:
Individual nicotine self-administration sessions during Lorcaserin + Chronic Nocotine treatment study (mean±sem).
Discussion
The results of this study provide promising insight into new lines of combining drug treatments to aid in smoking cessation. Replicating prior findings (Hall et al., 2015a; Higgins et al., 2012; Levin et al., 2011b; Levin et al., 2016), individual treatments with SCH-22390, lorcaserin and nicotine replacement therapy significantly reduced nicotine self-administration when compared to vehicle treated controls. Importantly these studies showed that the combination treatments with SCH-23390 plus nicotine or lorcaserin plus nicotine caused more robust decreases in nicotine self-administration.
Important was the persistent effect of the combined treatments even after treatment stopped. With all of the individual treatments, nicotine, SCH-23390 and lorcaserin, nicotine self-administration rebounded to levels comparable pre-treatment levels once treatment was removed in each group. This phase if testing models the relapse in smoking behavior in humans that often occurs after the end of therapeutic treatment. Ideally, people will not have to take a drug over the course of their lifetime in order to prevent their smoking habit, and ultimately a treatment should prevent the rate of relapse even after its use has been discontinued. Our findings suggest that that prolonged effects may be possible with the combination treatment. In this study SCH 23390 alone also showed a persisting effect of reducing nicotine self-administration after the end of treatment. Access to nicotine self-administration while under the influence of the combined treatment diminished the conditioned reinforcement driving self-administration likely accounted for the persisting effects after treatment withdrawal. However, further research is needed to see if the effects of combination therapy persist even after the termination if therapies are given over a longer period of time.
Since the rats had initial lever pressing training with food reinforcement, there was the possibility that continued lever pressing with the food was replaced with nicotine may have been a residual effect of the previous food delivery and not nicotine driven response. However, we view this as unlikely. The fact that the rats show increasing nicotine self-administration during the pretraining period indicates that nicotine served as a reinforcer for behavior. If it was just residual responding for prior food reinforcement then there should have been higher rates of responding and declining responding after the food reinforcers were no longer available.
It is possible that the lowered nicotine self-administration with the drug treatments may have resulted from drug effects of sedation rather than reduced nicotine reinforcement. For lorcaserin this was seen as being unlikely since lorcaserin treatment had no observed effect on responding for the inactive lever. There was a different result with SCH-23390. With this D1 antagonist there was a significant decrease in responding on the inactive lever during the time of treatment. Thus, there could have been a more general effect of reduced activity with SCH-23390. Interesting during the post treatment phase there was no longer a reduction in inactive lever pressing with the groups previously given SCH-23390 alone or SCH-23390 together with nicotine. However, there continued to be a significantly lower rate of nicotine self-administration. With chronic nicotine exposure there was a mixed effect on inactive lever pressing. In the study with lorcaserin, no effect of chronic nicotine treatment on inactive lever pressing was seen, but in the SCH-23390 study there was a significant chronic nicotine-induced reduction in inactive lever pressing. Whatever the effect of chronic nicotine on inactive lever pressing it did not appear to be very robust.
The aim of this research is to determine how persistent the effect of the drug treatment is in reducing nicotine self-administration. Of course, as with any chronic treatment over repeated measures the effects on the repeated measure is a result of both the continuing effect of the treatment and the carryover from the previous effects. The persistence of the effect is a result of both the continuing effect of the drug and the continuing effect of the prior effect of the drug. The clinical efficacy of continued reduction of self-administration is an important result regardless of the specific manner by which it occurred. Interestingly, despite the weeks of experience with lower nicotine self-administration with lorcaserin alone, upon removal of the treatment nicotine self-administration quickly rose to earlier levels.
Although long examined solely for its effect on nicotinic receptors, nicotine is now known to stimulate the release of a wide variety of neurotransmitters (Corrigall et al.,1992; Wonnacott et al., 1990). The increased release of dopamine in the mesolimbic pathway is widely considered to underlie the reinforcing effects of nicotine that lead to addiction Both antagonism of DA D1 receptors and activation of 5-HT2C receptors have previously demonstrated effectiveness in reducing nicotine’s effects in reinforcement (Corrigall and Coen, 1991; Hall et al., 2015a; Higgins et al., 2012; Kutlu et al., 2013). In this study, the dopamine D1 receptor antagonist SCH-23390 significantly reduced nicotine self-administration in rats, supporting previous research examining its effect on nicotine self-administration (Corrigall and Coen, 1991). Our findings also build on the idea expressed in previous papers to combine cessation treatment therapies to attempt to potentiate the effects of both treatments (Hall et al., 2015c).
Previous studies have investigated the combination of varenicline and bupropion as a possible treatment option (Ebbert et al., 2009; Hall et al., 2015c), concluding, ultimately, that a combination therapy of both drugs shows greater promise in treating nicotine addiction than either treatment alone. New treatment options have also been explored. The serotonergic 5-HT2c agonist lorcaserin, and the NMDA glutamate receptor antagonist dextromethorphan have all previously demonstrated efficacy in reducing nicotine self-administration by themselves (Glick et al., 2001; Levin et al., 2010; Levin et al., 2011b) and have been investigated as possible tobacco addiction therapies. Previous interaction studies between pyrilamine and dextromethorphan revealed no significant interaction; however, the combination of lorcaserin and dextromethorphan demonstrated increased effectiveness when taken concurrently (Briggs et al., 2016). Furthermore, it has recently been shown that the combination of pyrilamine and nicotine replacement therapy reduced nicotine self-administration greater than either treatment alone (Levin et al., 2016). These promising results encourage the continued exploration of both new targets for nicotine abuse treatment, as well as the effectiveness of combination therapies.
In addition to its antagonism of DA D1 receptors, SCH-23390 also has known agonist effects on a subset of serotonergic (5HT2C) receptors (Millan et al., 2001). While the concentration of administered dose was managed to ensure off-target effects of SCH-23390 were minimized, its action on 5-HT2C receptors may be relevant to nicotine self-administration. Supporting this, previous studies have shown that the selective 5-HT2C agonist lorcaserin can significantly decrease nicotine self-administration in rats (Levin et al., 2011b). Further studies would have to be performed to parse the separable effects SCH-23390 has on the two sets of receptors, and how each relates to nicotine self-administration. Further research is needed to explore the drug dose-response interaction and determine the selectivity of these compounds on nicotine self-administration. Also, the current studies were conducted with nicotine self-administration starting in young adulthood. Future studies will see if different drug effects on adplescent-onset nicotine self-administration.
Altogether, our findings demonstrate that a combination pharmacotherapy of SCH-23390 and nicotine replacement therapy, as well as a combination of lorcaserin and nicotine replacement therapy, had greater effect in reducing nicotine self-administration than each individual drug. Importantly, these combination treatments had more long-lasting effects, remaining effective even after treatment ended. Combination therapies show promise in attenuating nicotine intake even after the end of therapy and should be studied further in order to explore new and more effective treatment options for smoking cessation.
Highlights.
Reproducing earlier findings SCH-23390, lorcaserin and nicotine reduced nicotine self-administration.
The 5HT2C agonist treatment had additive effects with chronic nicotine infusion.
This demonstrates the feasibility of combination of chronic nicotine with therapies targeting non-nicotinic receptors.
Acknowledgement
This research was supported by the P50 grant DA027840 from NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bourne JA, 2001. SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Reviews. 7, 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs S, et al. , 2016. Dextromethorphan interactions with serotonergic and histaminergic treatments to reduce nicotine self-administration. Pharmacology, Biochemistry and Behavior. 142, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. Smoking and tobacco use—fact sheet: health effects of cigarette smoking; 2014. Vol. 2015, ed.^eds. [Google Scholar]
- Corrigall WA, Coen KM, 1991. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology. 104, 171–176. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, et al. , 1992. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 107, 285–289. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, 2000. Role of dopamine in the behavioural actions of nicotine related to addiction. European Journal of Pharmacologyl. 393, 295–314. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, et al. , 2000. Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin2C/2B receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse. 35, 53–61. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, et al. , 1999. SB 242 084, a selective serotonin 2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 38, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, et al. , 2009. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine & Tobacco Research. 11, 234–239. [DOI] [PubMed] [Google Scholar]
- Glick SD, et al. , 2001. Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. European Journal of Pharmacology. 422, 87–90. [DOI] [PubMed] [Google Scholar]
- Gonzales D, et al. , 2006. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Journal of The Americal Medical Association. 296, 47–55. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, McNeil JJ, 1990. Antismoking products. Medical Journal of Australia. 153, 699–707. [DOI] [PubMed] [Google Scholar]
- Hall BJ, et al. , 2015a. Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology. 99, 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, et al. , 2015b. Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology. 99, 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, et al. , 2015c. Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacology, Biochemistry and Behavior. 130, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, et al. , 2012. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 37, 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, et al. , 2013. Role of insular cortex D(1) and D(2) dopamine receptors in nicotine self-administration in rats. Behav Brain Res. 256, 273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, et al. , 2010. Sazetidine-A, a selective alpha4beta2 nicotinic receptor desensitizing agent and partial agonist, reduces nicotine self-administration in rats. Joural of Pharmacology and Experimental Therapeutics. 332, 933–939. [DOI] [PubMed] [Google Scholar]
- Levin ED, et al. , 2011a. Lorcaserin decreases nicotine self-administration in female rats. Journal of Pharmacology and Experimental Therapeutics. 338, 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, et al. , 2011b. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. Joural of Pharmacology and Experimental Therapeutics. 338, 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, et al. , 2016. Reduction of nicotine self-administration by chronic nicotine infusion with H1 histamine blockade. Psychopharmacology. 233, 3009–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. , 2007. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 146, 1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW, 2006. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Molecular Pharmacology. 70, 801–805. [DOI] [PubMed] [Google Scholar]
- Millan MJ, et al. , 2001. The "selective" dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology. 156,58–62. [DOI] [PubMed] [Google Scholar]
- Murrin LC, et al. , 1987. Nicotine administration to rats: methodological considerations. Life Sciences. 40, 1699–1708. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED, 2014. Lorcaserin, a selective 5-HT2c receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacology, Biochemical and Behavior. 125, 1–8. [DOI] [PubMed] [Google Scholar]
- Rollema H, et al. , 2007. Pharmacological profile of the α 4 β 2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 52, 985–994. [DOI] [PubMed] [Google Scholar]
- Smith BM, et al. , 2005. Discovery and SAR of new benzazepines as potent and selective 5-HT2C receptor agonists for the treatment of obesity. Bioorganic & medicinal chemistry letters. 15, 1467–1470. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG, 1967. Statistical Methods, Vol., Iowa State University Press, Ames, Iowa. [Google Scholar]
- Sonntag KC, et al. , 2014. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacology. 231, 1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford I, et al. , 2005. 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: action at 5-HT 2C, 5-HT 4 and 5-HT 1A receptors. Neuropharmacology. 49, 1228–1234. [DOI] [PubMed] [Google Scholar]
- Stead L, et al. , 2012. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. Art. No.: CD000146. [DOI] [PubMed] [Google Scholar]
- General Surgeon, 2014. In: The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Reports of the Surgeon General, Vol., ed.^eds., Atlanta (GA). [Google Scholar]
- Trauth JA, et al. , 1999. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Research. 851, 9–19. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, et al. , 1990. Presynaptic nicotinic receptors and the modulation of transmitter release. Ciba Foundation Symposium. 152, 87–105. [DOI] [PubMed] [Google Scholar]