Abstract
A 5-year-old neutered female toy Poodle chronically treated with systemic and topical azoles to control recurrent Malassezia dermatitis/otitis was presented because of the loss of treatment efficacy. Minimum Inhibitory Concentrations (MICs) obtained in vitro for various azoles (especially itraconazole and ketoconazole) against Malassezia strains isolated from the dog were increased by several-fold compared with MICs obtained for control isolates. These results reinforced the assumption based on clinical observation, i.e. the development of azole resistance
Keywords: Malassezia pachydermatis, Dog, Resistance, Azoles
1. Introduction
Malassezia pachydermatis is a lipophilic yeast that colonizes the stratum corneum and mucosal sites of dogs. Favorable growth conditions in the local environment allow excessive multiplication of this organism, which may then function as an opportunistic secondary pathogen [1]. Dogs may present with Malassezia otitis, dermatitis (either localized or generalized) or both. Clinical signs are variable and include erythema, alopecia, mild–to-severe pruritus, greasy exudation and scaling. Secondary lesions include excoriations, lichenification, hyperpigmentation and exudation. In generalized cases, a rancid odor is commonly reported [1].
The yeast overgrowth is generally secondary to an ongoing disorder, predominantly hypersensitivity diseases (especially atopy), keratinization defects, recurrent bacterial pyoderma and endocrine diseases (especially hypothyroidism) [1]. Both in the case of dermatitis and otitis, a hypersensitivity response to the yeast itself is likely to occur in many allergic dogs [1]. Breed predisposition appears to be an important risk factor and cases without apparent underlying causes have also been reported [2].
Therapeutic options for treating Malassezia otitis/dermatitis include systemic and/or topical antifungal agents belonging to various chemical classes. Azole compounds, which inhibit the fungal biosynthesis of ergosterol by interacting with sterol-14α-demethylase [3], are widely employed [1]. Various antiseptics, such as chlorhexidine, are also effective treatments [1].
The possibility of developing azole resistance by M. pachydermatis has been frequently claimed in the literature on the basis of in vitro tests (e.g.in the studies by Nijima et al. [4] and Jesus et al.[5]), but only one case of in vivo resistance (i.e. treatment failure) has been reported, to date, in the dog [6]. Here we describe another case of M. pachydermatis that displayed in vivo resistance to azole compounds.
2. Case
A 2.5-year-old neutered female toy Poodle was evaluated (day 0) for severe, recurrent, generalized Malassezia dermatitis and otitis. Possible predisposing causes (allergic dermatitis, hypothyroidism, leishmaniasis, ecto-parasite infestation etc.) were investigated and ruled out. Due to the “idiopathic” nature of the yeast overgrowth, the following protocol was adopted: itraconazole oral solution (Itrafungol® Eli Lilly Italia Spa, Sesto Fiorentino, Italy) 5 mg/kg twice a week on 2 consecutive days; 2% chlorhexidine and 2% miconazole shampoo (Malaseb®, Dechra Veterinary Products A/S, Denmark) once a week; ear drops containing miconazole, prednisone and polymyxin B (Surolan®, Elanco Animal Health; Basingstoke, UK), applied twice a day for 2 weeks whenever needed. This protocol allowed an excellent control of clinical signs, as well as a reduction in the number of yeast cells detected on skin and ear cytological samples. The treatment protocol had to be maintained on a long-term basis, since dermatological problems recurred when withdrawal was attempted.
About two years and a half later (day + 900), although the therapy had not been changed, severe scaling dermatitis, pruritus and offensive odor developed, associated with an increase in the number of yeast cells in the skin and ear cytological samples. Treatment frequency was increased, as follows: itraconazole oral solution 5 mg/kg once daily; 2% chlorhexidine and 2% miconazole shampoo every 2 days. However, there was neither improvement in dermatological signs, nor a reduction in the number of yeast cells in skin and ear cytological samples.
The dog was presented for a new evaluation (day + 930). The dermatological picture consisted of numerous small white and yellow scales distributed over the entire surface of the body, including the limbs and the head, which were loosely attached to the hairs or stuck to the skin surface, partial alopecia together with pruritus and marked offensive odor (Fig. 1). Bilateral ceruminous otitis was also present (Fig. 2). Numerous Malassezia yeasts (> 30× high-power field) were detected on multiple skin and ear cytological samples (Fig. 3).
Fig. 1.
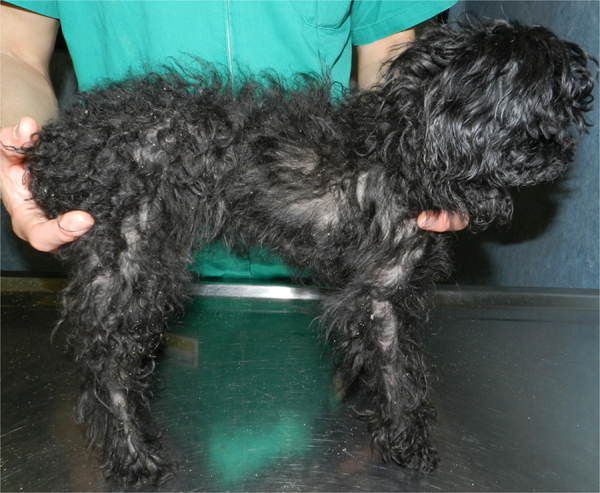
Severe generalized scaling dermatitis due to M. pachydermatis in a 5-year-old neutered female toy Poodle. A large amount of scales has been shed on the surface of the consulting table.
Fig. 2.
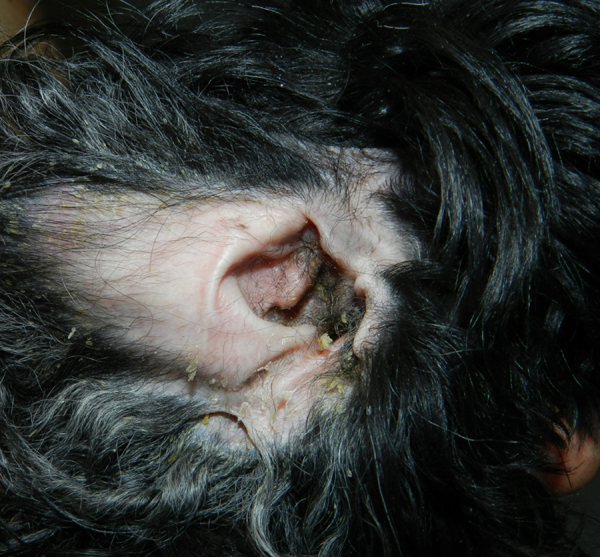
Scaling dermatitis with very mild erythema of the inner aspect of the pinna in the same dog as in picture 1. Ceruminous otitis was also present.
Fig. 3.
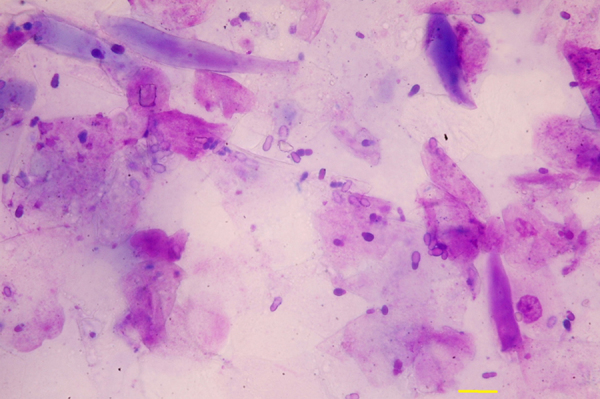
Numerous Malassezia yeasts visible on skin cytological sample (adhesive tape preparation, 40×) from a toy Poodle dog with severe generalized scaling dermatitis (Bar 10 µm).
Parasitic diseases and concurrent bacterial or dermatophyte infections were ruled out based on the administration of selamectin spot on (Stronghold® Zoetis 1348 Louvain-la-Neuve, Belgium) skin scrapings, direct hair examination, cytology and fungal cultures.
The worsening of an unidentified predisposing condition, a concomitant immunosuppressive disease, or the presence of azole resistance were considered as possible causes for the loss of treatment efficacy. Concomitant systemic or dermatological problems were not detected despite extensive clinical and laboratory investigations, including complete blood cell count, serum biochemistry panel, urinalysis, haemostatic profile, thyroid hormones and TSH measurement. In order to investigate possible azole resistance, acetate tape and swab samples were collected from different body areas and both ear canals and sent to a mycological research laboratory (day + 940).
All samples, cultured on Sabouraud dextrose agar (SDA) with chloramphenicol and gentamycin at 30 °C, yielded a profuse growth of yeast colonies with microscopic appearance typical of M. pachydermatis. The isolates employed for the in vitro tests (see below) were deposited in the collection of the Westerdijk Institute (formerly the Fungal Biodiversity Center – CBS) of Utrecht, where identification was confirmed at the molecular level.
2.1. Antifungal susceptibility testing (day + 950)
Three isolates were tested for the susceptibility to the following antifungal agents:
-
-
miconazole (MCZ), clotrimazole (CTZ) and terbinafine (TER) using a microplate broth-dilution (MiB) method modified from the reference method for in vitro testing of yeasts by the Clinical and Laboratory Standard Institute (CLSI) [7] (method described in document S1).
-
-
fluconazole (FCZ) and posaconazole (PSZ) using a commercial kit (E-test®, bioMérieux) (method described in document S1).
-
-
itraconazole (ITZ) and ketoconazole (KTZ), using both methods.
Results were expressed as MICs (minimum inhibitory concentrations of a drug able to inhibit fungal growth) [7]. For comparison, a reference strain (strain CBS 1879, considered the M. pachydermatis “type strain”) was included in the tests. For ITZ, KTZ, MCZ and CTZ, MICs were also compared with MICs – available in the database of the laboratory - previously obtained for 10 isolates of the yeast coming from dogs never subjected to antifungal therapies.
MICs of different azoles – in particular of ITZ, KTZ and MCZ – were increased by several-fold compared with MICs obtained for the control isolates (Table 1; Fig. 4).
Table 1.
MICs (µg/ml) for six isolates of M. pachydermatis obtained from the toy Poddle dog, a reference strain and 10 isolates of the yeast coming from dogs never subjected to antifungal therapies.
|
ITZ |
KTZ |
MCZ | TER | CTZ | PSZ | FCZ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Provenance | MiB | E-test | MiB | E-test | MiB | MiB | MiB | E-test | E-test |
| CBS 15214a | skin (abdomen) | 0.5 | 0.75 | 2 | 0.75 | 16 | 1 | 32 | 1.5 | > 256 |
| CBS 15215a | skin (dorsum) | 1 | 0.75 | 2 | 1 | 16 | 1 | 16 | 1.5 | 128 |
| CBS 15216a | right ear | 8 | > 32 | 16 | 1.5 | > 32 | 4 | 16 | 4 | > 256 |
| CBS 15208b | right ear | 1 | 0.75 | 4 | 1 | 16 | 1 | 32 | 2 | 128 |
| CBS 15212b | skin (neck) | 1 | 0.5 | 4 | 0.75 | 16 | 2 | 32 | 1.5 | > 256 |
| CBS 15211b | skin (left thigh) | 1 | 0.75 | 4 | 0.5 | 16 | 2 | 32 | 1.5 | > 256 |
| CBS 1879 | reference strain | 0.03 | 0.125 | 0.25 | 0.125 | 2 | 0.5 | 4 | 0.25 | 12 |
| / | dogs never subjected to antifungal therapiesc | 0.016–0.06 | 0.023–0.094 | 0.03–0.125 | 0.19–0.125 | 1–4 | / | 1–16 | / | / |
a,b = obtained from the toy Poddle dog, a) on day + 940; b) at the occasion of a follow up visit (day + 1350); c = for these isolates (n = 10) the range of MICs obtained is provided.
Number = the accession number assigned by the fungal collection of the Westerdijk Institute (formerly the Fungal Biodiversity Center – CBS) of Utrecht. MiB = microplate broth-dilution method. ITZ = Itraconazole; MCZ = Miconazole; TER = Terbinafine; KTZ = Ketoconazole; CTZ = Clotrimazole; PSZ = Posaconazole; FCZ = Fluconazole.
Fig. 4.
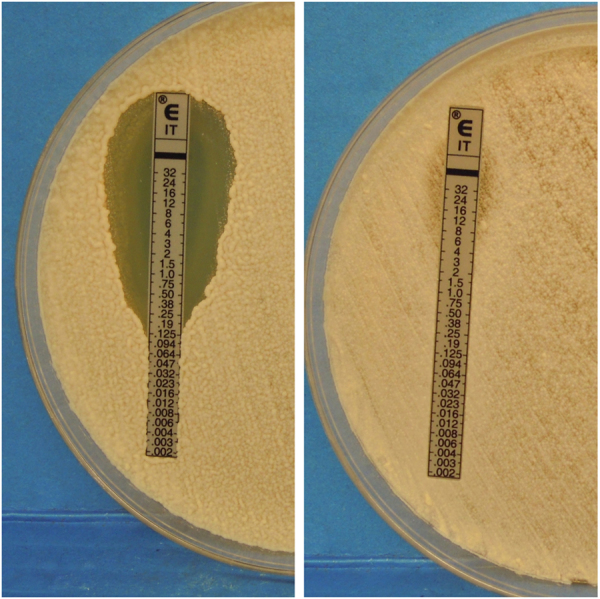
Results of the E-test assay for two isolates of M. pachydermatis against itraconazole. Left: reference strain CBS 1079 (MIC 0.125 µg/ml); right: isolate CBS 15216 - sampled from the toy Poddle dog - (MIC > 32 µg/ml).
These results reinforced the assumption based on clinical observation, i.e. the development of azole resistance.
Topical and systemic azoles were replaced with TER (Lamisil®, Novartis Pharma AG; Basel, Switzerland; 30 mg/Kg per os once a day) [8], [9]. However, during the following month (day + 960–990), clinical signs worsened. Oral TER was thus suspended and an exclusively topical approach was attempted with 4% chlorhexidine digluconate shampoo (Chlorexyderm® 4%, ICF, Cremona, Italy) performed every 2 days and an otic gel containing TER, dexamethasone and florphenicol (Osurnia®, Elanco Animal Health; Basingstoke, UK, twice a day). After 1 month (day + 1020), skin and ear lesions had significantly improved, but moderate pruritus and offensive odor were still present. Unfortunately, at that time cytology was not performed. Since then, different shampoos containing chlorhexidine, miconazole, zinc gluconate and colloidal sulphur are being employed on a rotating basis, with a fair control of the clinical signs. Moreover, during rechecks, a large number of Malassezia yeasts are always detected at cytology.
Eleven months later (day + 1350), other isolates were obtained in culture and tested in vitro. MICs for these isolates were consistently higher than those for the control isolates (Table 1).
3. Discussion
This case confirms what has been reported recently [6], namely that azole resistance can be the cause of treatment failure in a dog with Malassezia dermatitis/otitis. The previous report of resistance [6] concerned a 15-year old Miniature Dachshund dog in Japan, where resistance was suspected because of the lack of response despite 23 days of once-daily oral therapy with ITZ (8 mg/kg) and weekly shampooing with 2% miconazole/2% chlorhexidine shampoo. The clinical history regarding the dog was not provided, in particular nothing was said about previous antifungal treatments [6]. On the contrary, our dog was followed over an extended period, which allowed us to document that a prolonged exposure (years of treatment) to azole agents was required to develop resistance.
Antifungal susceptibility testing for M. pachydermatis must be interpreted with caution, as reference methods are not available [10]. To overcome this limit we used two methods (i.e. MiB and E-test) [11], [12], [13] and we compared the MICs of the isolates from the index case with the MICs obtained for some field isolates and a reference strain of the yeast. Not unexpectedly, the absolute values were somewhat different between the two methods (Table 1), but the same trend was observed, i.e. MICs of different azoles were increased for the six isolates compared with the control ones. One isolate in particular (strain CBS 15216) was highly resistant to MCZ and ITZ (where, in some cases, no MIC was detected, Fig. 4). These findings suggest that the skin of a given dog may be colonized by strains of M. pachydermatis with different antifungal susceptibility profiles. This possibility has already been shown for different bacterial species involved in canine otitis externa [14].
Interestingly, azole agents that had not been used in this dog (PSZ, FCZ, KTZ) showed a reduced activity in vitro, suggesting a possible cross-resistance of M. pachydermatis to different azoles. This phenomenon has been hypothesized in previous studies based on in vitro results [5].
Oral TER was ineffective in controlling clinical signs, despite there being a less marked difference between the MICs of the isolates and the reference strain (Table 1). This could be due to the lower concentrations of TER in canine stratum corneum or sebum compared to serum [15]. However, TER was active when employed topically, which can be explained by the concentration of active principle in the commercial formulation (Osurnia®), which greatly exceeds the MIC, being in the order of mg/ml.
The fact that chlorhexidine only allowed partial control of clinical signs is surprising as an antiseptic should also maintain its activity against an azole-resistant strain of M. pachydermatis. This possible incongruence could be explained by considering that the dog suffered from an “idiopathic” form of Malassezia dermatitis and otitis, and in such cases topical therapy alone is often insufficient [2].
Another important consideration is that the MICs for the isolates sampled at a later date were consistently higher than those for the control isolates (Table 1). This may indicate that resistance in M. pachydermatis, once established, is stable.
Very little is known regarding possible mechanisms of resistance to azoles in M. pachydermatis. Iatta et al. [16] reported that resistance may depend on increased efflux by pumps, particularly those belonging to the “major facilitator superfamily”. The underlying cause may also be due to mutations in the gene encoding the enzyme sterol-14α-demethylase. These mutations were found in the resistant strain isolated in Japan [6]. This theory could benefit from the availability of the resistant strains we have deposited in a culture collection, which can be used for further experiments.
In conclusion, this case shows that the development of azole resistance of clinical relevance is possible for strains of M. pachydermatis harbored by dogs. However, it is important to emphasize that the unique conditions that lead to resistance (an unusually long azole treatment necessary to treat an “idiopathic” and chronic overgrowth of Malassezia) are uncommon in clinical practice.
Acknowledgements
None.
Acknowledgments
Conflict of interest
There are none.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.mmcr.2018.12.004.
Appendix A. Supplementary material
Supplementary material
References
- 1.Chen T.-A., Hill P.B. The biology of Malassezia organisms and their ability to induce immune responses and skin disease. Vet. Dermatol. 2005;16:4–26. doi: 10.1111/j.1365-3164.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 2.Bond R., Ferguson E.A., Curtis C.F., Craig J.M., Lloyd D.H. Factors associated with elevated cutaneous Malassezia pachydermatis populations in dogs with pruritic skin disease. J. Small Anim. Pract. 1996;37:103–107. doi: 10.1111/j.1748-5827.1996.tb02353.x. [DOI] [PubMed] [Google Scholar]
- 3.Vanden Bossche H., Engelen M., Rochette F. Antifungal agents of use in animal health--chemical, biochemical and pharmacological aspects. J. Vet. Pharmacol. Ther. 2003;26:5–29. doi: 10.1046/j.1365-2885.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 4.Nijima M., Kano R., Nagata M., Hasegawa A., Kamata H. An azole-resistant isolate of Malassezia pachydermatis. Vet. Microbiol. 2011;149:288–290. doi: 10.1016/j.vetmic.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Jesus F.P.K., Lautert C., Zanette R.A., Mahl D.L., Azevedo M.I., Machado M.L. In vitro susceptibility of fluconazole-susceptible and -resistant isolates of Malassezia pachydermatis against azoles. Vet. Microbiol. 2011;152:161–164. doi: 10.1016/j.vetmic.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Kano R., Yokoi S., Kariya N., Oshimo K., Kamata H. Multi-azole-resistant strain of Malassezia pachydermatis isolated from a canine Malassezia dermatitis. Med. Mycol. 2018 doi: 10.1093/mmy/myy035. [DOI] [PubMed] [Google Scholar]
- 7.Arikan S. Current status of antifungal susceptibility testing methods. Med. Mycol. 2007;45:569–587. doi: 10.1080/13693780701436794. [DOI] [PubMed] [Google Scholar]
- 8.Rosales M.S., Marsella R., Kunkle G., Harris B.L., Nicklin C.F., Lopez J. Comparison of the clinical efficacy of oral terbinafine and ketoconazole combined with cephalexin in the treatment of Malassezia dermatitis in dogs - a pilot study. Vet. Dermatol. 2005;16:171–176. doi: 10.1111/j.1365-3164.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 9.Guillot J., Bensignor E., Jankowski F., Seewald W., Chermette R., Steffan J. Comparative efficacies of oral ketoconazole and terbinafine for reducing Malassezia population sizes on the skin of Basset Hounds. Vet. Dermatol. 2003;14:153–157. doi: 10.1046/j.1365-3164.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 10.Peano A., Pasquetti M., Tizzani P., Chiavassa E., Guillot J., Johnson E. Methodological Issues in Antifungal Susceptibility Testing of Malassezia pachydermatis. J. Fungi. 2017;3:37. doi: 10.3390/jof3030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cafarchia C., Figueredo L.A., Iatta R., Colao V., Montagna M.T., Otranto D. In vitro evaluation of Malassezia pachydermatis susceptibility to azole compounds using E-test and CLSI microdilution methods. Med. Mycol. 2012;50:795–801. doi: 10.3109/13693786.2012.674219. [DOI] [PubMed] [Google Scholar]
- 12.Peano A., Beccati M., Chiavassa E., Pasquetti M. Evaluation of the antifungal susceptibility of Malassezia pachydermatis to clotrimazole, miconazole and thiabendazole using a modified CLSI M27-A3 microdilution method. Vet. Dermatol. 2012;23:131–135. doi: 10.1111/j.1365-3164.2011.01025.x. (, e29) [DOI] [PubMed] [Google Scholar]
- 13.Pasquetti M., Chiavassa E., Tizzani P., Danesi P., Peano A. Agar diffusion procedures for susceptibility testing of Malassezia pachydermatis: evaluation of Mueller-Hinton Agar Plus 2% glucose and 0.5 µg/ml methylene blue as the test medium. Mycopathologia. 2015;180:153–158. doi: 10.1007/s11046-015-9913-2. [DOI] [PubMed] [Google Scholar]
- 14.Graham-Mize C.A., Rosser E.J. Comparison of microbial isolates and susceptibility patterns from the external ear canal of dogs with otitis externa. J. Am. Anim. Hosp. Assoc. 2004;40:102–108. doi: 10.5326/0400102. [DOI] [PubMed] [Google Scholar]
- 15.Gimmler J.R., White A.G., Kennis R.A., Cruz-Espindola C., Boothe D.M. Determining canine skin concentrations of terbinafine to guide the treatment of Malassezia dermatitis. Vet. Dermatol. 2015;26 doi: 10.1111/vde.12245. [DOI] [PubMed] [Google Scholar]
- 16.Iatta R., Puttilli M.R., Immediato D., Otranto D., Cafarchia C. The role of drug efflux pumps in Malassezia pachydermatis and Malassezia furfur defence against azoles. Mycoses. 2016;60(3):178–182. doi: 10.1111/myc.12577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


