Abstract
Adoptive T cell therapy (ACT) refers to the therapeutic use of T cells. T cells genetically engineered to express chimeric antigen receptors (CAR) constitute the most clinically advanced form of ACT approved to date for the treatment of CD19-positive leukaemias and lymphomas. CARs are synthetic receptors that are able to confer antigen-binding and activating functions on T cells with the aim of therapeutically targeting cancer cells. Several factors are essential for CAR T cell therapy to be effective, such as recruitment, activation, expansion and persistence of bioengineered T cells at the tumour site. Despite the advances made in CAR T cell therapy, however, most tumour entities still escape immune detection and elimination. A number of strategies counteracting these problems will need to be addressed in order to render T cell therapy effective in more situations than currently possible. Non-haematological tumours are also the subject of active investigation, but ACT has so far shown only marginal success rates in these cases. New approaches are needed to enhance the ability of ACT to target solid tumours without increasing toxicity, by improving recognition, infiltration, and persistence within tumours, as well as an enhanced resistance to the suppressive tumour microenvironment.
Subject terms: Cancer immunotherapy, Cancer therapeutic resistance
Introduction
Immunotherapy has become an established part of the standard care for a number of different cancers, including melanoma, non-small cell lung cancer and kidney cancer.1 The implementation of T cell-activating strategies followed the discovery that tumour-mediated immunosuppression occurs across most tumour entities.2 The development of antibodies to components of inhibitory checkpoints such as programmed cell death protein 1 (PD-1) has led to unparalleled response rates and efficacy in patients who would otherwise be refractory to treatment.3,4 Consequently, there has been a paradigm shift in oncology with the establishment of T cells as both a therapeutic target of antibodies and an effector mechanism against the cancer cell; in this latter context, T cells are able to directly trigger apoptosis of tumour cells through granule exocytosis (perforin, granzyme) or death ligand–death receptor (Fas–FasL, TRAIL) systems.5
Building on this success, the direct therapeutic use of T cells, in a therapy referred to as adoptive T cell therapy (ACT), would appear as a logical progression. Three forms of ACT currently exist: first, the use of tumour-specific T cells (tumour-infiltrating lymphocytes, TILs) isolated from a patient’s resected tumour, which are expanded in vitro and therapeutically reinfused;6 second, the genetic engineering of T cells isolated from peripheral blood to express a T cell receptor (TCR) that recognises a specific cancer antigen;7 or, third, the genetic engineering of T cells using fully synthetic chimeric antigen receptors (CAR) consisting of an antigen-binding domain fused to T cell-activating moieties.8
The use of TILs was first reported in the late 1980s,6 but the difficulties in reproducibly yielding TILs across different patients and types of cancer, as well as the burden of standardisation, given the variance in quantity and quality of the starting tissue, explains the slow clinical development of this approach. By contrast, CAR T cells have been tested in a wide range of cancer types, especially haematological malignancies. CARs specific for CD19 (also known as B-lymphocyte antigen CD19) have induced high remission rates (over 80%) in patients with treatment-refractory acute lymphocytic leukaemia (ALL) or diffuse large B-cell lymphoma.9,10 Although it still remains to be proven, it is very likely that these remission rates will also prolong overall survival for these otherwise untreatable patient populations. These findings have prompted the FDA to approve anti-CD19 CAR T cells as the first T cell therapy even for refractory ALL and diffuse large B-cell lymphoma.11,12 More CARs are currently undergoing clinical development for the treatment of other haematological entities, such as chronic lymphocytic leukaemia, or low grade lymphomas and myeloma.13,14 It is consequently likely that there will be an increase in approvals for the use of CAR T cells across a wider array of malignancies.
However, although the efficacy of CAR T cell therapy is unchallenged for a number of haematological malignancies, it is important to realise that, with over 100 types of cancer, haematological cancers comprise only a small fraction of diagnosed cancers and are responsible for only 6% of all reported deaths.15 Attempts over the past couple of years to treat solid malignancies with CAR T cells have resulted in elevated toxicities and a minimal observable therapeutic benefit for patients,16,17 highlighting the heterogeneity inherent in the therapeutic response of different cancer types. Here, we review the evolution of the CAR T cell over time, with a particular focus on the current limitations of CAR T cell therapy in solid tumours and strategies to overcome these limitations.
Evolution of CAR T cell development
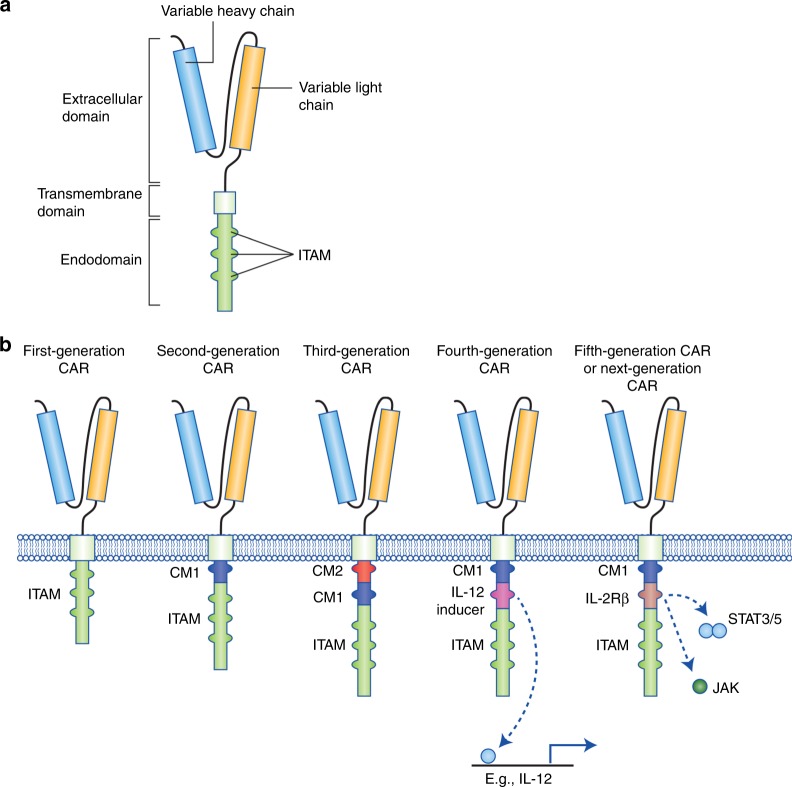
CARs are bioengineered receptors with specificity directed towards a desired antigen. The first CARs were generated some 30 years ago and have subsequently undergone a stepwise evolution in their development.1,18 The versatility of CARs stems from the fact that, unlike innate TCRs, they can recognise antigens in the absence of presentation by the major histocompatibility complex (MHC).18 This is a particular advantage when MHC expression is lost as a result of the immunosuppressive network of cancer cells.19 CARs comprise three main components: the extracellular domain, which is responsible for antigen recognition, the transmembrane domain, and the intracellular signalling domain.20 The extracellular region can be further segmented into the signal peptide, which is cleaved from the mature CAR expressed at the cell surface,21 and the antigen-recognition domain. The antigen-recognition domain is a single-chain fragment variant (scFV) predominantly composed of the variable light and heavy chain regions of an antigen-specific immunoglobulin separated by a flexible linker, and is tethered to the transmembrane domain through the spacer, which transmits the receptor-binding signal.20 The transmembrane domain is usually a hydrophobic alpha helix that spans the cell membrane and is fundamental for surface expression and stability of the receptor.20,22 The third region is the intracellular domain (or endodomain). Following antigen binding, the intracellular domain clusters and undergoes conformational changes, which enables downstream signalling proteins to be recruited and phosphorylated.23,24 The endodomain can contain several functional units. The intracellular domain of the T cell co-receptor CD3ζ, which contains three immunoreceptor tyrosine-based activation motifs (ITAMs) that are important for signal transduction, is the core component of most CARs20 (Fig. 1a).
Fig. 1.
Structure of different chimeric antigen receptor (CAR) generations. a The core structure of a CAR, highlighting the major components of the extracellular domain, the transmembrane domain and the intracellular domain (endodomain). b Evolution of the development of CARs from the first generation, which contained only ITAM motifs in the intracellular domain. Second-generation CARs included one co-stimulatory molecule (CM)1, and third-generation CARs contained a second CM. The fourth generation of CARs was based on second-generation CARs (containing 1–3 ITAMs) paired with a constitutively or inducibly expressed chemokine (e.g. IL-12). These T cells are also referred to as T cell redirected for universal cytokine-mediated killing (TRUCKs). The fifth, or ‘next generation’, is also based on the second generation of CARs, with the addition of intracellular domains of cytokine receptors (e.g. IL-2Rβ chain fragment). ITAM immunoreceptor tyrosine-based activation motifs, CD co-stimulatory domain, IL-12 activation of interleukin 12 transcription, IL-2Rβ truncated intracellular interleukin 2β chain receptor with a STAT3/5 binding motif
The progress in the development of CARs over the past three decades can be roughly grouped into five CAR generations based on the structure and composition of the endodomain.20 The first generation of CARs contained a single CD3ζ intracellular domain. Initial experiments with first-generation CAR T cells showed low cytotoxicity and proliferation owing to the lack of co-stimulatory (e.g. CD27, CD28, CD134, CD137) and cytokine (e.g. interleukin (IL)-2) signalling.20,25 A second generation of CARs was generated to enhance T cell proliferation and cytotoxicity by adding a co-stimulatory domain such as sections of CD28 or CD137 to the intracellular signalling domain.26–28 The third generation of CARs further expanded on the second generation by adding a third intracellular signalling sequence of an additional co-stimulatory domain such as CD134 or CD137.20 The fourth generation of CARs is based on second-generation CARs, but includes a protein, such as interleukin 12 (IL-12) that is constitutively or inducibly expressed upon CAR activation. T cells transduced with these fourth-generation CARs are referred to as T cells redirected for universal cytokine-mediated killing (TRUCKs). Activation of these CARs promotes the production and secretion of the desired cytokine to promote tumour killing though several synergistic mechanisms such as exocytosis (perforin, granzyme) or death ligand–death receptor (Fas–FasL, TRAIL) systems.5,25 TRUCKs will be further discussed in a separate section below. A fifth generation of CARs is currently being explored; these are based on the second generation of CARs, but they contain a truncated cytoplasmic IL-2 receptor β-chain domain with a binding site for the transcription factor STAT3. The antigen-specific activation of this receptor simultaneously triggers TCR (through the CD3ζ domains), co-stimulatory (CD28 domain) and cytokine (JAK–STAT3/5) signalling,29 which effectively provides all three synergistic signals (further discussed below) required physiologically to drive full T cell activation and proliferation. Additional variants of the aforementioned CARs, such as dual CARs, split CARs and inducible-split CARs, have been generated to further enhance the specificity and control of the transfused T cells. These CARs will be discussed in greater detail below18 (Fig. 1b).
In the past three decades, CARs have progressed from their initial characterisation to FDA approval for use in patients. In spite of these advances, however, it is important to bear in mind that the novel CAR designs and improvements in recent generations as seen in in vitro or in animal models have not been further corroborated in patients. In other words, no study has yet compared first or subsequent generation CARs specific for a single antigen in a clinical study, which prevents a proper comparison across the different generations of CARs and obscures the selection of optimal combinations for future clinical trials. Thus, any consideration of the use of a given CAR generation is based on preclinical animal models and not clinical data. Currently it is unclear which design would provide the best clinical benefit for patient outcome.
Limitations to CAR T cell therapy in solid tumours
A recent clinical trial treating patients suffering from acute B-cell lymphoblastic leukaemia with CD19-specific CAR T cells have shown an unprecedented clinical outcome, with 81% of 75 treated patients going into complete remission and minimal residual disease negativity.11,30 By contrast, several attempts to treat solid cancers with first-generation CAR T cells directed against several antigens (carbonic anhydrase IX (CAIX), CD171, folate receptor alpha (FR-α), GD2, human epidermal growth factor receptor 2 (HER2), mesothelin, EGFRvIII or vascular endothelial growth factor receptor 2 (VEGF-R2))31 did not significantly benefit patients, showing limited activity and frequent toxicity.32–34 Using TCR-modified T cells, clinical trials have also reported severe, even lethal toxicities.35 A TCR targeting the cancer testis antigen NY-ESO-1 showed promising results across trials and entities, and objective responses were observed in melanoma, sarcoma and myeloma, but otherwise responses have been anecdotal.36
In any case, targeting solid tumours with either approach presents a number of challenges not encountered when targeting blood malignancies. The hypoxic, poorly vascularised and extracellular matrix-rich tumour microenvironment prevents T cells from infiltrating the tumour tissue. Even for such T cells that do reach the tumour, the loss of tumour antigens prevents their specific recognition. Furthermore, inhibitory surface proteins, cytokines or soluble products of disrupted cell metabolism within the tumour can impair the activation and persistence of T cells. The need to enhance T cell recruitment into solid malignancies is highlighted by the observation that elevated lymphocyte infiltration is a powerful positive prognostic marker in various cancer subtypes (e.g. breast cancer, colorectal cancer, ovarian cancer, non-small cell lung cancer, melanoma and others).37,38 Accordingly, there is an extensive body of literature which is focused on transforming immune-scarce ‘cold’ tumours into immune-abundant ‘hot’ tumours,39 a subject which will be discussed in greater detail below. A detailed collection of overall strategies outside of ACT has been recently reviewed.40,41
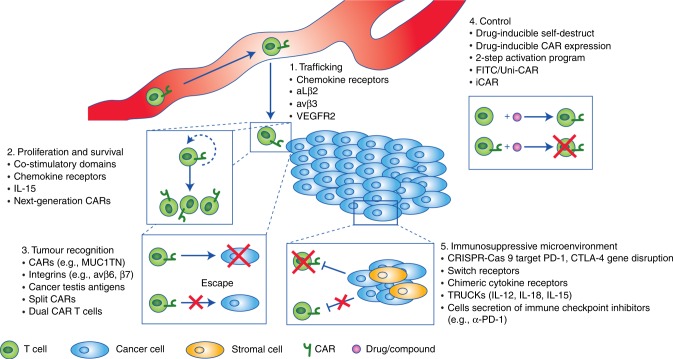
The importance of increased tumour recognition by T cells is underscored by several adaptive techniques adopted by cancer cells to circumvent immune detection, such as the aforementioned downregulation of MHC-associated antigen presentation.19 This is also further exemplified by encouraging results of phase II clinical trials of some cancer vaccines such as Canvaxin and GVAX, which were used to treat melanoma and prostate cancer, respectively.42,43 The mechanism of action of cancer vaccines is thought to drive antigen cross presentation and diversity, enabling the establishment of a protective immunity against cancer.44 Although the phase II clinical trials of Canvaxin and GVAX showed encouraging results, the phase III clinical trials of both cancer vaccines failed to demonstrate a survival benefit. Along the same lines, a multipeptide vaccine showed promising activity in phase I/II trials, paralleled by the induction of immune responses,45 but the phase III trial failed to achieve its primary endpoint, presumably due to low immunogenicity.45 These exemplary results indicate the correlation between the induction of a specific immune response and benefit to vaccine therapy, but at the same time question the overall therapeutic benefit of this approach to treat solid malignancies.46,47 In addition, the need to enhance T cell activation and persistence has been illustrated by the unprecedented success rate of trials of therapies that target immune inhibitory checkpoint proteins such as PD-1.3,4,48 Finally, many of the aforementioned notions are related to the ability of a cancer to directly alter its microenvironment, driving immune cell exclusion and reducing antigen presentation and lymphocyte activation.49 Based on these experiences and on preclinical studies, five important concepts have been identified which will need to be addressed to employ engineered T cells as a viable therapy for solid tumours (Fig. 2): improving T cell recruitment to tumours; enhancing T cell survival and activation; increasing tumour cell antigen recognition; implementing control strategies; and counteracting the immunosuppressive microenvironment.
Fig. 2.
Strategies to address limitations to CAR T cell therapy in solid tumours. Overview of the five major challenges facing adoptive T cell therapy in treating solid tumours—trafficking, proliferation and survival, tumour cell recognition/discrimination, control of transfused T cells and circumventing the immune-suppressive tumour microenvironment—and some approaches currently under investigation to improve them. IL interleukin, VEGFR2 vascular endothelial growth factor receptor 2, Uni-CAR Universal CAR, iCAR inhibitory CAR, TRUCKs T cell redirected for universal cytokine-mediated killing
Improvement of T cell trafficking to solid tumours
Immune infiltrate is typically low or absent in tumour biopsy samples from patients who do not respond to immune checkpoint blockade therapy,18 and is a prognostic factor for poor overall survival.50 For immune therapy to be effective, effector T cells need to reach their target cells. Unsurprisingly, if CAR T cells cannot access their target cells, it is very unlikely that they will be effective at controlling tumour growth. A recent strategy to promote immune cell recruitment to tumours takes advantage of cancer chemokine signalling.50 Chemokines are cytokines with chemotactic abilities, and are involved in regulating migration and trafficking of various immune cells and some somatic cells.50 Several studies have shown that tumour cells can produce chemokines, either themselves or through their stroma, which signal back to the cancer cells to promote cancer cell proliferation, survival, progression and migration.50,51 Some of these chemokines can promote the recruitment of immune-suppressive cells, which further enhances the immune-suppressive tumour environment.50 Approaches by us and others have sought to utilise the tumour chemokine signalling network to drive T cell recruitment by engineering the expression of a cognate chemokine receptor—CCR2,52 CCR2b,53,54 CCR4,55,56 CCR7,57 CXCR258 or CXCR459—on the surface of CAR T cells (Fig. 1). Recently, CXCR3 has also gained considerable attention in enhancing ACT.60 This interest stems from the observation that PD-1 blockade and/or chemotherapy has been shown to promote ACT recruitment in a CXCR3-ligand-dependent manner.61,62 A growing body of evidence corroborates the observation that the chemokine landscape within a given tumour can be extremely heterogenous,63 illustrating the need to identify specific candidates and strategies to enhance T cell infiltration into different cancers, which will vary from patient to patient.
While transgenic chemokine receptors come with the promise of directed recruitment to a desired site, the non-tumour specificity of chemokines might ‘distract’ T cells from their intended tumour target.64 Undesired effects might thus range from reduced activity through to novel toxicities, which is a particular risk when the primary target of the T cell is not tumour-specific. T cell entry into non-inflamed or non-tumour tissues is low under steady-state conditions.65 One might hypothesise that true added toxicities might especially arise when this state is disrupted, in the case of injury or autoimmune disorders.66,67 Such patients will need to be excluded from the first clinical trials dealing with chemokine-receptor-transduced T cells. Along the same lines, T cells will naturally utilise endogenously expressed chemokine receptors to traffic, and this process might compete with the newly introduced receptor.68 Although not yet seen in studies published so far, a careful analysis of the homing behaviour of such cells will need to be considered. From the safety perspective, the consequences of aberrant chemokine receptor signalling in T cells will need to be considered to prevent unwanted side effects stemming from this axis. On the other hand, the attraction of CAR T cells to distinct chemokine gradients might promote the development of chemokine loss or downregulation as a novel escape mechanism and thereby alter cancer biology, eventually rendering subsequent lines of immunotherapeutic treatment less likely to impact on the patient’s condition. Heterogeneity of chemokine expression will also need to be clarified both within entities and patients, as some disease sites with no or lower chemokine expression might remain inaccessible to the modified T cells. Currently, novel strategies to induce tumours to express a desired chemokine ligand through several intratumoural delivery methods (further discussed below) are being explored to circumvent this issue.
A recent approach to further enhance CAR T cell infiltration into solid tumours exploits the process of T cell egress: for example, by using α4 integrin mutant (S988A), protein kinase A (PKA)-mediated phosphorylation can be inhibited, stabilising the α4 (S988A)–paxillin interaction and resulting in an increase in α4 integrin signalling. The inhibition of PKA-mediated α4 integrin phosphorylation enhances integrin αLβ2 (LFA-1)-mediated migration, a phenomenon termed integrin transregulation.69 Together, increased α4 and αLβ2 integrin signalling promotes T cell extravasation from the vasculature and into the tissue, promoting T cell adhesion to the vasculature of inflamed tissue in an ICAM-1- and VCAM-1-dependent manner. In in vitro experiments, the inhibition of α4 integrin phosphorylation promoted αLβ2-mediated T cell migration, while in vivo, the α4 (S988A) mutant mice showed a marked increase in T cell entry into ectopically transplanted melanoma tumours and reduced the growth of implanted B16 melanoma tumours.70 CAR T cells have also been engineered to target antigens such as αvβ3 integrin, which are selectively enriched in the tumour microenvironment,71 or vascular endothelial growth factor (VEGF) receptor-2 (VEGFR2), a highly expressed antigen enriched in the tumour vasculature.72 Limited T cell infiltration into solid tumours will need to be addressed in order to drive CAR T cells into tumours that are not readily accessible or are hidden in dense extracellular matrices.
Alternatively, a technologically less challenging and clinically feasible strategy to overcome the issue of CAR T cell migration and tumour infiltration is to employ local chemokine delivery or production: several trials have investigated intracompartmental or intratumoural delivery of adenovirus chemokine expressing virus (CCL17)73 or DNA plasmids encoding the desired chemokine ligand (CCL5).74–76 Although these approaches are clinically feasible and do not influence the safety profile of CAR T cells, the results obtained from the trials were modest. Local delivery also comes with the major caveat that most patients are not candidates for such a treatment due to the position and technical difficulty of reaching the tumour site; furthermore, metastatic sites will generally remain unaffected by this approach.
T cell recognition and targeting of tumour cells
The ability to transduce ex vivo T cells with antigen-specific TCRs or CARs provides ACT with the powerful ability to selectively target desired antigen(s). Theoretically, this ability to select and target an antigen, or antigens, should provide the transfused T cells with an elevated level of antigen discrimination and, consequently, safety. At the same time, antigen recognition is the prerequisite for any efficacy expected from T cell therapy. However, several obstacles hinder proper targeting of antigen-specific CAR T cells. First, for T cell-mediated therapy to be effective, the targeted tumour antigen must be expressed on the cell surface, but most known tumour specific antigens are intracellular molecules or mutated versions of them.77,78 Second, the targeted antigen needs to be ideally expressed only on tumour cells—for example, truncated or mutated proteins. This is in contrast to tumour-associated antigens (TAA), which have an enriched expression in tumour cells but are also expressed in healthy tissues.79,80 A separate category comprises antigens expressed only in embryonic or otherwise immune privileged tissues, such as cancer testis antigens.81 A comprehensive overview of antigens currently targeted by CAR T cells has been recently published.16
In the absence of antigens that are truly tumour-specific, most T cell therapies in clinical trials are facing toxicity issues due to ‘on target off tumour’ recognition of the targeted antigen on normal cells.18 Several approaches are under investigation to mitigate the effects of on target off tumour recognition (discussed below). On the other hand, the major reason for treatment failure of the most advanced CAR T cells targeting CD19 is loss of antigen expression or selection by B-cell acute lymphoblastic leukaemia cells for mutants or variants that cannot be recognised by the anti-CD19 CAR T cells.82 Accordingly, highly specific antigen targeting by CAR T cells is accompanied by an inherent dilemma that applies selective pressure for the development of variants which are responsible for the relapse. Significant work has focused on reducing on target off tumour cross reaction by identifying more specific cancer antigens, such as αvβ6 or MUC1TN, which are highly expressed on several cancer subtypes (colon, lung, breast, cervix, pancreas and others) and can arise from post-translational cancer-specific modifications such as glycol modifications.83,84 In other cases, novel epitopes are exposed upon conformational changes induced by integrin activation, as seen for integrin β7, which is a candidate for targeting in multiple myeloma85 (Fig. 2). Currently, both on target off tumour and off target cross reaction are very difficult to predict in vitro and in silico, as shown by several examples in which preclinical models did not adequately predict effects or side effects.86,87 Along these lines, novel CARs need to be carefully assessed and clinicians need to favour a minimal anticipated biological effect level (MABEL) approach over the no observed adverse effect levels (NOAEL) for T cell dosing. This is of particular importance as the absence of evidence of toxicity does not equate to evidence of absence.
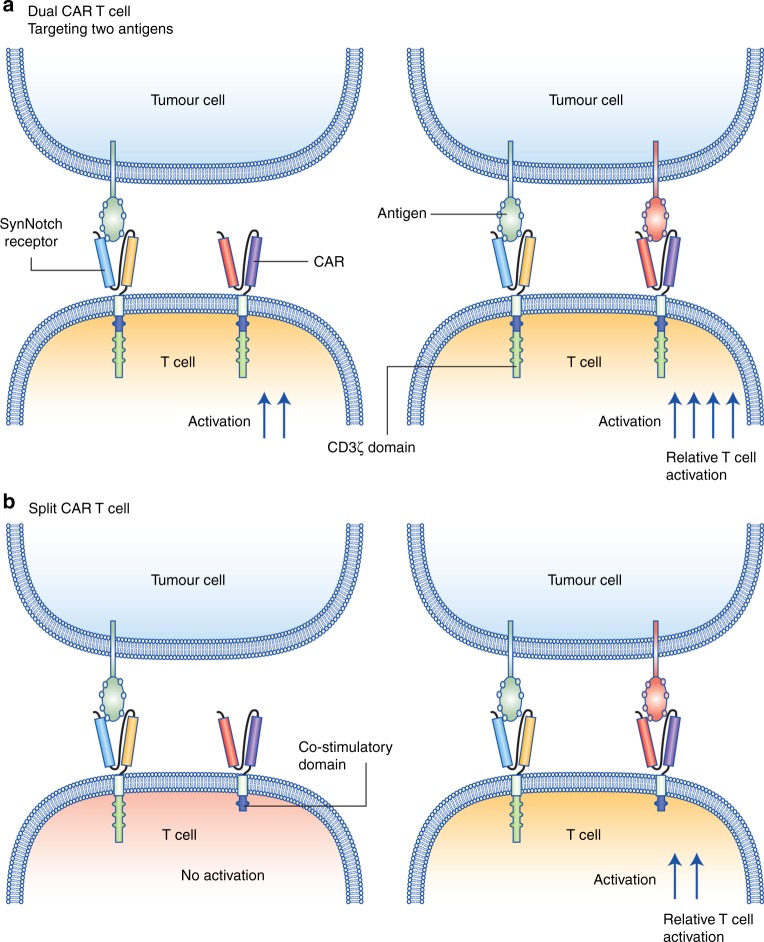
With the lack of bona fide cancer-specific antigens, efficacy and safety might be further enhanced by simultaneously targeting two or more different cancer-associated antigens. The advantage of this approach is that fully fledged T cell activation would only occur when both antigens are present, which should ideally be a very rare event outside of a tumour. With this in mind, two avenues of investigation are being pursued. The first combines the expression of two identical—except for the targeted antigen—CARs (referred to as dual CARs),88 enabling an enhancement of efficacy when both antigens are engaged (Fig. 3a). The second involves separation of the co-stimulatory domains (e.g. CD28 and 41BB) from CD3ζ on two different CARs (referred to as split CARs),89 requiring the simultaneous engagement of both CARs to complete T cell activation (Fig. 3b). Both approaches have demonstrated strong efficacy in preclinical models.90–92 Their clinical relevance is currently being investigated in clinical trials (including NCT03289455, NCT03125577, NCT03258047, NCT03198052). Additional strategies to enhance antigen discrimination and to reduce on target off tumour killing have also been engineered and are further explored in the section on programmed T cell control below.
Fig. 3.
Schematic representation of selected strategies to enhance CAR T cells recognition and activation. Recent strategies to enhance the activation of CAR T cells include the use of dual CARs, targeting two surface antigens (a) and split CARs (b). Legend indicated in the figure
T cell proliferation and survival
A major predictor of the efficacy of CAR T cell therapy is the extent of T cell expansion and persistence in a given patient.93 Expansion and persistence need to occur in order for T cells to reach a sufficient number to effectively eliminate the target tumour cells. Fundamental immunology indicates that T cells require three synergistic signals to drive proliferation and survival: TCR engagement, co-stimulatory signalling and cytokine signalling.94 The solid tumour microenvironment, however, despite containing plenty of antigens, lacks activating molecules and instead exposes effector cells to an abundance of suppressive molecules. This environment drives T cell anergy and dysfunction.94 A strategy to promote CAR T cell function involves the addition of co-stimulatory signalling moieties such as CD28 or 41BB to the CAR itself to promote T cell expansion and survival in these non-permissive environments.18 Mechanistically, however, the addition of one or more co-stimulatory domains might not be sufficient when promitotic signals are rare, prompting additional investigations into alternative mechanisms which can further enhance T cell proliferation and maintenance. One strategy is to fortify the T cells with cytokine signalling in such a T cell hostile environment (Fig. 2). Such an approach using repetitive dosing with biologically active IL-15 in mice enhanced antigen-specific CAR T cell recruitment, infiltration, proliferation and cytotoxic capabilities.95 This concept of ‘fortification’ is further realised in fifth-generation CARs, which include a truncated IL-2 receptor β chain and a STAT3-binding moiety. Activation of this kind of CAR can drive comprehensive TCR signalling complete with co-stimulatory and cytokine-driven JAK–STAT signalling to enhance proliferation and survival of the bioengineered T cells.29 These approaches to promote T cell proliferation and survival are still in the early stages of investigation and their potential contribution to future clinical settings is uncertain.
Programmed T cell control mechanisms
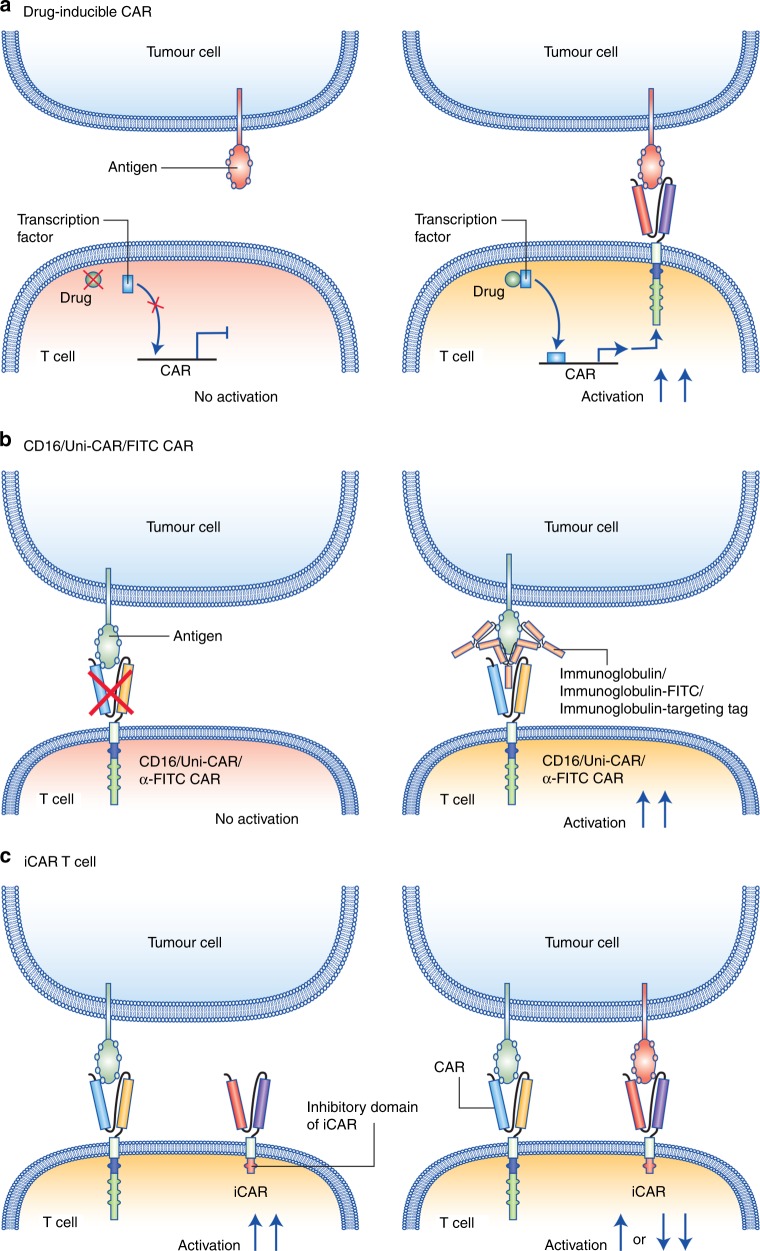
As highlighted above, compromised safety and CAR-related side effects are major hurdles to CAR T cell application.18,86,87 Once transfused into patients, CAR T cells are biologically active and difficult to control. With several CAR-associated deaths in clinical trials, there is a critical need to enhance and control CAR safety. In principle, safety could be enhanced in three ways: conditional and controllable activation of CAR T cells through switch compounds, such as a modified version of rapamycin (referred to as ‘rapalog’)96 (Fig. 4a); depletion of CAR T cells upon the occurrence of undesired and uncontrolled side effects; and suppression of CAR activity in the vicinity of non-tumour cells through receptors that recognise the latter. The most advanced modalities are strategies to deplete T cells upon the occurrence of side effects, using a suicide gene or agents to deplete cells bearing specific markers through monoclonal antibodies. An example of the latter is CAR T cells that express a truncated human EGFR polypeptide (huEGFRt), which is devoid of the extracellular N-terminal ligand-binding domains and intracellular receptor tyrosine kinase domain but can be targeted by cetuximab. Infusion of cetuximab, which is an IgG1 antibody, will bind to the truncated EGFR expressed on the transfused T cells and be eliminated by other immune cells through the recognition of the constant domain of cetuximab in a fragment crystallisable (Fc) receptor-dependent manner.97,98 Other examples include ganciclovir-mediated targeting of T cells transduced with the herpes simplex tyrosine kinase99 or a chemical inducer of dimerisation to deplete T cells transduced with an inducible caspase 9.100 Although in the context of allogeneic stem cell transplantation, some evidence suggests T cell depletion to be sufficient to reverse graft-versus-host-disease, such a relationship has not yet been proven for CAR T cell side effects.97 It remains to be seen whether depletion of CAR T cells upon the occurrence of severe toxicities could revert or prevent lethal or long-lasting tissue damage. An important side effect of such an approach, however, is the removal of the biologically active CAR T cells, which could in effect promote cancer recurrences.
Fig. 4.
Schematic representation of selected strategies to inhibit CAR T cell activation and prevent off tumour on target activity. Strategies to enhance CAR T cell safety include: a drug-inducible CAR T cells, b CAR T cells that require antigen-specific antibodies (CD16/FITC or Uni-CAR T cells) and c inhibitory CARs (iCARs), which moderate or inhibit T cell activation when antigens expressed on bystander cells are encountered. Legend indicated in the figure
Another attractive option is to render CAR T cell function dependent on the provision of additional substances such as antibodies or derivatives thereof. Along these lines, CARs targeting the Fc part of antibodies (e.g. anti-CD16-CAR),101 or targeting tags appended to antibodies (Uni-CAR)102 or derivatives such as FITC (anti-FITC CAR),103 have been developed (Fig. 4b). A similar strategy is the use of bispecific antibodies that specifically recruit CAR T cells by targeting a co-transduced marker antigen decorating the CAR T cell for enhanced CAR activity.104 These approaches allow precise control of CAR T cell reactivity based on the antibodies’ half-life, thus lowering the risk of side effects while preserving efficacy. Another advantage of aforementioned therapies is the possibility of using a single cellular product, irrespective of the TAA to be targeted, in combination with an approved monoclonal antibody.
Lastly, CAR T cells can utilise a NOT-gate circuits system to enhance efficacy and reduce off target targeting (Fig. 4c). A NOT-gate circuit is a CAR T cell that expresses (either inducibly or constitutively) two or more different CARs. The first CAR would target a tumour-specific antigen and contain the mandatory stimulatory (CD3ζ) and co-stimulatory (e.g. CD28 or CD137) domains, while the second CAR would be specific to an antigen which is typically expressed on normal healthy tissue or bystander cells and linked to inhibitory (iCAR) signalling domains (e.g. PD-1 and CTLA-4). The simultaneous engagement of both the CAR and the iCAR within the same immune synapse would prevent or dampen the activation of the T cell, and result in poor activation or T cell anergy,42 thus enhancing discrimination between tumour cells and healthy cells. These novel strategies to improve the specificity and control of CAR T cells could potentially increase the discrimination of normal versus tumoural cells, but additional investigation into the clinical significance is needed.
Counteracting the immunosuppressive microenvironment
Solid tumours are typically composed of a heterogeneous population of cells in a microenvironment which is hypoxic, poorly vascularised, has an elevated interstitial pressure and is often surrounded by a dense extracellular matrix.51 These physical and metabolic barriers prevent immune cell recruitment, activation and persistence, while simultaneously promoting the recruitment of immune suppressor cells.49,51 This enables tumour cells to escape immune detection and destruction and to dampen CAR T cell activity.105 Accordingly, immune suppression needs to be overcome for optimal CAR T cell action. In principle, three main avenues have been explored to enable CAR T cell activity in spite of immune suppression: deletion of selected immune-suppressive factors in the therapeutic T cells; introduction of molecules able to circumvent T cell suppression in CAR T cells; and combination therapies providing exogenous immune suppression reversion (Fig. 2).
Novel approaches to circumvent tumour-driven immune suppression have been explored by using the clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated 9 (Cas9) system. CRISPR–Cas9 technology has been used to enhance CAR T cell function by knocking out genes encoding T cell inhibitory receptors or signalling molecules such as PD-1 or CTLA-4.106–108 PD-1-deficient CD19 CAR T cells show improved killing of a CD19-positive and programmed cell death ligand 1 (PD-L1)-positive cell lines in vitro and enhanced clearance of tumour xenografts in vivo.106 Similarly, disruption of PD-1 enhanced in vivo anti-tumour activity in a model of prostate cancer using CAR T cells specific for prostate stem cell antigen (PSCA).108 Interestingly, the feasibility of generating PD-1 and CTLA-4 dual inhibitory pathway-resistant PSCA-specific CAR T cells has been demonstrated.107 However, the deletion of inhibitory molecules can be a double-edged sword, as the removal of some suppressive pathways may lead to uncontrolled proliferation or to an increased risk of autoimmunity. Immune checkpoints are molecules normally involved in the maintenance of peripheral tolerance to self-molecules by preventing over-reactivity of self-antigen-specific T cells. Deletion of such important molecules might induce, aside from overactivation, off-target activation of T cells, as observed previously in studies with systemic PD-1 antibody blockade.109 Although the use of CRISPR-Cas9 technology to knock out genes in CAR T cells is still in preclinical studies, a clinical trial that will evaluate the effect of PD-1 knockout by this technology in NY-ESO-1-specific TCR-transduced T cells has recently been approved (NCT03399448).110
An alternative approach to prolong the responsiveness of CAR-modified T cells is to equip them with activating chimeric switch receptors (CSRs), also referred to as immunomodulatory fusion proteins. Activating CSRs combine the extracellular ligand-binding domain of an inhibitory receptor (PD-1 or CTLA-4) fused through a transmembrane domain with the cytoplasmic co-stimulatory signalling domain of CD28.111–115 The engagement of the extracellular portion of this fusion receptor transmits an activating signal instead of the normal physiological inhibitory signal. T cells transduced with PD-1–CD28 CSR have been shown to have increased extracellular signal-regulated kinase (ERK) phosphorylation and granzyme B expression, increased proliferation and cytokine secretion—indicative of T cell co-stimulation—upon PD-L1 binding.112 The potent anti-tumour activity of CTLA-4–CD28 CSR T cells has been further validated in a murine model of melanoma.113 Two phase I clinical trials have already been initiated to determine the safety and efficacy of autologous PD-1–CD28 CSR-modified T cells in the treatment of PD-L1-positive, recurrent and metastatic malignant tumours (NCT02930967) and recurrent glioblastoma (NCT02937844). In line with the encouraging results from studies combining TCR-modified T cells and CSR,111,113,114 PD-1–CD28 can improve the therapeutic effects of anti-mesothelin CARs and anti-PSCA CARs in tumour xenograft models.115 The improved therapeutic effect was attributed to an increase in the number of infiltrating CAR T cells, a decrease in susceptibility of the cells to tumour-induced hypofunction, and an attenuation of inhibitory receptor expression. Together, these results provide a foundation for further clinical studies using CAR-T cell therapy in combination with a CSR strategy.
A further embodiment of CSRs is provided by chimeric cytokine receptors, which can transform secreted immunosuppressive signals into immune-activating signals. This notion has been demonstrated for the chimeric cytokine receptor IL-4R–IL-7R, which comprises the anti-inflammatory IL-4 receptor (IL-4R) exodomain fused to the pro-inflammatory IL-7 receptor (IL-7R) endodomain.116 Upon receptor engagement by tumour-derived IL-4, immunosuppressive effects were abrogated, increasing proliferation and activation of tumour-directed cytotoxic T cells and enhancing the anti-tumour activity in vivo. Optimal activation of tumour-antigen-specific T cells overexpressing IL-4R–IL-7R CSRs occurred only when engineered T cells encountered their specific tumour antigen and elevated IL-4 was present in the tumour microenvironment. In such a system, IL-4 would mimic beneficial IL-7 signalling through the CSR. Most cytokine signals mainly act in conjunction with TCR or related signalling, which is provided in this system by the CAR. Along these lines, full activation will only be seen in the presence of both IL-4 and the CAR target.117 These results encourage further adaptation of CSRs as a supplementary method to fine-tune CAR T cell therapies. It remains unclear whether targeting one of such inhibitory pathways would be sufficient to achieve therapeutic efficacy in a clinical setting.
Another approach involves the use of truncated suppressive receptors such as a dominant-negative form of TGF-β receptor II or a dominant-negative PD-1, which shield T cells from the negative effects of TGF-β and PD-L1, respectively.118–120 Apart from the upregulation of inhibitory ligands such as PD-L1, the tumour microenvironment is additionally enriched with immunosuppressive cytokines, one of which is TGF-β. TGF-β is produced in excess by tumour cells themselves, as well as cells of the tumour stroma.121 TGF-β has been shown to impair both innate and adaptive cellular immunity, generating a favourable microenvironment for tumour growth and metastasis, and is deemed an essential component of the tumour’s anti-immune defences.122,123 Following this line of thought, strategies that combine CAR T cell therapy with blocking immune-suppressive axes such as PD-1–PD-L1 or providing important cytokines depleted in the tumour environment, have been investigated. Combining PD-1-blocking antibodies with CAR T cell therapy has dramatically enhanced CAR T cell function in different preclinical models and prompted the initiation of clinical trials in different indications.124,125 Cytokine support, the third signal required for T cell activation, can also be a means of breaking through T cell suppression: provision of IL-2 or IL-15 has yielded significant improvements in different models,126,127 although a major caveat is the side effects of exogenous cytokine application. It remains to be seen if these deleterious effects might be outweighed by the clinical benefits of CAR T cell therapy.
CAR T cells as factories
A more complex strategy for overcoming tumour-driven immune suppression involves the use of CAR T cells that are capable of transforming the immunosuppressive microenvironment into an immune-permissive one. This strategy involves the use of the fourth generation of CARs, or TRUCKs, which are CAR T cells engineered to constitutively or inducibly express pro-inflammatory cytokines. One such candidate is IL-12, which strongly enhances the response of innate and adoptive immune cells to cancer cells.128 IL-12 increases interferon (IFN)-γ secretion and the expression of granzyme B and perforin by T cells129 and NK cells,130 and suppresses tumour-induced T-regulatory (T-reg) cell proliferation.131 As a consequence, these mechanisms may enhance additional tumour clearance by bystander NK cells and conventional T cells, and counteract the ability of T-reg cells to promote tumour growth. These effects will naturally synergise with CAR T cells for enhanced anti-tumoural activity. For this reason, the combination of CAR T cell therapy with constitutive or inducible IL-12 expression has been extensively explored for the treatment of several malignancies in preclinical models.132–134 IL-12-expressing TRUCKs exhibited a remarkable efficacy against solid tumours in preclinical models, with no observable signs of toxicity, as compared to clinical trials studying the use of recombinant human IL-12 as a therapy.135 Furthermore, a clinically relevant advantage of IL-12-producing TRUCKs is the elimination of established cancer without the requirement for cyclophosphamide preconditioning.132 The first dose-escalation trials of autologous TILs transduced with a gene encoding IL-12 driven by a T cell nuclear factor showed anti-tumour activity in melanoma, but also displayed dose-limiting severe toxicities including liver dysfunction, high fevers, and sporadic life-threatening haemodynamic instability.136 A phase I clinical trial of CAR T cells targeting the Mucin 1 antigen and co-expressing IL-12 provided only little therapeutic benefit, although no adverse side effects were reported.137 More clinical trials examining the safety and efficacy of IL-12-armoured CAR T cells are currently ongoing.138
A safer alternative to TRUCK IL-12 T cells might be CAR T cells secreting IL-18. IL-18 is a cytokine characterised as an inducer of IFN-γ expression in T cells139 and has been shown to activate monocytes and lymphocytes without causing severe toxicity in clinical trials.140 Recently, two studies revealed that inducible expression of IL-18 in CAR T cells enhances proliferation and anti-tumour activity of monocytes and lymphocytes.141,142 Interestingly, IL-18-producing TRUCK T cells induced acute inflammatory reactions and altered the balance of pro-inflammatory and anti-inflammatory cells in established, large pancreatic and lung tumours. Specifically, IL-18 polarises CAR T cells towards an effector phenotype paired with an acute inflammatory response. IL-18 CAR T cell treatment was accompanied by an increase in the number of M1 macrophages and NK cells, whereas a decrease in M2 macrophages, T regs and suppressive dendritic cells was observed. These effects go beyond the CAR T cell-only effects, and synergise with their activity.142
Another cytokine that has been overexpressed in CAR T cells is IL-15. IL-15 is a regulator of T cell homoeostasis, prolonging T cell survival. Additionally, it increases the lytic capacity of T cells by stimulating granzyme B expression.143 Anti-CD19 CAR T cells engineered to secrete IL-15 showed improved antigen-driven expansion, reduced PD-1 expression and cell death, and improved anti-leukaemic efficacy.144 In another approach, anti-CD19 CAR T cells have been designed to secrete a soluble form of herpes virus entry mediator (HVEM, TNFRSF14).145 The HVEM gene is frequently mutated in germinal centre (GC) lymphomas.146 The loss of inhibitory cell–cell interactions between HVEM and B and T Lymphocyte Attenuator (BTLA) leads to autonomous activation of B-cell proliferation and drives the development of GC lymphomas in vivo.145 HVEM-deficient lymphoma B cells also induce a tumour-supportive microenvironment. Accordingly, HVEM protein secreted by modified CAR T cells binds BTLA and restores tumour suppression.
Another strategy that exploits CAR T cells as local delivery agents or ‘micro-pharmacies’ is combinatorial immunotherapy, in which engineered CAR T cells secrete immune checkpoint inhibitors. For example, CAR T cells engineered to secrete human anti-PD-L1 antibodies to block T cell exhaustion have been shown to clear renal cell carcinoma in a humanised mouse model.147 Anti-PD-L1 antibody delivery to the tumour site led to a five-fold reduction in tumour growth and a 50–80% reduction in tumour weight in comparison to treatment with parental CAR T cells. Moreover, expression of PD-L1 and the cell proliferation marker Ki67 in the tumours decreased and levels of secreted granzyme B by modified CAR T cells increased. Anti-CD19 CAR T cells engineered to secrete anti-PD1 antibody enhanced anti-tumour activity and prolonged overall survival in a xenograft mouse model.148 Interestingly, a comparison of combinatorial therapy using CAR T cells engineered to secrete anti-PD1 antibodies versus CAR T cell therapy administered in conjunction with anti-PD1 antibodies revealed that systemically injected anti-PD-1 antibody had little effect on CD8+ T cell function.148 This result suggests that, given the low concentration of secreted anti-PD-1 in comparison to systemic injection (15-fold lower than the amount detected in the group in which antibodies were systemically injected,148) the anti-PD-1 antibody secreted by CAR T cells might provide a safer and more potent approach to enhancing the functional capacity of CAR T cells.
Taken together, the delivery of different payloads to the tumour through CAR T cells has shown promise in preclinical studies. Several clinical trials have been initiated to test the safety and efficacy of CAR T cells that, in addition to targeting a specific tumour antigen, secrete either anti-PD-1 alone or anti-PD-1 in combination with anti-CTLA-4 or anti-PD-L1 antibodies (NCT03179007, NCT03182816, NCT03182803, NCT03030001, NCT02873390, NCT02862028, NCT03170141). Further development of these combination therapies may become possible by new strategies to engineer T cells.
Conclusions
CAR T cells designed to express CD19 have shown unprecedented clinical success in otherwise refractory patients suffering from ALL or diffuse large B-cell lymphoma, frequently accompanied by severe adverse toxicity. These results exemplify the power of the approach and have revolutionised the concept of future blood-borne cancer treatments. By contrast, little or no clinical efficacy has so far been reported using CAR T cells for solid malignancies. Based on published clinical and preclinical trials with CAR-modified T cells, we have identified five important limitations to CAR therapy that need to be overcome for optimal treatment efficacy and safety: T cell recruitment, activation and proliferation, tumour cell targeting, control mechanisms, and circumventing the immune-suppressive microenvironment. These limitations will all have to be tackled in some way in order to increase T cell efficacy in solid tumours and to broaden the applicability of the strategy. An important approach will be the combination of several layers of engineering in one cellular product to address these limitations. This is an as yet unresolved issue, as most of the advances so far have been made in the area of tumour targeting, or on individually addressing these limitations as separate entities. Ongoing and future trials will reveal if the promise of cellular and, more specifically, CAR T cell therapy will benefit a broader population of tumour patients than those suffering from rare refractory haematological malignancies.
Acknowledgements
This study was supported by grants from the international doctoral programme “i-Target: Immunotargeting of cancer” funded by the Elite Network of Bavaria (to S.K. and S.E.), the Melanoma Research Alliance (grant number 409510 to S.K.), the Marie-Sklodowska-Curie “Training Network for the Immunotherapy of Cancer (IMMUTRAIN)” funded by the H2020 programme of the European Union (to S.E. and S.K.), the Else Kröner-Fresenius-Stiftung (to S.K.), the German Cancer Aid (to S.K.), the Ernst-Jung-Stiftung (to S.K.), the LMU Munich’s Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative (to S.E. and S.K.), the Bundesministerium für Bildung und Forschung VIP+ grant ONKATTRACT (to S.E. and S.K.) and the European Research Council Starting Grant (grant number 756017 to S.K.).
Author contributions
All authors contributed to the preparation, writing and proof reading of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Nicholas Tokarew, Justyna Ogonek
References
- 1.Kobold S, et al. Immunotherapy in tumors: activated T cells as a new treatment modality. Dtsch. Ärzteblatt Int. 2015;112:809–815. doi: 10.3238/arztebl.2015.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decker WK, et al. Cancer immunotherapy: historical perspective of a clinical revolution and emerging preclinical animal models. Front. Immunol. 2017;8:829. doi: 10.3389/fimmu.2017.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin J, et al. Combined Nivolumab and Ipilimumab or monotherapy in previously untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, et al. Nivolumab versus Everolimus in advanced renal cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. 2015;21:5047–5056. doi: 10.1158/1078-0432.CCR-15-0685. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 7.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buechner J, et al. Global registration trial of efficacy and safety of CTL019 in pediatric and young adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL): update to the interim analysis. Clin. Lymphoma, Myeloma Leuk. 2017;17:S263-S4. doi: 10.1016/j.clml.2017.07.030. [DOI] [Google Scholar]
- 10.Locke FL, et al. Clinical and biologic covariates of outcomes in ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (r-NHL) J. Clin. Oncol. 2017;35(15_suppl):7512. doi: 10.1200/JCO.2017.35.15_suppl.7512. [DOI] [Google Scholar]
- 11.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neelapu SS, et al. Axicabtagene Ciloleucel CAR T cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2017;15:31. doi: 10.1038/nrclinonc.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen AD, et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood. 2016;128:1147. [Google Scholar]
- 15.Chizuka A, et al. Difference between hematological malignancy and solid tumor research articles published in four major medical journals. Leukemia. 2006;20:1655. doi: 10.1038/sj.leu.2404369. [DOI] [PubMed] [Google Scholar]
- 16.D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 2018;9:282. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Rourke Donald M., Nasrallah MacLean P., Desai Arati, Melenhorst Jan J., Mansfield Keith, Morrissette Jennifer J. D., Martinez-Lage Maria, Brem Steven, Maloney Eileen, Shen Angela, Isaacs Randi, Mohan Suyash, Plesa Gabriela, Lacey Simon F., Navenot Jean-Marc, Zheng Zhaohui, Levine Bruce L., Okada Hideho, June Carl H., Brogdon Jennifer L., Maus Marcela V. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Science Translational Medicine. 2017;9(399):eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016;39:44–51. doi: 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark. Res. 2017;5:22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luiz Ricardo G, Paula Souza S, Ana Paula C, Barbara Brasil S, Antonio CVaTGA. Unraveling antibody display: systems biology and personalized medicine. Curr. Pharm. Des. 2016;22:6560–6576. doi: 10.2174/1381612822666160923112816. [DOI] [PubMed] [Google Scholar]
- 22.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin. Biol. Ther. 2011;11:855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantrell DA. T cell antigen receptor signal transduction. Immunology. 2002;105:369–374. doi: 10.1046/j.1365-2567.2002.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X, Vale R. Mechanisms of chimeric antigen receptor (CAR) signaling during T cell activation. Biophys. J. 2018;114:107a-8a. [Google Scholar]
- 25.Smith AJ, Oertle J, Warren D, Prato D. Chimeric antigen receptor (CAR) T cell therapy for malignant cancers: summary and perspective. J. Cell. Immunother. 2016;2:59–68. doi: 10.1016/j.jocit.2016.08.001. [DOI] [Google Scholar]
- 26.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 2003;3:939. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 27.Hombach A, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3ζ signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule. J. Immunol. 2001;167:6123. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 28.Finney HM, Lawson ADG, Bebbington CR, Weir ANC. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998;161:2791. [PubMed] [Google Scholar]
- 29.Kagoya Y, et al. A novel chimeric antigen receptor containing a JAK–STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018;24:352. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turtle CJ, et al. CD19 CAR–T cells of defined CD4(+):CD8(+) composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. (Sudbury, Mass). 2014;20:151–155. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JR, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 33.Lamers CHJ, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamers CHJ, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–e2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 35.Morgan RA, et al. Cancer regression and neurologic toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins PF, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T cell receptor: long term follow up and correlates with response. Clin. Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br. J. Cancer. 2017;117:451. doi: 10.1038/bjc.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br. J. Cancer. 2011;105:93. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haanen JBAG. Converting cold into hot tumors by combining immunotherapies. Cell. 2017;170:1055–1056. doi: 10.1016/j.cell.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 40.van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM. Migrating into the tumor: a roadmap for T cells. Trends Cancer. 2017;3:797–808. doi: 10.1016/j.trecan.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Turan T, et al. Immune oncology, immune responsiveness and the theory of everything. J. Immunother. Cancer. 2018;6:50. doi: 10.1186/s40425-018-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsueh Eddy C, Morton Donald L. Antigen-based immunotherapy of melanoma: Canvaxin therapeutic polyvalent cancer vaccine. Seminars in Cancer Biology. 2003;13(6):401–407. doi: 10.1016/j.semcancer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Small EJ, et al. Granulocyte macrophage colony-stimulating factor—secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 2007;13:3883. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 44.Gilboa E. A quantum leap in cancer vaccines? J. Immunother. Cancer. 2016;4:87. doi: 10.1186/s40425-016-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter S, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012;18:1254. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 46.Sondak VK, Sabel MS, Mulé JJ. Allogeneic and autologous melanoma vaccines: where have we been and where are we going? Clin. Cancer Res. 2006;12:2337s. doi: 10.1158/1078-0432.CCR-05-2555. [DOI] [PubMed] [Google Scholar]
- 47.Drake CG, Antonarakis ES. Current status of immunological approaches for the treatment of prostate cancer. Curr. Opin. Urol. 2010;20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brahmer J, et al. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:e1016700. doi: 10.1080/2162402X.2015.1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol. Res. 2014;2:1125–1131. doi: 10.1158/2326-6066.CIR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell144, 646–74 (2011). [DOI] [PubMed]
- 52.Brown CE, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J. Immunol. 2007;179:3332. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 53.Craddock JA, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. (1991) 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon EK, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapp M, et al. C-C chemokine receptor type-4 transduction of T cells enhances interaction with dendritic cells, tumor infiltration and therapeutic efficacy of adoptive T cell transfer. Oncoimmunology. 2016;5:e1105428. doi: 10.1080/2162402X.2015.1105428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Stasi A, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlsten M, et al. Efficient mRNA-based genetic engineering of human NK cells with high-affinity CD16 and CCR7 augments rituximab-induced ADCC against lymphoma and targets NK cell migration toward the lymph node-sssociated chemokine CCL19. Front. Immunol. 2016;7:105. doi: 10.3389/fimmu.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kershaw MH, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum. Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 59.Hillerdal V, Essand M. Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs. 2015;29:75–89. doi: 10.1007/s40259-015-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vignali D, Kallikourdis M. Improving homing in T cell therapy. Cytokine Growth Factor Rev. 2017;36:107–116. doi: 10.1016/j.cytogfr.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Peng W, et al. PD-1 blockade enhances T cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong M, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T cell infiltration and tumor control. Cancer Res. 2011;71:6997. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 63.Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA, Sarukhan A. Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Front. Immunol. 2014;5:127. doi: 10.3389/fimmu.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hillyer P, Mordelet E, Flynn G, Male D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin. Exp. Immunol. 2003;134:431–441. doi: 10.1111/j.1365-2249.2003.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang, Y. et al. CD4+and CD8+T cells have opposing roles in breast cancer progression and outcome. Oncotarget6, 17462–78 (2015). [DOI] [PMC free article] [PubMed]
- 66.Hoyer KK, Kuswanto WF, Gallo E, Abbas AK. Distinct roles of helper T cell subsets in a systemic autoimmune disease. Blood. 2009;113:389–395. doi: 10.1182/blood-2008-04-153346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rani M, Schwacha MG. The composition of T cell subsets are altered in the burn wound early after injury. PLoS ONE. 2017;12:e0179015. doi: 10.1371/journal.pone.0179015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garrood T, Lee L, Pitzalis C. Molecular mechanisms of cell recruitment to inflammatory sites: general and tissue-specific pathways. Rheumatology. 2006;45:250–260. doi: 10.1093/rheumatology/kei207. [DOI] [PubMed] [Google Scholar]
- 69.Rose DM, et al. Paxillin binding to the α4 integrin subunit stimulates LFA-1 (integrin αL β2-dependent T cell migration by augmenting the activation of focal adhesion kinase/proline-rich tyrosine kinase-2. J. Immunol. 2003;170:5912. doi: 10.4049/jimmunol.170.12.5912. [DOI] [PubMed] [Google Scholar]
- 70.Cantor JM, Rose DM, Slepak M, Ginsberg MH. Fine-tuning tumor immunity with integrin trans-regulation. Cancer Immunol. Res. 2015;3:661. doi: 10.1158/2326-6066.CIR-13-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu X, Rivera A, Tao L, Zhang X. Genetically modified T cells targeting neovasculature efficiently destroy tumor blood vessels, shrink established solid tumors, and increase nanoparticle delivery. Int. J. Cancer. 2013;133:2483–2492. doi: 10.1002/ijc.28269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chinnasamy D, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 2012;18:1672. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanagawa N, et al. CC-chemokine ligand 17 gene therapy induces tumor regression through augmentation of tumor-infiltrating immune cells in a murine model of preexisting CT26 colon carcinoma. Int. J. Cancer. 2007;121:2013–2022. doi: 10.1002/ijc.22908. [DOI] [PubMed] [Google Scholar]
- 74.Lavergne E, et al. Intratumoral CC chemokine ligand 5 overexpression delays tumor growth and increases tumor cell infiltration. J. Immunol. 2004;173:3755. doi: 10.4049/jimmunol.173.6.3755. [DOI] [PubMed] [Google Scholar]
- 75.Adusumilli PS, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tchou J, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 2017;5:1152. doi: 10.1158/2326-6066.CIR-17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noguchi T, et al. Intracellular tumor-associated antigens represent effective targets for passive immunotherapy. Cancer Res. 2012;72:1672. doi: 10.1158/0008-5472.CAN-11-3072. [DOI] [PubMed] [Google Scholar]
- 78.Donnard E, et al. Mutational analysis of genes coding for cell surface proteins in colorectal cancer cell lines reveal novel altered pathways, druggable mutations and mutated epitopes for targeted therapy. Oncotarget. 2014;5:9199–9213. doi: 10.18632/oncotarget.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting CD8 T cell immunity for more effective cancer immunotherapy. Front. Immunol. 2018;9:14. doi: 10.3389/fimmu.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T cell therapy. Nat. Biotechnol. 2013;31:999–1008. doi: 10.1038/nbt.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6:15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sotillo E, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whilding Lynsey M, Vallath S, Maher J. The integrin αvβ6: a novel target for CAR T cell immunotherapy? Biochem Soc. Trans. 2016;44:349. doi: 10.1042/BST20150249. [DOI] [PubMed] [Google Scholar]
- 84.Posey AD, et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane Mucin MUC1 control adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hosen N, et al. The activated conformation of integrin β7 is a novel multiple myeloma–specific target for CAR T cell therapy. Nat. Med. 2017;23:1436. doi: 10.1038/nm.4431. [DOI] [PubMed] [Google Scholar]
- 86.Dudal S, et al. Application of a MABEL approach for a T cell-bispecific monoclonal antibody: CEA TCB. J. Immunother. 2016;39:279–289. doi: 10.1097/CJI.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 87.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 88.Chen KH, et al. A compound chimeric antigen receptor strategy for targeting multiple myeloma. Leukemia. 2017;32:402. doi: 10.1038/leu.2017.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2012;31:71. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilkie S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 91.Duong CPM, Westwood JA, Berry LJ, Darcy PK, Kershaw MH. Enhancing the specificity of T cell cultures for adoptive immunotherapy of cancer. Immunotherapy. 2010;3:33–48. doi: 10.2217/imt.10.81. [DOI] [PubMed] [Google Scholar]
- 92.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI(3)kinase/AKT/Bcl-X(L) activation and CD8(+) T cell-mediated tumor eradication. Mol. Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fraietta JA, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melero I, Rouzaut A, Motz G, Coukos G. T cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ng SSM, et al. Heterodimeric IL15 treatment enhances tumor infiltration, persistence, and effector functions of adoptively transferred tumor-specific T cells in the absence of lymphodepletion. Clin. Cancer Res. 2017;23:2817. doi: 10.1158/1078-0432.CCR-16-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077-aab. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qasim W, et al. T cell transduction and suicide with an enhanced mutant thymidine kinase. Gene Ther. 2002;9:824. doi: 10.1038/sj.gt.3301690. [DOI] [PubMed] [Google Scholar]
- 100.Straathof KC, et al. An inducible caspase 9 safety switch for T cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caratelli S, et al. FCγ chimeric receptor-engineered T cells: methodology, advantages, limitations, and clinical relevance. Front. Immunol. 2017;8:457. doi: 10.3389/fimmu.2017.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173:1–13. doi: 10.1016/j.cell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tamada K, et al. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin. Cancer Res. 2012;18:6436. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- 104.Kobold S, et al. Selective bispecific T cell recruiting antibody and antitumor activity of adoptive T cell transfer. J. Natl. Cancer Inst. 2015;107:dju364-dju. doi: 10.1093/jnci/dju364. [DOI] [PubMed] [Google Scholar]
- 105.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rupp LJ, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017;7:737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren J, et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017;8:17002–17011. doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ren J, et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naidoo J, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baylis F, McLeod M. First-in-human phase 1 CRISPR gene editing cancer trials: are we ready? Curr. Gene Ther. 2017;17:309–319. doi: 10.2174/1566523217666171121165935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ankri C, Shamalov K, Horovitz-Fried M, Mauer S, Cohen CJ. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J. Immunol. 2013;191:4121–4129. doi: 10.4049/jimmunol.1203085. [DOI] [PubMed] [Google Scholar]
- 112.Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8+cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol. Immunol. 2012;51:263–272. doi: 10.1016/j.molimm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 113.Shin JH, et al. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T cell therapy in murine tumor models. Blood. 2012;119:5678–5687. doi: 10.1182/blood-2011-09-380519. [DOI] [PubMed] [Google Scholar]
- 114.Kobold S, et al. Impact of a new fusion receptor on PD-1–mediated immunosuppression in adoptive T cell therapy. J. Natl. Cancer Inst. 2015;107:djv146. doi: 10.1093/jnci/djv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu X, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second generation CAR T-Cells in advanced solid tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leen AM, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roybal Kole T, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Foster AE, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-β receptor. J. Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen N, Morello A, Tano Z, Adusumilli PS. CAR T cell intrinsic PD-1 checkpoint blockade: a two-in-one approach for solid tumor immunotherapy. Oncoimmunology. 2017;6:e1273302. doi: 10.1080/2162402X.2016.1273302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cherkassky L, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Massagué J. TGFβ in. Cancer Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat. Med. 2001;7:1118. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 123.Dasgupta S, Bhattacharya-Chatterjee M, O’Malley BW, Chatterjee SK. Inhibition of NK cell activity through TGF-β1 by downregulation of NKG2D in a murine model of head and neck cancer. J. Immunol. 2005;175:5541–5550. doi: 10.4049/jimmunol.175.8.5541. [DOI] [PubMed] [Google Scholar]
- 124.John LB, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 125.Chong EA, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)–modified T cells: refueling the CAR. Blood. 2017;129:1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu XJ, et al. Multiparameter comparative analysis reveals differential impacts of various cytokines on CART cell phenotype and function ex vivo and in vivo. Oncotarget. 2016;7:82354–82368. doi: 10.18632/oncotarget.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 129.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J. Exp. Med. 1994;180:211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cao X, et al. IL-12 stimulates interferon-gamma mediated inhibition of tumor-induced regulatory T cell proliferation and enhances tumor clearance. Cancer Res. 2009;69:8700–8709. doi: 10.1158/0008-5472.CAN-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pegram HJ, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 2017;7:10541. doi: 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leonard JP, et al. Effects of single-dose interleukin-12 exposure on interleukin-12–associated toxicity and interferon-γ production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 136.Zhang L, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 2015;21:2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.You F, et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Science China. Life Sci. 2016;59:386–397. doi: 10.1007/s11427-016-5024-7. [DOI] [PubMed] [Google Scholar]
- 138.Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl. Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect. Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robertson MJ, et al. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin. Cancer Res. 2008;14:3462–3469. doi: 10.1158/1078-0432.CCR-07-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu B, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chmielewski M, Abken H. CAR T cells releasing IL-18 convert to T-Bet high FoxO1 low effectors that exhibit augmented activity against advanced solid tumors. Cell Rep. 2017;21:3205–3219. doi: 10.1016/j.celrep.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 143.Tamang DL, et al. Induction of granzyme B and T cell cytotoxic capacity by IL-2 or IL-15 without antigens: multiclonal responses that are extremely lytic if triggered and short-lived after cytokine withdrawal. Cytokine. 2006;36:148–159. doi: 10.1016/j.cyto.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoyos V, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boice M, et al. Loss of the HVEM tumor suppressor in lymphoma and restoration by modified CAR-T cells. Cell. 2016;167:405–418. doi: 10.1016/j.cell.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheung KJJ, et al. Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res. 2010;70:9166–9174. doi: 10.1158/0008-5472.CAN-10-2460. [DOI] [PubMed] [Google Scholar]
- 147.Suarez ER, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li S, et al. Enhanced cancer immunotherapy by chimeric antigen receptor–modified T cells engineered to secrete checkpoint inhibitors. Clin. Cancer Res. 2017;23:6982–6992. doi: 10.1158/1078-0432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]