Abstract
Adipogenesis is a process which induces or represses many genes in a way to drive irreversible changes of cell phenotypes; lipid accumulation, round cell-shape, secreting many adipokines. As a master transcription factor (TF), PPARγ2 induces several target genes to orchestrate these adipogenic changes. Thus induction of Pparg2 gene is tightly regulated by many adipogenic and also anti-adipogenic factors. Four hours after the treatment of adipogenic hormones, more than fifteen TFs including glucocorticoid receptor (GR), C/EBPβ and AP-1 cooperatively bind the promoter of Pparg2 gene covering 400 bps, termed “hotspot”. In this study, we show that TEA domain family transcription factor (TEAD)4 reinforces occupancy of both GR and C/EBPβ on the hotspot of Pparg2 during early adipogenesis. Our findings that TEAD4 requires GR for its expression and for the ability to bind its own promoter and the hotspot region of Pparg2 gene indicate that GR is a common component of two positive circuits, which regulates the expression of both Tead4 and Pparg2. Wnt3a disrupts these mutually related positive circuits by limiting the nuclear location of GR in a β-catenin dependent manner. The antagonistic effects of β-catenin extend to cytoskeletal remodeling during the early phase of adipogenesis. GR is necessary for the rearrangements of both cytoskeleton and chromatin of Pparg2, whereas Wnt3a inhibits both processes in a β-catenin-dependent manner. Our results suggest that hotspot formation during early adipogenesis is related to cytoskeletal remodeling, which is regulated by the antagonistic action of GR and β-catenin, and that Wnt3a reinforces β-catenin function.
Introduction
The mouse 3T3-L1 cells have been widely used as an in vitro model system to investigate molecular mechanisms of adipogenesis1. The 3T3-L1 cells can be differentiated into mature adipocytes by two-day exposure to 3-isobutyl-1-methylxanthine (IBMX/M), dexamethasone (Dex/D), and insulin (I)2–4. Recently, genome wide analyses of DNase I hypersensitive regions revealed that after 4 h MDI treatment, approximately three times more DNA regions became accessible5. ChIP-seq analyses revealed that during such dynamic chromatin remodeling (within 4 h after MDI treatment), hundreds of DNA sequences covering 400 bps, termed “hotspots”, including the promoter of Pparg2, are cooperatively occupied with at least five TFs including C/EBPβ/δ, GR (also known as NR3C1), STAT5A, and other TFs. These early TFs recruit coregulators to induce chromatin remodeling and form early enhanceosomes at the hotspots1,5,6. Hotspots are enriched with enhancer histone marks, namely, H3K4me1, H3K4me2, and H3K27ac, suggesting that hotspots are key enhancers1,7–9. Similarly, intracellular and extracellular structures were also remodeled during adipogenesis when preadipocytes changed to round and lipid-laden adipocytes4,10–12. Within 24 h, MDI treatment rearranged the actin cytoskeleton from stress fibers to cortical structures in preadipocytes13. Thus, MDI dramatically rearranged both chromatin and cytoskeleton in this short period.
Canonical Wnt signaling represses adipogenesis, but enhances osteogenesis of mesenchymal stem cells14,15. 3T3-L1 preadipocytes secrete Wnt10b, which maintains preadipocytes in undifferentiated state via autocrine and paracrine signaling16–19. After binding to the frizzled receptor and coreceptor LRP5/6, the canonical Wnt ligand stabilizes β-catenin (also known as CTNNB1) by protecting it from the APC/AXIN/GSK-3β destruction complex. Stabilized β-catenin localizes in the nuclei and is recruited on Wnt target genes via protein-protein interactions with DNA-bound T-cell factor/lymphoid enhancer factor (TCF/LEF)20–23. As a coactivator, β-catenin increases the expression of TCF/LEF target genes such as Axin2 and Ccnd124,25. In addition, β-catenin maintains cytoskeletal integrity by interacting with membrane bound cadherins and α-catenin, which are connected to actin fibers26–28.
Previous studies on the anti-adipogenic effects of Wnt/β-catenin signaling focused on the inhibition of PPARγ activity by β-catenin29–34. However, how Wnt signaling prevents adipogenic hormones from de-repressing and activating the transcription of Pparg2 during early adipogenesis (within 48 h after MDI treatment) remains unclear. Here, we showed for the first time that canonical Wnt signaling inhibits not only hotspot formation of Pparg2, but also cytoskeletal rearrangement in a β-catenin-dependent manner. These two events are regulated by the antagonistic actions of GR and β-catenin, and Wnt3a reinforces β-catenin function.
Materials and methods
Materials and antibodies
Insulin, dexamethasone, 3-isobutyl-1-methylxanthine, Hoechst33258, Oil Red-O and RU486 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant mouse Wnt3a was purchased from R&D Systems (Minneapolis, MN, USA). Wnt3a-conditioned medium was obtained from confluent Wnt3a-expressing L929 cells35. Anti-PPARγ (E-8), C/EBPα (14AA), C/EBPβ (H-7), GR (M-20, G-5), p300 (C-20), CBP (A-22), KLF4 (H-180), c-Jun (H-79), and β-catenin (H-102) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-TAZ (M2-616) antibody was purchased from BD Biosciences (San Jose, CA, USA), anti-lamin A/C and anti-STAT5 antibodies from Cell Signaling Technology (Danvers, MA, USA), anti-FLAG (5A8E5), anti-Myc (2G8D5), and anti-HA (5E11D8) antibodies from GenScript (Piscataway, NJ, USA), anti-TEAD4 and anti-H3 antibodies from Abcam (Cambridge, MA, USA), anti-14-3-3γ (CG31-2B6) from Millipore (Billerica, MA, USA), and anti-β-actin (AC-15) from Sigma-Aldrich, fluorescent phalloidin conjugate from Invitrogen (Waltham, MA, USA). cDNAs of HA-GR or Myc-Tead4 was subcloned into the pLenti-vector. pBABE-3× FLAG-C/EBPβ and pCMV-3× FLAG vector have been described previously36. Primer sequences for qRT-PCR and ChIP-qPCR are summarized in Supplementary Table S1 and S2, respectively.
Cells and adipocyte differentiation
3T3-L1 mouse preadipocytes (ATCC, Rockville, MD, USA) and mouse NIH-3T3 cells were maintained in DMEM containing 10% (v/v) bovine serum as described35. AmphoPackTM 293 cells (BD Biosciences, San Jose, CA, USA), HEK-293T cells, C3H10T1/2 cells (ATCC, Rockville, MD, USA) and Wnt3a-L929 cells were maintained in DMEM containing 10% (v/v) FBS. For adipogenesis, post-confluent 3T3-L1 cells and C3H10T1/2 cells were exposed to the adipogenic cocktail (MDI) containing 2 μM D, 0.5 mM M, and 5 μg/ml I in DMEM supplemented with 10% FBS as described previously35. Lipid droplets were stained using Oil Red-O as described previously37.
Generation of stable cell lines using retrovirus or lentivirus infection
C/β-NIH cells and EV-NIH cells were generated by infecting mouse NIH-3T3 cells with retroviruses encoding FLAG-tagged C/EBPβ and empty vector, respectively, using the pBABE retroviral vector system and HEK293-based packaging cells (AmphoPackTM 293 cell line)35. GR-L1, Cβ-L1, or Tead4-L1 cells were generated by infecting mouse 3T3-L1 preadipocytes with lentiviruses expressing HA-tagged GR, FLAG-tagged C/EBPβ, or Myc-tagged TEAD4, respectively. GR/Cβ-L1 or EV/EV-L1 cells were generated by infecting Cβ-L1 and EV-L1 cells with lentiviruses encoding HA-tagged GR or empty vector, respectively, using the pWZL retroviral vector system. shTead4-L1, shβ-cat-L1, shGR-L1, and shCtrl-L1 cells were generated by infecting mouse 3T3-L1 cells with lentivirus encoding shRNA against mouse Tead4 (5′-GCTGAAACACTTACCCGAGAA-3′), mouse Ctnnb1 (5′-CCCAAGCCTTAGTAAACATAA-3′), mouse Nr3c1 (encoding GR) (5′-TGAGATTCGAATGACTTATAT-3′) and control (5′-CCTAAGGTTAAGTCGCCCTCG-3′), respectively, using the pLKO.1 lentiviral vector system (Addgene, Cambridge, MA, USA).
Chromatin immunoprecipitation (ChIP) and formaldehyde-assisted isolation of regulatory elements (FAIRE)
ChIP analyses were performed as described previously38. FAIRE analyses were performed using ChIP lysates (30 μg chromatin) as described previously39. Briefly, sonicated chromatin lysates were phase-separated by two rounds of phenol/chloroform extraction. Nucleosome-free DNA in the upper aqueous phase was obtained using ethanol precipitation. DNA was further treated with 10 μg RNase A and 20 μg proteinase K, and extracted using the QIAquick PCR purification kit (QIAGEN, Chatsworth, CA, USA). The isolated genomic DNAs were used for FAIRE-qPCR. The Ct value of a target gene in the isolated DNA sample of a ChIP or FAIRE experiment was normalized to the Ct value of the target gene in the input DNA (ΔCt = Ct sample−Ct input). The percentage of input indicates the value of 100 × 2ΔCt.
Western blot analyses, nuclear extraction, and reporter assay
Western blot analyses were performed as described previously35. To obtain nuclear extracts, the cells were washed twice with ice-cold phosphate buffered saline (PBS), harvested, and then lysed with hypotonic buffer (20 mM Tris-HCl (pH 8.0), 10 mM NaCl, 0.2% NP-40, 10 mM β-glycerophosphate, 10 mM NaF, 1 mM Na3VO4, and protease inhibitors) and incubated for 10 min on ice. The supernatant (cytosol extracts) was removed and the nuclear pellet was washed with hypotonic buffer and lysed with NETN buffer (20 mM Tris-HCl (pH 8.0), 140 mM NaCl, 0.5% NP-40, 10 mM β-glycerophosphate, 10 mM NaF, 1 mM Na3VO4, and protease inhibitors), followed by 10 cycles of sonication (1 cycle; 30 s on, 30 s off). Nuclear extracts (supernatant) were obtained by centrifugation at 13,000 × g for 10 min at 4 °C. The reporter plasmids, C/EBP-Luc and GRE-Luc, contained the luciferase gene under the regulation of three copies of C/EBP binding sequences (GTTGCGCAAG) and one copy of glucocorticoid responsive element (GRE) (AGAACACTGTGTTCT), respectively. Reporter assays were performed using Lipofectamine reagent as described previously40. The pRL-TK plasmid encoding Renilla luciferase was cotransfected for normalizing transfection efficiency.
Immunofluorescence of F-actin
The cellular F-actin was stained with fluorescent phalloidin conjugates (25 μΜ) for 40 min at room temperature prior to Hoechst staining. The stained cells were observed under a Zeiss LSM510 inverted confocal microscope according to the manufacturer’s instructions. F-actin structures in individual cells were categorized into three groups; S (stress) fiber, where F-actin stress fibers were observed both in the nucleus and cytoplasm; T (transition state) fiber, where F-actin stress fibers were observed in the cytoplasm but not in the nucleus; C (cortical structure), where F-actin stress fibers were observed neither in the nucleus nor in the cytoplasm, but were observed as cortical structures near the cellular membrane. Cells (13–48) in each treatment were observed and categorized into three groups.
Statistical analysis
All quantitative measurements were performed in at least three independent experiments. Two-tailed unpaired Student’s t-tests were used to compare the data between controls and indicated experimental groups. *p-values < 0.05; **p-values < 0.01; ***p-values < 0.001 were considered statistically significant.
Results
Wnt3a inhibits early induction of Pparg2
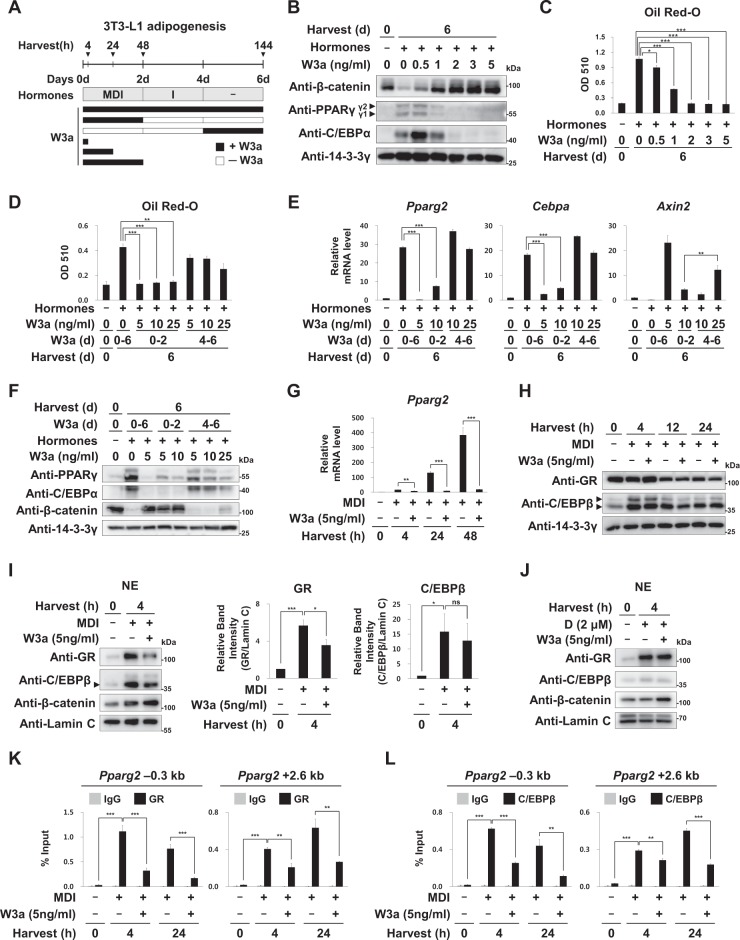
We treated 3T3-L1 preadipocytes with adipogenic hormones (MDI) in the presence of recombinant Wnt3a (Fig. 1a). MDI induced the protein levels of both PPARγ and C/EBPα, but reduced β-catenin proteins. Two ng/ml of Wnt3a was sufficient to block the induction of both PPARγ and C/EBPα proteins and lipid accumulation but increased β-catenin proteins (Fig. 1b, c, and S1A). Although Wnt3a repressed both PPARγ1 and PPARγ2 proteins, PPARγ2 is major target for C/EBPβ and GR in response to MDI (Fig. S1C). Early temporal treatment of Wnt3a (5 ng/ml, for 0–2 days) was sufficient to block the early and later processes of adipogenesis, whereas more Wnt3a (>25 ng/ml) was required to block adipogenesis during the later processes (4–6 days time points) (Fig. 1d–g and S1B). Although late temporal treatment (for 4–6 days) of Wnt3a (25 ng/ml) increased the mRNA levels of Axin2, a Wnt target gene, it did not effectively reduce the mRNA and protein levels of Pparg2 and Cebpa (Fig. 1e, f). These results confirmed the previous findings that the early period (within 0–2 days) is more sensitive to Wnt3a inhibition than the late period17.
Fig. 1. Effects of Wnt3a on early induction of Pparg2.
a Post-confluent 3T3-L1 preadipocytes were induced to undergo adipogenesis by treatment with adipogenic hormones, IBMX (M), dexamethasone (D), and insulin (I) in the presence or absence of recombinant mouse Wnt3a (W3a). The treated cells were harvested at the indicated time points after induction. b Western blot analyses of 3T3-L1 cells using the indicated antibodies. 14-3-3γ was used as the loading control. Arrows indicate two PPARγ proteins (γ1, 54 kDa; γ2, 57 kDa). c, d Optical densities (510 nm) of Oil Red-O stained lipid in the 3T3-L1 cells at 6 days after the hormone treatment. The images of the Oil Red-O stained 3T3-L1 cells are shown in the Supplementary Fig. S1. e qRT-PCR analyses of Pparg2, Cebpa, and Axin2 mRNA levels, which were normalized to 18S rRNA levels as described previously35. f Western blot analyses of 3T3-L1 cells using the indicated antibodies. g Relative mRNA levels of Pparg2 to 18S rRNA levels. h Western blot analyses of 3T3-L1 cells using the indicated antibodies. Arrows indicate two active forms of C/EBPβ (36 and 38 kDa respectively). i, j Western analyses of nuclear extracts (NE) of 3T3-L1 cells treated with either MDI or Dex (D; 2 μM) in the presence or absence of W3a. Lamin C was used as the loading control for the nuclear proteins. The relative band intensities of GR, C/EBPβ (36 kDa, the lower band with a black arrowhead), and lamin C were determined using the ImageJ software from four independent western analyses (details in Supplementary Fig. S2A). k, l ChIP-qPCR analyses of GR or C/EBPβ occupancy on Pparg2 (–0.3 kb or +2.6 kb from TSS) in 3T3-L1 cells. qPCR data show mean ± S.E. All data were repeated at least three independent same or similar experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 by Students’ t-test; ns, not significant
Wnt3a significantly reduced the nuclear protein level of GR, but not C/EBPβ during early adipogenesis, although Wnt3a did not reduce the total protein levels of either GR or C/EBPβ (Fig. 1h, i and S2A). Interestingly, Wnt3a did not reduce GR protein level in the nuclei of the 3T3-L1 cells treated with only Dex, suggesting that Wnt3a specifically reduced nuclear GR only during early adipogenesis (Fig. 1j). Consistently, Wnt3a reduced GR binding at two major hotspots (−0.3 or +2.6 kb) located near the Pparg2 transcription start site (TSS) (Fig. 1k). Although Wnt3a did not reduce C/EBPβ protein level, it inhibited the binding of C/EBPβ on the hotspots in Pparg2 (Fig. 1l). We selected seven genes (Acsl1, Hp, Tpcn2, Slc10a6, Krt13, Tsc22d3 and Megf9), the promoters (−5 to +1 kb from TSS) of which contain GR binding peaks, as identified in the ChIP-seq analyses5. We found that Wnt3a reduced GR occupancy in the promoter of all seven genes, which are also occupied by C/EBPβ; interestingly, Wnt3a also reduced C/EBPβ binding to these promoters (Fig. S2B). These results suggest that Wnt3a reduced GR and C/EBPβ occupancies on other MDI induced genes.
Wnt3a disrupts cooperation between C/EBPβ and GR on the Pparg2 promoter
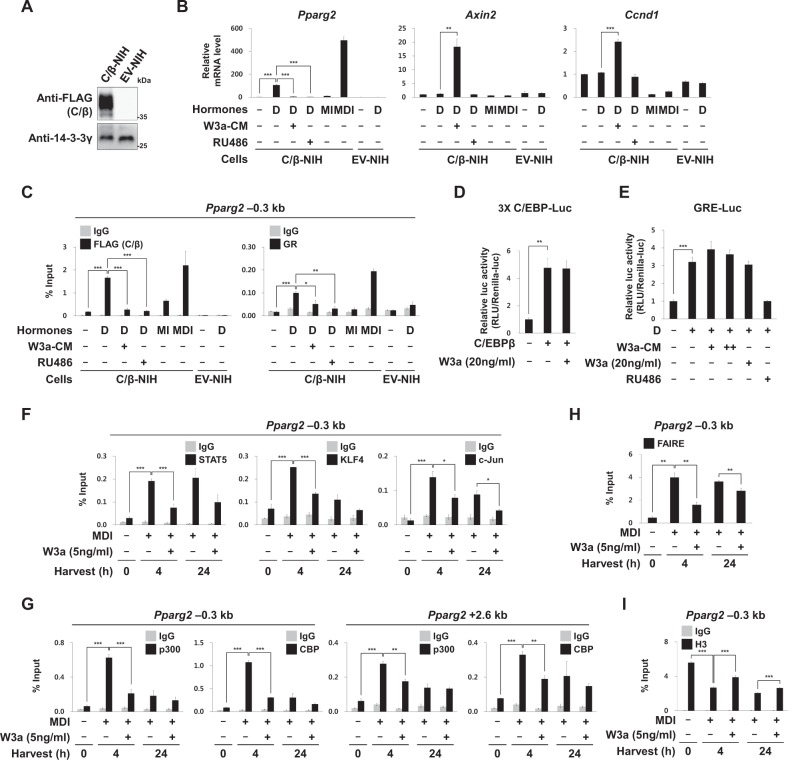
To investigate whether the reduction in nuclear GR destabilized C/EBPβ binding to the Pparg2 promoter, we generated C/β-NIH cells that ectopically express FLAG-tagged C/EBPβ (Fig. 2a). Dex alone (without IBMX) can induce Pparg2 expression in C/β-NIH cells, as IBMX is required for the induction of C/EBPβ (Fig. 2b). Furthermore, the constitutively expressed C/EBPβ can bind the Pparg2 promoter only when GR binds it in response to Dex treatment (Fig. 2c), and vice versa, GR binds to the Pparg2 promoter only in C/β-NIH cells but not in EV-NIH cells. These results confirmed that C/EBPβ and GR interdependently bind the Pparg2 promoter. Similar to Wnt3a, a GR antagonist, RU486, prevented not only GR but also C/EBPβ from binding to the Pparg2 promoter (Fig. 2c).
Fig. 2. Effects of Wnt3a on cooperative binding of C/EBPβ and GR on Pparg2.
a–c NIH-3T3 cells were infected with retrovirus encoding FLAG-tagged C/EBPβ (C/β-NIH cells) or empty vector as a control (EV-NIH cells). a Western blot analyses using anti-FLAG and 14-3-3γ antibodies. b, c Post-confluent C/β-NIH or EV-NIH cells were treated with the indicated adipogenic hormones, Wnt3a conditioned media (W3a-CM; 75%), or RU486 (10 μΜ) for 24 h. b qRT-PCR analyses of Pparg2, Axin2, and Ccnd1 mRNA levels, which were normalized to 18S rRNA levels. c ChIP-qPCR analyses of FLAG (C/EBPβ) or GR occupancy on the –0.3 kb region from TSS of Pparg2. d, e Reporter analyses using C/EBP-Luc or GRE-Luc. The transfected 3T3-L1 cells were treated with Dex (D, 2 μM), W3a-CM (50 or 100%), recombinant mouse Wnt3a (W3a; 20 ng/ml), or RU486 (10 μM) for 24 h. f, g ChIP-qPCR analyses of STAT5, KLF4, c-Jun, p300, or CBP occupancy on Pparg2 (–0.3 or +2.6 kb from TSS) in 3T3-L1 cells. h FAIRE-qPCR analyses on the –0.3 kb region of Pparg2 in 3T3-L1 cells (details in Materials and methods). i ChIP-qPCR analyses of histone H3 on the –0.3 kb region from TSS of Pparg2 in 3T3-L1 cells. qPCR data show mean ± S.E. All data were repeated at least three independent same or similar experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 by Students’ t-test
In addition to the Pparg2 promoter, C/EBPβ and GR can bind to different sets of their own target sequences. Analyses of the luciferase reporter gene show that Wnt3a does not inhibit C/EBPβ to induce the luciferase gene regulated by C/EBP binding sites (Fig. 2d). Similarly, RU486, but not Wnt3a, prevented Dex from inducing the luciferase gene, which was under the regulation of the glucocorticoid response element (GRE) (Fig. 2e). These results suggest that Wnt3a blocks neither C/EBPβ nor GR binding to their consensus motifs but disrupts the cooperativity between C/EBPβ and GR on the promoter of Pparg2 by reducing GR level in the nuclei during early adipogenesis. We investigated whether Wnt3a also disrupted the cooperative binding of other hotspot TFs. We found that Wnt3a also reduced binding of STAT5, KLF4, and c-Jun to Pparg2 (Fig. 2f). The cooperative bindings of several TFs significantly increased the recruitment of enhancer-associated coregulators, p300 and CBP, to form enhanceosomes8,41 and increased the accessibility of the chromatin structure of Pparg21. As expected, Wnt3a blocked the recruitment of p300 and CBP on Pparg2 in the MDI-treated cells (Fig. 2g). FAIRE (that detects nucleosome-depleted regions in the genome) and H3 ChIP analyses showed that MDI treatment reduced histone occupancy at the Pparg2 promoter, but not in the presence of Wnt3a, suggesting that Wnt3a inhibits MDI-induced chromatin opening of Pparg2 (Fig. 2h, i).
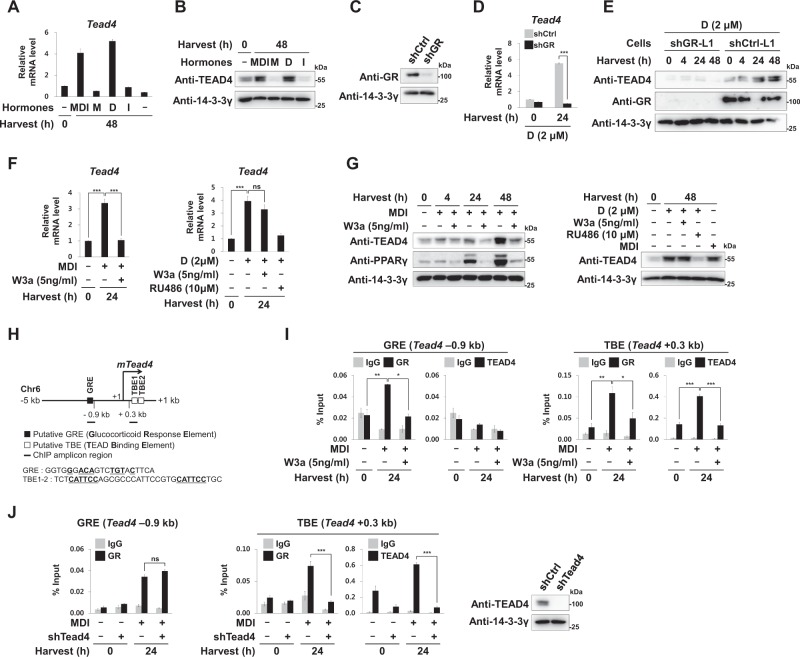
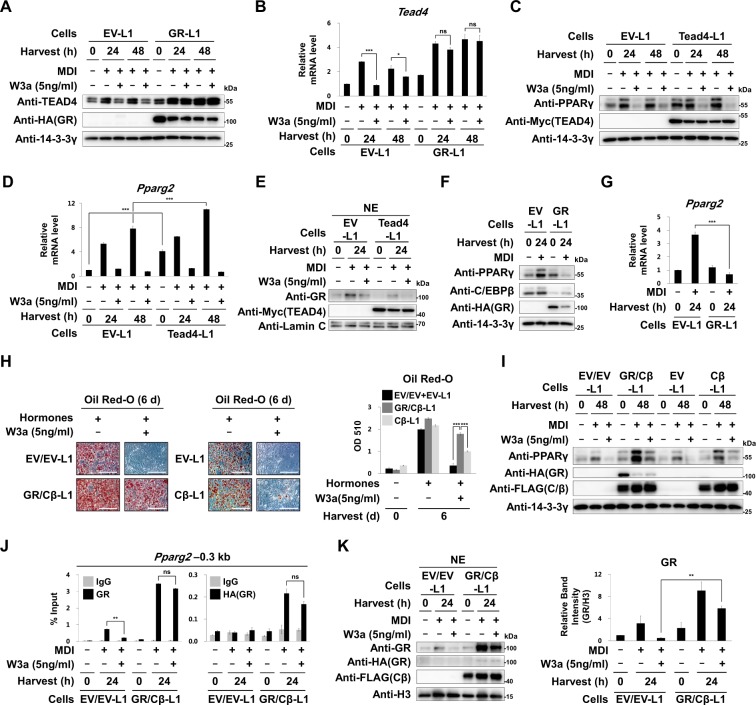
Wnt3a prevents GR from inducing Tead4, a novel hotspot TF of Pparg2
Starick et al. showed that TEA domain transcription factors (TEADs) reinforced GR binding to a subset of GR target genes as a heterodimer binding partner of GR42. For the first time, we found that Dex is responsible for inducing the mRNA and protein levels of Tead4 in a GR-dependent manner (Fig. 3a–e and S3A to S3C). Interestingly, Wnt3a completely blocked MDI from inducing the mRNA and protein levels of Tead4, but did not block Dex’s ability to induce Tead4 (Fig. 3f, g). These results are consistent with the findings that Wnt3a inhibits GR function in MDI-treated cells with higher sensitivity than in Dex-treated 3T3-L1 cells (Fig. 1i, j). ChIP revealed that both GR and TEAD4 occupied their putative binding sites in Tead4 after MDI treatment (Fig. 3h, i). Interestingly, we found that GR bound to not only GRE (−0.9 kb) but also to TEAD binding elements (TBE) (+0.3 kb) in Tead4 (Fig. 3h, i), and that knockdown of Tead4 reduced GR binding on the TBE of Tead4 (Fig. 3j). These results suggest that GR and TEAD4 can cooperatively bind to the TBE of Tead4, and that Tead4 is the target of TEAD4 itself as well as GR. Wnt3a prevented both GR and TEAD4 from binding to the GRE and the TBE of Tead4 during early adipogenesis (Fig. 3i).
Fig. 3. GR-induced TEAD4.
a qRT-PCR analyses of Tead4 mRNA of 3T3-L1 cells treated with adipogenic hormones as indicated. b Western blot analyses of 3T3-L1 cells using anti-TEAD4 and 14-3-3γ antibodies. c 3T3-L1 preadipocytes were infected with lentiviruses encoding shRNAs against mouse GR (shGR-L1 cells) or control shRNA (shCtrl-L1 cells). Western blot analyses showing GR protein levels in the shCtrl-L1 cells or the shGR-L1 cells. d, e The shCtrl-L1 cells or the shGR-L1 cells were treated with Dex (D, 2 μΜ) for the indicated time points. d Relative mRNA levels of Tead4 to 18S rRNA levels. e Western analyses showing TEAD4 protein levels. f, g The 3T3-L1 cells were treated with MDI or Dex in the presence or absence of W3a or RU486 for the indicated time points. f Relative mRNA levels of Tead4 to 18S rRNA levels. g Western analyses showing TEAD4 protein levels. h Indication of a putative GRE site (closed box) and TBE sites (open boxes) on Tead4 (–5 to +1 kb) at chromosome 6. Black bars indicate ChIP amplicon regions (–0.9 and +0.3 kb from TSS of Tead4 gene). i, j ChIP-qPCR analyses of GR or TEAD4 occupancy on Tead4 (–0.9 or +0.3 kb from TSS) in 3T3-L1, shCtrl-L1, or shTead4-L1 cells. Western analyses showing TEAD4 protein levels of shCtrl-L1 cells or shTead4-L1 cells (right panel). qPCR data show mean ± SE. All data were repeated at least three independent same or similar experiments. *p < 0.05, ** p < 0.01, and *** p < 0.001 by Students’ t-test; ns, not significant
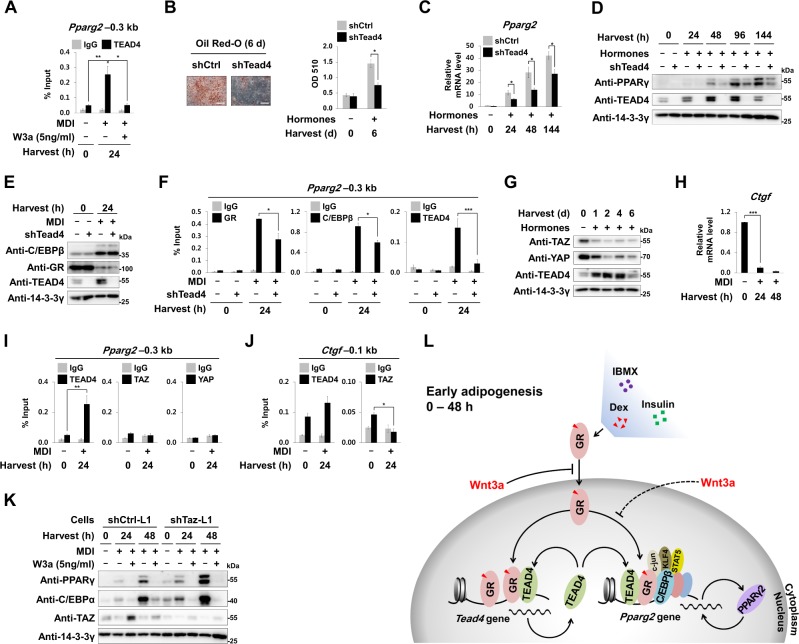
Furthermore, TEAD4 also bound to the Pparg2 promoter during early adipogenesis. We found that Tead4 knockdown reduced Pparg2 induction and adipogenesis (Fig. 4a–d). Interestingly, Tead4 knockdown did not reduce GR and C/EBPβ protein levels but reduced their binding to the Pparg2 promoter (Fig. 4e, f), suggesting that TEAD4 reinforced GR binding not only to the Tead4 promoter, but also to the Pparg2 promoter, for strong induction of both Pparg2 and Tead4. Although TEADs are major transcription factors that convey the Hippo signal by recruiting their coactivators TAZ/YAP, TEAD4 recruited neither YAP nor TAZ on Pparg2 (Fig. 4g–j). We also found that TEAD4 binds on the promoter of other MDI-induced genes, but do not recruit TAZ or YAP (Fig. S4A). Furthermore, TAZ knockdown did not block Wnt3a inhibitory effects suggesting that TAZ is not essential for the anti-adipogenic function of Wnt3a (Fig. 4k and Fig. S4B to S4E). We showed for the first time that TEAD4 and TAZ/YAP oppositely regulate the expression of Pparg2. These findings indicated that TEAD4 and GR form a positive circuit for induction of both Tead4 and Pparg2. Thus, Wnt3a disrupted two mutually related positive circuits by blocking GR binding to the promoters of Tead4 and Pparg2 (Fig. 4l).
Fig. 4. TEAD4 as a novel hotspot TF for Pparg2 induction.
a ChIP-qPCR analyses of TEAD4 occupancy on the –0.3 kb region from TSS of Pparg2 in 3T3-L1 cells. b–f The shCtrl-L1 cells or the shTead4-L1 cells were induced to undergo adipogenesis by treating with adipogenic hormones for the indicated time points as described in Fig. 1a. b Images and optical densities (510 nm) of Oil Red-O stained lipid. Scale bars, 200 μm. c Relative mRNA levels of Pparg2 to 18S rRNA levels. d, e Western blot analyses using the indicated antibodies. f ChIP-qPCR analyses of GR, C/EBPβ, or TEAD4 occupancy on the –0.3 kb region from TSS of Pparg2. g–j 3T3-L1 cells were treated with MDI for the indicated time points. g Western blot analyses of 3T3-L1 cells using the indicated antibodies. h Relative mRNA levels of Ctgf to 18S rRNA levels. i, j ChIP-qPCR analyses of TEAD4, TAZ, or YAP occupancy on the –0.3 kb region from TSS of Pparg2 and the –0.1 kb region from TSS of Ctgf. k Western blot analyses using the indicated antibodies in the shCtrl-L1 cells and shTaz-L1 cells. l Schematic diagram showing GR-TEAD4-PPARγ2 positive circuits during early adipogenesis. Wnt3a disrupted two mutually related positive circuits by limiting the nuclear localization of GR. qPCR data show mean ± SE. All data were repeated at least three independent same or similar experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 by Students’ t-test
Overexpression of GR is sufficient for blocking the inhibitory effects of Wnt3a
We investigated whether overexpression of ectopic GR is sufficient to resume the MDI-mediated induction of Tead4 in Wnt3a-treated 3T3-L1 cells. We found that GR overexpression prevented Wnt3a from reducing the mRNA and protein levels of Tead4. These results confirmed that Wnt3a reduced the expression of Tead4 by limiting the GR nuclear protein level during early adipogenesis (Figs. 1i, 5a, b). Overexpression of ectopic TEAD4 further increased the mRNA and protein levels of Pparg2 in MDI-treated as well as untreated 3T3-L1 cells, confirming that TEAD4 is a positive regulator of Pparg2 (Fig. 5c, d). However, in TEAD4 overexpressing cells, Wnt3a still limited GR protein level in the nuclei and reduced MDI-mediated induction of Pparg2, suggesting that TEAD4 cannot substitute GR for Pparg2 induction (Fig. 5c–e). Interestingly, overexpression of GR reduced the amount of C/EBPβ protein, which limited the MDI-mediated induction of PPARγ (Fig. 5f, g). This finding is consistent with previous study, which showed that liganded GR inhibits cAMP-activated CREB, an important transcription factor required for the induction of Cebpb43. We overexpressed GR together with C/EBPβ in 3T3-L1 cells (GR/Cβ-L1 cells). Unlike the ectopic expression of only C/EBPβ (Cβ-L1 cells), overexpression of both GR and C/EBPβ resulted in reinitiation of adipogenesis in 3T3-L1 cells even in the presence of Wnt3a (Fig. 5h). Although Wnt3a still reduced early induction of PPARγ, GR/Cβ-L1 cells maintained sufficiently high PPARγ protein level to enable completion of adipogenesis even in the presence of Wnt3a (Fig. 5h, i). ChIP analyses also showed that in GR/Cβ-L1 cells, Wnt3a did not reduce GR bindings to the Pparg2 promoter (Fig. 5j). These results corroborated the observation that GR protein level remained high in the nuclei of GR/Cβ-L1 cells even in the presence of Wnt3a (Fig. 5k) and demonstrated that Wnt3a blocked MDI-mediated induction of both Tead4 and Pparg2 by limiting GR in nuclei during early adipogenesis.
Fig. 5. Effects of GR and C/EBPβ overexpression.
a–g 3T3-L1 preadipocytes were infected with lentiviruses encoding HA-tagged GR (GR-L1), Myc-tagged TEAD4 (Tead4-L1) or empty vector as a control (EV-L1 cells). These cells were treated with MDI in the presence or absence of W3a for the indicated time points. a Western analyses of TEAD4 and HA-tagged GR protein. b Relative levels of Tead4 mRNA to 18S rRNA. c Western analyses of PPARγ and Myc-tagged TEAD4. d Relative levels of Pparg2 mRNA to 18S rRNA. e Western analyses of nuclear extracts (NE) of the EV-L1 cells or the Tead4-L1 cells treated with MDI in the presence or absence of W3a for 24 h using the indicated antibodies. Lamin C was used as the loading control for the nuclear proteins. f Western blot analyses using the indicated antibodies. g Relative levels of Pparg2 mRNA to 18S rRNA. h–k 3T3-L1 preadipocytes were infected with lentiviruses encoding FLAG-tagged C/EBPβ (Cβ-L1) or empty vector as a control (EV-L1 cells). Cβ-L1 cells and EV-L1 cells were further infected with retrovirus encoding HA-tagged GR (GR/Cβ-L1) or empty vector (EV/EV-L1 cells), respectively. These cells were treated with MDI in the presence or absence of W3a for the indicated time points. h Images and optical densities (510 nm) of Oil Red-O stained lipid. Scale bars, 200 μm. i Western analyses using the indicated antibodies. j ChIP-qPCR analyses of GR or HA-tagged GR occupancy on the –0.3 kb region from TSS of Pparg2. p = 0.012 for GR and p = 0.085 for HA (GR). k Western analyses of nuclear extracts (NE) of the EV/EV-L1 cells or GR/Cβ-L1 cells treated with MDI in the presence or absence of W3a. H3 was used as the loading control for nuclear proteins. The relative band intensities of GR and H3 were determined using the ImageJ software from two independent western blot analyses. qPCR data show mean ± SE. All data were repeated at least three independent same or similar experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 by Students’ t-test; ns, not significant
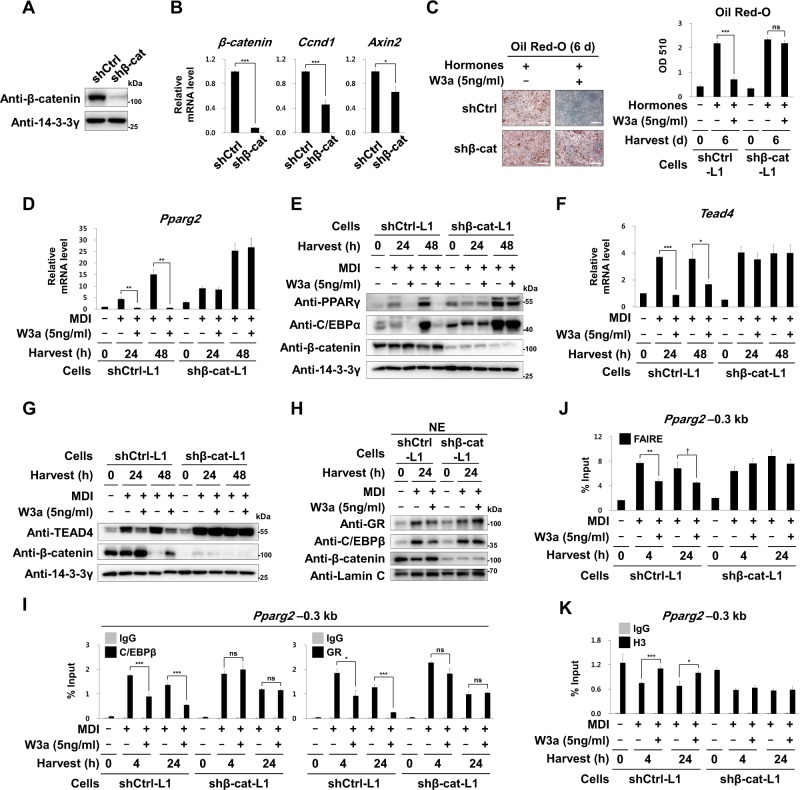
β-Catenin is necessary for conveying the Wnt3a signal to the Pparg2 promoter
Knockdown of β-catenin reduced the mRNA levels of its target genes such as Axin2 and Ccnd1 in 3T3-L1 cells (Fig. 6a, b) and completely blocked the inhibitory effects of Wnt3a on adipogenesis and induction of both Pparg2 and Tead4 (Fig. 6c–g). We found that Wnt3a did not reduce the nuclear amounts of GR proteins in shβ-cat-L1 cells (Fig. 6h). Consequently, in 3T3-L1 cells with β-catenin knockdown, Wnt3a did not block the occupancies of C/EBPβ and GR on the Pparg2 promoter (Fig. 6i) and did not inhibit the opening of chromatin structure (Fig. 6j, k). These results indicate that β-catenin is necessary for conveying the Wnt3a signal to the chromatin of Tead4 and Pparg2 by limiting the nuclear localization of GR.
Fig. 6. Effects of β-catenin knockdown.
a–k 3T3-L1 preadipocytes were infected with a lentivirus encoding shRNA against mouse β-catenin (shβ-cat-L1 cells) or control shRNA (shCtrl-L1 cells). a Western blot analyses showing β-catenin level. b qRT-PCR analyses showing relative mRNA levels of β-catenin, Ccnd1, and Axin2 to 18S rRNA levels. c–k The shCtrl-L1 or shβ-cat-L1 cells were induced to undergo adipogenesis by treating with adipogenic hormones for the indicated time points in the presence or absence of W3a (5 ng/ml). c Images and optical densities (510 nm) of Oil Red-O stained lipid. Scale bars, 200 μm. d, f Relative mRNA levels of Pparg2 and Tead4 to 18S rRNA levels. e, g Western blot analyses using the indicated antibodies. h Western blot analyses of nuclear extracts (NE) of the shCtrl-L1 or shβ-cat-L1 cells using the indicated antibodies. Lamin C was used as the loading control for nuclear proteins. i, k ChIP-qPCR analyses of C/EBPβ, GR, or H3 occupancy on the –0.3 kb region from TSS of Pparg2. j FAIRE-qPCR analyses on the –0.3 kb region from TSS of Pparg2. qPCR data show mean ± SE. All data were repeated at least three independent same or similar experiments. *p < 0.05, **p < 0.01, and ***p < 0.001, † p = 0.065 by Students’ t-test; ns, not significant
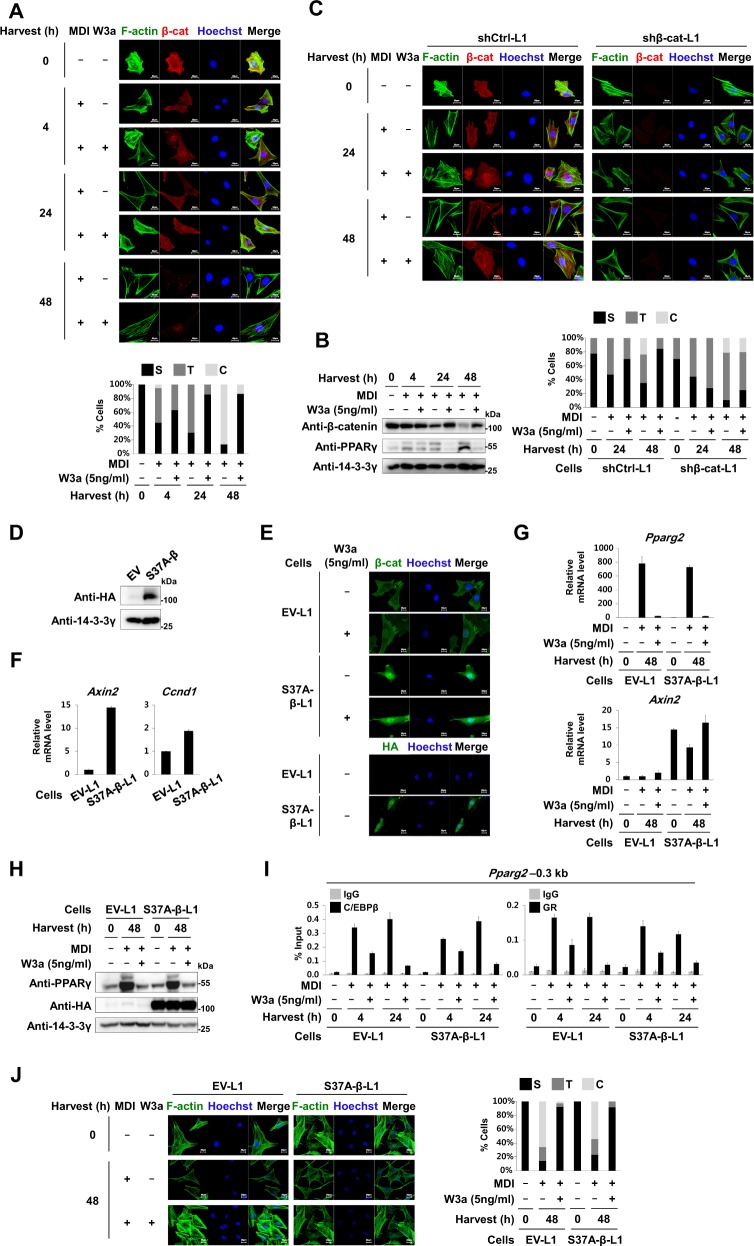
Wnt3a blocks remodeling of actin cytoskeleton in a β-catenin-dependent manner
β-Catenin is connected to the actin cytoskeleton via interactions with α-catenin and cadherin proteins in cytoplasm26. Upon exposure to adipogenic hormones, 3T3-L1 cells differentiate into round and lipid-laden adipocytes, accompanied by changes in the actin cytoskeleton from stress fibers to cortical structures4. Time course staining with fluorescent phalloidin, a probe specific for filamentous actin (F-actin), revealed that MDI reduced F-actin stress fibers in 50% cells from 4 h, whereas they disappeared in 86% cells within 48 h; instead, F-actin reorganized only at the cortical region. The amount of β-catenin protein decreased during the rearrangement of F-actin from stress fibers to cortical structures. Wnt3a prevented MDI from reducing β-catenin protein and disrupting F-actin stress fibers (Fig. 7a, b). However, this was not observed in shβ-cat-L1 cells, suggesting that β-catenin is required for Wnt3a to prevent F-actin rearrangement (Fig. 7c). However, we observed that active S37A-β-catenin which is constitutively present in the nuclei, is not sufficient to elicit the inhibitory effects of Wnt3a on remodeling of chromatin and cytoskeleton during early adipogenesis although at late phase of adipogenesis, ectopic expression of S37A-β-catenin can reduce the mRNA and protein levels of Pparg2 even in the absence of Wnt3a (Fig. 7d–j and S5A to S5C).
Fig. 7. Cytoskeletal rearrangement during early adipogenesis.
3T3-L1 cells were treated with MDI for the indicated time points in the presence or absence of W3a (5 ng/ml) as described in Fig. 1a. a Confocal microscopic images of cellular filamentous actin (F-actin) and β-catenin (upper panel). F-actin structures in individual cells were categorized into three groups. S (stress fiber), where F-actin stress fibers were observed in both nuclei and cytoplasm; T (transition status), where F-actin stress fibers were observed in the cytoplasm but not in the nucleus; C (cortical structure), where F-actin stress fibers were observed neither in the nucleus nor in the cytoplasm, but F-actin was observed near the cellular membrane. Cells (13–48) in each treatment were observed and categorized into three groups. The graph indicates the percentage of cells in each category (lower panel). b Western blot analyses of 3T3-L1 cells using the indicated antibodies. c Confocal microscopic images of cellular F-actin and β-catenin (upper panel). The graph indicates the percentage of cells in each category (S, T, and C described in Fig. 7a) (lower panel). d–j 3T3-L1 preadipocytes were infected with retrovirus encoding HA-tagged S37A-β-catenin (S37A-β-L1 cells) or empty vector (EV-L1 cells) as a control. d Western blot analyses of the EV-L1 cells or the S37A-β-L1 cells using anti-HA and 14-3-3γ antibodies. e Confocal microscopic images of the EV-L1 cells or the S37A-β-L1 cells immunostained with either anti-β-catenin antibody or anti-HA antibody. The cells were treated with W3a (5 ng/ml) for 24 h. The nuclei were stained with Hoechst 33258 (blue). f qRT-PCR analyses showing relative mRNA levels of Axin2 and Ccnd1 to 18S rRNA. g–j The EV-L1 cells or the S37A-β-L1 cells were treated with MDI for the indicated time points in the presence or absence of W3a (5 ng/ml). g Relative mRNA levels of Pparg2 and Axin2 to 18S rRNA. h Western blot analyses using the indicated antibodies. i ChIP-qPCR analyses of C/EBPβ or GR occupancy on the –0.3 kb region from TSS of Pparg2. j Confocal microscopic images of the cells immunostained with fluorescent phalloidin conjugates (green) and with Hoechst 33258 (blue) (left panel). Graph indicating the percentage of cells in each category (S, T, and C described in Fig. 7a) (right panel). qPCR data show mean ± SE. All data were repeated at least three independent same or similar experiments
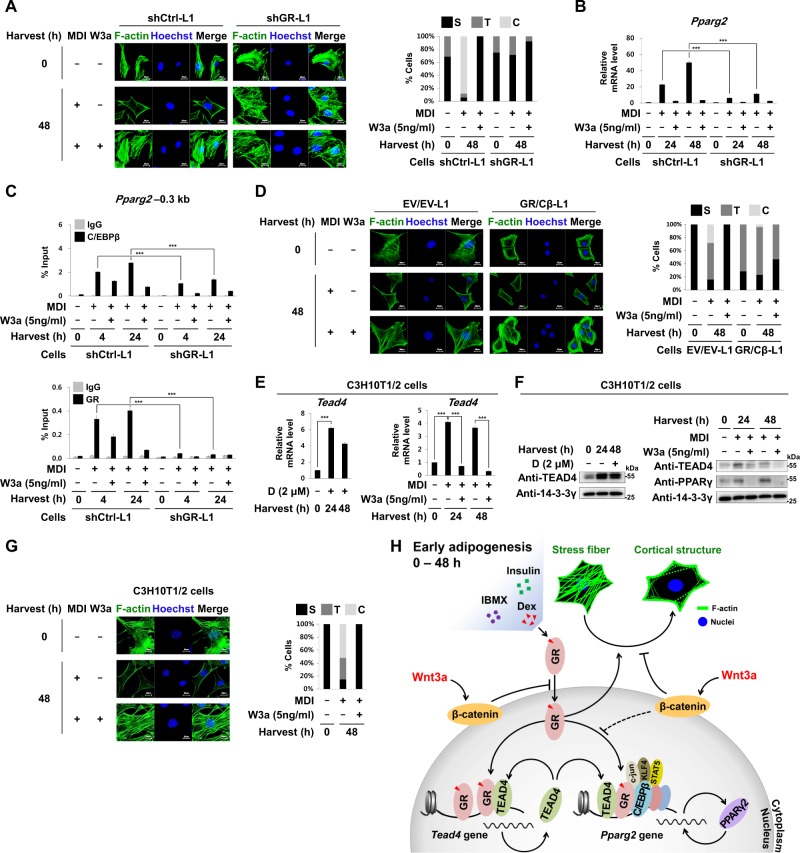
As Wnt3a reduced nuclear GR protein level in a β-catenin-dependent manner (Fig. 6h), we investigated whether GR is necessary for MDI to rearrange F-actin. In shGR-L1 cells with GR knockdown, MDI could neither rearrange F-actin nor induce the mRNA level of Pparg2 (Fig. 8a, b). GR knockdown reduced MDI-induced binding of C/EBPβ to the Pparg2 promoter (Fig. 8c). In contrast, in GR/Cβ-L1 cells without MDI treatment, F-actin stress fibers were observed only around the peripheral region but not in the nuclei of 3T3-L1 cells (Fig. 8d). Wnt3a did not recover F-actin stress fibers in GR/Cβ-L1 cells. Like 3T3-L1 cells, in C3H10T1/2, mouse mesenchymal stem cells, both Dex and MDI increased the mRNA and protein levels of TEAD4 and PPARγ which were also inhibited by Wnt3a (Fig. 8e, f). Furthermore, Wnt3a prevented MDI from rearranging F-actin stress fibers in C3H10T1/2 cells (Fig. 8g). These findings suggest that during early adipogenesis, GR is necessary both for the rearrangement of F-actin and hotspot formation on the Pparg2 promoter, and that Wnt3a blocks these two events by limiting the nuclear level of GR in a β-catenin-dependent manner (Fig. 8h).
Fig. 8. Effects of GR on cytoskeletal rearrangement.
The shCtrl-L1 or shGR-L1 cells were induced to undergo adipogenesis by treating with adipogenic hormones for the indicated time points in the presence or absence of W3a (5 ng/ml). a Confocal microscopic images of F-actin stress fibers (left panel). The graph indicates the percentage of cells in each category (S, T, and C described in Fig. 7a) (right panel). b qRT-PCR analyses of Pparg2 mRNA levels to 18S rRNA levels. c ChIP-qPCR analyses of C/EBPβ or GR occupancy on the –0.3 kb region from TSS of Pparg2. d Confocal microscopic images of cellular F-actin stress fibers (left panel) in the EV/EV-L1 or GR/Cβ-L1 cells. The graph indicates the percentage of cells in each category (S, T, and C described in Fig. 7a) (right panel). e–g C3H10T1/2 cells were treated with Dex (2 μM) or MDI for the indicated time points in the presence or absence of W3a (5 ng/ml). e qRT-PCR analyses of Tead4 and Pparg2 mRNA levels to 18S rRNA levels. f Western blot analyses showing TEAD4 and PPARγ protein levels. g Confocal microscopic images of cellular F-actin stress fibers (left panel) in C3H10T1/2 cells. The graph indicates the percentage of cells in each category (S, T, and C described in Fig. 7a) (right panel). h Schematic diagram showing the inhibitory effects of Wnt3a/β-catenin on positive circuits of GR-TEAD4-PPARγ2 and cytoskeletal remodeling during early adipogenesis (details in Results). qPCR data show mean ± SE. All data were repeated at least three independent same or similar experiments. ***p < 0.001 by Students' t-tests
Discussion
The findings that treatment of Dex followed by IBMX was sufficient for adipogenesis, but that IBMX treatment followed by Dex treatment did not recapitulate this effect suggested that Dex primed preadipocytes to a novel commitment state for adipogenesis44. Adipogenesis of mouse embryonic fibroblasts (MEFs) isolated from GR (Nr3c1) knockout and GR dimerization-defective mutant mice was impaired45. Knockdown of GR or omission of Dex from MDI did not induce Pparg2 in 3T3-L1 cells8,46. These previous studies indicated that GR is essential for Pparg2 induction.
Using extensive genome-wide profiling of 15 TFs at 4 h after MDI treatment, Mandrup and colleagues demonstrated that during early adipogenesis, hotspots co-occupied by more TFs recruited more coactivators such as p300/CBP to constitute super-enhancers1. Furthermore, enhancers with large numbers of TFs are more sensitive to small changes in TF concentration compared to those with smaller numbers of TFs47–49. This is consistent with our findings that reduction in nuclear GR level by β-catenin sensitively changes super-enhancer formation on Pparg2 and other genes (Fig. S2B). Our findings that GR amplifies MDI signals to Pparg2 by inducing TEAD4, a novel TF for Pparg2, highlighted that changes in GR activity affect Pparg2 expression (Fig. 3).
ChIP-exo sequencing using GR antibody in IMR90 cells revealed that TEAD4 and GR co-occupied GR target genes as a heterodimer and that TEAD knockdown decreased the expression of several GR target genes42. Biochemical CAP-SELEX analyses which identify cooperative interactions between TF pairs and heterodimeric DNA motifs, revealed that TEAD4 is the most common partner that cooperatively binds diverse DNA sequences with 32 TFs among 100 tested TFs. TEAD4 and its partner TFs recognize composite sequences that were considerably different from the individual TF motifs, suggesting that in vivo functions of TEAD4 may encompass diverse biological functions depending on its partner TFs50. This is the first study to show that TEAD4 cooperatively binds to Pparg2 together with other hotspot TFs, and that Tead4 knockdown reduced Pparg2 expression. Since GR and TEAD4 cooperatively bind and induce Tead4, GR is a key TF that persistently drives expression of both Tead4 and Pparg2. Wnt3a disrupted two mutually related positive circuits by limiting GR binding to the promoters of these two genes.
Goentoro and Kirschner have showed that fold-change, but not the absolute level of β-catenin, regulates Wnt signaling in an experimental model of Xenopus dorsal-anterior development51. Our findings that the nuclear form of S37A-β-catenin failed to deliver Wnt3a signal imply that Wnt3a disrupts hotspot formation and cytoskeletal rearrangement but not by increasing the coactivator activity of β-catenin in the nuclei (Fig. 7d–j). Similar to β-catenin, the TAZ coactivator activated by the canonical Wnt pathway, inhibits adipogenesis by inhibiting PPARγ protein52,53. We found that the protein level of TAZ decreased during early adipogenesis, but not in the presence of Wnt3a, suggesting that TAZ can be a mediator that delivers the Wnt signal to Pparg2. However, our finding that TAZ knockdown did not block Wnt3a inhibitory effects indicates that TAZ is not essential for the anti-adipogenic function of Wnt3a (Fig. 4k).
Unlike lipogenesis in other cells such as hepatocytes and myocytes, adipogenesis requires dramatic cytoskeletal remodeling of F-actin stress fibers to cortical actin structures, which is required to hold a large lipid vacuole in the center, relocate the nucleus and other organelles, and attain a round shape. Cytoskeletal remodeling starts within 24 h after adipogenic hormone treatments13, suggesting that cytoskeletal remodeling is followed by complete induction of lipogenic genes as a feed-forward mechanism. These nuclear and cytoplasmic events should interdependently regulate each other to prevent metabolic and structural catastrophes during adipogenesis54,55. In addition to adipogenic hormones, the stiffness of extracellular matrices (ECM) is involved in commitment for adipogenesis. Primary preadipocytes embedded in stiffer matrices showed reduced rates of adipogenesis10. ECM stiffness increases tissue tension, which leads to increased actin and myosin fiber and cell stretching56,57. Interestingly, mesenchymal stem cells exposed to mechanical strain show increase in β-catenin level and could not differentiate into adipocytes, suggesting that Wnt/β-catenin conveys signals from intracellular tension to the nuclei58. In agreement with this result, we found that Wnt signal could not prevent MDI from changing F-actin stress fibers to cortical F-actin structures during adipogenesis in the absence of β-catenin.
Interestingly, Wnt3a reduced GR level in the nuclei during the early phase of adipogenesis, but not in Dex-treated preadipocytes, suggesting that the antagonistic effect of Wnt/β-catenin on GR is specific for adipogenesis (Figs. 1i, j, 6h). Extensive studies have revealed that the chaperone complex and intact cytoskeleton are required for the nuclear transport of GR59. Furthermore, GR is connected to actin filaments through HSP90, a main component of GR-chaperone complex suggesting that the nuclear transport of GR and cytoskeletal rearrangement are closely related60. Upon ligand binding, the GR-chaperone complex recruits a motor protein dynein to form the liganded GR-HSP90-FKBP52-dynein complex, which is able to move along the microtubules through the nuclear pore complex (NPC) to the nucleus61,62. However, when the cytoskeleton is disrupted like adipogenesis, liganded GR simply diffuses in and out of the nucleus mainly via importins, exportins, and the RanGTPase system59,63. The mechanism via which the liganded-GR translocates into the nucleus during adipogenic cytoskeletal rearrangement remains unknown. In contrast, β-catenin harbors armadillo repeats, which are similar to the importin-β HEAT repeats64–66. Therefore, β-catenin can pass through NPC like importin and equilibrates between the nucleus and the cytoplasm by passive diffusion. Therefore subcellular distribution of β-catenin in the nuclei, cytoplasm, and the membrane can be determined from its location and the levels of diverse interacting complexes, namely, the TCF/LEF transcription factors in the nucleus, APC and AXIN in the cytoplasm, and cadherin complex in the membrane67. It remains to be investigated whether GR and β-catenin compete with each other for nuclear translocation during adipogenic cytoskeletal rearrangement.
In conclusion, this study provides insights into an intriguing question regarding chromatin remodeling and actin rearrangement, which are interdependently regulated during adipogenesis. Our findings that GR is necessary for the rearrangement of both cytoskeleton and chromatin, and that canonical Wnt3a inhibited both processes in a β-catenin-dependent manner, suggest that rearrangements of both chromatin and cytoskeleton are related in ways that involve the antagonistic activities of GR and β-catenin, and that Wnt3a reinforced β-catenin function.
Supplementary information
Acknowledgements
We thank Prof. Eek-hoon Jho (University of Seoul) for providing cDNA of S37A-β-catenin-HA and Wnt3a-L929 cells. We thank Prof. Rik Derynck (University of California, San Francisco) and Prof. Young Joo Lee (Sejong University) for providing reporter plasmids of 3X C/EBP-Luc and GRE-Luc, respectively. We thank Prof. Hueng-Sik Choi (Chonnam National University) and Prof. Hyun Woo Park (Yonsei University) for providing cDNAs of HA-GR and Myc-Tead4, respectively. We also thank Prof. Jae-Bong Park (Hallym University College of Medicine) and Prof. Jeong-Ho Hong (Korea University) for their advice and technical support. We thank Sungwook Yoo for technical support. This work was supported by the National Research Foundation of Korea [NRF-2014R1A1A2056917, NRF-2017M3A9D5A01052447, NRF-2016R1A2B4012840, NRF-2018R1A4A1025985].
Authors contributions
H.P. and B.P. conceived the study, designed the experiments, and analyzed data; B.P. performed most of the experiments; S.C. and G-J.L. performed the immunofluorescence assay. J.K.K. and B.K. analyzed the bioinformatics data; H.P. and B.P. wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by S. Inoue
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41419-018-1249-7).
References
- 1.Siersbaek R, et al. Transcription factor cooperativity in early adipogenic hotspots and super-enhancers. Cell Rep. 2014;7:1443–1455. doi: 10.1016/j.celrep.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 2.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 3.Farmer SR. Transcriptional control of adipocyte formation. Cell. Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siersbaek R, et al. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011;30:1459–1472. doi: 10.1038/emboj.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siersbaek R, Nielsen R, Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. 2012;23:56–64. doi: 10.1016/j.tem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Siersbaek R, et al. Molecular architecture of transcription factor hotspots in early adipogenesis. Cell Rep. 2014;7:1434–1442. doi: 10.1016/j.celrep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steger DJ, et al. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 2010;24:1035–1044. doi: 10.1101/gad.1907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siersbaek R, et al. Dynamic rewiring of promoter-anchored chromatin loops during adipocyte differentiation. Mol. Cell. 2017;66:420–435 e425. doi: 10.1016/j.molcel.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Chun TH, et al. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng. Part. A. 2009;15:147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- 13.Nobusue H, et al. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 2014;5:3368. doi: 10.1038/ncomms4368. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl Acad. Sci. USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 16.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CN, et al. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 18.Kawai M, et al. Wnt/Lrp/beta-catenin signaling suppresses adipogenesis by inhibiting mutual activation of PPARgamma and C/EBPalpha. Biochem. Biophys. Res. Commun. 2007;363:276–282. doi: 10.1016/j.bbrc.2007.08.088. [DOI] [PubMed] [Google Scholar]
- 19.Cawthorn WP, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50:477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 24.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 25.Lasota J, et al. Nuclear expression and gain-of-function beta-catenin mutation in glomangiopericytoma (sinonasal-type hemangiopericytoma): insight into pathogenesis and a diagnostic marker. Mod. Pathol. 2015;28:715–720. doi: 10.1038/modpathol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrea PD, Gottardi CJ. Beyond beta-catenin: prospects for a larger catenin network in the nucleus. Nat. Rev. Mol. Cell Biol. 2016;17:55–64. doi: 10.1038/nrm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marie PJ, Hay E. Cadherins and Wnt signalling: a functional link controlling bone formation. Bone Rep. 2013;2:330. doi: 10.1038/bonekey.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freytag SO, Geddes TJ. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science. 1992;256:379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, et al. Cyclin D1 repression of peroxisome proliferator-activated receptor gamma expression and transactivation. Mol. Cell. Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu M, et al. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J. Biol. Chem. 2005;280:16934–16941. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J. Biol. Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 33.Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int. J. Obes. 2005;29:S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol. Cell. Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park YK, et al. Hypoxia-inducible factor-2alpha-dependent hypoxic induction of Wnt10b expression in adipogenic cells. J. Biol. Chem. 2013;288:26311–26322. doi: 10.1074/jbc.M113.500835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park YK, Park H. Differentiated embryo chondrocyte 1 (DEC1) represses PPARgamma2 gene through interacting with CCAAT/enhancer binding protein beta (C/EBPbeta) Mol. Cells. 2012;33:575–581. doi: 10.1007/s10059-012-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YK, Park H. Prevention of CCAAT/enhancer-binding protein beta DNA binding by hypoxia during adipogenesis. J. Biol. Chem. 2010;285:3289–3299. doi: 10.1074/jbc.M109.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, et al. Multi-dimensional histone methylations for coordinated regulation of gene expression under hypoxia. Nucleic Acids Res. 2017;45:11643–11657. doi: 10.1093/nar/gkx747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon JM, Giresi PG, Davis IJ, Lieb JD. Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA. Nat. Protoc. 2012;7:256–267. doi: 10.1038/nprot.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon Y, Lee S, Park B, Park H. Distinct hypoxic regulation of preadipocyte factor-1 (Pref-1) in preadipocytes and mature adipocytes. Biochim. Biophys. Acta. 2018;1865:334–342. doi: 10.1016/j.bbamcr.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Park YK, Ge K. Glucocorticoid receptor accelerates, but is dispensable for, adipogenesis. Mol. Cell. Biol. 2017;37:e00260–16. doi: 10.1128/MCB.00260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starick SR, et al. ChIP-exo signal associated with DNA-binding motifs provides insight into the genomic binding of the glucocorticoid receptor and cooperating transcription factors. Genome Res. 2015;25:825–835. doi: 10.1101/gr.185157.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Focking M, Holker I, Trapp T. Chronic glucocorticoid receptor activation impairs CREB transcriptional activity in clonal neurons. Biochem. Biophys. Res. Commun. 2003;304:720–723. doi: 10.1016/S0006-291X(03)00665-X. [DOI] [PubMed] [Google Scholar]
- 44.Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell. 2008;19:4032–4041. doi: 10.1091/mbc.e08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asada M, et al. DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Kruppel-like factor 15 gene expression. Lab. Invest. 2011;91:203–215. doi: 10.1038/labinvest.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cha JY, et al. Dexras1 mediates glucocorticoid-associated adipogenesis and diet-induced obesity. Proc. Natl Acad. Sci. USA. 2013;110:20575–20580. doi: 10.1073/pnas.1320454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giniger E, Ptashne M. Cooperative DNA binding of the yeast transcriptional activator GAL4. Proc. Natl Acad. Sci. USA. 1988;85:382–386. doi: 10.1073/pnas.85.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griggs DW, Johnston M. Regulated expression of the GAL4 activator gene in yeast provides a sensitive genetic switch for glucose repression. Proc. Natl Acad. Sci. USA. 1991;88:8597–8601. doi: 10.1073/pnas.88.19.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt SF, Larsen BD, Loft A, Mandrup S. Cofactor squelching: artifact or fact? Bioessays. 2016;38:618–626. doi: 10.1002/bies.201600034. [DOI] [PubMed] [Google Scholar]
- 50.Jolma A, et al. DNA-dependent formation of transcription factor pairs alters their binding specificity. Nature. 2015;527:384–388. doi: 10.1038/nature15518. [DOI] [PubMed] [Google Scholar]
- 51.Goentoro L, Kirschner MW. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol. Cell. 2009;36:872–884. doi: 10.1016/j.molcel.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong JH, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 53.Azzolin L, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Yang W, et al. Arp2/3 complex regulates adipogenesis by controlling cortical actin remodelling. Biochem. J. 2014;464:179–192. doi: 10.1042/BJ20140805. [DOI] [PubMed] [Google Scholar]
- 55.Yang W, et al. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum. Mol. Genet. 2014;23:502–513. doi: 10.1093/hmg/ddt444. [DOI] [PubMed] [Google Scholar]
- 56.Akimoto T, et al. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem. Biophys. Res. Commun. 2005;329:381–385. doi: 10.1016/j.bbrc.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 57.Jakkaraju S, Zhe X, Pan D, Choudhury R, Schuger L. TIPs are tension-responsive proteins involved in myogenic versus adipogenic differentiation. Dev. Cell. 2005;9:39–49. doi: 10.1016/j.devcel.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Sen B, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandevyver S, Dejager L, Libert C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic. 2012;13:364–374. doi: 10.1111/j.1600-0854.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 60.Miyata Y, Yahara I. Cytoplasmic 8 S glucocorticoid receptor binds to actin filaments through the 90-kDa heat shock protein moiety. J. Biol. Chem. 1991;266:8779–8783. [PubMed] [Google Scholar]
- 61.Silverstein AM, et al. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J. Biol. Chem. 1999;274:36980–36986. doi: 10.1074/jbc.274.52.36980. [DOI] [PubMed] [Google Scholar]
- 62.Wochnik GM, et al. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 63.Galigniana MD, et al. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol. Endocrinol. 1998;12:1903–1913. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- 64.Fagotto F. Looking beyond the Wnt pathway for the deep nature of beta-catenin. EMBO Rep. 2013;14:422–433. doi: 10.1038/embor.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eleftheriou A, Yoshida M, Henderson BR. Nuclear export of human beta-catenin can occur independent of CRM1 and the adenomatous polyposis coli tumor suppressor. J. Biol. Chem. 2001;276:25883–25888. doi: 10.1074/jbc.M102656200. [DOI] [PubMed] [Google Scholar]
- 66.Lee SJ, et al. The adoption of a twisted structure of importin-beta is essential for the protein–protein interaction required for nuclear transport. J. Mol. Biol. 2000;302:251–264. doi: 10.1006/jmbi.2000.4055. [DOI] [PubMed] [Google Scholar]
- 67.Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J. Cell. Sci. 2006;119:1453–1463. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.