Abstract
Context
Menstrual irregularity after menarche has been attributed to immature estrogen positive feedback activity (E+FB) but data are conflicting.
Objective
To determine the hypothalamic–pituitary–ovarian contributions to menstrual irregularity in adolescents.
Methods
Twenty-three healthy girls [aged 12.8 to 17.6 years; 0.4 to 3.5 years postmenarche; body mass index (BMI) percentile, 41.0 to 99.3] underwent serial hormone measurements and pelvic ultrasounds during two consecutive menstrual cycles. Hormones and follicle growth were compared with 65 adult historic controls with ovulatory cycles (OVs).
Results
Girls had anovulatory cycles (ANOVs; 30%), OVs with a short luteal phase (short OVs; 22%), or OVs with normal luteal phase (normal OVs; 48%) without differences in cycle length, chronologic or gynecologic age, or BMI. Adolescents showed a spectrum of E+FB [midcycle LH adjusted for preovulatory estradiol (E2)]; only normal OV girls were comparable to adults. All OV girls had lower E2, progesterone, and gonadotropins during the luteal phase and luteal-follicular transition compared with adults. Normal OV girls also had lower follicular phase LH and FSH levels, a longer follicular phase, a slower dominant follicle growth rate, and smaller estimated preovulatory follicle size than adults. Follicular phase E2 and inhibin B levels were lower in normal OV girls than in adults even after adjusting for differences in FSH and follicle size.
Conclusions
Early postmenarchal girls with normal OVs demonstrate mature E+FB but continue to have lower gonadotropin levels, diminished ovarian responsiveness, and decreased corpus luteum sex steroid synthesis compared with adults, indicating that reproductive axis maturity requires coordinated development of all components of the hypothalamic–pituitary–ovarian axis.
Investigation of hormone dynamics and follicle growth in early post-menarchal girls reveals intact estrogen positive feedback but decreased LH and FSH levels and ovarian responsiveness.
During the first few years after menarche, the hypothalamic–pituitary–ovarian axis is not fully mature and previous studies suggest that approximately half of menstrual cycles are anovulatory (1). The reported prevalence of ovulatory cycles (OVs) in adolescents at more advanced gynecologic ages (years since menarche) is inconsistent across studies (2–13), possibly due to differences in the methods used to identify OV, sample size, as well as subject ethnicity, nutritional status, and age at menarche (14). Importantly, few studies (7, 14, 15) have investigated hormone dynamics in contemporary adolescent girls who have matured in an era of increasing rates of obesity and earlier puberty, two factors that may influence the tempo of hypothalamic–pituitary–ovarian axis maturity.
Estrogen positive feedback activity was traditionally considered to be the major limiting factor in establishing regular OVs after menarche (13, 16–18). However, this concept is at odds with some more recent reports of spontaneous or estrogen-induced LH surges in premenarchal girls that are of comparable magnitude to surges in adult women (7, 14, 19–23). In addition, small increases in pregnanediol (Pd) glucuronide, the primary urinary metabolite of progesterone (P4), have been observed in many early adolescent menstrual cycles, suggesting ovulation with residual immaturity in corpus luteum (CL) formation and/or function (9, 24). We therefore hypothesized that other components of hypothalamic–pituitary–ovarian axis regulation contribute to reproductive axis immaturity. For example, the dynamic secretory profile of FSH, particularly during the luteal-follicular transition (LFT), when levels rise fourfold in adult women (25), is essential for initiating folliculogenesis and controlling the number of follicles reaching maturity. Moreover, for an LH surge to induce ovulation, it must be precisely coordinated with follicle growth and estradiol (E2) production, and the granulosa cells of the preovulatory follicle must express LH receptors to luteinize (26).
The current study was designed to investigate the reproductive hormone profiles that underlie the diverse menstrual cycle patterns of early postmenarchal girls and to contrast these profiles with those of normal adult women.
Materials and Methods
Subjects
Healthy girls, aged 12.8 to 17.6 years, 0.4 to 3.5 years postmenarche, were studied. A maximum gynecologic age of 3.5 years was chosen based on the longitudinal study by Vihko and Apter (27) of 200 girls demonstrating that 41% of girls had achieved OVs by the end of the fourth gynecologic year. We reasoned that we would capture a sufficient number of girls with OVs and anovulatory cycles (ANOVs) by including girls within or just after the third gynecologic year.
Subjects were recruited from the community by advertising in newspapers, on community bulletin boards, on the Internet, or through direct mail (postcard) marketing. Subjects were not on any medications known to interfere with reproductive hormones and had never been treated for a menstrual condition. Two subjects were on centrally acting medications (methylphenidate, a catecholamine reuptake inhibitor, and sertraline, a selective serotonin reuptake inhibitor). All subjects had normal thyroid, prolactin, and androgen levels (28); no inflammatory acne; and were nonhirsute (Ferriman-Gallwey score <8). They did not exercise excessively [defined as running >20 miles per week or its equivalent as determined using the Compendium of Physical Activities (29, 30)] and had no history of an eating disorder, stress fracture, or osteopenia/osteoporosis. All subjects were nonsmokers. Relevant medical history was obtained in person from the subject and a parent.
The Partners Human Research Committee approved the study and signed informed assent and consent were obtained from each subject and her parent, respectively.
Protocol
Method comparison and validation
Recognizing the impracticality of obtaining daily blood specimens from adolescents, we first performed a pilot study to determine the feasibility of using saliva, urine, and dried blood spots (DBS) for hormone assays. Seven subjects participated in five study visits (one visit/week) to contemporaneously measure LH and FSH in serum and DBS and E2 and P4 (or its primary urinary metabolite, Pd) in serum, DBS, urine, and saliva (n = 35 samples). Twenty-one additional samples were obtained from 12 subjects who completed blood draws and collected DBS and/or urine on the same day during intensive cycle monitoring (see below).
Menstrual cycle monitoring
Seven subjects from the pilot study and 16 additional subjects participated. Hormone levels were measured during two consecutive menstrual cycles from approximately cycle day 10 in cycle 1 to cycle day 20 in cycle 2. Subjects underwent one to three blood draws per week (for LH, FSH, E2, P4) in clinic according to their availability and were asked to collect DBS (for LH, FSH) and dried urine strips (for E2, Pd) at home on the remaining days. Levels from DBS and urine samples, with the exception of urine Pd, were then converted to serum-equivalents (see Data Analysis). Note that saliva samples were not used in the full protocol because of poor assay precision at the low salivary hormone levels found in adolescents (see “Results”). Inhibin B (INHB) was measured in a subset of serum samples (those with adequate sample volume) in the early follicular phase (EFP) (two to six samples per subject; 91 in total). Samples were obtained every 1 to 2 days during cycle 1 and week 1 of cycle 2. Nineteen of the 23 girls had additional hormone (17.5 ± 5.6 days) and sonographic data (2.1 ± 0.9 scans) that allowed us to determine ovulatory status in cycle 2. Data were available for 87.2% ± 3.0% of days in cycle 1 and 77.7% ± 4.3% of days in cycle 2.
One investigator (J.M.A.) performed all trans-abdominal pelvic ultrasounds (two to five/subject) using a 5-2 MHz curved transducer (Phillips HDII XE system, Andover, MA) and recorded follicle counts, maximum follicle diameter, the presence of a CL and free fluid in the posterior cul-de-sac, and ovarian volume. Follicles were scanned in multiple planes to determine maximum diameter. Ovarian volume was calculated using the formula for the volume of an ellipsoid (L × H × W × 0.523).
Subjects took daily iron supplements and hemoglobin was monitored throughout the study at four time points.
Assays
Serum
Serum was analyzed for LH, FSH, E2, P4, and total testosterone (TT) using a chemiluminescent microparticle immunoassay (CMIA) (Architect; Abbott Diagnostics, Abbott Park, IL) (31). The E2 (32) and TT (33) assays have been calibrated against liquid chromatography/tandem mass spectrometry (LC/MS). To confirm the accuracy of low P4 values by CMIA in this study, 23 samples with P4 of 0.4 to 10.3 ng/mL were reanalyzed using LC/MS (Esoterix, Inc., Endocrine Sciences, LabCorp, Calabasas Hills, CA). All P4 values <0.6 ng/mL (n = 3) by CMIA were undetectable by LC/MS. After excluding these undetectable values, however, there was a tight correlation between the two methods (slope = 0.9, intercept = 0.06, r2 = 0.9). INHB was measured at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core using an enzyme-linked immunoassay (Beckman Coulter, Inc., Brea, CA).
DBS
LH and FSH were analyzed using a solid-phase, two-site chemiluminescent immunometric assay (Siemens, Berlin, Germany). E2 and P4 were analyzed using an enzyme immunoassay (DRG International, Inc., Springfield, NJ) (34, 35).
Dried urine strips
E2 and Pd levels were determined by gas chromatography-MS/MS (Agilent 7000B) after extraction and enzymatic hydrolysis of sulfate and glucuronide conjugates (36).
Saliva
E2 and P4 were measured using an enzyme immunoassay (DRG International, Inc.) with C-18 column extraction and concentration (34, 37).
Gonadotropins are expressed in international units per liter, as equivalents of the Pituitary Second International Reference Standard 80/552. See Supplementary Table 1 and Supplementary Methods for additional details pertaining to assay performance and sample collection and processing.
Data analysis
Comparison of serum with saliva, urine, and DBS measurements
To identify the best noninvasive methods of hormone measurement, we fit log-transformed hormone levels derived from DBS and serum (FSH, LH) or urine and serum (E2) using Passing-Bablok nonparametric regression models (Analyze-it 3.90.7, Leeds, UK) (Supplementary Figs. 1 and 2). The strongest correlations were between DBS and serum LH and FSH (both r = 0.9, P < 0.001) and between urine and serum E2 (r = 0.8, P < 0.001). The fitted models then allowed conversion of DBS and urine levels to serum-equivalents for use in the full protocol [serum FSH = 0.89*(DBS FSH0.88); serum LH = 0.43*(DBS LH1.16); serum E2 = 41.85*(urine E21.08)]. P4 in DBS and saliva and Pd in urine did not correlate well with serum P4, particularly at low levels (0 to 2 ng/mL). In the full protocol, P4 was expressed only as serum P4 [12.3 ± 1.0 (mean ± SE) samples per subject]. Urine Pd was also used (without interconversion) in the full protocol (18.6 ± 2.2 samples per subject) because the assay appeared to perform well in the range indicative of ovulation according to serum P4 (i.e., P4 > 1.65 ng/mL as described below).
Menstrual cycle classification
To establish biochemical criteria for OVs in adolescents, we examined serum P4 and urine Pd measurements from 37 cycles with (i) sonographic evidence of ovulation based on collapse of the dominant follicle, detection of a CL, and/or increased free fluid in the posterior cul-de-sac or (ii) sonographic evidence of anovulation, including absent follicle growth, regression of the dominant follicle without subsequent follicle growth, or identification of a large (≥30 mm) unruptured follicle within 2 days of menses (38–40). We then performed receiver-operator characteristic curve analysis and identified cut-offs with the highest combined sensitivity and specificity: P4 = 1.65 ng/mL (sensitivity 85%, specificity 96%) and Pd = 390.5 µg/g Cr (sensitivity 73%, specificity 96%) (Supplementary Fig. 3). Areas under the curve were 0.96 for P4 (95% CI, 0.89 to 1.0; P < 0.0001) and 0.93 for Pd (95% CI, 0.85 to 1.0; P < 0.0001).
Cycles were determined to be ovulatory if they met the following criteria: (i) a midcycle (MC) LH peak; (ii) a preovulatory E2 peak; and (iii) a serum P4 >1.65 ng/mL or urine Pd >390.5 µg/g Cr. A peak was defined as the first LH value ≥mean + 2 SD of the six preceding values, as previously described (41). Sonographic signs of ovulation also provided supporting evidence. The day of ovulation was defined as the cycle day meeting at least three of the following criteria (42): (i) day of the MC LH peak; (ii) day of the MC FSH peak; (iii) day of/day after the preovulatory E2 peak (defined as highest E2 value); (iv) day on which serum P4 doubled or exceeded 0.8 ng/mL (41), (v) 3 days before a sustained, threefold increase in urine Pd above mean follicular phase (FP) levels (modified Kassam algorithm) (43); and (vi) day of/day before follicle collapse on ultrasound. Urine Pd levels were lagged by 1 day to account for hormone metabolism and excretion (44). The length of the FP was calculated as the number of days from menses onset up to and including the estimated day of ovulation. The length of the luteal phase (LP) was calculated as the number of days after ovulation until the day before next menses.
In ANOVs, an LH peak was also identified by an LH value at least two SDs above the mean of the six preceding values (41). The length of the FP was equal to total cycle length. Luteinization of a dominant follicle was defined as a serum P ≥ 1 ng/mL (based on the average P4 within 36 hours of LH surge initiation in adults) (41) or a threefold rise in urine Pd above the mean FP level (45).
Comparisons among adolescent subgroups and with historic adult controls
Gonadotropin and sex steroids in adolescents were compared with corresponding data from 65 women, ages 18 to 34 years, with regular OVs who underwent daily blood sampling for one to two cycles (94 cycles total) (46). INHB measurements were available in 11 of these women (47). Follicle growth rates and E2 levels relative to follicle size were compared between adolescents and 45 ovulatory women who underwent two to six serial transvaginal ultrasounds and a subset (n = 21) who had concurrent daily blood samples in the week before ovulation (48).
The same assay platforms for serum gonadotropins and sex steroids in adolescents were used previously in adult controls (see Supplementary Methods). INHB in adults was measured using a different enzyme-linked immunoassay (Serotec, Oxford, UK) and levels were converted to values from the current assay (49).
Baseline characteristics were compared among adolescent groups using one-way analysis of variance. FP hormones were normalized to a 14-day FP (42) and all hormones were natural-log-transformed before analysis. For LH, FSH, and E2, cycle day numbering was centered on the MC LH peak of cycle 1 (day 0) and compared across all groups during the late FP (LFP, −5 to −2), MC (−1 to +1), LP (+2 to menses) of cycle 1 and in days 1 to 7 of cycle 2 (EFP). P4 levels were compared at MC and during the LP.
ANOVs were either centered to MC (day 0) using the LH peak (n = 3; LH 13.9, 16.9, and 24.2 IU/L, respectively), the day with the highest E2 level (n = 3; E2 84, 91, or 232 pg/mL, respectively), or the first day with evidence of luteinization of the dominant follicle (n = 1; defined as serum P4 ≥1 ng/mL or threefold rise in urine Pd) to allow for comparisons with OVs.
All group comparisons were performed using linear mixed models to account for repeated measures. Prespecified pairwise comparisons of adolescent groups and adults were performed using the two-sample t test. To capture the preovulatory E2 rise that starts ~60 hours before the MC surge (50, 51), E2 from day −3 to 0 was also compared by linear mixed models. Mean LH at MC, adjusted for the mean preovulatory E2 level, was used as an index of E2 positive feedback and was compared between groups using analysis of covariance.
Dominant follicle growth rates and maximum preovulatory follicle size during cycle 1 (day −7 to 0) were derived from a linear mixed model (52). The day on which a dominant follicle (≥10 mm) first appeared was predicted from the model and corresponding CIs estimated by the Fieller method (53). Two indices of ovarian maturity were compared across groups using linear mixed models: (i) cycle 2 EFP INHB adjusted for FSH; and (ii) FP E2 adjusted for same-day FSH and follicle diameter (cycles 1 and 2).
All analyses were conducted in JMP Pro 13.2 and SAS 9.4 (Cary, NC). Results are reported as geometric mean ± SE or the geometric mean difference (95% CI) for log-transformed data.
Results
Subject characteristics
Twenty-three healthy adolescents participated (Table 1 and Supplementary Table 2). Subjects had Tanner V breast development and a gynecologic age of 0.4 to 3.5 years (1.7 ± 0.2). They were 14.4 ± 0.2 years old and racially diverse (15 white, 5 black, 3 Asian; 25% Hispanic). Four girls (17%) were overweight and nine (39%) were obese; the remaining 10 girls (44%) were of normal weight. Subjects were nonhirsute, had no more than mild acne, and had normal TT levels (determined at the screening visit), with no differences between the groups [(mean ± SE) normal OV, 19.2 ± 3.0 ng/dL; short OV 20.0 ± 3.5 ng/dL; and ANOV 22.0 ± 5.9 ng/dL; P = 0.9].
Table 1.
Baseline Characteristics of Subjects and Comparisons of Cycle 1 Subgroups
| Ovulatory, Normal Luteal (Normal OV) | Ovulatory, Short Luteal (Short OV) | Anovulatory (ANOV) | Difference Among Groups, P-Valuea | |
|---|---|---|---|---|
| n | 11 | 5 | 7 | |
| Chronologic age, y | 14.8 ± 0.8 | 14.0 ± 0.3 | 14.0 ± 0.5 | 0.48 |
| Age at menarche, y | 12.6 ± 0.2 | 12.9 ± 0.3 | 12.5 ± 0.2 | 0.58 |
| Gynecologic age, y | 1.9 ± 0.3 | 1.9 ± 0.5 | 1.5 ± 0.4 | 0.68 |
| BMI percentile | 79.8 ± 9.3 | 78.9 ± 5.7 | 83.3 ± 8.2 | 0.91 |
| Cycle length, d | 39.4 ± 3.9 | 25.2 ± 1.1 | 35.9 ± 4.4 | 0.10 |
| FP length, d | 27.8 ± 4.0 | 17.4 ± 1.2 | – | 0.11 |
| LP length, d | 11.6 ± 0.4 | 7.8 ± 0.8 | – | –b |
| Peak serum P4, ng/mLc | 7.1 ± 1.3 | 3.4 ± 0.4 | 1.4 ± 1.1 | –b |
| Peak urine Pd, µg/g Crc | 1276.7 ± 153.9 | 588.0 ± 89.0 | 316.4 ± 145.5 | –b |
One-way ANOVA.
Used to define the groups of adolescents.
To convert P4 to SI units (nmol/L), multiply by 3.18; for Pd (nmol/mmol creatinine), multiply by 0.35.
Menstrual cycle patterns
Using intensive biochemical and sonographic monitoring of cycle 1, we grouped cycles into three different patterns: (i) OVs with normal (10 to 14 days) LP length (designated “normal OV”); (ii) OVs with short (5 to 9 days) LP length (“short OV”) (54, 55); and (iii) anovulatory cycles (“ANOV”) (Table 1 and Supplementary Table 2). All but one OV (cycle length, 77 days) fell within the 21- to 45-day range typical of adolescent menstrual cycles (1), as did all but two ANOVs (cycle lengths, 20 and 60 days) (Supplementary Fig. 4a). There was significant overlap in cycle length among these groups, indicating that cycle length, when within this range, is a poor predictor of ovulatory status in adolescents. In this sample, chronologic age, gynecologic age, and body mass index (BMI) percentile did not differ significantly among groups (Table 1), and there was no correlation between gynecologic age and cycle length (slope = 2.9, intercept = 30.0, r2 = 0.07, P = 0.2) (Supplementary Fig. 4b). The presence of one OV did not necessarily signal reproductive maturity as four (21.1%) subjects had one OV and one ANOV during the monitoring period (57.9% had two OVs and 21.1% had two ANOVs). Cycle dynamics also appeared to influence the chance of ovulation in the subsequent cycle: subjects with an OV in cycle 1 were more likely to have another OV (in cycle 2) than subjects with an ANOV in cycle 1 (84.6% vs 33.3%, P = 0.046) (Supplementary Table 2). One subject with ANOVs and two subjects with normal OVs had enlarged ovaries (12.8 to 17.3 cm3), defined as an average ovarian volume >12 cm3 in adolescents (56).
Comparisons of hormone dynamics and folliculogenesis
Girls with OVs in cycle 1
Girls with normal OVs and short OVs demonstrated the dynamic changes in LH, FSH, E2, and P4 across the menstrual cycle that typify mature OVs in adults, including an increase in FSH during the LFT, a biphasic increase in E2, a MC LH peak, and a peri-ovulatory and postovulatory increase in P4 or Pd (Table 2; Fig. 1). However, the level of hormonal detail afforded by the current studies revealed significant differences in the magnitude of these hormone changes between adult women and adolescents.
Table 2.
Mixed Model Estimatesa of Hormone Dynamics by Phase
| LFP |
MC |
LP |
EFP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | P | Mean | 95% CI | P | Mean | 95% CI | P | Mean | 95% CI | P | |
| LH, IU/L | ||||||||||||
| Adult | 6.0 | 5.4–6.7 | Reference | 18.2 | 16.1–20.5 | Reference | 3.8 | 3.5–4.2 | Reference | 4.4 | 4.0–4.9 | Reference |
| Normal OV | 6.8 | 5.1–9.2 | 0.43 | 16.7 | 11.3–24.6 | 0.67 | 2.5 | 1.9–3.3 | 0.005 | 2.8 | 2.1–3.8 | 0.006 |
| Short OV | 3.4 | 1.9–6.1 | 0.06 | 12.7 | 7.3–22.1 | 0.21 | 1.5 | 0.9–2.3 | <0.001 | 2.7 | 1.8–4.3 | 0.04 |
| ANOV | 7.6 | 5.2–11.1 | 0.26 | 7.2 | 4.5–11.7 | <0.001 | 2.2 | 1.4–3.3 | 0.009 | 2.3 | 1.6–3.4 | 0.002 |
| FSH, IU/L | ||||||||||||
| Adult | 4.8 | 4.5–5.2 | Reference | 7.3 | 6.7–7.8 | Reference | 4.0 | 3.7–4.2 | Reference | 6.5 | 6.1–7.0 | Reference |
| Normal OV | 4.6 | 3.8–5.5 | 0.62 | 5.7 | 4.5–7.2 | 0.06 | 1.5 | 1.2–1.9 | <0.001 | 4.1 | 3.4–5.1 | <0.001 |
| Short OV | 3.5 | 2.4–5.0 | 0.08 | 5.8 | 4.1–8.1 | 0.20 | 1.7 | 1.3–2.3 | <0.001 | 4.3 | 3.2–5.7 | 0.005 |
| ANOV | 4.1 | 3.2–5.2 | 0.21 | 3.4 | 2.5–4.5 | <0.001 | 1.8 | 1.3–2.3 | <0.001 | 2.7 | 2.1–3.5 | <0.001 |
| E2,b pg/mL | ||||||||||||
| Adult | 133.2 | 120.7–147.0 | Reference | 154.4 | 139.2–171.2 | Reference | 98.2 | 90.8–106.3 | Reference | 54.0 | 49.3–59.2 | Reference |
| Normal OV | 51.2 | 40.0–65.5 | <0.001 | 123.5 | 89.5–170.3 | 0.20 | 59.2 | 46.3–75.5 | <0.001 | 16.9 | 12.9–22.2 | <0.001 |
| Short OV | 77.9 | 48.5–125.1 | 0.03 | 133.4 | 82.6–215.5 | 0.56 | 64.1 | 43.5–94.6 | 0.04 | 26.8 | 18.2–39.4 | <0.001 |
| ANOV | 47.7 | 34.5–66.0 | <0.001 | 108.0 | 73.5–158.7 | 0.08 | 87.4 | 61.9–123.2 | 0.51 | 35.2 | 25.0–49.6 | 0.02 |
| P4,b ng/mL | ||||||||||||
| Adult | 1.0 | 0.9–1.3 | Reference | 5.1 | 4.4–5.9 | Reference | ||||||
| Normal OV | 0.7 | 0.4–1.4 | 0.29 | 3.2 | 1.9–5.4 | 0.09 | ||||||
| Short OV | 0.5 | 0.2–1.7 | 0.23 | 2.0 | 0.9–4.4 | 0.02 | ||||||
| ANOV | 0.4 | 0.2–1. | 0.06 | 1.0 | 0.4–2.5 | <0.001 | ||||||
Means and 95% CIs are estimated values from the mixed model, taking into account the repeated measures across participants. Log-transformed values were transformed back to original units for presentation here.
To convert E2 to SI units (pmol/L), multiply by 3.67; for P4 (nmol/L), multiply by 3.18.
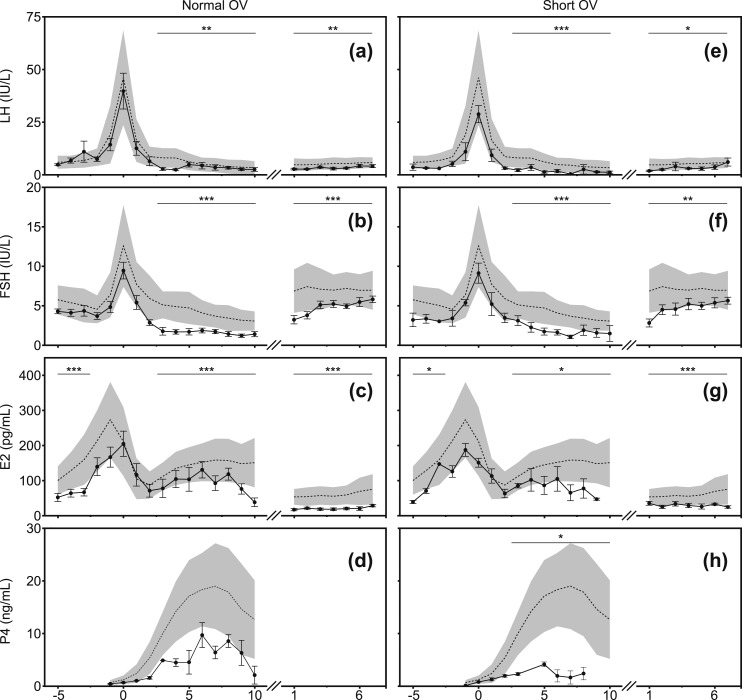
Figure 1.
LH, FSH, E2, and P4 levels during two consecutive menstrual cycles in adolescent girls with either normal OV (left, n = 11) or short OV (right, n = 5) cycles. Levels represent serum or serum equivalents as determined from DBS or urine samples. Cycle days are centered to the MC LH peak of cycle 1 (day 0) and shown during the first week of cycle 2 (day 1 = menses). Adolescents: filled circles are arithmetic mean values and error bars are ±1 SE. Both groups are compared with historic adult controls (n = 65). Adults: Dashed line denotes arithmetic mean level and shaded area is ±1 SD. *P < 0.05; **P < 0.01; ***P < 0.001 for cycle phase (i.e., LFP, MC, LP, EFP). To convert E2 to SI units (pmol/L), multiply by 3.67; for P4 (nmol/L), multiply by 3.18.
In normal OV adolescents (Figs. 1a–1d; Table 2; Supplementary Figs. 5a and 6a), E2 was lower compared with adults during the LFP (days −5 to −2; P < 0.001) and in the immediate preovulatory period [days−3 to 0; percent difference in means (95% CI): −35.3% (−51.5 to −13.7), P = 0.003; modeled estimate of E2 means (95% CI): normal OV, 115.0 pg/mL (87.3 to 151.4); adult, 117.7 (162.9 to 194.0)], but the MC LH peak remained robust (P = 0.2). Indeed, the magnitude of the LH peak controlled for preovulatory E2 was not different from adult cycles [percent difference in means −12.4% (−36.6 to 21.0), P = 0.4], consistent with mature estrogen positive feedback activity in normal OV girls. Despite a normal LH peak and LP length, however, normal OV girls showed a tendency toward lower P4 levels (P = 0.09) and did not attain adult levels of E2 (P < 0.001) during the LP. Normal OV adolescents showed a precipitous drop in FSH and LH after the MC LH peak. FSH and LH remained lower in normal OV than in adults during the LP (FSH, P < 0.001; LH, P = 0.005) and subsequent EFP of cycle 2 (FSH, P < 0.001; LH, P = 0.006) (i.e., the LFT). E2 levels were also lower (P < 0.001) in adolescents during the EFP of cycle 2 compared with adults.
In short OV adolescents (Figs. 1e–1h; Table 2; Supplementary Figs. 5b and 6b), E2 was not different from adults during the immediate preovulatory period [percent difference in E2 means (95% CI): −20.7% (−50.7 to 27.7), P = 0.3; modeled estimate of mean in short OV, 141.0 pg/mL (88.3 to 225.1)] but was lower than in adults when the entire LFP was included (P = 0.03). The MC LH peak was not statistically lower (P = 0.2). However, the LH peak adjusted for preovulatory E2 was less in short OV girls than adults [−37.1% (−60.2 to −0.7); P = 0.047], raising the possibility of immature positive feedback activity in this group. FSH, LH, and E2 fell rapidly after MC in short OV girls and remained lower than in adults during the LP (P < 0.05 for all) and EFP of cycle 2 (P < 0.05 for all). P4 in short OV girls was lower (P = 0.02) than in adults during a LP of 5 to 9 days’ duration.
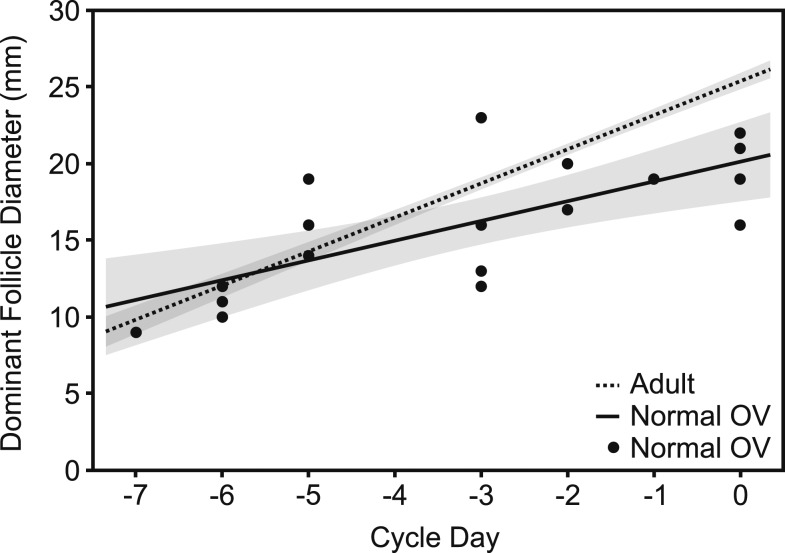
In normal OV adolescents and adults, the dominant follicle first appeared 6 to 7 days before MC in cycle 1 [estimated median (interquartile range): 7.0 days (5.5 to 7.5) vs 7.0 days (6.0 to 7.0) in adolescents vs adults; P = 0.8], indicating that the longer FP observed in the adolescents was due to a longer time required for small antral follicle growth and/or selection of the dominant follicle rather than for dominant follicle growth [FP: 27 days (20 to 28) vs 16 days (14 to 16.5) in adolescents vs adults; P < 0.001]. The dominant follicle grew more slowly in normal OV adolescents than in adults (1.5 ± 0.2 vs 2.2 ± 0.1 mm/d; P < 0.001; Fig. 2) such that the predicted size of the preovulatory follicle was smaller in adolescents than adults (mean preovulatory follicle diameter, 20.3 ± 1.1 vs 25.2 ± 0.4 mm; P < 0.001). Follicular growth in short OV girls (1.4 ± 1.2 mm/d; preovulatory diameter, 20.6 ± 4.8 mm) largely mirrored that of normal OV girls (test for difference in regression lines for folliculogenesis, P = 0.9).
Figure 2.
Dominant follicle diameter plotted against cycle day for adults and normal OV adolescents. Lines and 95% confidence bands were estimated from a linear mixed model. Adults, dashed line; normal OV adolescents, solid line. Filled circles are individual data points for normal OV adolescents. The slope of a line is the estimated follicle growth rate; the intercept at cycle day 0 is the estimated follicle size at ovulation. The dominant follicle grew more slowly in adolescent girls with normal OV cycles than in adults resulting in a smaller estimated follicle size at ovulation in cycle 1.
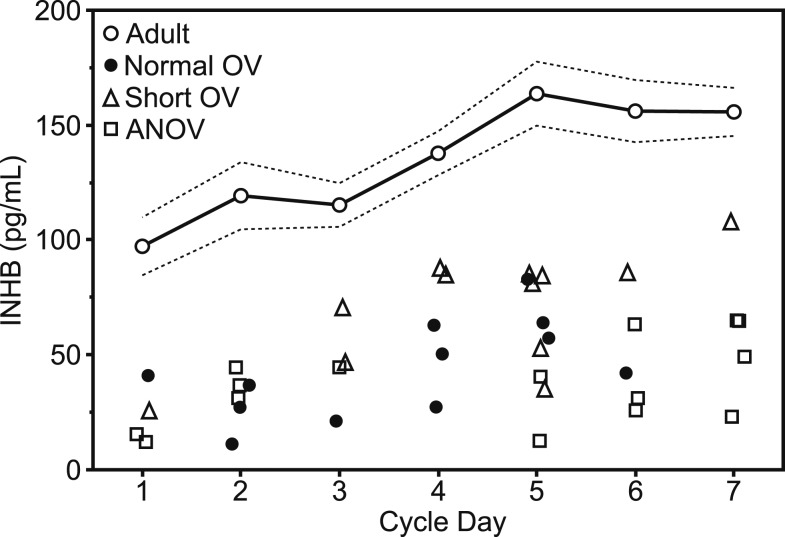
Both normal and short OV adolescents had signs of ovarian immaturity that were unrelated to diminished FSH stimulation. Mid- to late-follicular E2 was lower in normal and short OV girls compared with adults even after adjusting for both lower FSH and smaller follicle size [percent difference in means: normal OV vs adult, −39.4% (−60.1 to −8.0), P = 0.02; short OV vs adult, −46.6% (−71.0 to −1.9), P = 0.04]. Cycle 2 EFP INHB was lower than in adults before (P < 0.01 for normal and short OV; Fig. 3) and after adjusting for lower FSH levels [percent difference in means: normal OV vs adult, −58.0% (−75.0 to −29.6), P = 0.002; short OV vs adult, −42.6% (−64.5, −7.3), P = 0.02].
Figure 3.
INHB levels plotted against cycle day for adults and adolescents during the EFP of cycle 2. Adults: arithmetic mean levels, (○); dashed lines delineate ±1 SE. Adolescents: individual data points, normal OV (●), short OV (△), or ANOV (□). Adult and adolescent subjects each contributed data to only a subset of cycle days. INHB levels were lower in all adolescent groups compared with adults during the first 7 d of cycle 2. INHB values are equivalent in SI units (ng/L).
Girls with ANOVs in cycle 1
ANOV adolescents demonstrated heterogeneous hormone profiles (Table 2; Supplementary Figs. 5c and 6c). Two girls had normal follicle development with a corresponding increase in E2 and a relatively robust increase in LH without ensuing follicle rupture; instead, the dominant follicle(s) continued to secrete E2 and a small amount of P4 before menses occurred. One of these subjects demonstrated persistent growth of three follicles for at least 5 days after MC, consistent with the presence of luteinized unruptured follicles (Supplementary Fig. 7). The second subject grew one dominant follicle (maximum diameter 30 mm) and had a MC LH peak of 24.2 IU/L; after MC, follicle growth ceased but E2 remained in the 130 to 150 pg/mL range and P4 rose to 3.9 ng/mL before menses occurred 12 days later. Note that without serial ultrasounds demonstrating a luteinized unruptured follicle, this cycle would have been misclassified as ovulatory. The remaining ANOV girls had either minimal follicle growth (n = 2; maximum diameter 7 and 11 mm, respectively), formed a luteinized preovulatory follicle that did not ovulate (n = 2; 27 mm and P4 1.3 ng/mL; 39 mm and P4 1.0 ng/mL), or formed a preovulatory follicle that did not luteinize or ovulate (n = 1; 22 mm, P4 0.5 ng/mL).
As a group, ANOV girls had lower E2 before MC and lower LH and FSH at MC compared with adults (Table 2). MC LH in ANOV girls remained attenuated compared with adults after adjustment for E2 [difference: −57.9% (−71.1 to −38.8), P < 0.001]. FSH and LH levels in ANOV girls fell after MC but E2 secretion was preserved relative to adults. In these ANOV girls, E2 was presumably not derived from the CL, but from one or more luteinized follicles that continued to produce E2 for several days until menses (Supplementary Fig. 7). In the EFP of cycle 2, LH, FSH, and E2 were lower in ANOV girls than in adults (Table 2).
ANOV girls as a whole did not differ from the two OV groups in dominant follicle growth rate or follicle size at MC (0.9 ± 0.3 mm/d; 20.0 ± 1.5 mm; test for difference in regression lines, P = 0.5), but EFP INHB was lower in ANOV girls than in adults both before (P < 0.01; Fig. 3) and after adjusting for lower FSH [difference: ANOV vs adult −61.7% (−76.9% to −36.7%), P < 0.001], suggesting ovarian immaturity.
Discussion
We performed detailed reproductive phenotyping of a contemporary cohort of 23 healthy early postmenarchal girls to gain insight into the normal development of the hypothalamic–pituitary–ovarian axis. Approximately three-quarters demonstrated at least one cycle in which ovulation occurred, but many of these OVs were characterized by luteal insufficiency (short LP length and/or lower P4 or Pd compared with adult women). Hormone dynamics in ANOVs were variable, as has been noted by others (14, 57), and included minimal or absent follicle growth, growth of a luteinized preovulatory follicle that was not followed by ovulation and CL formation, and/or a diminished LH peak at MC. We demonstrate that the luteinized unruptured follicle syndrome is an abnormality that falls within the adolescent luteal insufficiency spectrum. Importantly, we found that the threshold P4 value indicative of ovulation in adolescents is ~1.65 ng/mL, well below the commonly used adult standard of 4 to 5 ng/mL, suggesting that many adolescent cycles previously classified as anovulatory may have instead been ovulatory with luteal insufficiency. The episodic release of P4 by the CL (58) in the setting of slow frequency LH pulses during the LP suggests further that more frequent measurement of P4 in the LP, complemented by pelvic ultrasounds, will be necessary to accurately classify adolescent cycles in future studies, and in particular, to distinguish adolescents with short OV cycles from those with ANOV cycles with luteinized unruptured follicles. OV and ANOV cycles appear to be indistinguishable based on any routine parameters readily available to the clinician (e.g., patient’s cycle length, gynecologic age, chronologic age, or BMI).
The ultimate step in female reproductive axis maturation has traditionally been deemed the acquisition of estrogen positive feedback: mature GnRH secretion leads to mature gonadotropin secretion and normal folliculogenesis; growth of a healthy dominant follicle then provides the exponential increase in estrogen levels required to induce an LH surge and consequent ovulation (59, 60). The current studies, however, demonstrate that even adolescent girls who have achieved this milestone (i.e., OVs, a robust LH peak at MC, and normal LP length) continue to have immature FSH and LH dynamics compared with normal adults, suggesting the GnRH secretory profile or pituitary responsiveness is not fully mature. Previous studies reported that FSH increases more slowly in ovulatory adolescents than in adults during the FP but did not compare absolute FSH levels (or LH levels) between adolescents and adults or assess gonadotropin secretion during the LFT (61). A small study of daily urinary hormone measurements in early postmenarchal girls compared with adults found no differences in FSH and LH, but groups were compared using a 3- to 6-month integrated measure without consideration of cycle phase (14). In the current study, we observed a profound decrease in FSH and LH levels compared with adults during the LFT, even in girls with normal OV cycles, which was presumably responsible for lower E2 and INHB in the EFP of the subsequent cycle in girls. The slower growth rate of the dominant follicle and smaller predicted preovulatory follicle size in adolescents compared with adults in the current study and in previous studies (61, 62) are also consistent with diminished trophic stimulation by FSH in adolescents. Thus, although the robust LH peak at MC in ovulatory adolescents demonstrates that the pituitary is capable of storing and releasing LH in response to GnRH, lower FSH (and also LH) during the LFT suggest residual hypothalamic and/or pituitary immaturity. No previous studies have rigorously investigated hormone dynamics during the LFT in adolescents. While several studies carefully documented hormone profiles in girls with frequent blood or urine sampling, they did not examine intercycle hormone dynamics (13, 14, 61, 63–67). The only reference to a potential immaturity of FSH dynamics during the LFT was made by Hayes and Johanson (63) who noted that the late luteal rise in FSH characteristic of adult cycles was only observed in girls with a gynecologic age of at least 3 years.
Although our data suggest that diminished FSH stimulation contributes to the decreased follicular growth rate in adolescents, there is also evidence for intrinsic ovarian immaturity. E2 was significantly lower during the FP in adolescents even after adjusting for their smaller follicle sizes, suggesting a developmental discordance between FSH-driven follicle growth and aromatization and/or LH-driven androgen synthesis. We had insufficient data on other potential markers of follicle maturity (P4, inhibin A) relative to follicle size to further address the possibility of ovarian immaturity. These data provide support for previous in vitro studies: Anderson et al. (68) demonstrated that secondary follicles and oocytes isolated from ovarian cortical biopsies from early postmenarchal girls grow more slowly than follicles from adult women under the same culture conditions of insulin and recombinant FSH. Inadequate follicle development is also suggested by lower luteal P4 in ovulatory girls compared with adults as the quality of the CL is thought to reflect the quality of its predecessor, the dominant follicle (69).
This study has several limitations. Given the intensive nature of these studies, particularly in pediatric subjects, we used several noninvasive, in-home sampling modalities to augment clinic blood draws and ultrasounds. Even with these additional methods, we were unable to capture hormone data and follicle size on every day. Our pilot study demonstrated strong correlations between serum and DBS for gonadotropins and between serum and urine for E2. Although P4 salivary assays have gained increasing popularity in the fertility industry, in our hands, salivary P4 could not distinguish ANOVs from OVs in adolescents. It is unclear if this was related to the assay itself or to poor collection technique (i.e., recent chewing gum, food, or liquids), but it does suggest that studies that classified adolescent menstrual cycles based solely on salivary P4 using adult cutoffs should be interpreted with caution. Similarly, we found that urine Pd was only a reliable indicator of serum P4 in the mid- to upper range (≈6 to 20 ng/mL). We compared adolescents with historic as opposed to contemporary adult controls because we had access to a large database of daily blood samples across the menstrual cycle in these very well-characterized healthy women. Although assays of serum gonadotropins and sex steroids were conducted in the same laboratory (the Reproductive Endocrine Unit Reference Laboratory at Massachusetts General Hospital), we cannot rule out the possibility that the differences observed between the two age groups may be related, in part, to different assay conditions or to differences in gonadotropin glycosylation patterns. Ultrasounds were performed trans-vaginally in adult controls and trans-abdominally in the current subjects; however, this procedural change was unlikely to introduce bias, as all scans in adolescents and in adults were performed by the same senior ultrasonographer (J.M.A.). Furthermore, antral follicle counts, which require a transvaginal approach, were neither attempted nor relevant to the current study. We studied each subject over 2 months with intensive monitoring but captured only a snapshot of the pattern of changes that occur between menarche and the establishment of mature OVs. Finally, this study was not powered to determine whether chronologic age, gynecologic age, race, or BMI influence cycle dynamics in adolescent girls although each of these questions is of interest.
In conclusion, the current studies demonstrate the heterogeneity of hormone profiles that underlie irregular menses in the early postmenarchal years and highlight immaturity of both FSH secretion and ovarian responses in adolescents. These data suggest further that maturation of all components of the hypothalamic-pituitary-ovarian axis is required to achieve one of the most critical milestones of adolescence: the mature OV of a fertile woman.
Supplementary Material
Acknowledgments
We thank the nurses of the Massachusetts General Hospital Clinical Research Center for their support in conducting these studies, Ms. Johanna Clair and Ms. Annette Rice for assistance in sample collection and/or processing, and the Ligand Assay and Analysis Core at the University of Virginia Center for Research in Reproduction for performing the inhibin B assays.
Financial Support: This work was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES103315; to N.D.S.) and by Grant Number 1UL1TR001102. N.D.S. is also supported as a Lasker Clinical Research Scholar (1SI2ES025429-01). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the NICHD/NIH (P50-HD28934). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Center for Advancing Translational Science or the National Institutes of Health.
Clinical Trial Information: ClinicalTrials.gov no. NCT02486757 (registered 1 July 2015).
Author Contributions: N.D.S. (study PI) designed and implemented the study and supervised all aspects of data collection and analysis. N.D.S. and B.Z.S. wrote the manuscript with editorial contributions by J.E.H. and D.M.U. B.Z.S. carried out the data analysis, provided interpretation, and prepared all graphics. T.K. recruited subjects, coordinated study visits, obtained study samples, and collected and organized the data. J.M.A. performed all ultrasounds and analyzed and interpreted the corresponding data. P.M.S., D.W.C., and D.T.Z. oversaw all laboratory studies. D.M.U. directed statistical analyses; B.Z.S., J.A.M., and D.M.U. conducted all statistical analyses. C.K.W. and J.E.H. provided data on adult controls. C.K.W. assisted in data interpretation. J.E.H. contributed to the current study design, data analysis, and manuscript preparation. All authors read, edited, and approved the final manuscript.
Disclosure Summary: D.W.C. is an employee and shareholder of LabCorp. D.T.Z. owns ZRT Laboratory. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- ANOV
anovulatory cycle
- BMI
body mass index
- CL
corpus luteum
- CMIA
chemiluminescent microparticle immunoassay
- DBS
dried blood spot
- E2
estradiol
- EFP
early follicular phase
- FP
follicular phase
- INHB
inhibin B
- LC/MS
liquid chromatography/tandem mass spectrometry
- LFP
late follicular phase
- LFT
luteal-follicular transition
- LP
luteal phase
- MC
midcycle
- OV
ovulatory cycle
- P4
progesterone
- Pd
pregnanediol
- TT
total testosterone
References
- 1. Rosenfield RL. Clinical review: adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab. 2013;98(9):3572–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flug D, Largo RH, Prader A. Menstrual patterns in adolescent Swiss girls: a longitudinal study. Ann Hum Biol. 1984;11(6):495–508. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization Task Force on Adolescent Reproductive Health World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. Longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. J Adolesc Health Care. 1986;7(4):236–244. [PubMed] [Google Scholar]
- 4. Apter D. Serum steroids and pituitary hormones in female puberty: a partly longitudinal study. Clin Endocrinol (Oxf). 1980;12(2):107–120. [DOI] [PubMed] [Google Scholar]
- 5. Borsos A, Lampé L, Balogh A, Csoknyay J, Ditròi F, Székely P. Ovarian function after the menarche and hormonal contraception. Int J Gynaecol Obstet. 1988;27(2):249–253. [DOI] [PubMed] [Google Scholar]
- 6. Doring GK. The incidence of anovular cycles in women. J Reprod Fertil. 1969;6:77–81. [Google Scholar]
- 7. Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab. 2000;85(3):1021–1025. [DOI] [PubMed] [Google Scholar]
- 8. Lemarchand-Béraud T, Zufferey MM, Reymond M, Rey I. Maturation of the hypothalamo-pituitary-ovarian axis in adolescent girls. J Clin Endocrinol Metab. 1982;54(2):241–246. [DOI] [PubMed] [Google Scholar]
- 9. Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol. 1983;97(2):213–219. [DOI] [PubMed] [Google Scholar]
- 10. Wilson DW, Read GF, Hughes IA, Walker RF, Griffiths K. Hormone rhythms and breast cancer chronoepidemiology: salivary progesterone concentrations in pre- and post-menarchal girls and in normal premenopausal women. Chronobiol Int. 1984;1(2):159–165. [DOI] [PubMed] [Google Scholar]
- 11. Vollman RF. The menstrual cycle. In: Friedman EA, ed. Major Problems in Obstetrics and Gynecology. Vol 7. Philadelphia, PA: W.B. Saunders Company; 1977:11–193. [PubMed] [Google Scholar]
- 12. Vuorento T, Huhtaniemi I. Daily levels of salivary progesterone during menstrual cycle in adolescent girls. Fertil Steril. 1992;58(4):685–690. [PubMed] [Google Scholar]
- 13. Winter JS, Faiman C. The development of cyclic pituitary-gonadal function in adolescent females. J Clin Endocrinol Metab. 1973;37(5):714–718. [DOI] [PubMed] [Google Scholar]
- 14. Zhang K, Pollack S, Ghods A, Dicken C, Isaac B, Adel G, Zeitlian G, Santoro N. Onset of ovulation after menarche in girls: a longitudinal study. J Clin Endocrinol Metab. 2008;93(4):1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod. 2004;19(2):383–392. [DOI] [PubMed] [Google Scholar]
- 16. Rosenfield RL, Barnes RB. Menstrual disorders in adolescence. Endocrinol Metab Clin North Am. 1993;22(3):491–505. [PubMed] [Google Scholar]
- 17. Resko JA, Goy RW, Robinson JA, Norman RL. The pubescent rhesus monkey: some characteristics of the menstrual cycle. Biol Reprod. 1982;27(2):354–361. [DOI] [PubMed] [Google Scholar]
- 18. Dierschke DJ, Weiss G, Knobil E. Sexual maturation in the female rhesus monkey and the development of estrogen-induced gonadotropic hormone release. Endocrinology. 1974;94(1):198–206. [DOI] [PubMed] [Google Scholar]
- 19. Presl J, Horejsi J, Stroufova A, Herzmann J. Sexual maturation in girls and the development of estrogen induced gonadotropic hormone release. Ann Biol Anim Biochim Biophys. 1976;16(3):377–383. [Google Scholar]
- 20. Reiter EO, Kulin HE, Hamwood SM. The absence of positive feedback between estrogen and luteinizing hormone in sexually immature girls. Pediatr Res. 1974;8(8):740–745. [DOI] [PubMed] [Google Scholar]
- 21. Wennink JM, Delemarre-van de Waal HA, Schoemaker R, Schoemaker H, Schoemaker J. Luteinizing hormone and follicle stimulating hormone secretion patterns in girls throughout puberty measured using highly sensitive immunoradiometric assays. Clin Endocrinol (Oxf). 1990;33(3):333–344. [DOI] [PubMed] [Google Scholar]
- 22. Hansen JW, Hoffman HJ, Ross GT. Monthly gonadotropin cycles in premenarcheal girls. Science. 1975;190(4210):161–163. [DOI] [PubMed] [Google Scholar]
- 23. Rovner P, Keltz J, Allshouse A, Isaac B, Hickmon C, Lesh J, Chosich J, Santoro N. Induction of the LH surge in premenarchal girls confirms early maturation of the hypothalamic-pituitary-ovarian axis. Reprod Sci. 2018;25(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics. 2015;136(6):1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall JE, Schoenfeld DA, Martin KA, Crowley WF Jr. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab. 1992;74(3):600–607. [DOI] [PubMed] [Google Scholar]
- 26. Erickson GF, Hsueh AJ, Quigley ME, Rebar RW, Yen SS. Functional studies of aromatase activity in human granulosa cells from normal and polycystic ovaries. J Clin Endocrinol Metab. 1979;49(4):514–519. [DOI] [PubMed] [Google Scholar]
- 27. Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem. 1984;20(1):231–236. [DOI] [PubMed] [Google Scholar]
- 28. Silfen ME, Denburg MR, Manibo AM, Lobo RA, Jaffe R, Ferin M, Levine LS, Oberfield SE. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88(10):4682–4688. [DOI] [PubMed] [Google Scholar]
- 29. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. [DOI] [PubMed] [Google Scholar]
- 30. Feicht CB, Johnson TS, Martin BJ, Sparkes KE, Wagner WW Jr. Secondary amenorrhoea in athletes. Lancet. 1978;2(8100):1145–1146. [DOI] [PubMed] [Google Scholar]
- 31. Shaw ND, Butler JP, Nemati S, Kangarloo T, Ghassemi M, Malhotra A, Hall JE. Accumulated deep sleep is a powerful predictor of LH pulse onset in pubertal children. J Clin Endocrinol Metab. 2015;100(3):1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sluss PM, Hayes FJ, Adams JM, Barnes W, Williams G, Frost S, Ramp J, Pacenti D, Lehotay DC, George S, Ramsay C, Doss RC, Crowley WF Jr. Mass spectrometric and physiological validation of a sensitive, automated, direct immunoassay for serum estradiol using the Architect. Clin Chim Acta. 2008;388(1-2):99–105. [DOI] [PubMed] [Google Scholar]
- 33. Bui HN, Sluss PM, Blincko S, Knol DL, Blankenstein MA, Heijboer AC. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids. 2013;78(1):96–101. [DOI] [PubMed] [Google Scholar]
- 34. Du JY, Sanchez P, Kim L, Azen CG, Zava DT, Stanczyk FZ. Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum, whole blood, saliva, and capillary blood. Menopause. 2013;20(11):1169–1175. [DOI] [PubMed] [Google Scholar]
- 35. Edelman A, Stouffer R, Zava DT, Jensen JT. A comparison of blood spot vs. plasma analysis of gonadotropin and ovarian steroid hormone levels in reproductive-age women. Fertil Steril. 2007;88(5):1404–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu Q, Chen J, Wei Z, Brandon TR, Zava DT, Shi YE, Cao Y. Sex hormone metabolism and threatened abortion. Med Sci Monit. 2017;23:5041–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glaser RL, Zava DT, Wurtzbacher D. Pilot study: absorption and efficacy of multiple hormones delivered in a single cream applied to the mucous membranes of the labia and vagina. Gynecol Obstet Invest. 2008;66(2):111–118. [DOI] [PubMed] [Google Scholar]
- 38. Hackelöer BJ, Sallam HN. Ultrasound scanning of ovarian follicles. Clin Obstet Gynaecol. 1983;10(3):603–620. [PubMed] [Google Scholar]
- 39. O’Herlihy C, De Crespigny LJ, Robinson HP. Monitoring ovarian follicular development with real-time ultrasound. Br J Obstet Gynaecol. 1980;87(7):613–618. [DOI] [PubMed] [Google Scholar]
- 40. Queenan JT, O’Brien GD, Bains LM, Simpson J, Collins WP, Campbell S. Ultrasound scanning of ovaries to detect ovulation in women. Fertil Steril. 1980;34(2):99–105. [PubMed] [Google Scholar]
- 41. Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57(4):792–796. [DOI] [PubMed] [Google Scholar]
- 42. Filicori M, Santoro N, Merriam GR, Crowley WF Jr. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(6):1136–1144. [DOI] [PubMed] [Google Scholar]
- 43. O’Connor KA, Brindle E, Miller RC, Shofer JB, Ferrell RJ, Klein NA, Soules MR, Holman DJ, Mansfield PK, Wood JW. Ovulation detection methods for urinary hormones: precision, daily and intermittent sampling and a combined hierarchical method. Hum Reprod. 2006;21(6):1442–1452. [DOI] [PubMed] [Google Scholar]
- 44. Moghissi KS, Syner FN, Evans TN. A composite picture of the menstrual cycle. Am J Obstet Gynecol. 1972;114(3):405–418. [DOI] [PubMed] [Google Scholar]
- 45. Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect. 1996;104(4):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taylor AE, Whitney H, Hall JE, Martin K, Crowley WF Jr. Midcycle levels of sex steroids are sufficient to recreate the follicle-stimulating hormone but not the luteinizing hormone midcycle surge: evidence for the contribution of other ovarian factors to the surge in normal women. J Clin Endocrinol Metab. 1995;80(5):1541–1547. [DOI] [PubMed] [Google Scholar]
- 47. Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84(1):105–111. [DOI] [PubMed] [Google Scholar]
- 48. Adams JM, Taylor AE, Crowley WF Jr, Hall JE. Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab. 2004;89(9):4343–4350. [DOI] [PubMed] [Google Scholar]
- 49. Randolph JF Jr, Harlow SD, Helmuth ME, Zheng H, McConnell DS. Updated assays for inhibin B and AMH provide evidence for regular episodic secretion of inhibin B but not AMH in the follicular phase of the normal menstrual cycle. Hum Reprod. 2014;29(3):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu JH, Yen SS. Induction of midcycle gonadotropin surge by ovarian steroids in women: a critical evaluation. J Clin Endocrinol Metab. 1983;57(4):797–802. [DOI] [PubMed] [Google Scholar]
- 51. Simon JA, Bustillo M, Thorneycroft IH, Cohen SW, Buster JE. Variability of midcycle estradiol positive feedback: evidence for unique pituitary responses in individual women. J Clin Endocrinol Metab. 1987;64(4):789–793. [DOI] [PubMed] [Google Scholar]
- 52. Mikolajczyk RT, Stanford JB, Ecochard R. Multilevel model to assess sources of variation in follicular growth close to the time of ovulation in women with normal fertility: a multicenter observational study. Reprod Biol Endocrinol. 2008;6(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fieller EC. Some problems in interval estimation. J R Stat Soc B. 1954;16:175–185. [Google Scholar]
- 54. Jones GE. Some newer aspects of the management of infertility. J Am Med Assoc. 1949;141(16):1123–1129, illust. [DOI] [PubMed] [Google Scholar]
- 55. Sonntag B, Ludwig M. An integrated view on the luteal phase: diagnosis and treatment in subfertility. Clin Endocrinol (Oxf). 2012;77(4):500–507. [DOI] [PubMed] [Google Scholar]
- 56. Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, Pena A, Horikawa R, Gomez-Lobo V, Joel D, Tfayli H, Arslanian S, Dabadghao P, Garcia Rudaz C, Lee PA. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83(6):376–389. [DOI] [PubMed] [Google Scholar]
- 57. Apter D, Viinikka L, Vihko R. Hormonal pattern of adolescent menstrual cycles. J Clin Endocrinol Metab. 1978;47(5):944–954. [DOI] [PubMed] [Google Scholar]
- 58. Filicori M, Butler JP, Crowley WF Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73(6):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grumbach MM, Roth JC, Kaplan SL, Kelch RP. Hypothalamic-pituitary regulation of puberty in man: evidence and concepts derived from clinical research In: Grumbach MM, Grave GD, Mayer FE, eds. Control of the Onset of Puberty. New York, NY: Wiley; 1974:115–166. [Google Scholar]
- 60. Kulin HE. The maturation of ovulatory potential in man. Horm Res. 1980;12(1):46–62. [DOI] [PubMed] [Google Scholar]
- 61. Apter D, Räisänen I, Ylöstalo P, Vihko R. Follicular growth in relation to serum hormonal patterns in adolescent compared with adult menstrual cycles. Fertil Steril. 1987;47(1):82–88. [DOI] [PubMed] [Google Scholar]
- 62. Cabral ZA, de Medeiros SF. Follicular growth pattern in normal-cycling Brazilian adolescents. Fertil Steril. 2007;88(6):1625–1631. [DOI] [PubMed] [Google Scholar]
- 63. Hayes A, Johanson A. Excretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in urine by pubertal girls. Pediatr Res. 1972;6(1):18–25. [DOI] [PubMed] [Google Scholar]
- 64. Penny R, Olambiwonnu NO, Frasier SD. Episodic fluctuations of serum gonadotropins in pre- and post-pubertal girls and boys. J Clin Endocrinol Metab. 1977;45(2):307–311. [DOI] [PubMed] [Google Scholar]
- 65. Venturoli S, Porcu E, Fabbri R, Paradisi R, Ruggeri S, Bolelli G, Orsini LF, Gabbi D, Flamigni C. Menstrual irregularities in adolescents: hormonal pattern and ovarian morphology. Horm Res. 1986;24(4):269–279. [DOI] [PubMed] [Google Scholar]
- 66. Venturoli S, Porcu E, Fabbri R, Magrini O, Gammi L, Paradisi R, Flamigni C. Longitudinal evaluation of the different gonadotropin pulsatile patterns in anovulatory cycles of young girls. J Clin Endocrinol Metab. 1992;74(4):836–841. [DOI] [PubMed] [Google Scholar]
- 67. Lunenfeld B, Kraiem Z, Eshkol A, Werner-Zodrow I. The ovary learns to ovulate. J Biosoc Sci Suppl. 1978;10(5):27–42. [DOI] [PubMed] [Google Scholar]
- 68. Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McNeely MJ, Soules MR. The diagnosis of luteal phase deficiency: a critical review. Fertil Steril. 1988;50(1):1–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.