Nephrotoxicity is the major limiting factor for the clinical use of vancomycin (VCM) for treatment of serious infections caused by multiresistant Gram-positive bacteria. This study investigated the renal protective activity of rutin in a rat model of VCM-induced kidney injury in male Wistar rats.
KEYWORDS: NF-κB, nephrotoxicity, oxidative stress, rutin, vancomycin
ABSTRACT
Nephrotoxicity is the major limiting factor for the clinical use of vancomycin (VCM) for treatment of serious infections caused by multiresistant Gram-positive bacteria. This study investigated the renal protective activity of rutin in a rat model of VCM-induced kidney injury in male Wistar rats. VCM administered intraperitoneally at 200 mg/kg twice daily for 7 successive days resulted in significant elevation of blood urea nitrogen and creatinine, as well as urinary N-acetyl-β-D-glucosaminidase. Coadministration of VCM with oral rutin at 150 mg/kg significantly reduced these markers of kidney damage. Rutin also significantly attenuated VCM-induced oxidative stress, inflammatory cell infiltration, apoptosis, and decreased interleukin-1β and tumor necrosis factor alpha levels (all P < 0.05 or 0.01) in kidneys. Renal recovery from VCM injury was achieved by rutin through increases in Nrf2 and HO-1 and a decrease in NF-κB expression. Our results demonstrated a protective effect of rutin on VCM-induced kidney injury through suppression of oxidative stress, apoptosis, and downregulation of the inflammatory response. This study highlights a role for oral rutin as an effective intervention to ameliorate nephrotoxicity in patients undergoing VCM therapy.
INTRODUCTION
Vancomycin (VCM) is a glycopeptide antibiotic commonly used to treat methicillin-resistant Gram-positive bacteria, especially Staphylococcus aureus (1). However, between 5 and 35% of all VCM-treated patients suffer some form of nephrotoxicity (2–5). Current animal experiments suggest that VCM alters energy-dependent renal reabsorption in the proximal tubule that leads to mitochondrial dysfunction and that coadministration of antioxidants can prevent nephrotoxicity (6–12).
Reactive oxygen species (ROS) have been implicated in models of toxic renal failure due to cisplatin and aminoglycoside exposure. These compounds, as well as VCM, perturb proximal renal tubule epithelial cells that are the sites of reabsorption (6). The affects attributed to VCM involve renal tubular cell apoptosis through an increase in mitochondrial superoxide production, leading to a loss of mitochondrial membrane potential and elevated caspase activity (13). The filtration and energy transport mechanisms in the proximal tubule epithelia render the kidney highly susceptible to toxicant-induced renal injury (14). For instance, one of the most sensitive biomarkers of cell injury to the proximal tubules is the excretion of urinary N-acetyl-β-d-glucosaminidase (NAG) (15). Urinary NAG activity is also a sensitive indicator for glycopeptide antibiotic-induced nephrotoxicity (16).
Rutin is a citrus flavonoid glycoside consisting of the flavonol quercetin and the disaccharide rutinose (17). This polyphenolic bioflavonoid possesses a wide range of pharmacological activities and has substantial antioxidant properties. It has been utilized as an antioxidant as well as an anticancer, anti-inflammatory, antidiabetic and antihyperpietic agent (18). Its toxicity is significantly less than other bioflavonoids, and it is a nonoxidizable molecule. These qualities suggest that rutin could be a highly potent and relatively safe antioxidant.
Rutin supplementation in experimental diets has demonstrated its in vivo antioxidant, anti-inflammatory, antiapoptotic, and antiautophagic properties and has been used to mitigate gentamicin-induced nephrotoxicity in a rat model (19). This protective effect has been linked to NF-κB, tumor necrosis factor alpha (TNF-α), and Bcl-2 against benzo(a)pyrene-induced lung toxicity and genotoxicity in mice (20). Rutin administration also lowered plasma glucose and decreased oxidative stress via the Nrf2 signaling pathway in a rat model of diabetic neuropathy (21). Coincidently, rutin significantly increased NF-E2-related factor 2 (Nrf2) and heme oxygenase (HO-1) expression in biliary obstruction-induced liver injury in a rat model (22). A protective effect of rutin was also demonstrated in a rheumatoid arthritis model that involved the downregulation of the NF-κB p65 protein (19).
In the present study, activities of rutin against VCM-induced nephrotoxicity, along with potential molecular mechanisms for its mode of action were evaluated in a rat model.
RESULTS
Assessment of kidney function.
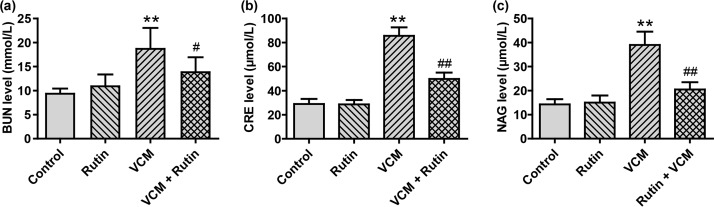
Serum blood urea nitrogen (BUN) and creatinine (CRE) levels have been reported as an initial indication of VCM-induced nephrotoxicity (7). We found no differences between groups 1 and 2. However, both BUN and CRE levels in the VCM-treated rats (group 3) were significantly increased compared to groups 1 and 2 (P < 0.01). Interestingly, BUN and CRE levels in group 4 (VCM plus rutin) were significantly decreased in comparison with group 3 (P < 0.05 and P < 0.01, respectively) but still higher than controls (Fig. 1a and b). In addition, urinary NAG excretion in group 3 was higher than in controls (P < 0.01), and rutin coadministration significantly reduced urinary NAG activity (P < 0.01) (Fig. 1c).
FIG 1.
Rutin attenuates VCM-induced nephrotoxicity in rats. (a to c) Serum BUN, CRE, and urinary NAG levels, respectively, in rats treated with VCM in the presence or absence of rutin. The results are presented as group means ± the SD (n = 10 in each group). **, P < 0.01 compared to group 1; # and ##, P < 0.05 and P < 0.01, respectively, compared to the VCM treatment group.
Rutin ameliorates VCM-induced oxidative stress in kidney tissue.
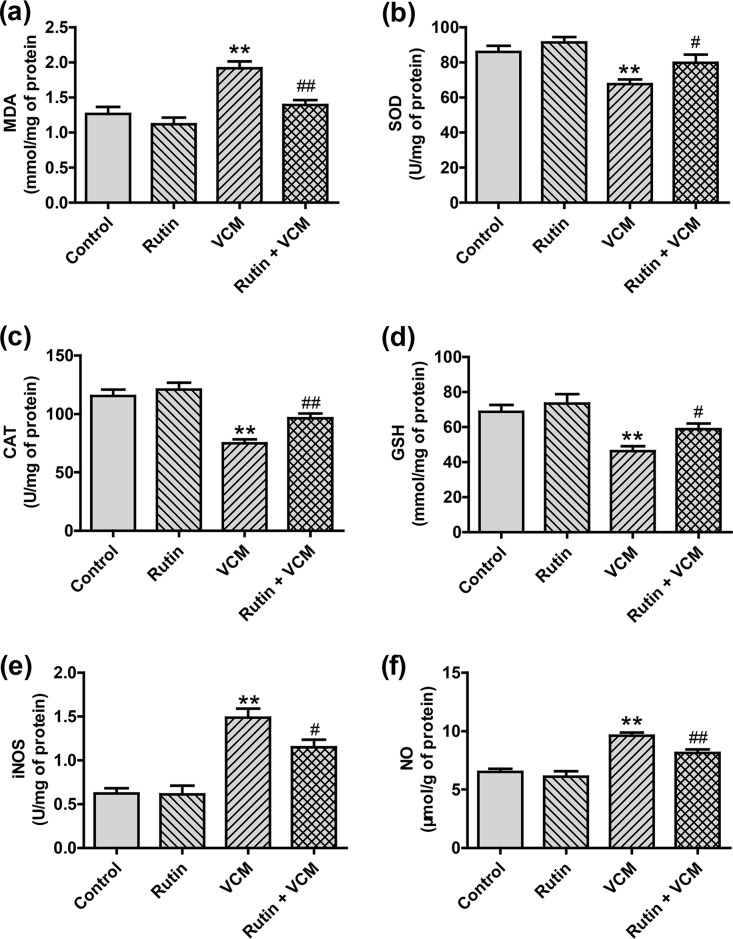
In rats treated with VCM alone (group 3), we found a significant induction of oxidative stress in kidneys with a marked increase in malondialdehyde (MDA), inducible nitric oxide synthase (iNOS), and nitric oxide (NO) levels (all P < 0.01). This was accompanied by significant decreases in superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) activities (all P < 0.01). In the rutin-plus-VCM group 4, the oxidative stress markers were significantly attenuated (P < 0.05 and P < 0.01, respectively). These oxidative stress indicators did not noticeably change between the rutin and control groups (Fig. 2).
FIG 2.
Impact of rutin on the levels of oxidative and nitrosative stress markers in the kidney tissues of rats treated with VCM. The results are presented as group means ± the SD (n = 10 in each group). **, P < 0.01 compared to group 1; # and ##, P < 0.05 and P < 0.01, respectively, compared to the VCM treatment group.
Rutin attenuates VCM-induced activation of caspase-9 and -3 and inflammatory mediators in kidneys.
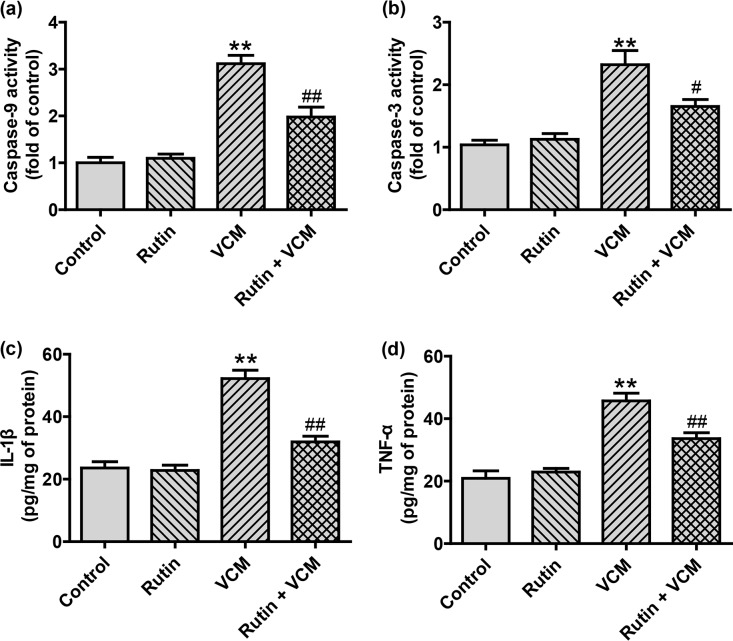
Because VCM can activate renal caspase-9 and -3, we examined whether rutin can alter this process or not (23). Rats that received VCM alone (group 3) had significant increases in caspase-9 (3.14-fold) and caspase-3 (2.36-fold) activities (both P < 0.01) compared to control group 1. Rutin prophylaxis resulted in a marked attenuation of VCM-induced caspase activation caspase-9 levels decreased by 1.97-fold and caspase-3 decreased by 1.66-fold compared to VCM group 3. The administration of rutin alone (group 2) did not alter caspase levels from control values (group 1) (Fig. 3a and b).
FIG 3.
Rutin attenuates VCM-induced activation of caspase-9 (a) and caspase-3 (b) and the inflammatory mediators IL-1β (c) and TNF-α (d) in the kidneys of rats treated with VCM. ELISA results are presented as group means ± the SD (n = 10 for each group). **, P < 0.01 compared to group 1; # and ##, P < 0.05 and P < 0.01, respectively, compared to the VCM treatment group.
The levels of the inflammatory mediators intereleukin-1β (IL-1β) and TNF-α were also significantly increased by VCM treatment. However, rutin coadministration with VCM decreased IL-1β 38% and TNF-α 27% (Fig. 3c and d).
Histopathological evaluation.
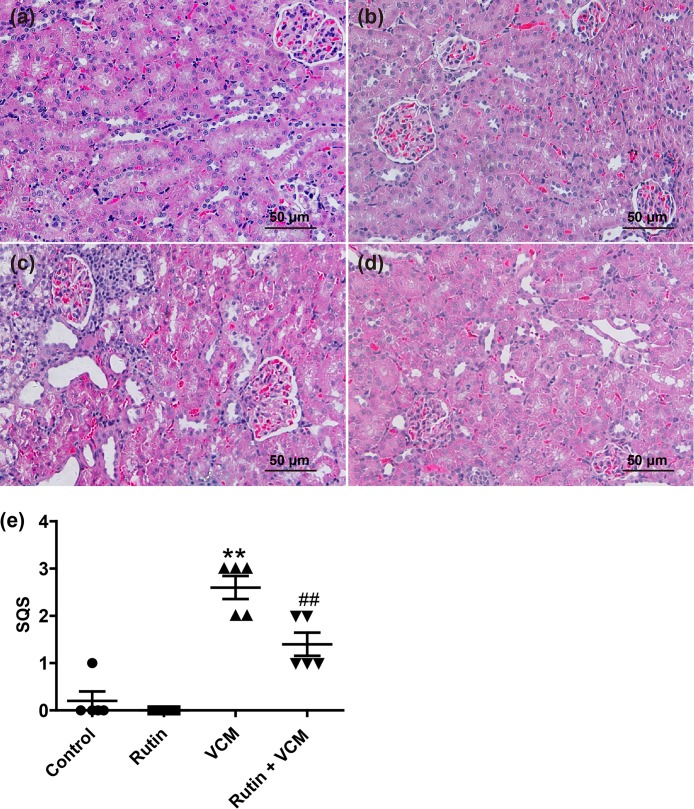
Kidney histopathological examination showed that rutin prophylaxis markedly attenuated VCM-induced tissue damage (Fig. 4). The kidney tissues of rats treated with VCM alone displayed extensive damage that included tubular degeneration, tubular dilation, necrosis, cast formation, and infiltration of inflammatory cells (Fig. 4c). Rutin prophylaxis attenuated the VCM-induced kidney damage, as evidenced by a marked attenuation of the infiltration of inflammatory cells and decreased tubular necrosis in the renal cortex (Fig. 4d). A semiquantitative scoring reinforced these findings and revealed a significant attenuation of damage with rutin coadministration with VCM (P < 0.01) (Fig. 4e). There were no marked histopathological changes in the rutin alone group, and the histology was similar to the saline vehicle control group 1 (Fig. 4a and b).
FIG 4.
Representative histopathological changes in kidneys of rats treated with VCM and rutin. (a to d) H&E-stained rat kidney sections from control group 1 (a), rutin group 2 (b), VCM group 3 (c), and the rutin-plus-VCM group (d). (e) Semiquantitative scores of kidney damage (group means ± the SD, n = 5). **, P < 0.01 compared to group 1; ##, P < 0.01 compared to the VCM treatment group.
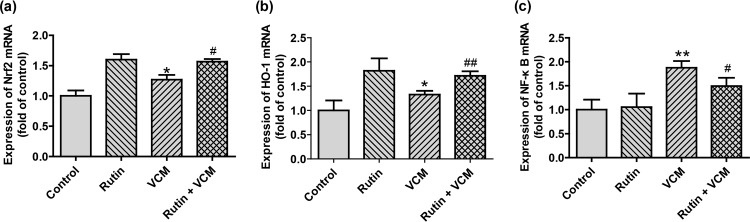
Expression of HO-1, Nrf2, and NF-κB mRNA in kidney tissues.
To examine the effects of rutin more closely, we measured steady-state mRNA levels of genes that are induced in kidneys by VCM. Both Nrf2 and HO-1 mRNAs were markedly increased in the VCM group. Interestingly, these levels were even greater in all rutin-treated groups compared to that in the saline-treated control rats (Fig. 5) (P < 0.05 and P < 0.01, respectively). In contrast, the expression of NF-κB mRNA was significantly decreased in the VCM-plus-rutin group compared to the VCM group. Unlike the results of upregulation of Nrf2 and HO-1, NF-κB displayed no marked changes following rutin treatment.
FIG 5.
(a to c) Steady-state mRNA levels of Nrf2 (a), HO-1 (b), and NF-κB (c) in rat kidney samples. Values are presented as group means ± the SD (n = 10 in each group). * and **, P < 0.05 and P < 0.01, respectively, compared to group 1; # and ##, P < 0.05 and P < 0.01, respectively, compared to the VCM treatment group.
DISCUSSION
VCM is used for treatment of serious infections, and its therapeutic effects are significantly improved by dose escalation. However, high-dose therapy with VCM is limited by its cumulative risk of nephrotoxicity (24). Although there are studies indicating the molecular mechanisms of VCM nephrotoxicity, no definitive conclusions have been drawn. Accordingly, the development of strategies to avoid this unwanted side effect is of the utmost importance.
Rutin is an active citrus flavonoid compound that possesses a variety of pharmacological activities (25, 26). In the present study, we provided evidence that oral rutin prophylaxis significantly reduces VCM-induced nephrotoxicity in a rat model. Rats that were coadministered oral rutin (150 mg/kg) had decreased serum BUN and CRE levels. This indicated that rutin decreased the overall damage caused by high-dose VCM. This was corroborated by a decrease in kidney damage (Fig. 1 and 4). In addition, the significant increase in urinary NAG excretion detected after VCM administration was neutralized with rutin coadministration (Fig. 2). There are newer glycopeptide biomarkers for acute kidney injury that are more sensitive than NAG, and these can be tested in future trials of VCM-induced tubular injury (27–29).
Previous studies have indicated that oxidative stress plays a critical role in VCM-induced kidney damage in cultured proximal tubular cells and in vivo in rats (30). In support of this, we found decreased SOD and CAT activities and lower levels of GSH in the kidneys of VCM-treated rats. Moreover, the peroxidized lipid decomposition marker MDA and nitrosative stress-related NO and iNOS activities were significantly elevated in the kidney tissues of the VCM-treated rats. Pretreatment with rutin strongly diminished damage from both these factors, indicating that oral rutin has a nephroprotective effect (Fig. 2).
Nrf2 is a transcription factor that centrally regulates oxidative stress response genes (31). The upregulation of Nrf2 effectively prolongs the cellular oxidative damage response by activating genes that provide phase II detoxifying enzymes and antioxidant enzymes, including CAT, SOD, HO-1, and glutathione peroxidase (32). In the present study, we found that rutin treatment increased Nrf2 mRNA levels, as well as one of its downstream target genes HO-1. Rutin also increased SOD and CAT activities (Fig. 2). These data indicated that the Nrf2/HO-1 pathway contributes to the nephroprotective effect of rutin against VCM.
Previous studies have shown that rutin coadministration alleviated VCM-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Rutin also ameliorated intestinal toxicity induced by methotrexate linked with antioxidative and anti-inflammatory effects (33, 34). Furthermore, testicular degeneration and damage and decreased sperm viability due to cisplatin treatment in rats was attenuated by rutin (30). In contrast, the NF-κB p65 subunit can inhibit the Nrf2-antioxidant response element pathway at the transcriptional level by depriving the CREB binding protein from the Nrf2 promoter and recruiting histone deacetylase 3 to MafK (35). Our results indicated that the activation of the Nrf2/HO-1 pathway contributes to the ability of rutin to inhibit the NF-κB-mediated inflammatory response (Fig. 5).
Rutin is also an effective antioxidant flavonoid that prevents the adverse effects of ethanol on cadmium-induced oxidative stress by increasing glutathione and glutathione peroxidase activities in the testes of adult rats (36). Rutin was a significant inhibitor of iron-dependent lipid peroxidation systems by acting as an iron chelator that formed an inert iron complex unable to initiate lipid peroxidation. Rutin was able to suppress free radical processes at three stages: the formation of superoxide ion, the generation of hydroxyl (or crypto-hydroxyl) radicals in the Fenton reaction, and the formation of lipid peroxy radical (37). Furthermore, rutin effectively protected the human hepatoma cell line from H2O2-induced oxidative stress and apoptosis in a dose-dependent manner. This ability involved mechanisms related to the regulation of ROS production, the inhibition of lipid peroxidation, the protection of the intracellular antioxidant system, and its modulation of the Bcl-2/Bax and NF-кB/p65 signaling pathways (38). Overall, the direct radical scavenging activity of rutin and its ability to increase the resistance of the kidneys by activating their intrinsic antioxidant defense mechanisms are all activities attributed to rutin and are responsible for dampening the VCM-induced nephrotoxicity.
Previous studies identified that the mitochondrial, oxidative stress, and PAR and PARP-1 protein expression pathways are involved in VCM-induced nephrotoxicity in rats and in human kidney proximal tubular cells (30, 39). Both the intrinsic (mitochondrial) and the extrinsic (death receptor) pathways could activate the key apoptotic mediator caspase-3 (40). Caspase-9 plays a key role in the mitochondrial apoptosis pathway (40). We have demonstrated that rutin supplementation significantly attenuated VCM-induced increased activity of caspase-3 and -9 in kidney tissues. Indeed, several other studies have indicated that the majority of pharmacological activities of rutin are closely related to its role in modulating mitochondrial function and dynamics (41, 42).
Inflammation is an additional factor that facilitated VCM-induced nephrotoxicity (43). NF-κB is a crucial transcriptional mediator of the proinflammatory cytokine response that is regulated by ROS and plays a pivotal part in damage to tubular epithelial cells (44). In the present study, we found that VCM-evoked inflammatory responses in kidneys resulted in infiltration of inflammatory cells and elevated NF-κB expression, as well as the production of the proinflammatory cytokines IL-1β and TNF-α (Fig. 3c and d). Rutin supplementation markedly diminished NF-κB expression and attenuated IL-1β and TNF-α generation to control levels (Fig. 3c and d). Coincidently, rutin suppressed the production of TNF-α and IL-6 and the activation of NF-κB and extracellular signal-regulated kinases 1 and 2 (ERK1/2) by the high-mobility group box 1 (HMGB) factor. This indicates that rutin could be a candidate therapeutic agent for treatment of various severe vascular inflammatory diseases (45). In order to improve anti-inflammatory activity of the rutin, it was microencapsulated in a chitosan matrix using the spray-drying technique, and the resulting drug delivery system was investigated (46). We used a high dose of the nephrotoxic agent VCM, rending a poor clinical relevance to this particular study. Our data demonstrate that a high dose of rutin provides valuable protection against VCM nephrotoxicity, while developing new drug delivery systems to improve bioavailability could be a great approach. Notably, various formulations such as a rutin-loaded nanostructured lipid carrier have improved the stability and bioavailability of oral rutin (47).
In conclusion, we found that rutin had significant prophylactic effects on VCM-evoked nephrotoxicity. Rutin inhibited oxidative/nitrosative stress, inflammation, and apoptosis in the kidneys of rats. Rutin did not have any undesirable effects on normal rats, indicating that this compound should be considered as a candidate of novel agents to mitigate the nephrotoxic effects of VCM therapy.
MATERIALS AND METHODS
Chemicals.
Abbott Laboratories (North Chicago, IL) provided vancomycin. Rutin (purity 95%), Tris-HCl, EDTA-Na2, sucrose, and saline were purchased from Sigma (St. Louis, MO).
Animal experiments.
This animal study followed the Guidelines of Experimental Animal Care issued by the Animal Welfare and Research Ethics Committee at Qingdao Agriculture University. We used male Wistar rats weighing 250 to 300 g (Vital River Animal Technology, Beijing, China). The rats were kept in a controlled environment at 23 ± 2°C with humidity at 60% ± 5% and a controlled light cycle (12-h light/12-h dark). The rats were acclimated to this environment for 5 days prior to the start of the experiments and had free access to food and water during the experiments.
The animals were randomly divided into four groups (n = 10 in each group). Group 1 served as control that received intraperitoneal (i.p.) injections of physiological saline at 1 ml/kg. Group 2 was the rutin control group that was administered rutin (150 mg/kg, p.o.). Group 3 was the VCM group that received VCM (200 mg/kg, i.p. twice daily), and group 4 was treated with rutin (150 mg/kg, p.o.) 2 h prior to VCM (200 mg/kg, i.p., twice daily) administration. All groups were treated for 7 consecutive days (6, 19). At 12 h following the last dose, the rats were transferred to metabolic cages for spontaneous micturition to collect urine samples. Rats were then euthanized by i.p. sodium pentobarbital injection (80 mg/kg; Sigma-Aldrich, St. Louis, MO). Blood samples were obtained from renal veins into polypropylene centrifuge tubes and allowed to clot spontaneously at room temperature. Serum was then separated by centrifugation at 2,000 × g at 4°C for 10 min and stored at –80°C until analysis. CRE, BUN, and urinary NAG levels were assayed using commercial assay kits according to the manufacturer’s instructions (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). Kidney tissue samples were collected, either formalin fixed or frozen for biochemical, histopathological, enzyme-linked immunosorbent assay (ELISA), and quantitative RT-PCR (qRT-PCR) analysis as described below.
Measurement of markers of oxidative stress in kidney tissues.
Sections of kidney tissue were homogenized in 9 volumes of cold Tris buffer (0.01 M Tris-HCl, 0.1 mM EDTA-Na2, 0.01 M sucrose, 0.9% saline [pH 7.4]) and a 10% (wt/vol) tissue homogenate was prepared. The supernatant obtained from 3,000 × g at 4°C for 15 min of centrifugation was used to measure MDA, CAT, NO, iNOS, GSH, and SOD using commercial assay kits (Nanjing Jiancheng). Protein concentrations were measured using a bicinchoninic acid protein assay kit (Beyotime, Haimen, China).
Histopathological examination.
For light microscopy examination of histological sections, the right kidneys from 5 rats in each group were processed and fixed in 10% neutral buffered formalin. The formalin fixed tissue was embedded in paraffin, sliced into 4-μm tissue sections and stained with hematoxylin-eosin (H&E) (48, 49). Five coded sections from each group were evaluated blindly by a pathologist who was not revealed of the treatment scheme implemented as described previously (50). A semiquantitative evaluation of kidney injury was conducted and a semiquantitative score was given to grade the pathological changes severity for each kidney sample. Tissues were examined for tubular epithelial alterations (dilatation, desquamation, vacuolization, and casts) and interstitial inflammatory cell infiltration. All histopathological parameters were graded as follows: 0, no meaningful histopathological damage; +1, mild degree of damage; +2, mild to moderate degree of damage; +3, moderate degree of damage; +4, moderate to severe degree of damage; and +5, severe degree of damage.
Measurement of caspase-9 and -3 activities and TNF-α and IL-1β levels.
The supernatants (stored at –80°C) were used to determine the activities of caspase-3 and caspase-9 (Nanjing Jian Cheng Biotechnology Co., Ltd., Nanjing, China) and TNF-α and IL-1β (R&D Systems, Minneapolis, MN) using commercial ELISA kits according to manufacturers’ instructions (51).
qRT-PCR analysis.
For the determination of mRNA levels of genes of interest, total RNA from kidney tissue samples was isolated using TRIzol reagent (Life Technologies, Grand Island, NY) following manufacturer’s instructions. Total RNA (2 µg) was reverse transcribed using a Prime Script RT-PCR kit (TaKaRa, Dalian, China). The quality of RNA was verified by measuring the OD at 260/280 nm. Primer sets used for amplification of target genes are detailed in Table 1. Real-time qRT-PCR was performed using an AB7500 real-time PCR instrument (Applied Biosystems, Foster City, CA). Target genes expression was analyzed relative to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) levels.
TABLE 1.
Primer sequences used for real-time qRT-PCR
| Gene | Primer sequence (5′–3′) |
|---|---|
| Nrf2 | CAC ATT CCC AAA CAA GAT GC |
| TCT TTT TCC AGC GAG GAG AT | |
| HO-1 | CGT GCT CGA ATG AAC ACT CT |
| GGA AGC TGA GAG TGA GGA CC | |
| NF-кB | CAC TGT CTG CCT CTC TCG TCT |
| AAG GAT GTC TCC ACA CCA CTG | |
| GAPDH | ACA GTC CAT GCC ATC ACT GCC |
| GCC TGC TTC ACC ACC TTC TTG |
Statistical analyses.
Values are presented as means ± the standard deviation (SD) of at least five experiments. Data were analyzed using SPSS v15.0 (SPSS, Inc., Chicago, IL), and the differences between groups were compared via one-way analysis of variance, followed by Dunnett’s multiple-comparison tests. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Plan (2016YFD0501309) and the Startup and Innovation Leader Talent Plan of Qingdao [15-10-3-15-(41)-zch].
The authors declare no conflicting financial interest.
REFERENCES
- 1.Ingram P, Lye D, Tambyah P, Goh W, Tam V, Fisher D. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother 62:168–171. doi: 10.1093/jac/dkn080. [DOI] [PubMed] [Google Scholar]
- 2.Moellering R. 2006. Vancomycin: a 50-year reassessment. Clin Infect Dis 42:S3–S4. doi: 10.1086/491708. [DOI] [PubMed] [Google Scholar]
- 3.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. 2012. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations: a literature review. Eur J Clin Pharmacol 68:1243–1255. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 4.Lodise T, Lomaestro B, Graves J, Drusano G. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elting L, Rubenstein E, Kurtin D, Rolston K, Fangtang J, Martin C, Raad I, Whimbey E, Manzullo E, Bodey G. 1998. Mississippi mud in the 1990s: risks and outcomes of vancomycin-associated toxicity in general oncology practice. Cancer 83:2597–2607. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Ogino T, Inoue M, Okada S, Kinoshita H. 2003. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 37:373–379. doi: 10.1080/1071576031000061002. [DOI] [PubMed] [Google Scholar]
- 7.Oktem F, Arslan M, Ozguner F, Candir O, Yilmaz H, Ciris M, Uz E. 2005. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 215:227–233. doi: 10.1016/j.tox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Hodoshima N, Nakano Y, Izumi M, Mitomi N, Nakamura Y, Aoki M, Gyobu A, Shibasaki S, Kurosawa T. 2004. Protective effect of inactive ingredients against nephrotoxicity of vancomycin hydrochloride in rats. Drug Metab Pharmacokinet 19:68–75. doi: 10.2133/dmpk.19.68. [DOI] [PubMed] [Google Scholar]
- 9.Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut H. 2005. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin Pharmacol Toxicol 97:325–332. doi: 10.1111/j.1742-7843.2005.pto_153.x. [DOI] [PubMed] [Google Scholar]
- 10.Abraham N, Asija A, Drummond G, Peterson S. 2007. Heme oxygenase-1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther 7:89–108. doi: 10.2174/156652307780363134. [DOI] [PubMed] [Google Scholar]
- 11.Dieterich C, Puey A, Lin S, Lyn S, Swezey R, Furimsky A, Fairchild D, Mirsalis J, Ng H. 2009. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci 107:258–269. doi: 10.1093/toxsci/kfn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanos V, Cataldi L. 2001. Renal transport of antibiotics and nephrotoxicity: a review. J Chemother 13:461–472. doi: 10.1179/joc.2001.13.5.461. [DOI] [PubMed] [Google Scholar]
- 13.Arimura Y, Yano T, Hirano M, Sakamoto Y, Egashira N, Oishi R. 2012. Mitochondrial superoxide production contributes to vancomycin-induced renal tubular cell apoptosis. Free Radic Biol Med 52:1865–1873. doi: 10.1016/j.freeradbiomed.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, Nishikawa M, Sato E, Matsuno K, Sasaki J. 1999. Synthesis of superoxide dismutase derivative that specifically accumulates in renal proximal tubule cells. Arch Biochem Biophys 368:354–360. doi: 10.1006/abbi.1999.1329. [DOI] [PubMed] [Google Scholar]
- 15.Miyakita H, Puri P. 1994. Urinary levels of N-acetyl-β-d-glucosaminidase: a simple marker for predicting tubular damage in higher grades of vesicoureteric reflux. Eur Urol 25:135–137. [DOI] [PubMed] [Google Scholar]
- 16.Yoshiyama Y, Yazaki T, Wong P, Beauchamp D, Kanke M. 2001. The effect of fosfomycin on glycopeptide antibiotic-induced nephrotoxicity in rats. J Infect Chemother 7:243–246. doi: 10.1007/s101560100043. [DOI] [PubMed] [Google Scholar]
- 17.Calabrò M, Tommasini S, Donato P, Stancanelli R, Raneri D, Catania S, Costa C, Villari V, Ficarra P, Ficarra R. 2005. The rutin/beta-cyclodextrin interactions in fully aqueous solution: spectroscopic studies and biological assays. J Pharm Biomed Anal 36:1019–1027. doi: 10.1016/j.jpba.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Hertog M, Hollman P, Katan M, Kromhout D. 1993. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer 20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 19.Kandemir FM, Ozkaraca M, Yildirim BA, Hanedan B, Kirbas A, Kilic K, Aktas E, Benzer F. 2015. Rutin attenuates gentamicin-induced renal damage by reducing oxidative stress, inflammation, apoptosis, and autophagy in rats. Renal Failure 37:518–525. doi: 10.3109/0886022X.2015.1006100. [DOI] [PubMed] [Google Scholar]
- 20.Shahid A, Ali R, Ali N, Hasan S, Rashid S, Majed F, Sultana S. 2016. Attenuation of genotoxicity, oxidative stress, apoptosis, and inflammation by rutin in benzo(a)pyrene exposed lungs of mice: plausible role of NF-κB, TNF-α, and Bcl-2. J Complement Integr Med 13:17–29. doi: 10.1515/jcim-2015-0078. [DOI] [PubMed] [Google Scholar]
- 21.Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, Chen X. 2016. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol 771:84–92. doi: 10.1016/j.ejphar.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Pan P, Lin S, Wang Y, Chen W, Chuang Y, Wu C, Chen C. 2014. Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Radic Biol Med 73:106–116. doi: 10.1016/j.freeradbiomed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Im D. s, Shin H. j, Yang KJ, Jung SY, Song H. y, Hwang HS, Gil H-W. 2017. Cilastatin attenuates vancomycin-induced nephrotoxicity via P-glycoprotein. Toxicol Lett 277:9–17. doi: 10.1016/j.toxlet.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Toyoguchi T, Takahashi S, Hosoya J, Nakagawa Y, Watanabe H. 1997. Nephrotoxicity of vancomycin and drug interaction study with cilastatin in rabbits. Antimicrob Agents Chemother 41:1985–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh C, Yang J, Yang M, Li Y, Kuan Y. 2014. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free Radic Biol Med 69:249–257. doi: 10.1016/j.freeradbiomed.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Hohmann M, Cardoso R, Fattori V, Arakawa N, Tomaz J, Lopes N, Casagrande R, Verri W. 2015. Hypericum perforatum reduces paracetamol-induced hepatotoxicity and lethality in mice by modulating inflammation and oxidative stress. Phytother Res 29:1097–1101. doi: 10.1002/ptr.5350. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell J, Rhodes N, Lodise T, Prozialeck W, Miglis C, Joshi M. 2017. 24-Hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 61:e00416-17. doi: 10.1128/AAC.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O’Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. doi: 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalaklioglu S, Tekcan M, Gungor N, Celik-Ozenci C, Aksoy N, Baykal A, Tasatargil A. 2010. Role of the poly(ADP-ribose)polymerase activity in vancomycin-induced renal injury. Toxicol Lett 192:91–96. doi: 10.1016/j.toxlet.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X. 2017. Nrf2: a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol 54:6006–6017. doi: 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- 32.Kerasioti E, Stagos D, Tzimi A, Kouretas D. 2016. Increase in antioxidant activity by sheep/goat whey protein through nuclear factor-like 2 (Nrf2) is cell type dependent. Food Chem Toxicol 97:47–56. doi: 10.1016/j.fct.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Aksu E, Kandemir F, Özkaraca M, Ömür A, Küçükler S, Çomaklı S. 2017. Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia 49:e12593. [DOI] [PubMed] [Google Scholar]
- 34.Gautam R, Singh M, Gautam S, Rawat J, Saraf S, Kaithwas G. 2016. Rutin attenuates intestinal toxicity induced by methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement Altern Med 16:99. doi: 10.1186/s12906-016-1069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Qu J, Shen X. 2008. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta 1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Abarikwu S, Olufemi P, Lawrence C, Wekere F, Ochulor A, Barikuma A. 2017. Rutin, an antioxidant flavonoid, induces glutathione and glutathione peroxidase activities to protect against ethanol effects in cadmium-induced oxidative stress in the testis of adult rats. Andrologia 49:e12696. [DOI] [PubMed] [Google Scholar]
- 37.Afanas’ev IB, Dcrozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. 1989. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol 38:1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Guo B, Ye M, Liao R, Li S. 2016. Protective effect of rutin against H2O2-induced oxidative stress and apoptosis in human lens epithelial cells. Curr Eye Res 41:933–942. doi: 10.3109/02713683.2015.1082186. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto Y, Yano T, Hanada Y, Takeshita A, Inagaki F, Masuda S, Matsunaga N, Koyanagi S, Ohdo S. 2017. Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur J Pharmacol 800:48–56. doi: 10.1016/j.ejphar.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Ozkan G, Ulusoy S, Orem A, Alkanat M, Mungan S, Yulug E, Yucesan F. 2013. How does colistin-induced nephropathy develop and can it be treated? Antimicrob Agents Chemother 57:3463–3469. doi: 10.1128/AAC.00343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo S, Lee M, Chang E, Shin Y, Oh S, Kim I, Kim Y. 2015. Rutin increases muscle mitochondrial biogenesis with AMPK activation in high-fat diet-induced obese rats. Nutrients 7:8152–8169. doi: 10.3390/nu7095385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrasco-Pozo C, Mizgier M, Speisky H, Gotteland M. 2012. Differential protective effects of quercetin, resveratrol, rutin, and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem Biol Interact 195:199–205. doi: 10.1016/j.cbi.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Li H, Qiu S, Dong Z, Xiang X, Zhang D. 2017. MBD2 upregulates miR-301a-5p to induce kidney cell apoptosis during vancomycin-induced AKI. Cell Death Dis 8:e3120. doi: 10.1038/cddis.2017.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantaluppi V, Quercia A, Dellepiane S, Ferrario S, Camussi G, Biancone L. 2014. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol Dial Transplant 29:2004–2011. doi: 10.1093/ndt/gfu046. [DOI] [PubMed] [Google Scholar]
- 45.Yoo H, Ku S, Baek Y, Bae J. 2014. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm Res 63:197–206. doi: 10.1007/s00011-013-0689-x. [DOI] [PubMed] [Google Scholar]
- 46.Cosco D, Failla P, Costa N, Pullano S, Fiorillo A, Mollace V, Fresta M, Paolino D. 2016. Rutin-loaded chitosan microspheres: characterization and evaluation of the anti-inflammatory activity. Carbohydr Polym 152:583–591. doi: 10.1016/j.carbpol.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 47.Ravi G, Charyulu R, Dubey A, Prabhu P, Hebbar S, Mathias A. 2018. Nano-lipid complex of rutin: development, characterization, and in vivo investigation of hepatoprotective, antioxidant activity, and bioavailability study in rats. AAPS Pharm Sci Tech 152:1–19. [DOI] [PubMed] [Google Scholar]
- 48.Qu S, Zhao L, Zhu J, Wang C, Dai C, Guo H, Hao Z. 2017. Preparation and testing of cefquinome-loaded poly lactic-co-glycolic acid microspheres for lung targeting. Drug Deliv 24:745–751. doi: 10.1080/10717544.2017.1321058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu S, Dai C, Yang F, Huang T, Xu T, Zhao L, Li Y, Hao Z. 2018. A comparison of two methods for the preparation cefquinome-loaded gelatin microspheres for lung targeting. Pharm Res 35:43. doi: 10.1007/s11095-018-2342-4. [DOI] [PubMed] [Google Scholar]
- 50.Baliga R, Zhang Z, Baliga M, Ueda N, Shah S. 1998. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 53:394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 51.Qu S, Dai C, Zhu J, Zhao L, Li Y, Hao Z. 2018. Cefquinome-loaded microsphere formulations against Klebsiella pneumoniae infection during experimental infections. Drug Deliv 25:909–915. doi: 10.1080/10717544.2018.1461958. [DOI] [PMC free article] [PubMed] [Google Scholar]