Herein, we describe the in vivo efficacy of human-simulated WCK 5222 (cefepime-zidebactam) exposure against carbapenem-resistant Acinetobacter baumannii strains in a neutropenic murine thigh infection model. Five of the six isolates examined expressed OXA-23 or OXA-24.
KEYWORDS: human simulated, pharmacodynamics, pharmacokinetics
ABSTRACT
Herein, we describe the in vivo efficacy of human-simulated WCK 5222 (cefepime-zidebactam) exposure against carbapenem-resistant Acinetobacter baumannii strains in a neutropenic murine thigh infection model. Five of the six isolates examined expressed OXA-23 or OXA-24. WCK 5222, despite showing MICs of 16 to 64 mg/liter, produced remarkable in vivo activity; human-simulated exposure showed a decline in the bacterial burden for all isolates (mean reduction, −2.09 ± 1.01 log10 CFU/thigh), while a lack of activity was observed with cefepime and zidebactam monotherapies.
INTRODUCTION
Nosocomial infections due to Acinetobacter baumannii are prevalent worldwide and include serious infections, such as ventilator-associated pneumonia and bloodstream infections (1, 2). A. baumannii has the capacity to acquire almost all bacterial resistance mechanisms, including those resulting in carbapenem resistance (1) and multidrug resistance. The main mechanisms contributing to carbapenem resistance in A. baumannii are the production of carbapenem-hydrolyzing β-lactamases (carbapenemases, predominantly oxacillinases belonging to Ambler class D enzymes, such as OXA-23-like and OXA-24-like), the overexpression of efflux pumps, as well as a reduced expression of outer membrane porins that modulate cellular permeability (3). Thus, A. baumannii is considered one of the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) organisms, representing one of the greatest challenges in clinical practice (4).
A novel diazabicyclooctane non-β-lactam antibiotic, zidebactam (Wockhardt Bio AG, Switzerland), has been found to exhibit a dual mode of action that includes a β-lactam-enhancing effect mediated via selective and high-affinity binding to penicillin binding protein 2 (PBP-2) and inhibitory activity against Ambler class A and C β-lactamases (5, 6). Cefepime is a fourth-generation cephalosporin with activity against A. baumannii through binding to PBP-1a and PBP-3 (7, 8). Compared with cefepime alone, the combination of cefepime with zidebactam (WCK 5222; Wockhardt Bio AG, Switzerland) exhibited improved in vitro and in vivo activity against A. baumannii (5, 9).
We evaluated the in vivo efficacy of the human-simulated regimen (HSR) of WCK 5222 (9) for 24 h against clinical carbapenem-resistant A. baumannii isolates (N = 6) in a neutropenic murine thigh infection model. The protocol was approved by the Hartford Hospital Institutional Animal Care and Use Committee. All examined isolates were meropenem resistant with MICs of 8 to >64 mg/liter, including 5 isolates expressing the carbapenem-hydrolyzing oxacillinases OXA-23 or OXA-24. Cefepime, zidebactam, and WCK 5222 (cefepime-zidebactam at 1:1 ratio) MICs were assessed in triplicate using the broth microdilution methodology as outlined by the Clinical and Laboratory Standards Institute (9, 10). Quality control isolate Pseudomonas aeruginosa ATCC 27853 was used for the validation of WCK 5222 (range, 0.5 to 2 mg/liter) and zidebactam (range, 1 to 8 mg/liter) MICs; additionally, Staphylococcus aureus ATCC 29213 was used for the validation of cefepime (range, 1 to 4 mg/liter). The modal MIC was utilized to characterize the isolates for the final analyses. Cefepime and WCK 5222 MICs were 128 to >512 and 16 to 64 mg/liter, respectively; zidebactam MICs were >512 mg/liter for all isolates (Table 1). Female ICR mice weighing 20 to 22 g (Envigo RMS, Inc., Frederick, MD) were rendered transiently neutropenic via intraperitoneal (i.p.) injections of 150 and 100 mg/kg cyclophosphamide (Sigma-Aldrich Co., St. Louis, MO) 4 days and 1 day prior to bacterial inoculation, respectively. In addition, a single i.p. injection of 5 mg/kg uranyl nitrate was administered 3 days prior to inoculation to induce a predictable degree of renal impairment. Mice were inoculated intramuscularly in each thigh with 0.1 ml of a bacterial suspension of 107 CFU/ml 2 h prior to antibiotic dosing to achieve an initial inoculum of ∼106 CFU/thigh. Treatment and control groups were composed of 3 mice each. The 0-h control groups were sacrificed 2 h postinoculation. Treatment groups received a previously established HSR of either cefepime (Qilu Antibiotics, Jinan, China) equivalent to an intravenous (i.v.) clinical dose of 2 g every 8 h (q8h) as a 1-h infusion (9), zidebactam (Wockhardt Bio AG, Switzerland) equivalent to an i.v. clinical dose of 1 g q8h as a 1-h infusion (9), or WCK 5222 (doses of cefepime HSR coadministered with those of zidebactam HSR). All treatments were administered by subcutaneous injections (0.1 ml/agent) for 24 h. All HSRs mimicked the exposures in human plasma on the basis of the percentage of the dosing interval during which the free drug concentrations remained above the MIC (%fT>MIC) (9, 11). The WCK 5222 %fT>MIC of cefepime and zidebactam for each isolate are reported in Table 1 (9). Control mice were vehicle dosed for 24 h. Treatment and 24-h control mice were sacrificed at the end of the study period, and thighs were harvested and processed as previously described (12). To assess efficacy, changes in the log10 CFU/ml at 24 h relative to the initial bacterial burdens of the 0-h groups were calculated.
TABLE 1.
Phenotypic profiles and resistance mechanisms of the Acinetobacter baumannii isolates selected for the in vivo efficacy studiesa
| Organism | β-Lactamases | Additional positive molecular test results | Modal MIC (mg/liter)b |
WCK 5222 %fT>MICc |
||
|---|---|---|---|---|---|---|
| Cefepime | WCK 5222 | Cefepime | Zidebactam | |||
| ACBN 194 | ADC-25, OXA-23, OXA-82 | aph(3′)-Ic, armA, catB8, mph(E), msr(E), strA, strB, sul1 | 512 | 16 | 66.25 | 41.25 |
| ACBN 179 | ADC-25, OXA-23, OXA-223 | aadA2, aadB, sul1 | 256 | 32 | 41.25 | 20.42 |
| ACBN 160 | OXA-24, OXA-65, TEM-1B | aac(3)-IIa, strA, strB, sul2 | >512 | 32 | 41.25 | 20.42 |
| ACBN 189 | OXA-24, OXA-65, TEM-1B | aac(3)-IIa, strA, strB, sul2 | 128 | 32 | 41.25 | 20.42 |
| ACBN JJ4-25 | ADC-30, OXA-66, OXA-72 | aac(3)-I, aacA16, aadA1, aph(6)-Ia, aph(6)-Id, sul2, tet(B) | 256 | 64 | 19.58 | 3.75 |
| ACBN 171 | ADC-25, OXA-23, OXA-66 | armA, catB8, mph(E), msr(E), strA, strB, sul1 | 256 | 64 | 19.58 | 3.75 |
Adapted from reference 9.
For all isolates studied, the modal zidebactam MIC was ≥512 mg/liter.
Estimated for the murine human-simulated regimens.
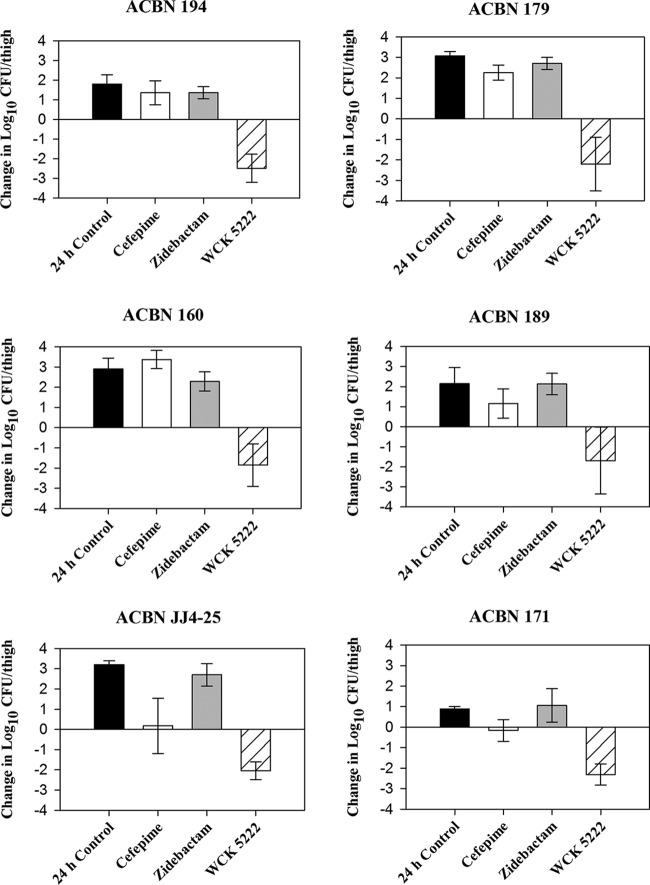
The average log10 CFU/thigh at 0 h across all isolates was 5.85 ± 0.22. Mean increases in bacterial burden at 24 h in the untreated control, cefepime HSR-treated, and zidebactam HSR-treated groups were 2.34 ± 0.93, 1.36 ± 1.40, and 2.04 ± 0.80 log10 CFU/thigh, respectively. A decline in bacterial burden was observed with the WCK 5222 HSR for all isolates, with a mean reduction of −2.09 ± 1.01 log10 CFU/thigh across all isolates. Four out of six isolates achieved a >2-log10 reduction with WCK 5222 HSR, while a >1-log10 reduction was attained in the remaining two isolates (Fig. 1).
FIG 1.
Mean bacterial growth or reduction in log10 CFU/thigh plus or minus the standard deviation (SD) at 24 h relative to the starting inoculum in a neutropenic murine thigh infection model.
Infections caused by carbapenem-resistant A. baumannii remain a challenge to treat effectively. In the present murine thigh study, WCK 5222 displayed potent in vivo activity against carbapenem-resistant A. baumannii expressing OXA carbapenemases. These in vivo potency results are in general agreement with those reported by Avery et al. using the murine lung model (9). In the neutropenic lung model, the authors demonstrated a >2-log10 reduction in bacterial burden upon the administration of WCK 5222 HSR against 12 meropenem-resistant A. baumannii isolates with WCK 5222 MICs of 16 to 64 mg/liter, despite achieving %fT>MICs of cefepime and zidebactam in plasma as low as ∼20% and ∼4%, respectively, as shown in Table 1 (9).
Previously published in vitro data demonstrated that zidebactam has the capability to potentiate the activity of cefepime against carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa isolates, as evidenced by the marked decline in WCK 5222 MICs relative to those of cefepime alone (13, 14). However, zidebactam has been shown to cause a modest potentiation of cefepime against A. baumannii in vitro, as the WCK 5222 MICs remained high (≥16 mg/liter). Nevertheless, our results suggested that zidebactam could effectively enhance cefepime activity in vivo, inclusive of isolates with a WCK 5222 MIC of 64 mg/liter. Similar observations were reported by Bhagwat et al. and Avery et al., where cefepime exposures below the typical threshold required for efficacy were shown to produce significant bactericidal effects in vivo when combined with zidebactam, suggestive of significant potentiation in vivo (9, 15). Notably, this enhancer effect has been previously reported for other β-lactam/β-lactamase inhibitor combinations, such as nacubactam-based combinations (16).
In summary, WCK 5222 HSR showed potent in vivo activity against carbapenem-resistant A. baumannii expressing OXA carbapenemases in the murine thigh infection model, which is attributed to the β-lactam-enhancing effect of zidebactam driven by the complementary PBP binding of cefepime and zidebactam. These results support the clinical evaluation of WCK 5222 for the management of infections due to carbapenem-resistant A. baumannii.
ACKNOWLEDGMENTS
This study was funded by Wockhardt Bio AG, Switzerland.
We acknowledge Sara Giovagnoli, Janice Cunningham, Elizabeth Cyr, Kim Greenwood, Michelle Insignares, Tomefa Asempa, James Kidd, Lauren McLellan, Ana Motos, Alissa Padgett, Debora Santini, Sean Stainton, Christina Sutherland, Courtney Bouchard, Nicole DeRosa, Elias Mullane, and Jennifer Tabor-Rennie from the Center for Anti-Infective Research and Development (Hartford, CT) for their dedication to exceptional technical quality and assistance in conducting the study.
REFERENCES
- 1.Lee C-R, Lee J, Park M, Park K, Bae IK, Kim Y, Cha C-J, Jeong B, Lee S. 2017. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol 7:1–35. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi PR, Khande HN, Takalkar SS, Kulkarni AM, Chavan RP, Zope VS, Palwe SR, Biniwale SS, Bhagwat SS, Patel MV. 2016. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: in vitro and in vivo coverage of OXA-carbapenemases expressing-acinetobacter (OXA-AB), abstr 440 Abstr Gen Meet Am Soc Microbiol. ASM Press, Washington, DC. [Google Scholar]
- 3.Hsu L-Y, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. 2017. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev 30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santajit S, Indrawattana N. 2016. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res Int 2016:2475067. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2017. Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline, including tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace MK, Bonomo RA, Oliver A. 2017. Potent β-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother 61:e01238. doi: 10.1128/AAC.01238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endimiani A, Perez F, Bonomo RA. 2008. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther 6:805–824. doi: 10.1586/14787210.6.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avery LM, Abdelraouf K, Nicolau DP. 2018. Assessment of the in vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother doi: 10.1128/AAC.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2018. Performance Standards for Antimicrobial Susceptibility Testing, M100, 28th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Rodvold KA, Gotfried MH, Chugh R, Gupta M, Patel A, Chavan R, Yeole R, Friedland HD, Bhatia A. 2018. Plasma and intrapulmonary concentrations of cefepime and zidebactam following intravenous administration of WCK 5222 to health adult subjects. Antimicrob Agents Chemother 62:e00682-18. doi: 10.1128/AAC.00682-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging Gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1138. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 14.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. doi: 10.1128/AAC.00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhagwat SS, Takalkar SS, Chavan RP, Friedland HD, Patel MV. 2017. WCK 5222 [cefepime (FEP) + WCK 5107 (zidebactam, ZID)]: in vivo demonstration of ZID-mediated β-lactam enhancer effect leading to lowering of FEP %f T>MIC against P. aeruginosa (PA) and A. baumannii (AB), abstr P284 Abstr Gen Meet Am Soc Microbiol ASM Press, Washington, DC. [Google Scholar]
- 16.Morinaka A, Tsutsumi Y, Yamada M, Suzuki K, Watanabe T, Abe T, Furuuchi T, Inamura S, Sakamaki Y, Mitsuhashi N, Ida T, Livermore DM. 2015. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam “enhancer.” J Antimicrob Chemother 70:2779–2786. doi: 10.1093/jac/dkv166. [DOI] [PubMed] [Google Scholar]