Data for a total of 164 bloodstream infection cases due to carbapenem-resistant Enterobacteriaceae (CRE) from 2013 to 2017 were retrospectively collected from 36 tertiary hospitals in 19 provinces in China to evaluate the outcomes and risk factors for mortality by univariable and multivariable analysis. The most frequent infecting species was Klebsiella pneumoniae (69.5%, 114/164).
KEYWORDS: bacteremia, carbapenem-resistant Enterobacteriaceae, combination therapy, in-hospital mortality, monotherapy
ABSTRACT
Data for a total of 164 bloodstream infection cases due to carbapenem-resistant Enterobacteriaceae (CRE) from 2013 to 2017 were retrospectively collected from 36 tertiary hospitals in 19 provinces in China to evaluate the outcomes and risk factors for mortality by univariable and multivariable analysis. The most frequent infecting species was Klebsiella pneumoniae (69.5%, 114/164). The overall in-hospital and 14-day mortality rates were 32.9% (54/164) and 31.1% (42/135), respectively. Multivariable analysis revealed that septic shock (adjusted odds ratio [aOR], 6.339; 95% confidence interval [CI], 1.586 to 25.332; P = 0.009), the Pitt bacteremia score (aOR, 1.300; 95% CI, 1.009 to 1.676; P = 0.042), and the Charlson comorbidity index (aOR, 1.392; 95% CI, 1.104 to 1.755; P = 0.005) were independently associated with a hazard effect on mortality. Combination therapy, especially tigecycline-based combination therapy, resulted in relatively low rates of in-hospital mortality and failure in clearance of CRE infection. Survival analysis revealed that appropriate therapy was associated with a lower 14-day mortality rate than inappropriate therapy (including nonactive therapy; P = 0.022), that combination therapy was superior to monotherapy (P = 0.036), that metallo-β-lactamase producers were associated with a lower 14-day mortality than strains without carbapenemases or KPC-2 producers (P = 0.009), and that strains with MICs of >8 mg/liter for meropenem were associated with a higher 14-day mortality rate than those with MICs of ≤8 mg/liter (P = 0.037). Collectively, the severity of illness, meropenem MICs of >8 mg/liter, and carbapenemase-producing types were associated with the clinical outcome. Early detection of the carbapenemase type and initiation of appropriate combination therapy within 96 h might be helpful for improving survival.
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae (CRE), which are a global public health issue due to rapidly transmissible carbapenem resistance elements and few remaining viable therapies, are associated with significant morbidity and mortality (1–4). Indeed, the all-cause mortality from severe bloodstream CRE infections is nearly 70% (5–8). In addition, polymyxin B, polymyxin E, and the new β-lactamase inhibitors avibactam (9) and relebactam (10) are not commercially available in China, where CRE is endemic (2), so treatments are limited to tigecycline, aminoglycosides, fosfomycin, and, possibly, carbapenem, although carbapenem MICs routinely exceed 8 mg/liter (11–14).
The superiority of combination therapy over other treatments remains controversial (15), although it is favored by the Chinese XDR Consensus Working Group (16) and in most retrospective studies (15, 17). In any case, selecting the optimal therapy is a tremendous challenge due to pervasive drug resistance. In addition, the optimal timing to initiate therapy remains uncertain and requires verification through cumulative clinical practice. Finally, investigations into whether clinical outcomes are associated with specific resistance genes or resistance levels are quite limited, because most studies focus on one pathogen type, e.g., carbapenem-resistant Klebsiella pneumoniae (CRKP) with KPC-2 and Enterobacteriaceae with VIM, an OXA-48-like carbapenemase, or are case-control studies comparing K. pneumoniae isolates resistant or susceptible to carbapenem or Enterobacteriaceae with or without carbapenemase production (18, 19). Noting that KPC-2 is the most prevalent mobile carbapenemase in mainland China (2), followed by NDM, which emerged in 2009 (20), data for 164 nosocomial bloodstream infections were retrospectively collected to address these problems.
RESULTS
Clinical characteristics.
Between 6 April 2013 and 28 July 2017, data for 177 patients with bacteremia due to CRE were enrolled in this study. Ultimately, data for 164 unique episodes were included in the analysis after applying inclusion and exclusion criteria (Fig. 1). The median patient age was 52.0 years (interquartile range, 34.3 to 73.0 years), and 53 of 164 patients (32.3%) were female (Table 1). The rates of death and survival, stratified by demographic and clinical characteristics, are listed in Table 1. The most frequent cases were bacteremia from pneumonia in 29/81 patients (35.8%), catheter-related bacteremia in 23/81 patients (28.4%), and intra-abdominal infection in 18/81 patients (22.2%). The median Charlson comorbidity index and Pitt bacteremia score at onset were 4.2 (interquartile range, 2.0 to 5.8) and 3.9 (interquartile range, 2.0 to 6.0), respectively.
FIG 1.
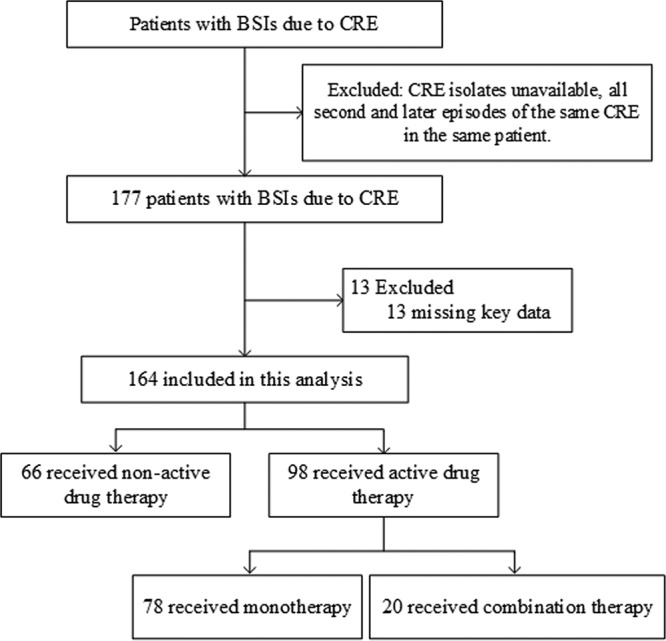
Flowchart of the included patients with bloodstream infections (BSIs) due to carbapenem-resistant Enterobacteriaceae (CRE), as identified according to CLSI breakpoints.
TABLE 1.
Demographic and clinical characteristics of patients with bloodstream infections due to carbapenem-resistant Enterobacteriaceae and stratified by in-hospital mortality
| Characteristic | Value(s) for the following patients: |
P value | ||
|---|---|---|---|---|
| Total (n = 164) | Patients who died (n = 54) | Patients who survived (n = 110) | ||
| Demographics | ||||
| No. of female patients/total no. of patients (%) | 53/164 (32.3) | 18/54 (33.3) | 35/110 (31.8) | 0.845 |
| Median (interquartile range) age (yr) | 52.0 (34.3–73.0) | 57.0 (44.0–74.0) | 50.0 (32.0–73.0) | 0.283 |
| No. of patients infected with the following CRE organisms/total no. of CRE-infected patients (%): | 0.020 | |||
| Klebsiella pneumoniae | 114/164 (69.5) | 44/54 (81.5) | 70/110 (63.6) | |
| blaKPC-2-producing ST11 K. pneumoniae | 76/110 (69.1) | 35/43 (81.4) | 41/67 (61.2) | 0.025 |
| Other organismsa | 50/164 (30.5) | 10/54 (18.5) | 40/110 (36.4) | |
| No. of patients with CRE causing BSIb with the following AST profile/total no. of patients (%): | ||||
| Levofloxacin resistance (MIC, ≥4 mg/liter) | 134/164 (81.7) | 49/54 (90.7) | 85/110 (77.3) | 0.036 |
| Amikacin resistance (MIC, ≥32 mg/liter) | 80/164 (48.8) | 36/54 (66.7) | 44/110 (40.0) | 0.001 |
| Tigecycline resistance (MIC, ≥4 mg/liter) | 22/164 (13.4) | 5/54 (9.3) | 17/110 (15.5) | 0.274 |
| Colistin resistance (MIC, ≥4 mg/liter) | 3/160 (1.9) | 2/53 (3.8) | 1/107 (0.9) | 0.255 |
| No. of patients with CRE with the following resistance mechanism/total no. of patients (%): | <0.001 | |||
| Non-CPE | 20/164 (12.2) | 9/54 (16.7) | 11/110 (10.0) | |
| KPC-2 productionc | 90/164 (54.9) | 39/54 (72.2) | 51/110 (46.4) | |
| Metallo-β-lactamase productiond | 54/164 (32.9) | 6/54 (11.1) | 48/110 (43.6) | |
| CRE meropenem MICs of >8 mg/liter | 99/164 (60.4) | 39/54 (72.2) | 60/110 (54.5) | 0.030 |
| No. of patients with the following likely source of bacteremia/total no. of patients (%): | ||||
| Catheter related | 23/81 (28.4) | 7/30 (23.3) | 16/51 (31.4) | 0.438 |
| Urinary tract | 4/81 (4.9) | 0/30 (0) | 4/51 (7.8) | |
| Biliary | 6/81 (7.4) | 3/30 (10.0) | 3/51 (5.9) | 0.665 |
| Intra-abdominal | 18/81 (22.2) | 7/30 (23.3) | 11/51 (21.6) | 0.854 |
| Pneumonia | 29/81 (35.8) | 13/30 (43.3) | 16/51 (31.4) | 0.278 |
| Skin and soft tissue | 1/81 (1.2) | 0/30 (0) | 1/51 (2.0) | |
| No. of patients transferring from other institutions/total no. of patients (%) | 39/164 (23.8) | 18/54 (33.3) | 21/110 (19.1) | 0.044 |
| Median (interquartile range) Pitt bacteremia score | 3.9 (2.0–6.0) | 5.0 (3.0–8.0) | 3.0 (1.0–5.0) | <0.001 |
| No. of patients with Pitt bacteremia score of ≥6/total no. of patients (%) | 41/154 (26.6) | 24/53 (45.3) | 17/101 (16.8) | <0.001 |
| Median (interquartile range) Charlson comorbidity index | 4.2 (2.0–5.8) | 5.0 (3.0–7.0) | 4.0 (1.0–5.0) | 0.004 |
| No. of patients with a Charlson index of ≥3/total no. of patients (%) | 106/164 (64.6) | 40/54 (74.1) | 66/110 (60.0) | 0.076 |
| No. of patients with a history of surgery/total no. of patients (%) | 14/164 (8.5) | 4/54 (7.4) | 10/110 (9.1) | 1 |
| No. of patients with a history of critical care in prior 1 yr/total no. of patients (%) | 34/164 (20.7) | 13/54 (24.1) | 21/110 (19.1) | 0.459 |
| No. of patients with the following invasive procedures or treatments (≤30 dayse)/total no. of patients (%): | ||||
| Thoracentesis | 9/164 (5.5) | 5/54 (9.3) | 4/110 (3.6) | 0.157 |
| Lumbar puncture | 10/164 (6.1) | 2/54 (3.7) | 8/110 (7.3) | 0.500 |
| Arterial cannula | 17/164 (10.4) | 5/54 (9.3) | 12/110 (10.9) | 0.745 |
| Central venous catheter | 64/164 (39.0) | 21/54 (38.9) | 43/110 (39.1) | 0.980 |
| Tracheal cannula | 54/164 (32.9) | 21/54 (38.9) | 33/110 (30.0) | 0.255 |
| Tracheotomy | 17/164 (10.4) | 7/54 (13.0) | 10/110 (9.1) | 0.445 |
| Urinary catheter | 54/164 (32.9) | 21/54 (38.9) | 33/110 (30.0) | 0.255 |
| Gastric tube | 49/164 (29.9) | 18/54 (33.3) | 31/110 (28.2) | 0.498 |
| Receipt of corticosteroids (≤30 dayse ) | 43/164 (26.2) | 14/54 (25.9) | 29/110 (26.4) | 0.952 |
| Receipt of immunosuppressive inhibitors (≤30 dayse ) | 15/162 (9.3) | 4/53 (7.5) | 11/109 (10.1) | 0.775 |
| ICU stay prior to BSI within 30 days | 75/154 (48.7) | 29/52 (55.8) | 46/102 (45.1) | 0.210 |
| Exposure to antimicrobials in prior 30 dayse | ||||
| None | 14/164 (8.5) | 4/54 (7.4) | 10/110 (9.1) | 1 |
| First- or second-generation cephalosporin | 15/164 (9.1) | 0/54 (0) | 15/110 (13.6) | |
| Third- or fourth-generation cephalosporin | 37/164 (22.6) | 12/54 (22.2) | 25/110 (22.7) | 0.942 |
| β-Lactam–β-lactamase inhibitor | 94/164 (57.3) | 33/54 (61.1) | 61/110 (55.5) | 0.491 |
| Carbapenem | 83/164 (50.6) | 37/54 (68.5) | 46/110 (41.8) | 0.001 |
| Quinolone | 44/164 (26.8) | 13/54 (24.1) | 31/110 (28.2) | 0.577 |
| Aminoglycosides | 15/164 (9.1) | 5/54 (9.3) | 10/110 (9.1) | 0.972 |
| Tigecycline | 15/164 (9.1) | 9/54 (16.7) | 6/110 (5.5) | 0.019 |
| No. of patients with septic shock/total no. of patients (%) | 63/159 (39.6) | 41/54 (75.9) | 22/105 (21.0) | <0.001 |
| No. of patients in ICU when blood culture was collected/total no. of patients (%) | 88/164 (53.7) | 39/54 (72.2) | 49/110 (44.5) | 0.001 |
| No. of patients with presumed CRE clearance/total no. of patients (%) | 96/133 (72.2) | 8/33 (24.2) | 88/100 (88.0) | <0.001 |
Including Escherichia coli, Enterobacter spp., Citrobacter freundii, and Serratia marcescens.
BSI, bloodstream infection.
Including isolates coproducing KPC, ESBLs, or AmpC.
Metallo-β-lactamases, including NDM-1/4/5/7, VIM-1, and IMP-1/4/26.
≤30 days or within 30 days or in prior 30 days indicated 30 days prior to collection of the first blood sample positive by culture.
Microbiological characteristics of CRE isolates.
The predominant infecting CRE was K. pneumoniae (69.5%, 114/164), followed by Escherichia coli (14.6%, 24/164) and Enterobacter spp. (12.2%, 20/164). Among isolates with (87.8%, 144/164) or without (12.2%, 20/164) carbapenemase, the predominant organism was also K. pneumoniae at 72.2% (104/144) and 50.0% (10/20), respectively. For all isolates, the rates of susceptibility to the tested antibiotics were as follows: meropenem, 9.1% (15/164); aztreonam, 12.8% (16/125); levofloxacin, 17.7% (29/164); amikacin, 51.8% (85/164); tigecycline, 86.6% (142/164); minocycline, 44.0% (70/159); and colistin, 98.1% (157/160) (Table 2). The rates of susceptibility to levofloxacin (3.3% versus 35.1%) and amikacin (22.2% versus 87.8%) were significantly lower for isolates producing blaKPC-2 than for isolates not producing blaKPC-2 (P < 0.001; data not shown). On the other hand, the rates of susceptibility to meropenem were much higher for isolates with blaVIM-1/IMP-1/4/26 (54.5%) than for isolates with carbapenemase (23.8%), isolates with blaKPC-2 (2.2%), and isolates with blaNDM-1/4/5/7 (4.7%) (P < 0.001) (Table 2).
TABLE 2.
Antimicrobial susceptibility profiles of carbapenem-resistant Enterobacteriaceae isolates
| Antimicrobial | No. of susceptible isolates/no. of isolates tested (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All strains | By organism |
By carbapenemase production |
|||||||
|
Klebsiella pneumoniae
(n = 114) |
Escherichia coli
(n = 24) |
Enterobacter spp. (n = 20) |
Other organismsa (n = 6) |
KPC-2 (n = 90) |
NDM-1/4/5/7 (n = 43) |
VIM-1 IMP-1/4/26 (n = 11) |
Non-CPEb
(n = 20) |
||
| Ertapenem | 0/78 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imipenem | 12/164 (7.3) | 5/114 (4.4) | 2/24 (8.3) | 3/20 (15.0) | 2/6 (33.3) | 1/90 (1.1) | 2/43 (4.7) | 7/11 (63.6) | 2/20 (10.0) |
| Meropenem | 15/164 (9.1) | 7/114 (6.1) | 2/24 (8.3) | 4/20 (20.0) | 2/6 (33.3) | 2/90 (2.2) | 2/43 (4.7) | 6/11 (54.5) | 5/20 (23.8) |
| Ceftazidime | 3/164 (1.8) | 1/114 (0.9) | 1/24 (4.2) | 0/20 (0) | 1/6 (16.7) | 0/90 (0) | 0/43 (0) | 0/11 (0) | 3/20 (15.0) |
| Ceftriaxone | 0/164 (0) | 0/114 (0) | 0/24 (0) | 0/20 (0) | 0/6 (0) | 0/90 (0) | 0/43 (0) | 0/11 (0) | 0/20 (0) |
| Cefotaxime | 1/160 (0.6) | 1/111 (0.9) | 0/24 (0) | 0/19 (0) | 0/6 (0) | 0/87 (0) | 0/43 (0) | 0/11 (0) | 1/19 (5.3) |
| Cefoxitin | 4/164 (2.4) | 3/114 (2.6) | 1/24 (4.2) | 0/20 (0) | 0/6 (0) | 2/90 (2.2) | 0/43 (0) | 0/11 (0) | 2/20 (10.0) |
| Aztreonam | 16/125 (12.8) | 0/81 (0) | 8/21 (38.1) | 5/17 (29.4) | 3/6 (50.0) | 0/64 (0) | 10/33 (30.3) | 5/10 (50.0) | 1/18 (5.6) |
| Cefepime | 13/164 (7.9) | 6/114 (5.3) | 2/24 (8.3) | 4/20 (20.0) | 1/6 (16.7) | 3/90 (3.3) | 0/43 (0) | 5/11 (45.5) | 5/20 (25.0) |
| Cefoperazone-sulbactam | 3/160 (1.9) | 0/111 (0) | 2/24 (8.3) | 1/19 (5.3) | 0/6 (0) | 0/87 (0) | 0/43 (0) | 1/11 (9.1) | 2/19 (10.5) |
| Piperacillin-tazobactam | 15/164 (9.1) | 3/114 (2.6) | 4/24 (16.7) | 6/20 (30.0) | 2/6 (33.3) | 0/90 (0) | 1/43 (2.3) | 8/11 (72.7) | 6/20 (30.0) |
| Ciprofloxacin | 21/164 (12.8) | 10/114 (8.8) | 4/24 (16.7) | 5/20 (25) | 2/6 (33.3) | 1/90 (1.1) | 8/43 (18.6) | 8/11 (72.7) | 4/20 (20.0) |
| Levofloxacin | 29/164 (17.7) | 14/114 (12.3) | 4/24 (16.7) | 8/20 (40.0) | 3/6 (50.0) | 3/90 (3.3) | 11/43 (25.6) | 9/11 (81.8) | 6/20 (30.0) |
| Amikacin | 85/164 (51.8) | 41/114 (36.0) | 20/24 (83.3) | 18/20 (90.0) | 6/6 (100.0) | 20/90 (22.2) | 36/43 (83.7) | 10/11 (90.9) | 19/20 (95.0) |
| Tigecycline | 142/164 (86.6) | 97/114 (85.1) | 23/24 (95.8) | 16/20 (80.0) | 6/6 (100.0) | 80/90 (88.9) | 36/43 (83.7) | 9/11 (81.8) | 17/20 (85.0) |
| Minocycline | 70/159 (44.0) | 41/111 (36.9) | 17/23 (73.9) | 8/19 (42.1) | 4/6 (66.7) | 36/87 (41.4) | 21/42 (50.0) | 5/11 (45.5) | 8/19 (42.1) |
| Colistin | 157/160 (98.1) | 109/111 (98.2) | 24/24 (100.0) | 19/19 (100.0) | 5/6c (83.3) | 87/87 (100.0) | 43/43 (100.0) | 11/11 (100.0) | 16/19 (84.2) |
Including Citrobacter freundii and Serratia marcescens.
CPE, carbapenemase-producing Enterobacteriaceae.
One of six isolates was S. marcescens, which is innately resistant to colistin.
The majority of CRE isolates produced blaKPC-2 (54.9%, 90/164), followed by blaNDM (26.2%, 43/164, including 20 isolates producing blaNDM-1, 20 producing blaNDM-5, 2 producing blaNDM-7, and 1 producing blaNDM-4) and blaIMP (6.1%, 10/164, including 7 isolates producing blaIMP-4, 2 producing blaIMP-26, and 1 producing blaIMP-1). The blaVIM-1 gene was present in only one CRE isolate. Of the 20 isolates without a carbapenemase, most produced blaCTX-M-type enzymes (35.0%, 7/20) and three produced both blaCTX-M and blaDHA. Extended-spectrum β-lactamases (ESBLs) or AmpC enzymes were not detected in nine isolates. The clinical characteristics of patients with infections caused by CRE isolates without carbapenemase production (non-CPE) and isolates with KPC-2 and metallo-β-lactamase production are listed in Table S1 in the supplemental material.
Among 114 carbapenem-resistant K. pneumoniae isolates, the most dominant sequence type (ST) was ST11 (66.7%, 76/114), followed by ST15 (7.0%, 8/114). As sequence types were diverse among 24 carbapenem-resistant E. coli and Enterobacter cloacae isolates, none were clearly dominant.
Therapeutic characteristics.
Definitive therapy was provided to 133 of 164 patients, while 147 patients received empirical therapy; among the patients in the latter group, 57 received at least one active antibiotic. However, 31 patients did not receive therapy with active drugs, because 23 died before antimicrobial susceptibility test reports became available and treatment was abandoned for 8 due to severe illness. A total of 57 patients (34.8%) received early appropriate therapy, as defined in Materials and Methods, and 75 (45.7%) received appropriate therapy. Of 89 patients who received inappropriate therapy, 66 received inactive drugs. Ninety-eight patients, including 78 patients who received monotherapy (79.6%, 78/98) and 20 who received combination therapy (20.4%, 20/98), received at least one active antibiotic. The clinical characteristics of patients who underwent monotherapy and combination therapy were summarized in Table S2. The most frequent antibiotic given in monotherapy was tigecycline (n = 31), followed by carbapenems (meropenem, imipenem, biapenem, or panipenem; n = 25) and aminoglycosides (amikacin or etimicin; n = 17). In combination therapy, tigecycline was also the most frequently used (n = 10).
In-hospital mortality.
The overall in-hospital crude mortality rate was 32.9% (54/164). K. pneumoniae isolates, especially ST11 K. pneumoniae isolates with blaKPC-2, as well as isolates with meropenem MICs of >8 mg/liter, were more common among patients who died than among survivors (P < 0.05; Table 1). Amikacin and levofloxacin resistance was also much more frequent among the former, along with cancer (55.6% versus 30.9%) as an underlying disease (P < 0.05; data not shown). Transfer from other institutions (33.3% versus 19.1%), Pitt bacteremia scores of ≥6 (45.3% versus 16.8%), exposure in the prior 30 days to intravenous carbapenem (68.5% versus 44.0%) or tigecycline (16.7% versus 5.5%), septic shock (75.9% versus 21.0%), and staying in an intensive care unit (ICU) when blood culture was collected (72.9% versus 44.5%) were also significantly different in frequency between the patient groups (Table 1).
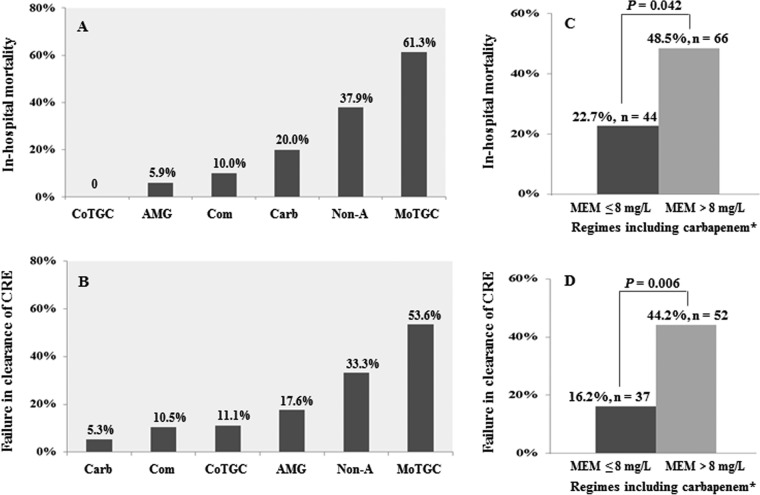
In-hospital mortality rates for patients who received active tigecycline-based combination therapy (0%, 0/10), active aminoglycoside monotherapy (5.9%, 1/17), active drug combination therapy (10.0%, 2/20, including 10 who received tigecycline-based combination therapy), active carbapenem monotherapy (20.0%, 5/25; carbapenem MICs for 25 cases were <8 mg/liter, and for these patients, carbapenem was administered at the conventional dose and by the conventional route), inactive drug therapy (37.9%, 25/66), and active tigecycline monotherapy (61.3%, 19/31) are displayed in Fig. 2A. The rates of failure in clearance of CRE infections among patients who received active tigecycline-based combination therapy (11.1%, 1/9), active aminoglycoside monotherapy (17.6%, 3/17), active drug combination therapy (10.5%, 2/19), active carbapenem monotherapy (5.3%, 1/19), inactive drug therapy (33.3%, 16/48), and active tigecycline monotherapy (53.6%, 15/28) are shown in Fig. 2B. Among patients treated with an active or inactive carbapenem, the in-hospital mortality rate was lower for patients with isolates with meropenem MICs of ≤8 mg/liter (22.7%, 10/44) than for patients with isolates with meropenem MICs of >8 mg/liter (48.5%, 32/66) (P = 0.042) (Fig. 2C). Accordingly, the latter isolates were not cleared from more cases (44.2%, 23/52) than the former (16.2%, 6/37) (P = 0.006) (Fig. 2D).
FIG 2.
Clinical and microbiological outcomes following various therapeutic regimes. CoTGC, active tigecycline-based combination therapy (n = 10); AMG, active aminoglycoside monotherapy (n = 17); Com, active drug combination therapy (including tigecycline-based combination therapy; n = 20); Carb, active carbapenem monotherapy (n = 25); Non-A, nonactive drug therapy (n = 66); MoTGC, active tigecycline monotherapy (n = 31); MEM, meropenem MIC values. *, including inactive and active carbapenem administration.
On multivariate analyses, septic shock (adjusted odds ratio [aOR], 6.339; 95% confidence interval [CI], 1.586 to 25.332; P = 0.009), the Pitt bacteremia score (aOR, 1.300; 95% CI, 1.009 to 1.676; P = 0.042), and the Charlson comorbidity index (aOR, 1.392; 95% CI, 1.104 to 1.755; P = 0.005) were independently associated with a hazard effect on in-hospital mortality (Table 3). On the other hand, appropriate therapy was not associated with a protective effect on in-hospital mortality by multivariate logistic regression analysis (Table 4). Similarly, the all-cause in-hospital mortality rate was not significantly higher after monotherapy (34.6%, 27/78) than after combination therapy (10.0%, 2/20) (P = 0.052) (Table 4). However, the all-cause 14-day mortality rate was significantly higher following monotherapy (30.4%, 21/69) than following combination therapy (5.0%, 1/20) (P = 0.034) (data not shown).
TABLE 3.
Univariate and multivariate logistic regression analysis of predictors of in-hospital mortality in patients with CRE bacteremia
| Characteristic | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | |
| CRE meropenem MIC > 8 mg/liter | 2.167 (1.072–4.380) | 0.031 | ||
| Pitt bacteremia score | 1.320 (1.163–1.498) | <0.001 | 1.300 (1.009–1.676) | 0.042 |
| Charlson comorbidity index | 1.199 (1.078–1.335) | 0.001 | 1.392 (1.104–1.755) | 0.005 |
| Prior carbapenem use | 3.028 (1.522–6.025) | 0.002 | 3.077 (0.838–11.307) | 0.090 |
| Prior tigecycline use | 3.467 (1.165–10.317) | 0.025 | ||
| Septic shock | 11.899 (5.449–25.985) | <0.001 | 6.339 (1.586–25.332) | 0.009 |
| In ICU when blood culture was obtained | 3.237 (1.600–6.546) | 0.001 | ||
| Appropriate therapy | 0.636 (0.328–1.234) | 0.181 | ||
| Combination therapy | 0.210 (0.045–0.973) | 0.046 | 0.169 (0.026–1.106) | 0.064 |
| Surgery or drainage of infected foci | 0.756 (0.378–1.512) | 0.429 | ||
TABLE 4.
In-hospital mortality among patients who received active therapy based on in vitro antimicrobial susceptibility test results
| Treatment | No. of patients with the following outcome/no. of patients (%): |
P value | ||
|---|---|---|---|---|
| All patients (n = 164) |
Death (n = 54) |
Survival (n = 110) |
||
| Nonactive drug therapy | 66/164 (40.2) | 25/54 (46.3) | 41/110 (37.3) | 0.268 |
| Active drug treatment | 98/164 (59.8) | 29/54 (53.7) | 69/110 (62.7) | 0.268 |
| Early appropriate therapy (≤2 days) | 57/164 (34.8) | 19/54 (35.2) | 38/110 (34.5) | 0.936 |
| Appropriate therapy (<5 days) | 76/164 (46.3) | 21/54 (38.9) | 55/110 (50.0) | 0.180 |
| Monotherapy | 78/98 (79.6) | 27/29 (93.1) | 51/69 (73.9) | 0.052 |
| Tigecycline | 31/78 (39.7) | 19/27 (70.4) | 12/51 (23.5) | |
| Carbapenem | 25/78 (32.1) | 5/27 (18.5) | 20/51 (39.2) | |
| Aminoglycosides | 17/78 (21.8) | 1/27 (3.7) | 16/51 (31.4) | |
| Quinolones | 2 | 2 | 0 | |
| Trimethoprim-sulfamethoxazole | 3 | 0 | 3 | |
| Combination therapy | 20/98 (20.4) | 2/29 (6.9) | 18/69 (26.1) | 0.052 |
| Tigecycline-based | 10/20 (50.0) | 0 | 10/18 (55.6) | |
| Tigecycline + aminoglycosides | 5 | 0 | 5 | |
| Tigecycline + carbapenem | 2 | 0 | 2 | |
| Tigecycline + polymyxin Ba | 2 | 0 | 2 | |
| Tigecycline + trimethoprim-sulfamethoxazole | 1 | 0 | 1 | |
| Carbapenem + quinolones | 3 | 0 | 3 | |
| Aminoglycoside-based | 3 | 2 | 1 | |
| Aminoglycosides + quinolones | 1 | 0 | 1 | |
| Aminoglycosides + fourth-generation cephalosporin | 1 | 1 | 0 | |
| Aminoglycosides + aztreonam + third-generation cephalosporin | 1 | 1 | 0 | |
| Including β-lactam–β-lactamases inhibitor | 4 | 0 | 4 | |
| β-Lactam–β-lactamases inhibitor + fourth-generation cephalosporin | 1 | 0 | 1 | |
| β-Lactam–β-lactamases inhibitor + aztreonam | 1 | 0 | 1 | |
| β-Lactam–β-lactamases inhibitor + minocycline | 1 | 0 | 1 | |
| β-Lactam–β-lactamases inhibitor + quinolones | 1 | 0 | 1 | |
Although polymyxin B is not available in China, two patients were treated with this drug, which was purchased from abroad.
Among 19 patients who died after tigecycline monotherapy, 16 were infected by CRE with meropenem MICs of >8 mg/liter, 18 were infected by blaKPC-2 producers, and 16 were infected by forms highly resistant to amikacin at MICs of more than 256 mg/liter. All isolates from this group were resistant to quinolones (data not shown). Among all patients who received tigecycline monotherapy, there was no statistically significant difference between survivors and patients who died in terms of age (P = 0.538), the Pitt bacteremia score (P = 0.124), the Charlson comorbidity index (P = 0.809), or the number of days of delay in tigecycline treatment (P = 0.401).
Fourteen-day mortality.
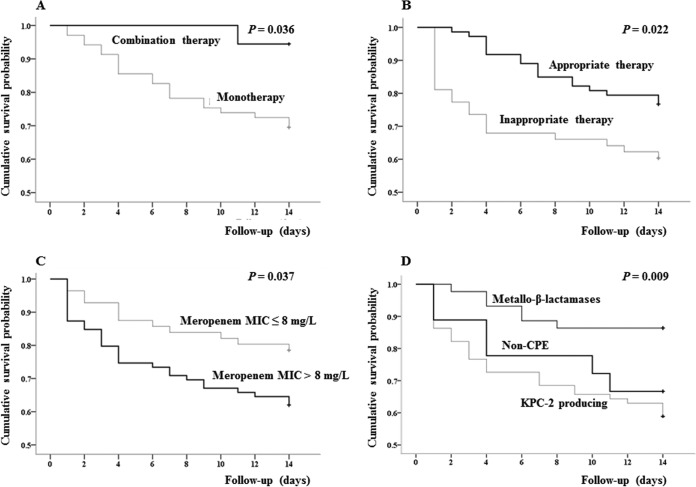
Excluding 29 of 164 cases for whom therapy was abandoned without a clinical cure or improvement and who were in the hospital for less than 14 days after the first positive blood culture, the 14-day mortality rate was 31.1% (42/135). Kaplan-Meier survival curves are shown in Fig. 3 and indicate that mortality was statistically significantly higher among patients infected by isolates with meropenem MICs of >8 mg/liter than among patients infected by isolates with meropenem MICs of ≤8 mg/liter (P = 0.037). The rate of mortality was significantly lower among patients infected by isolates with metallo-β-lactamase than among patients infected by isolates with KPC-2 and isolates without carbapenemase production (P = 0.009). The rate of mortality was also significantly higher after combination therapy than after monotherapy (P = 0.036), as well as after appropriate therapy than after inappropriate therapy (P = 0.022).
FIG 3.
Kaplan-Meier curves showing the impact on 14-day mortality of monotherapy and combination therapy (A), appropriate and inappropriate therapy (B), infection by isolates with meropenem MICs of >8 mg/liter and ≤8 mg/liter (C), and infection by isolates with or without KPC-2 (D). Patients (29/164) for whom therapy was abandoned without clinical cure or improvement were excluded from evaluation of the impact of therapy on 14-day mortality, as these 29 patients stayed in hospitals for less than 14 days after a blood sample for culture was collected. +, right censoring of data; Non-CPE, CRE isolates without carbapenemases.
DISCUSSION
Bacteremia remains a significant cause of morbidity and mortality in patients admitted to hospitals (21, 22). Indeed, the rates of in-hospital and 14-day mortality in our study were 32.9% (54/164) and 31.1% (42/135), respectively, which are at the lower end of previously reported rates of approximately 20% to 70% (23, 24). Critical illness, characterized by a Pitt bacteremia score of ≥6, a Charlson comorbidity index of ≥3, and the onset of septic shock, was independently associated with a hazard effect on in-hospital mortality. We also found that infections with K. pneumoniae, KPC-2 producers, and isolates with meropenem MICs of >8 mg/liter resulted in poor outcomes, in line with the findings of Wang et al. (25), who reported that infections by isolates with meropenem MICs of ≥8 mg/liter, regardless of species, were associated with poorer outcomes than infections by Enterobacteriaceae with meropenem MICs of 2 to 4 mg/liter or ≤1 mg/liter. In contrast, survival was better among patients with infections by isolates with metallo-β-lactamase than among patients with infections by isolates with KPC-2 or without a carbapenemase. The probable reason for these disparities in survival is that K. pneumoniae has always been highly resistant to carbapenems and is considered a multidrug- or even pan-drug-resistant organism that acquires several resistance elements from the environment and against which few treatments are left. We also noted that resistance to amikacin and quinolones was higher in KPC-2 producers than in non-KPC-2-producers, implying that amikacin- and/or quinolone-sparing regimes are preferable (15). We also recommend that for CRKP bacteremia, phenotyping by methods such as modified carbapenem inactivation with or without EDTA (3, 26, 27) is essential in order to detect KPC-2, a serine enzyme, or NDM, a metallo-β-lactamase.
Early interventions against CRE bacteremia are often emphasized (28), but the timing of initiation of therapy is controversial. One meta-analysis found that compared with the rate of survival from a carbapenem-susceptible K. pneumoniae infection, the rate of survival from a CRKP infection increases with therapy using ≥1 active drug within 48 h at the correct doses (28). In contrast, Gutiérrez-Gutiérrez et al. (29) noted that although therapy with at least one active drug at the correct doses enhances the 30-day mortality rate if it is administered within 5 days or less, similar therapy within 2 days does not seem to be any more effective. Similarly, we found that therapy with at least one active drug at conventional doses within 5 days or less after the onset of bacteremia resulted in outcomes superior to those of therapy with inactive drugs or therapy with active drugs ≥5 days after bacteremia. This result suggests that administration of active drugs within 96 h and timely antimicrobial susceptibility testing (AST) might improve the clinical outcomes.
In addition to reducing the development of resistance in the clinic and the environment (30), accumulating evidence shows that combination therapy boosts survival compared to monotherapy, especially in patients with bacteremia and patients who are critically ill (31–33). Similarly, we found that combination therapy seems to be more effective than monotherapy, although monotherapy is not any more effective than therapy with inactive drugs, as previously reported by Tzouvelekis et al. (34). Monotherapy was not superior to inactive therapy, probably because of the high rate of mortality in the tigecycline monotherapy group. Although an optimal combination therapy against CRE has not yet been established (30), combination therapy that includes meropenem was associated with significantly better survival. Indeed, regimens based on two carbapenems were previously recommended for CRKP infection when meropenem MICs were ≤8 mg/liter (11, 33). In addition, the rates of failure of CRE clearance were the lowest at 10.0% and 5.3%, respectively, following combination therapy and therapy with carbapenem alone or in combination with at least 1 inactive antibiotic. Similarly, the rate of treatment failure following tigecycline-based combination therapy was low at 11.1%, although the rate of treatment failure was 53.6% following therapy with tigecycline alone or in combination with at least 1 inactive drug. In-hospital mortality and the failure of CRE clearance following regimes that include carbapenem were higher for infections by isolates with MICs of >8 mg/liter than for infections by isolates with meropenem MICs of ≤8 mg/liter. These findings indicated that tigecycline monotherapy, as well as therapy with inactive drugs or other inappropriate therapy, might be avoided.
To the best of our knowledge, this is the first investigation in China of hazard factors and clinical outcomes from infections by CRE that were after various therapies and that had various resistance mechanisms and resistance levels. However, the data require further validation by randomized controlled trials, since this study was retrospective and observational. Moreover, data on colistin therapy are missing because this drug is not available in China, although data on colistin therapy were available for two patients who were able to purchase colistin abroad. Additionally, we did not analyze whether salvage high-dose carbapenem or prolonged infusion therapy was associated with improved outcomes of infections by strains highly resistant to carbapenem, since few such cases were present in the study. We intend to address these limitations and clarify the present results using a larger observational study or even a prospective cohort study.
In conclusion, patients with severe illness, patients infected with isolates highly resistant to meropenem (MICs >8 mg/liter), and patients infected with blaKPC-2 producers had poor outcomes. Appropriate therapy and combination therapy were associated with a protective effect on 14-day mortality among patients with CRE bacteremia.
MATERIALS AND METHODS
Setting.
A study of data for patients with bacteremia due to CRE in a CRE network of 36 tertiary hospitals in 19 provinces between 6 April 2013 and 28 July 2017 was conducted. Membership in the network was voluntary, although cases were included only if CRE isolates were preserved and were available along with clinical records collected until patient discharge or death. Also, only the first episode per CRE strain for each patient was considered. Data for emergency cases were excluded, along with those for outpatients, patients who died <24 h after collection of the first blood sample for culture, and cases with missing key data, especially the therapies provided and outcomes. K. pneumoniae, Escherichia coli, Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii, and Serratia marcescens were identified by local hospital laboratories as CREs if MICs were ≥2 mg/liter for meropenem or imipenem and ≥1 mg/liter for ertapenem, following the breakpoints set by Clinical and Laboratory Standards Institute in 2017 (http://www.clsi.org).
Bacterial isolates and microbiological detection.
Isolates were sent to the central laboratory at the Peking University People’s Hospital, a 1,884-bed tertiary hospital, for reidentification of CRE by both antimicrobial susceptibility testing (AST) and molecular analysis of resistance mechanisms. Bacteria were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker Daltonics Inc., Billerica, MA, USA) or by use of a Vitek GNI system (bioMérieux Vitek Inc., Hazelwood, MO, USA). MICs were determined by agar dilution or broth microdilution according to 2018 guidelines described in CLSI document M07-A10 (2015) (35) and CLSI document M100-S28 (http://www.clsi.org). Breakpoints for tigecycline and colistin were set according to the standards of the U.S. Food and Drug Administration and the guidelines of the European Committee on Antimicrobial Susceptibility Testing (http://www.eucast.org), respectively.
K. pneumoniae, E. coli, and E. cloacae were also characterized by PCR-based multilocus sequence typing according to protocols described at http://bigsdb.pasteur.fr/klebsiella/klebsiella.html, http://mlst.warwick.ac.uk/mlst/dbs/Ecoli, and https://pubmlst.org/ecloacae/, respectively. PCR was also used to detect common Chinese carbapenemase genes (blaKPC, blaNDM, blaIMP, blaOXA-48, and blaVIM) and other β-lactamase genes (blaSHV, blaTEM, blaCTX-M, blaDHA, and blaCMY) (36).
Clinical and epidemiological data.
Medical records were reviewed to determine whether infections were nosocomial (37) and to obtain the date and location of CRE isolation, demographic characteristics, a history of hospital or department transfer, the health care exposure history 1 year prior to the positive CRE result, the Charlson comorbidity index, and the severity of acute conditions as evaluated by the Pitt bacteremia score (38). The following were also determined: the source of bacteremia based on clinical and microbiological data (39); recent exposure to invasive procedures; antimicrobial treatment 30 days prior to collection of the first blood sample positive by culture; treatments, such as intravenous antimicrobial therapy and operations on infected foci; and clinical outcomes, including sustained microbiological clearance, i.e., at least one negative subsequent blood culture after the first positive blood culture. Overall in-hospital mortality and all-cause 14-day mortality were the primary and secondary endpoints, respectively. We note that antimicrobial treatment decisions were made based only on initial phenotypic resistance reports. Clinical and epidemiological data were reviewed by two clinical microbiological professionals at Peking University People’s Hospital, and queries were sent to participating hospitals and clinical professors were consulted as needed to clarify contradictory or inconsistent information.
Definitions.
Imipenem and meropenem were considered active if in vitro MICs were 8 mg/liter or less (28) and if the dose and the route of delivery of carbapenem were consistent with conventional medical standards or combined with other active drugs. We considered tigecycline to be active if the MICs were less than or equal to 4 mg/liter and if it was administered at a first dose of 100 mg followed by 50 mg every 12 h. Other antimicrobials were considered active if the infecting pathogens were found to be susceptible according to the breakpoints set by the Clinical and Laboratory Standards Institute and if the dose and the route of delivery were consistent with conventional medical standards. Early appropriate therapy was defined as treatment provided with at least one active drug within 48 h after the onset of CRE bacteremia, while appropriate therapy was defined as treatment provided with at least one active drug within 5 days of the first positive blood culture. Inappropriate therapy was defined as therapy that included at least one active drug but that started after more than 5 days after the onset of CRE bacteremia or therapy without an active drug after the onset of CRE bacteremia (28, 29). Empiric therapy and definitive therapy were defined as treatments provided at any time before and after AST results were available, respectively. Combination therapy was defined as treatment with more than one active drug, while monotherapy was defined as treatment with only one active antibiotic (28, 29). If treatments were modified, the final regime was considered in the analysis if the outcome was poor. However, the prior treatment was considered in the analysis if microbiological clearance, i.e., a negative blood culture within 2 weeks (28, 29), was achieved before treatments were altered. Clinical cure was defined as eradication of all signs or symptoms of bacteremia. Deaths within 14 days from the first positive blood culture were considered in determining 14-day mortality rates.
Statistical analysis.
Variables were described using median (interquartile range) or frequency count according to the type of variable. We compared categorical variables by Pearson’s χ2 or Fisher’s exact test if cells had a frequency of 5 or less and continuous variables by Wilcoxon-Mann-Whitney U tests for nonnormally distributed variables or Student’s t test for normally distributed variables. The subdivisions of survival and death were compared to predict the risk factors for mortality. The risk factors for CRE infection were evaluated using univariable logistic regression, as listed by odds ratios (ORs) and the corresponding 95% confidence intervals (95% CIs). All univariables with a P value of 0.20 or less for overall in-hospital mortality were included in the multivariate logistic regression models by manually selecting them in a backward stepwise manner. Survival for 14 days from the first positive CRE culture was plotted as Kaplan-Meier curves, and the rates of survival were compared by the log rank test to evaluate the effect of treatments, meropenem MICs of ≤8 mg/liter and >8 mg/liter, and resistance mechanisms. All tests were two-tailed, and significance was set at 0.05 in SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA). Susceptibility rates were determined using WHONET software (version 5.6).
Ethics approval.
The study was reviewed and granted ethical exemption by the medical ethics committee of the Peking University People’s Hospital and was approved by each participating hospital according to local requirements. Informed consent was not needed because the study was observational and retrospective. Data were anonymized.
Supplementary Material
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (grant no. 81625014 and 81661138006).
The National Natural Science Foundation of China did not participate in study design, data collection and interpretation, or decision to publish.
For providing CRE isolates and reviewing medical records, we thank Yang Li at the Department of Clinical Microbiology, Peking University International Hospital; Dawen Guo at the Department of Clinical Microbiology, The First Affiliated Hospital of Harbin Medical University; Zhiwu Liu at the Department of Clinical Microbiology, The First Hospital of Lanzhou University; Xiaoqian Zhang at the Department of Clinical Microbiology, Henan Province Hospital of TCM; Zhongju Chen at the Department of Clinical Microbiology, Tongji Medical College of Huazhong University of Science & Technology; Peihong Yang at the Department of Clinical Laboratory, The Fourth Military Medical University Xijing Hospital; Xiaobo Ma at the Department of Clinical Laboratory, The First Affiliated Hospital of Xiamen University; Fengbo Zhang at the Department of Clinical Laboratory, The First Affiliated Hospital of Xinjiang Medical University; Yanping Luo at the Department of Clinical Laboratory, Chinese PLA General Hospital; Na Zhang at the Department of Clinical Laboratory, Ordos City Central Hospital; Liangyi Xie at the Department of Clinical Laboratory, Hunan Provincial People’s Hospital; Wei Li at the Department of Clinical Laboratory, Qilu Hospital of Shandong University; Sijin Man at the Department of Clinical Laboratory, Tengzhou Central People's Hospital; Ji Zeng at the Department of Clinical Microbiology, Puai Hospital of Tongji Medical College of Huazhong University of Science & Technology; Hong Zou at the Department of Clinical Microbiology, The First People's Hospital of Xiangtan City; Wenen Liu at the Department of Clinical Microbiology, Xiangya Hospital Central South University; Cuimei Zou at the Department of Clinical Laboratory, The First Affiliated People’s Hospital of Yinchuan; Hongli Sun at the Department of Clinical Laboratory, Peking Union Medical College Hospital; Zhusheng Guo at the Department of Clinical Laboratory, Dongguan Tungwah Hospital; Jiaming Huang at the Department of Clinical Laboratory, The Second Affiliated Hospital of Fujian Medical University; Longjie Gan at the Department of Clinical Laboratory, The First Affiliated Hospital of Fujian Medical University; Chao Zhuo at the Department of Respiratory Medicine, The First Affiliated Hospital of Sun Yat-Sen University; Jinjing Tian at the Department of Clinical Laboratory, The Second People’s Hospital of Liaocheng; Yuanyuan Xu at the Department of Clinical Laboratory, Wanbei Coal-Electricity Group General Hospital; and Xiaoying Li at the Department of Clinical Laboratory, Weifang Traditional Chinese Hospital. For assistance with epidemiological statistics, we thank Huixin Liu of the Peking University People’s Hospital, Yanyan Shi of the Peking University Third Hospital, and Yu Xie of the Institute of Medical Information, Chinese Academy of Medical Sciences and Peking Union Medical College.
We thank Editage (www.editage.cn) for English language polishing.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01511-18.
REFERENCES
- 1.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers AJ, Peirano G, Pitout JD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, Schlaeffer F, Sherf M. 2009. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. 2014. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 20:1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman ND, Carmeli Y, Walton AL, Schwaber MJ. 2017. Carbapenem-resistant Enterobacteriaceae: a strategic roadmap for infection control. Infect Control Hosp Epidemiol 38:580–594. doi: 10.1017/ice.2017.42. [DOI] [PubMed] [Google Scholar]
- 9.Tuon FF, Rocha JL, Formigoni-Pinto MR. 2018. Pharmacological aspects and spectrum of action of ceftazidime-avibactam: a systematic review. Infection 46:165–181. doi: 10.1007/s15010-017-1096-y. [DOI] [PubMed] [Google Scholar]
- 10.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagacé-Wiens PRS, Walkty A, Denisuik A, Golden A, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2018. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs 78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. 2018. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev 31:e00079-17. doi: 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. 2016. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother 17:761–781. doi: 10.1517/14656566.2016.1145658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trecarichi EM, Tumbarello M. 2017. Therapeutic options for carbapenem-resistant Enterobacteriaceae infections. Virulence 8:470–484. doi: 10.1080/21505594.2017.1292196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassetti M, Peghin M, Pecori D. 2016. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis 29:583–594. doi: 10.1097/QCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 16.Chinese XDR Consensus Working Group, Guan X, He L, Hu B, Hu J, Huang X, Lai G, Li Y, Liu Y, Ni Y, Qiu H, Shao Z, Shi Y, Wang M, Wang R, Wu D, Xie C, Xu Y, Yang F, Yu K, Yu Y, Zhang J, Zhuo C. 2016. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect 22:S15–S25. doi: 10.1016/j.cmi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Nabarro LE, Veeraraghavan B. 2015. Combination therapy for carbapenem-resistant Enterobacteriaceae: increasing evidence, unanswered questions, potential solutions. Eur J Clin Microbiol Infect Dis 34:2307–2311. doi: 10.1007/s10096-015-2486-7. [DOI] [PubMed] [Google Scholar]
- 18.Tamma PD, Goodman KE, Harris AD, Tekle T, Roberts A, Taiwo A, Simner PJ. 2017. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 64:257–264. doi: 10.1093/cid/ciw741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villegas MV, Pallares CJ, Escandón-Vargas K, Hernández-Gómez C, Correa A, Álvarez C, Rosso F, Matta L, Luna C, Zurita J, Mejía-Villatoro C, Rodríguez-Noriega E, Seas C, Cortesía M, Guzmán-Suárez A, Guzmán-Blanco M. 2016. Characterization and clinical impact of bloodstream infection caused by carbapenemase-producing Enterobacteriaceae in seven Latin American countries. PLoS One 11:e0154092. doi: 10.1371/journal.pone.0154092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balkan II, Aygün G, Aydın S, Mutcalı SI, Kara Z, Kuşkucu M, Midilli K, Şemen V, Aras S, Yemişen M, Mete B, Özaras R, Saltoğlu N, Tabak F, Öztürk R. 2014. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis 26:51–56. doi: 10.1016/j.ijid.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Amit S, Mishali H, Kotlovsky T, Schwaber MJ, Carmeli Y. 2015. Bloodstream infections among carriers of carbapenem-resistant Klebsiella pneumoniae: etiology, incidence and predictors. Clin Microbiol Infect 21:30–34. doi: 10.1016/j.cmi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. 2012. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 24.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. 2009. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol 30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Zhang Y, Yao X, Xian H, Liu Y, Li H, Chen H, Wang X, Wang R, Zhao C, Cao B, Wang H. 2016. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis 35:1679–1689. doi: 10.1007/s10096-016-2710-0. [DOI] [PubMed] [Google Scholar]
- 26.Kuchibiro T, Komatsu M, Yamasaki K, Nakamura T, Nishio H, Nishi I, Kimura K, Niki M, Ono T, Sueyoshi N, Kita M, Kida K, Ohama M, Satoh K, Toda H, Mizutani T, Fukuda N, Sawa K, Nakai I, Kofuku T, Orita T, Watari H, Shimura S, Fukuda S, Nakamura A, Wada Y. 2018. Evaluation of the modified carbapenem inactivation method for the detection of carbapenemase-producing Enterobacteriaceae. J Infect Chemother 24:262–266. doi: 10.1016/j.jiac.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Zavascki AP, Bulitta JB, Landersdorfer CB. 2013. Combination therapy for carbapenem-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther 11:1333–1353. doi: 10.1586/14787210.2013.845523. [DOI] [PubMed] [Google Scholar]
- 28.Kohler PP, Volling C, Green K, Uleryk EM, Shah PS, McGeer A. 2017. Carbapenem resistance, initial antibiotic therapy, and mortality in Klebsiella pneumoniae bacteremia: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 38:1319–1328. doi: 10.1017/ice.2017.197. [DOI] [PubMed] [Google Scholar]
- 29.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap ÖK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J, REIPI/ESGBIS/INCREMENT Investigators. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 30.Alhashem F, Tiren-Verbeet NL, Alp E, Doganay M. 2017. Treatment of sepsis: what is the antibiotic choice in bacteremia due to carbapenem resistant Enterobacteriaceae? World J Clin Cases 5:324–332. doi: 10.12998/wjcc.v5.i8.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 32.Falcone M, Russo A, Iacovelli A, Restuccia G, Ceccarelli G, Giordano A, Farcomeni A, Morelli A, Venditti M. 2016. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 34.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 36.Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 38.Rhee JY, Kwon KT, Ki HK, Shin SY, Jung DS, Chung DR, Ha BC, Peck KR, Song JH. 2009. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock 31:146–150. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
- 39.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.