The determination of antibiotic potency against bacterial strains by assessment of their minimum inhibitory concentration normally uses a standardized broth microdilution assay procedure developed more than 50 years ago. However, certain antibiotics require modified assay conditions in order to observe optimal activity.
KEYWORDS: MIC, additive effects, broth microdilution assay, microtiter plate effects
ABSTRACT
The determination of antibiotic potency against bacterial strains by assessment of their minimum inhibitory concentration normally uses a standardized broth microdilution assay procedure developed more than 50 years ago. However, certain antibiotics require modified assay conditions in order to observe optimal activity. For example, daptomycin requires medium supplemented with Ca2+, and the lipoglycopeptides dalbavancin and oritavancin require Tween 80 to be added to the growth medium to prevent the depletion of free drug via adsorption to the plastic microplate. In this report, we examine systematically the effects of several different plate types on microdilution broth MIC values for a set of antibiotics against Gram-positive and Gram-negative bacteria, both in medium alone and in medium supplemented with the commonly used additives Tween 80, lysed horse blood, and 50% human serum. We observed very significant differences in measured MICs (up to 100-fold) for some lipophilic antibiotics, such as the Gram-positive lipoglycopeptide dalbavancin and the Gram-negative lipopeptide polymyxins, and found that nonspecific binding plates can replace the need for surfactant additives. Microtiter plate types and any additives should be specified when reporting broth dilution MIC values, as results can vary dramatically for some classes of antibiotics.
INTRODUCTION
Antibiotic effectiveness against bacterial strains is routinely assessed by a variety of methods, including disk diffusion assays, gradient diffusion methods (e.g., the commercial bioMérieux Etest or Thermo Scientific Oxoid M.I.C.Evaluator strip systems) and automated susceptibility testing (1) (such as the bioMérieux Vitek, BD Phoenix, Beckman Coulter MicroScan, and Thermo Fisher Scientific Sensititre systems). The gold standard reference assays are considered to be agar and broth dilution assays, in which the antibiotic of interest is serially diluted in 2-fold steps in agar or broth, with each plate or tube/well then inoculated with a defined number of bacteria. The MIC is then measured as the lowest concentration of drug that will inhibit visible growth of the organism after overnight incubation. A number of standardized procedures have been published. The most widely referenced is the Clinical and Laboratory Standards Institute (CLSI) M07-A11 (2) and accompanying M100-S28 supplement (3), which include procedures for agar and broth dilution assays. In 2006, the British Society for Antimicrobial Chemotherapy (BSAC) published a document available on their website (4) that also describes agar and broth dilution determinations, an updated version of a 2001 reference (5). Both the BSAC and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) have methods available on their websites for standardized disk diffusion assays. The EUCAST website refers to recommendations from the International Organization for Standardization (ISO) for broth MIC determination for nonfastidious organisms, with modified medium for fastidious organisms (http://www.eucast.org/ast_of_bacteria/mic_determination/?no_cache=1). The relevant ISO guidance document is ISO 20776-1 (6).
The published reference procedures for broth dilution assays do not generally specify the type and nature of the container in which the assays should be conducted, with the CLSI M07-A11 document listing “sterile 13 × 100-mm test tubes” for the macrodilution procedure and “plastic microdilution trays that have round or conical bottom wells” for the microdilution procedure (2). The one exception is a CLSI-EUCAST working group recommendation in 2016 that surfactants should not be included in the reference broth microdilution method for colistin, and that untreated polystyrene (PS) trays should be employed (7). Agar and broth dilution methods are also reported in a 2008 Nature Protocols article (8), which is one of the very few published protocols that mentions the potential for plate composition effects on MIC potency. This report showed that cationic antimicrobial peptides (AMPs) have reduced MICs in tissue culture-treated PS plates compared to those in polypropylene (PP) plates, and that the addition of acetic acid/bovine serum albumin (BSA) alleviated this effect (8). The adherence of cationic AMPs to PS (particularly tissue-culture treated PS) is also mentioned in a note in a 2007 Methods in Molecular Biology chapter, which recommended the use of PP plates (9).
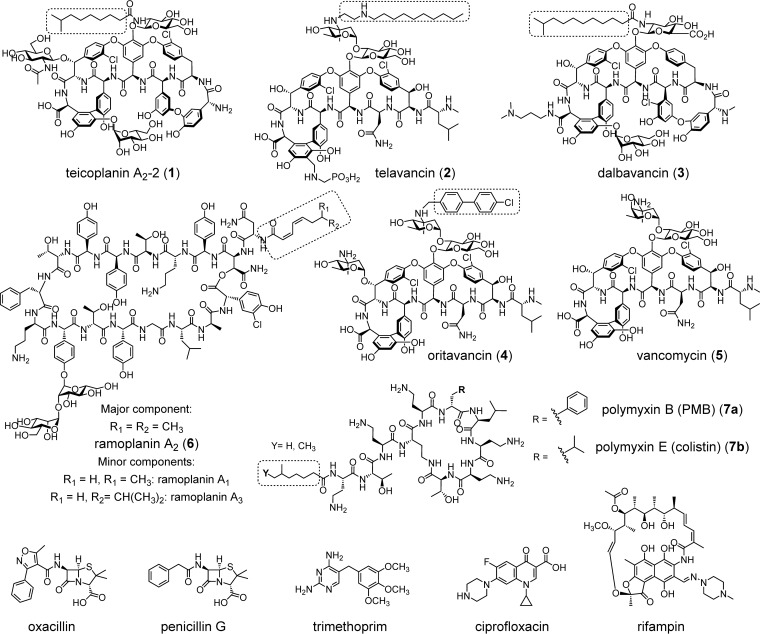
Some antibiotics, such as the lipoglycopeptides teicoplanin (compound 1), telavancin (compound 2), dalbavancin (compound 3), oritavancin (compound 4), and ramoplanin (compound 6) (Fig. 1), must be solubilized in dimethyl sulfoxide (DMSO) or 0.002% polysorbate 80 (Tween 80) in water to prevent adherence to plastic surfaces, including assay plates (10–14), with other additives, such as 2% lysed horse blood (LHB) (10) or 0.02% bovine serum albumin (BSA) (15, 16) found to have a similar blocking effect as surfactant. Notably, the closely related glycopeptide vancomycin (compound 5), without a lipophilic moiety, does not require surfactant supplement. Similarly, MIC determinations of the lipopeptide polymyxin class of antibiotics (polymyxin B [compound 7a] and polymyxin E or colistin, compound 7b) have been found to be affected by additives (0.002% polysorbate) (17–20) and assay container composition (21). During the preparation of this paper, two new reports described container effects on polymyxin MIC determinations (22, 23).
FIG 1.
Chemical structures of lipophilic antibiotics and nonlipophilic comparators: teicoplanin (compound 1), telavancin (compound 2), dalbavancin (compound 3), oritavancin (compound 4), vancomycin (compound 5), ramoplanin (compound 6), polymyxin B (compound 7a), colistin (compound 7b), oxacillin, penicillin G, trimethoprim, ciprofloxacin, and rifampin. Lipophilic groups are highlighted with a dashed box.
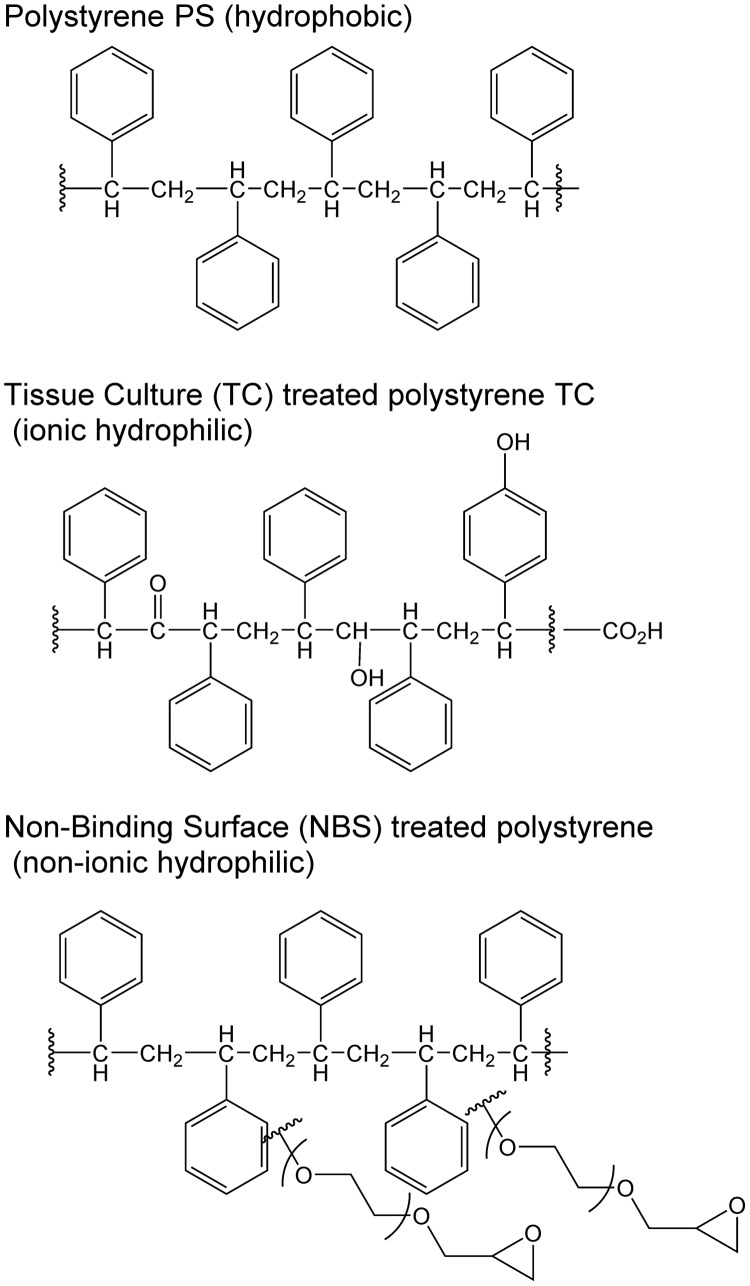
Microtiter plates used in broth microdilution assays are generally made from PS, but several different types of surface modifications are available. For example, Corning offers over 10 types of surface treatments for microplates, many of them designed to specifically bind cells or biomolecules. Untreated PS is considered a medium binding surface that is hydrophobic and binds biomolecules through passive interactions. The standard tissue culture-treated (TC-treated) surface, used for the attachment and growth of anchorage-dependent cells, is created by applying a corona discharge that grafts oxygen atoms onto the surface PS chains (Fig. 2) so that the surface becomes hydrophilic and negatively charged. Other binding surfaces include a high binding surface to bind biomolecules that possess ionic groups and/or hydrophobic regions, and surfaces coated with poly-d-lysine, sulfhydryl, carbohydrate, amine, or photoreactive groups that can be used to covalently immobilize biomolecules.
FIG 2.
Plate surface modifications of polystyrene for tissue culture (76) and nonbinding surface (77) plates.
Some surfaces are designed to minimize binding, including Corning’s nonbinding surface (NBS), a proprietary treatment technology that creates a polyethylene oxide-like nonionic hydrophilic surface to minimize nonspecific molecular interactions (Fig. 2). The NBS surface has been compared to untreated PS and PP for the binding of radiolabeled proteins; BSA bound to PS at 450 ng/cm2 and to PP at 440 ng/cm2 but to the NBS-coated PS at <2.5 ng/cm2 (78), so the NBS plate is recommended to reduce protein binding during assays.
Similarly, Thermo Scientific Nunc offers a range of treated plates, generally based on PS, with various degrees of absorption characteristics; the Nunc MiniSorp and GeNunc module surfaces have very low nonspecific binding characteristics, due to specially formulated polyethylene resin. Unfortunately, some plates have optical characteristics unsuitable for MIC determinations requiring optical density or visual readouts of turbidity.
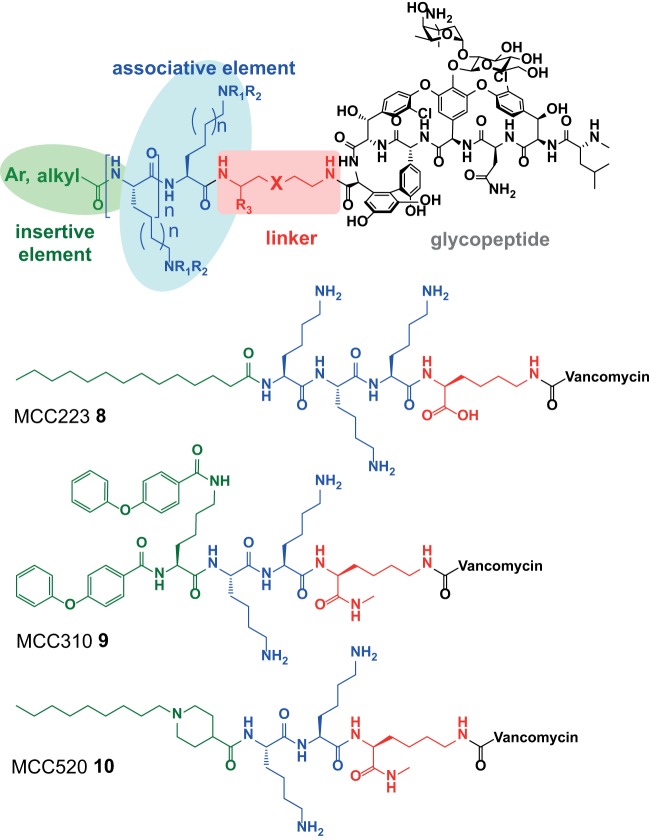
In the course of developing third-generation semisynthetic lipoglycopeptide antibiotics designed to selectively target bacterial membranes (24), our laboratory noted that nonbinding surface (NBS) plates provided significantly improved microdilution MIC values compared to other types of plates, and we also observed significant variations caused by added protein or surfactant depending on the plate type. As a result, we initiated a systematic comparison of several different plate types for microdilution assays, comparing various antibiotics against both Gram-positive and Gram-negative strains, and with/without a number of common additives. We were particularly interested in determining if a common plate type could prevent the need for specialized assay conditions for individual lipophilic antibiotics, driven by our internal drug discovery program on synthetic lipoglycopeptide vancomycin derivatives. These “vancapticins” increase the selectivity of vancomycin toward bacterial membranes by using an attached cationic “associative” peptide sequence terminated with an “insertive” lipophilic group (Fig. 3) (24). They possess high protein binding and a propensity to adhere to plastic surfaces, similar to the second-generation lipoglycopeptides telavancin (compound 2), dalbavancin (compound 3), and oritavancin (compound 4). The ability to avoid additives would simplify assay preparation, preventing errors due to incorrect concentrations of polysorbate. It would also avoid potential unexpected effects of added surfactants, given that a nonionic polyethylene glycol surfactant (Triton X-100) similar to Tween 80 (T80) used to avoid compound aggregation during other types of screening assays has been shown to unpredictably affect assay results (25). More importantly, it would enable better standardization of conditions and comparison of antimicrobial activity profiles between laboratories if a single plate type, with no need for broth additives, could be adopted.
FIG 3.
General design of the vancapticins, and specific structures of MCC223, MCC310, and MCC520.
RESULTS
Our studies were designed to examine the effects of different plate types on broth microdilution MIC determinations. Initially, we selected seven different PS-based plate types, as follows: Corning untreated flat-bottom, Corning TC-treated flat-bottom, Corning NBS-treated flat-bottom, Nunc untreated flat-bottom, Nunc TC-treated U-bottom, Nunc TC-treated flat-bottom, and Trek Diagnostics untreated flat-bottom plates. For each plate type, one Gram-positive organism was tested against seven antibiotics and one Gram-negative organism against seven antibiotics (see Fig. S1 in the supplemental material for experimental design). Antibiotics were selected to include those previously reported to exhibit plate- or surfactant-based MIC variations, as well as examples expected to not show an effect. All assays were conducted in both Mueller-Hinton broth (MHB) and in MHB supplemented with 0.002% T80 to see if the plate type could obviate surfactant. The second set of assays (Fig. S1) examined whether differences between plates seen in the first experiments were consistent across bacterial strains. Three plate types from one manufacturer (Corning untreated, TC treated, and NBS treated) were compared using the same sets of antibiotics but with six additional Gram-positive and three additional Gram-negative strains, again using MHB medium with and without T80. The final experiments (Fig. S1) compared the effects of different additive used to assess the effectiveness of antibiotics under physiological conditions, 50% human serum or 2% lung surfactant. These were done in four plate types (Trek PS untreated or Corning PS untreated, TC treated, and NBS treated), and were compared in MHB, MHB with added 0.002% T80, and MHB with added 2% LHB.
Experiment 1, initial plate comparison with or without Tween 80.
The first set of experiments compared MIC values determined in seven different plate types against one Gram-positive (methicillin-resistant Staphylococcus aureus [MRSA] ATCC 43300) and one Gram-negative (Escherichia coli ATCC 25922) organism conducted in MHB with and without 0.002% T80, using two sets of the following seven antibiotics for the two different classes of bacteria: vancomycin, ciprofloxacin, telavancin, dalbavancin, MCC233, MCC310, and MCC520 for MRSA; and colistin, ciprofloxacin, oxacillin, trimethoprim, polymyxin B, penicillin, and rifampin for E. coli (note that oxacillin and penicillin G generally have poor Gram-negative activity; they were included to assess if surfactant additives or plate types had synergistic effects, via damage to the bacterial membranes, that increased their potency by providing greater access to the periplasm/peptidoglycan). Literature value ranges for these antibiotics against MRSA and E. coli are listed in Table 1, with plate results tabulated in Table 2 and visualized in Fig. 4.
TABLE 1.
Literature MIC values for tested antibiotics
| Antibiotic | MIC (µg/ml) (reference[s]) |
|
|---|---|---|
| MRSA | E. coli | |
| Vancomycin | 2 (62) | |
| Ciprofloxacin | 0.5 (63) | 0.03–0.06 (64, 65) |
| Telavancin | 0.06 (66, 67) | |
| Dalbavancin | 0.06 (68, 69) | |
| Colistin | 0.5–2 (70) | |
| Oxacillin | >128 (71) | |
| Trimethoprim | 0.06–0.25 (72) | |
| Polymyxin B | 0.25–1 (73) | |
| Penicillin G | 16–64 (71) | |
| Rifampin | 0.015 (62) | 2 (74, 75) |
TABLE 2.
Comparison of 7 plate types regarding MIC versus MRSA ATCC 43300 in Mueller-Hinton broth in the presence and absence of Tween 80 (polysorbate 80)
| Plate | Medium | MIC (µg/ml) by antibiotic |
||||||
|---|---|---|---|---|---|---|---|---|
| Vancomycin | Ciprofloxacin | Telavancin | Dalbavancin | MCC223 | MCC310 | MCC520 | ||
| Nunc flat TC | −T80 | 1–2 | 1 | 0.5–1 | 0.25 | 2 | 4–8 | 4 |
| +T80 | 1 | 0.25–0.5 | 0.125– 0.25 | 0.125–0.25 | 1–2 | 2–4 | 2–4 | |
| Nunc U TC | −T80 | 0.5 | 0.125–0.25 | 0.5 | 0.25 | 2 | 2–4 | 4 |
| +T80 | 1 | 0.25–0.5 | 0.25–0.5 | 0.06–0.25 | 1–2 | 1–4 | 1–2 | |
| Nunc flat PS | −T80 | 1 | 0.125 | 0.06 | 0.5 | 0.5–1 | 1–2 | 0.25 |
| +T80 | 1 | 0.25–0.5 | 0.06 | 0.25 | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 | |
| Corning flat NBS | −T80 | 0.5 | 0.125–0.25 | 0.03–0.06 | 0.03–0.06 | ≤0.003 | ≤0.003 | 0.007 |
| +T80 | 1 | 0.5 | 0.125 | 0.125–0.5 | 0.003–0.007 | 0.007–0.015 | 0.015 | |
| Corning flat PS | −T80 | 0.5 | 0.25 | 0.06 | 0.5–1 | 0.5–1 | 4 | 0.125–0.25 |
| +T80 | 1–2 | 0.25–0.5 | 0.03–0.06 | 0.03–0.125 | 0.003–0.007 | ≤0.003 | 0.007 | |
| Corning flat TC | −T80 | 0.5 | 0.06–0.125 | 1 | 4 | 1–2 | 2–4 | 2–4 |
| +T80 | 1 | 0.125–0.5 | 0.125–0.25 | 0.125–0.25 | 1–2 | 2–4 | 4 | |
| Trek flat PS | −T80 | 0.5–1 | 0.125–0.5 | 0.03–0.06 | 0.25–0.5 | 0.5–1 | 4 | 0.125 |
| +T80 | 1 | 0.25–0.5 | 0.03–0.06 | 0.06 | ≤0.003 | ≤0.003 | 0.007–0.015 | |
FIG 4.
Comparison of antibiotic MICs versus MRSA (ATCC 43300) determined in seven different plate types, with and without the addition of 0.002% Tween 80.
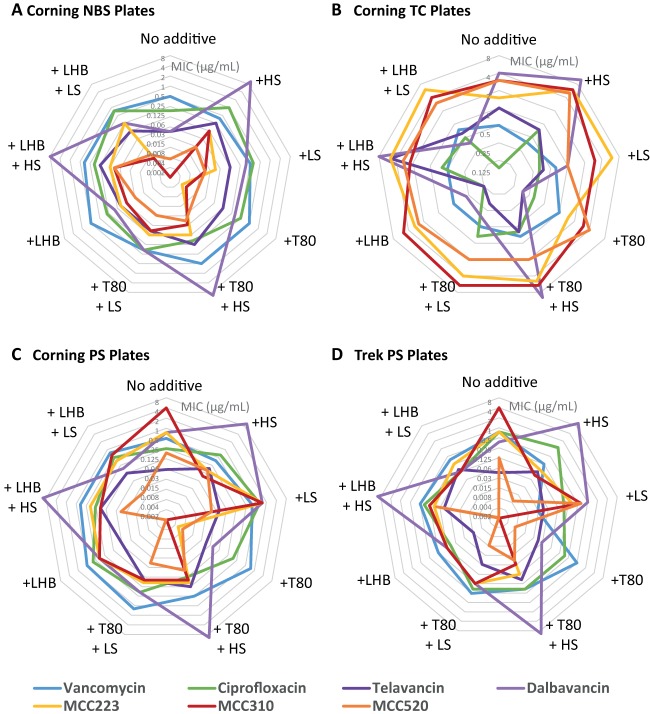
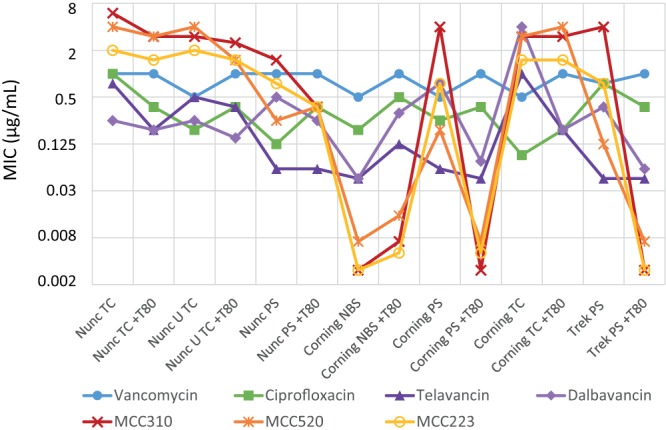
The control antibiotics with low protein binding and nonlipophilic characteristics, e.g., ciprofloxacin and vancomycin, showed little variations in MIC regardless of plate type or addition of T80 (vancomycin, around 1 µg/ml; ciprofloxacin, around 0.125 µg/ml). In contrast, large plate effects were observed for lipophilic antibiotics against both MRSA and E. coli, with some antibiotics (dalbavancin, the vancapticins, colistin, and polymyxin B) showing much more potent activity in NBS plates. The nontreated plates showed varied effects depending on the manufacturer, while the TC-coated plates uniformly showed decreased antibiotic activity. The presence of Tween 80 had little impact on NBS plate results (although MIC activity was slightly reduced in some cases), nor in Corning or Nunc TC plates, but resulted in much greater potency of some antibiotics in Corning and Trek PS plates, with less effect in Nunc PS plates. Notably, antibiotics that had improved potency with the addition of Tween 80 in untreated PS plates showed equivalent MIC values in NBS plates without added surfactant. Given that the control antibiotics with low protein binding and nonlipophilic characteristics showed little variation, it would appear to be unlikely that the plate or Tween itself is having a synergistic effect on antibacterial activity.
For the assays against MRSA, telavancin in all 3 untreated PS plates showed values around 0.06 µg/ml with and without added T80, similar to levels in NBS plates, while TC plates without additive produced higher values (0.5 to 1 µg/ml), which were reduced when T80 was added (0.125 µg/ml). Dalbavancin and the vancapticins showed much more pronounced variations, with >4-fold improvements (and up to >1,000-fold) when T80 was added to PS plates from two of the three manufacturers; improvements in Nunc PS plates were substantially less striking (1- to 8-fold). The Corning NBS plates without additive gave values comparable to those with PS plus T80 (e.g., ≤0.003 to 0.03 µg/ml). The addition of T80 to NBS plates resulted in 2-fold or greater reductions in potency. The TC plates consistently gave MICs of ≥2 µg/ml for these antibiotics, with minimal improvements upon the addition of T80.
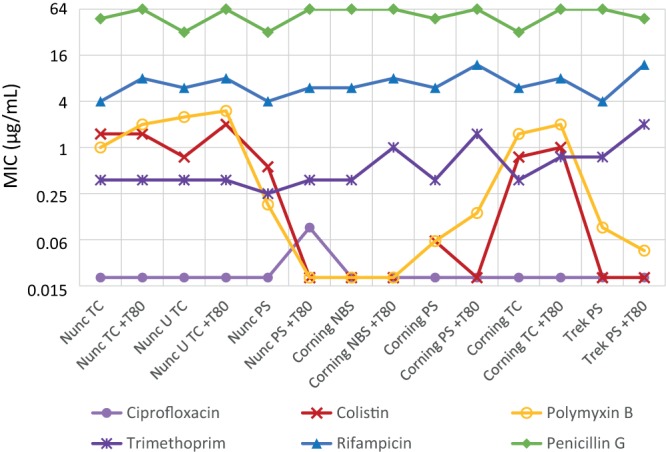
Similar variations were seen in the antibiotics tested against E. coli (Table 3 and Fig. 5). Ciprofloxacin, oxacillin, penicillin G, trimethoprim, and rifampin showed little variation across plate types, with or without added T80. In contrast, colistin (and, to a lesser extent, polymyxin B) showed the same trends as the lipoglycopeptides, as follows: PS plates gave more potent values (≤0.03 to 1 µg/ml) than TC plates (0.5 to 4 µg/ml), and the addition of T80 generally improved activity in PS plates (≤0.03 to 0.25 µg/ml) but not in TC or NBS plates. The NBS plates gave values equivalent to the best PS plus T80 results (≤0.03 µg/ml).
TABLE 3.
Comparison of 7 plate types regarding MIC versus E. coli ATCC 25922 in Mueller-Hinton broth in the presence and absence of Tween 80 (polysorbate 80)
| Plate | Medium | MIC (µg/ml) by antibiotic |
||||||
|---|---|---|---|---|---|---|---|---|
| Colistin | Ciprofloxacin | Oxacillin | Trimethoprim | Polymyxin B | Penicillin G | Rifampin | ||
| Nunc flat TC | −T80 | 1–2 | ≤0.03 | >64 | 0.25–0.5 | 1 | 32–64 | 4 |
| +T80 | 1–2 | ≤0.03 | >64 | 0.25–0.5 | 2 | 64 | 8 | |
| Nunc U TC | −T80 | 0.5–1 | ≤0.03 | >64 | 0.25–0.5 | 1–4 | 32 | 4–8 |
| +T80 | 2 | ≤0.03 | >64 | 0.25–0.5 | 2–4 | 64 | 8 | |
| Nunc PS | −T80 | 0.125–1 | ≤0.03 | >64 | 0.25 | 0.125–0.25 | 32 | 4 |
| +T80 | ≤0.03 | 0.06–0.125 | >64 | 0.25–0.5 | 0.03–0.06 | 64 | 4–8 | |
| Corning NBS | −T80 | ≤0.03 | ≤0.03 | >64 | 0.25–0.5 | ≤0.03 | 64 | 4–8 |
| +T80 | ≤0.03 | ≤0.03 | >64 | 1 | ≤0.03 | 64 | 8 | |
| Corning PS | −T80 | 0.06 | ≤0.03 | >64 | 0.25–0.5 | 0.06 | 32–64 | 4–8 |
| +T80 | ≤0.03 | ≤0.03 | >64 | 1–2 | 0.03–0.25 | 64 | 8–16 | |
| Corning TC | −T80 | 0.5–1 | ≤0.03 | >64 | 0.25–0.5 | 1–2 | 32 | 4–8 |
| +T80 | 1 | ≤0.03 | >64 | 0.5–1 | 2 | 64 | 8 | |
| Trek PS | −T80 | ≤0.03 | ≤0.03 | >64 | 0.5–1 | 0.06–0.125 | 64 | 4 |
| +T80 | ≤0.03 | ≤0.03 | >64 | 2 | 0.03–0.06 | 32–64 | 8–16 | |
FIG 5.
Comparison of antibiotic MICs versus E. coli (ATCC 25922) determined in seven different plate types, with and without the addition of 0.002% Tween 80 (note oxacillin not seen as >64 g/ml).
Experiment 2, plate plus Tween 80 comparison versus expanded set of bacteria.
In order to examine whether the plate variations in MIC extended across multiple types of bacteria, three plate types (PS, TC, and NBS) from the same manufacturer were compared, using the same combinations of seven antibiotics against either seven Gram-positive (Table S1 and Fig. S2) or four Gram-negative (Table S2 and Fig. S3) bacteria. The results were very similar to those observed from the first experiments across all strains tested, in that more polar antibiotics were generally unaffected by the plate type/additive, while lipoglycopeptides or lipopeptides were least potent in TC-without T80 plates and most potent in PS with T80, with equivalent activity in NBS without T80.
Experiment 3, plate plus additives comparison.
In assessing the activity of potential antibiotics, it is important to conduct assays in the presence of biological components that the antibiotics will encounter in the human body, namely, human serum (inactivation by protein binding) and lung surfactant (encountered when treating pneumonia). We therefore assessed four plate types (PS, TC, and NBS from Corning, and PS from Trek) in MHB, MHB plus 50% human serum (HS), and MHB plus 2% artificial lung surfactant (LS). For this experiment, in addition to testing the activity with no additive, and with T80, we also tested in the presence of 2% lysed horse blood (LHB), which was previously reported to have the same blocking effect as surfactant (10).
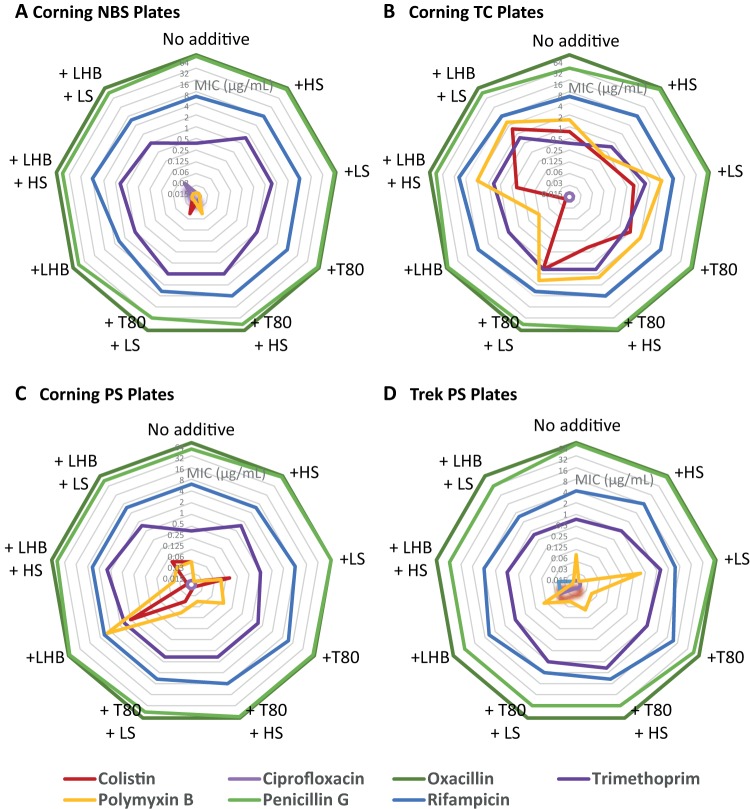
The Gram-positive antibiotic panel against MRSA ATCC 43300 (Table 4 and Fig. 6) demonstrated again that vancomycin and ciprofloxacin had little variation with plate type or any combination of T80, LHB, HS, or LS additive. Dalbavancin was strikingly inactivated in the presence of 50% HS under all plate type and additive conditions, with telavancin and vancapticin activities reduced to a lesser extent. In TC plates, T80 and LHB improved the activities of telavancin and dalbavancin but had little effect on vancapticin activity. In PS plates, telavancin was generally unaffected by the additives, while dalbavancin and the vancapticins showed significant improvement (>10-fold). NBS plates gave the most potent activity for all lipoglycopeptides but with reductions in activity when T80 or LHB was added.
TABLE 4.
Comparison of 4 plate types in Mueller-Hinton broth versus MRSA ATCC 43300 in the presence and absence of Tween 80 (polysorbate 80) or lysed horse blood (LHB), with added 50% human serum (HS) or 2% lung surfactant (LS)
| Plate | Medium | Additive | MIC (µg/ml) by antibiotic |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Vancomycin | Ciprofloxacin | Telavancin | Dalbavancin | MCC223 | MCC310 | MCC520 | |||
| Corning NBS | MH | None | 0.5 | 0.125 to 0.25 | 0.03 to 0.06 | 0.03 to 0.06 | ≤0.003 | ≤0.003 | 0.007 |
| 50% HS | 0.25 to 0.5 | 1 | 0.25 | >8 | 0.125 | 0.125 | 0.03 | ||
| 2% LS | 0.5 | 0.125 to 1 | 0.125 | 0.25 to 0.5 | 0.03 to 0.06 | 0.015 to 0.03 | 0.015 to 0.03 | ||
| MH + T80 | None | 1 | 0.5 | 0.125 | 0.125 to 0.5 | 0.003 to 0.007 | 0.007 | 0.015 | |
| 50% HS | 1 | 0.125 to 0.25 | 0.25 | >8 | 0.125 | 0.06 | 0.03 to 0.06 | ||
| 2% LS | 0.25 to 0.5 | 0.25 to 0.5 | 0.06 to 0.125 | 0.25 to 0.5 | 0.125 | 0.06 to 0.125 | 0.03 | ||
| MH + 2% LHB | None | 1 | 0.06 to 0.5 | 0.06 to 0.125 | 0.06 to 0.25 | 0.06 to 0.125 | 0.06 | 0.03 | |
| 50% HS | 0.5 to 1 | 0.25 to 0.5 | 0.25 | 8 | 0.125 | 0.06 to 0.125 | 0.06 to 0.125 | ||
| 2% LS | 0.5 to 1 | 0.5 to 1 | 0.125 | 0.25 | 0.25 | 0.015 to 0.06 | 0.015 | ||
| Corning PS | MH | None | 0.5 | 0.25 | 0.06 | 0.5 to 1 | 0.5 to 1 | 4 | 0.125 to 0.25 |
| 50% HS | 0.25 to 0.5 | 0.25 to 1 | 0.125 to 0.25 | >8 | 0.06 to 0.25 | 0.06 to 0.125 | 0.06 to 0.25 | ||
| 2% LS | 0.5 to 1 | 0.5 to 2 | 0.03 to 0.125 | 1 to 2 | 1 to 2 | 1 to 2 | 0.03 to 0.06 | ||
| MH + T80 | None | 1 to 2 | 0.25 to 0.5 | 0.03 to 0.06 | 0.03 to 0.125 | 0.003 to 0.007 | ≤0.003 | 0.007 | |
| 50% HS | 0.5 | 0.125 | 0.25 | >8 | 0.125 to 0.25 | 0.06 to 0.125 | 0.03 to 0.125 | ||
| 2% LS | 0.5 to 2 | 0.25 to 0.5 | 0.06 to 0.25 | 0.25 to 0.5 | 0.125 to 0.25 | 0.06 to 0.25 | 0.03 to 0.06 | ||
| MH + 2% LHB | None | 1 | 0.25 to 1 | 0.06 | 0.25 to 0.5 | 0.25 to 0.5 | 0.25 to 0.5 | ≤0.003 | |
| 50% HS | 0.5 to 1 | 0.03 to 0.5 | 0.125 to 0.25 | >8 | 0.25 to 0.5 | 0.125 to 0.25 | 0.03 to 0.06 | ||
| 2% LS | 0.5 to 1 | 0.5 | 0.125 | 0.125 to 0.25 | 0.25 to 0.5 | 0.25 to 1 | 0.015 to 0.03 | ||
| Corning TC | MH | None | 0.5 | 0.06 to 0.125 | 1 | 4 | 1 to 2 | 2 to 4 | 2 to 4 |
| 50% HS | 0.25 to 0.5 | 0.5 to 1 | 0.5 to 1 | >8 | 4 to >8 | 4 to >8 | 1 to >8 | ||
| 2% LS | 0.25 to 1 | 0.125 to 0.5 | 0.25 to 0.5 | 1 | 4 to 8 | 2 to 4 | 1 | ||
| MH + T80 | None | 1 | 0.125 to 0.5 | 0.125 to 0.25 | 0.125 to 0.25 | 1 to 2 | 2 to 4 | 4 | |
| 50% HS | 0.5 to 1 | 0.25 to 1 | 0.25 to 1 | >8 | 2 to 8 | 4 to 8 | 2 | ||
| 2% LS | 0.5 | 0.5 to 1 | 0.125 to 0.25 | 0.25 to 0.5 | 4 | 4 to 8 | 2 | ||
| MH + 2% LHB | None | 0.5 | 0.125 | 0.125 | 0.06 to 0.5 | 2 to 4 | 2 to 8 | 1 to 4 | |
| 50% HS | 0.5 | 0.25 to 1 | 2 to 8 | 8 | 2 to 8 | 0.5 to 4 | 2 to 4 | ||
| 2% LS | 0.5 to 1 | 0.5 | 0.25 to 1 | 0.25 to 0.5 | 4 to >8 | 4 | 2 to 4 | ||
| Trek PS | MH | None | 0.5 to 1 | 0.5 to 1 | 0.03 to 0.06 | 0.25 to 0.5 | 0.5 to 1 | 4 | 0.125 |
| 50% HS | 0.25 | 0.25 to 2 | 0.125 | >8 | 0.06 to 0.25 | 0.06 to 0.125 | 0.003 to 0.015 | ||
| 2% LS | 0.03 | 0.125 to 0.25 | 0.03 to 0.06 | 1 | 0.25 to 0.5 | 0.25 to 1 | 0.25 to 1 | ||
| MH + T80 | None | 1 | 0.25 to 0.5 | 0.03 to 0.06 | 0.06 | ≤0.003 | ≤0.003 | 0.007 | |
| 50% HS | 0.25 to 0.5 | 0.25 to 0.5 | 0.125 to 0.25 | 8 to >8 | 0.125 | 0.06 | 0.03 to 0.06 | ||
| 2% LS | 0.06 to 1 | 0.25 to 0.5 | 0.06 | 0.25 | 0.25 | 0.25 | 0.015 | ||
| MH + 2% LHB | None | 0.25 | 0.06 to 0.125 | 0.015 | 0.125 | 0.125 | 0.125 | 0.003 to 0.015 | |
| 50% HS | 0.5 | 0.25 to 0.5 | 0.06 to 0.125 | 8 to >8 | 0.125 to 0.25 | 0.06 to 0.5 | 0.06 to 0.25 | ||
| 2% LS | 0.25 to 0.5 | 0.125 | 0.06 to 0.25 | 0.125 | 0.25 | 0.125 | ≤0.003 | ||
FIG 6.
Comparison of antibiotic MICs against MRSA (ATCC 43300) determined in four plate types with and without the addition of 0.002% Tween 80 or LHB, with and without the addition of human serum or lung surfactant, as follows: Corning NBS (A), Corning TC (B), Corning PS (C), and Trek PS (D). Note that each hexagon represents one antibiotic-strain pair. If the MIC remains constant across plate types/conditions, the plot should be symmetrical, as is generally the case for vancomycin (blue).
In the Gram-negative antibiotic panel (Table 5 and Fig. 7), ciprofloxacin, oxacillin, penicillin G, trimethoprim, and rifampin again showed little variation even with HS or LS additives. Trimethoprim was marginally more active in their absence across multiple plate types, though this difference disappeared once T80 or LHB was added. Colistin and polymyxin B were most active in NBS plates and least active in TC plates regardless of T80, LHB, HS, or LS additive. Curiously, LHB in Corning PS plates greatly reduced colistin and polymyxin B activity compared to T80 but not in Trek PS plates, and in TC plates, the effect was reversed.
TABLE 5.
Comparison of 4 plate types in Mueller-Hinton broth versus E. coli ATCC 25922 in the presence and absence of Tween 80 (polysorbate 80) or lysed horse blood (LHB), with added 50% human serum (HS) or 2% lung surfactant (LS)
| Plate | Medium | Additive | MIC (µg/ml) by antibiotic |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Colistin | Ciprofloxacin | Oxacillin | Trimethoprim | Polymyxin B | Penicillin G | Rifampin | |||
| Corning NBS | MH | None | ≤0.03 | ≤0.03 | >64 | 0.25 to 0.5 | ≤0.03 | 64 | 4 to 8 |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 1 to 2 | ≤0.03 | 64 | 8 | ||
| 2% LS | ≤0.03 | ≤0.03 | >64 | 1 to 2 | ≤0.03 | 64 | 8 | ||
| MH + T80 | None | ≤0.03 | ≤0.03 | >64 | 1 | ≤0.03 | 64 | 8 | |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 2 | 0.03 to 0.06 | 32 to 64 | 8 | ||
| 2% LS | 0.03 to 0.06 | ≤0.03 | >64 | 2 | ≤0.03 | 32 | 4 to 8 | ||
| MH + 2% LHB | None | ≤0.03 | ≤0.03 | >64 | 1 | ≤0.03 | 32 to 64 | 2 to 4 | |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 1 to 2 | ≤0.03 | 32 to 64 | 8 | ||
| 2% LS | 0.03 to 0.06 | 0.03 to 0.06 | >64 | 1 | ≤0.03 | 32 to 64 | 4 to 8 | ||
| Corning PS | MH | None | 0.06 | ≤0.03 | >64 | 0.25 to 0.5 | 0.06 | 32 to 64 | 4 to 8 |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 1 to 2 | ≤0.03 | 64 | 4 to 8 | ||
| 2% LS | 0.06 to 0.25 | ≤0.03 | >64 | 1 | 0.06 to 0.125 | 64 to >64 | 8 | ||
| MH + T80 | None | ≤0.03 | ≤0.03 | >64 | 1 to 2 | 0.03 to 0.25 | 64 | 8 to 16 | |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 1 to 2 | 0.03 to 0.06 | 64 | 8 | ||
| 2% LS | 0.03 to 0.06 | ≤0.03 | >64 | 1 to 2 | 0.06 to 0.125 | 32 to 64 | 4 to 8 | ||
| MH + 2% LHB | None | 1 | ≤0.03 | >64 | 1 to 2 | 2 to 8 | 64 | 4 to 8 | |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 1 to 8 | 0.03 to 0.06 | 32 to >64 | 4 to 8 | ||
| 2% LS | 0.06 to 0.125 | ≤0.03 | >64 | 1 to 2 | 0.06 | 32 to 64 | 4 to 8 | ||
| Corning TC | MH | None | 0.5 to 1 | ≤0.03 | >64 | 0.25 to 0.5 | 1 to 2 | 32 | 4 to 8 |
| 50% HS | 0.125 to 0.5 | ≤0.03 | >64 | 0.5 to 1 | 0.25 to 0.5 | 64 | 8 | ||
| 2% LS | 0.5 to 1 | ≤0.03 | >64 | 1 to 2 | 4 | 64 | 8 | ||
| MH + T80 | None | 1 | ≤0.03 | >64 | 0.5 to 1 | 2 | 64 | 8 | |
| 50% HS | 0.25 to 0.5 | ≤0.03 | >64 | 1 to 2 | 1 to 4 | 64 | 8 | ||
| 2% LS | 1 to 2 | ≤0.03 | >64 | 1 to 2 | 2 to 4 | 32 to 64 | 4 to 8 | ||
| MH + 2% LHB | None | ≤0.03 | ≤0.03 | >64 | 1 | 0.125 | 64 | 8 | |
| 50% HS | 0.25 to 0.5 | ≤0.03 | >64 | 1 to 2 | 4 | 32 to 64 | 8 to 16 | ||
| 2% LS | 2 to 4 | ≤0.03 | >64 | 1 to 2 | 2 to 8 | 32 to 64 | 8 | ||
| Trek PS | MH | None | ≤0.03 | ≤0.03 | >64 | 0.5 to 1 | 0.06 to 0.125 | 64 | 4 |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 1 | ≤0.03 | 64 to >64 | 8 | ||
| 2% LS | ≤0.03 | ≤0.03 | >64 | 1 to 4 | 0.5 to 1 | 64 | 4 to 8 | ||
| MH + T80 | None | ≤0.03 | ≤0.03 | >64 | 2 | 0.03 to 0.06 | 32 to 64 | 8 to 16 | |
| 50% HS | ≤0.03 | ≤0.03 | >64 | 2 to 4 | 0.03 to 0.125 | 32 | 4 to 8 | ||
| 2% LS | ≤0.03 | ≤0.03 | >64 | 2 | 0.03 to 0.06 | 32 | 4 | ||
| MH + 2% LHB | None | 0.03 to 0.06 | 0.03 to 0.06 | >64 | 1 | 0.03 to 0.25 | 32 | 4 to 8 | |
| 50% HS | 0.03 to 0.06 | ≤0.03 | >64 | 1 | ≤0.03 | 32 | 4 | ||
| 2% LS | ≤0.03 | ≤0.03 | >64 | 0.5 to 1 | ≤0.03 | 32 | 2 to 4 | ||
FIG 7.
Comparison of antibiotic MICs against E. coli (ATCC 25922) determined in four plate types with and without the addition of 0.002% Tween 80 or LHB, with and without the addition of human serum or lung surfactant, as follows: Corning NBS (A), Corning TC (B), Corning PS (C), and Trek PS (D). Note that each hexagon represents one antibiotic-strain pair. If the MIC remains constant across plate types/conditions, the plot should be symmetrical, as is generally the case for rifampin (blue).
DISCUSSION
We have, for the first time, systematically evaluated plate-based effects on MIC determinations during broth microdilution assays. While MIC assay guidelines cover a range of experimental parameters, the composition of the assay vessel is generally not specified, other than the CLSI-EUCAST recommendations for colistin that state that plain PS should be employed (7).
The majority of antibiotic stock solutions for testing are prepared in water, phosphate buffer, or pH-adjusted aqueous solution (106 out of 139 reported in CLSI M100 [see Table 6A, “Solvents and diluents for preparation of stock solutions of antimicrobial agents”]) (3). However, several antibiotics, notably the lipophilic lipoglycopeptides telavancin (compound 2), dalbavancin (compound 3), and oritavancin (compound 4) (Fig. 1), must be solubilized in DMSO or 0.002% polysorbate 80 (Tween 80) in water (3). Furthermore, the CLSI reference MIC quality control range tabulated for dalbavancin and oritavancin are obtained in cation-adjusted Mueller-Hinton broth (CAMHB) supplemented with 0.002% polysorbate 80 (see Table 5A-1 in CLSI M100, “MIC QC ranges for nonfastidious organisms and antimcirobial agents”) (3). This requirement is consistent with published reports that 0.002% (final concentration) of polysorbate 80 is required for reproducible MIC testing of dalbavancin without substrate or medium constituent interference, due to poor antibiotic solubility and facile absorption to plastic surfaces (13). If the dalbavancin dilutions were in contact with plastic for as little as 30 min before inoculation with surfactant-containing media, the measured MIC against S. aureus ATCC 29123 rose from CLSI-consistent values of 0.06 µg/ml to values of 2 to 8 µg/ml. Similarly, oritavancin (compound 4) MIC values were underestimated by 16- to 32-fold in the absence of added polysorbate 80, again due to the depletion of free drug onto plastic surfaces (10, 11). The loss to PS microtiter plates was quantified using [14C]oritavancin; at 16 µg/ml, the concentration of oritavancin was approximately 70% of that expected, but at 4 µg/ml, it was only 35%, and at 1 µg/ml, it was <10% (10). The addition of 2% lysed horse blood (LHB) was found to have the same blocking effect as surfactant (10). A similar effect, with added protein reducing antibiotic adherence to plastic, was observed with the glycolipodepsipeptide complex ramoplanin (compound 6), where the addition of 0.02% bovine serum albumin (BSA) resulted in more potent MICs (15, 16). In 2014, the CLSI methods for determining the MIC of telavancin (compound 2) were revised to include the addition of polysorbate 80, with DMSO used during stock preparation (12, 14). Notably, the closely related glycopeptide vancomycin (compound 5), without a lipophilic moiety, does not require surfactant supplement. A recent report discussed the effects of solvent (DMSO, ethanol, and methanol) on bacterial growth and found 20% reductions in growth across five organisms at concentrations of >3% DMSO, >3% methanol, or 1% ethanol, so solubilizing additives may also affect assay results (26).
Members of the lipopeptide polymyxin class of antibiotics (polymyxin B [compound 7a] and polymyxin E [or colistin, compound 7b]) are important last-line therapeutic agents against many multidrug-resistant Gram-negative bacteria (27–31). They consist of an N-terminal fatty acid side chain that is attached to a polycationic deca-peptide backbone (Fig. 1) with physicochemical properties similar to those of the lipoglycopeptides. These structural features confer amphipathicity, which is a key feature of many other cationic antimicrobial peptides (CAMPs) (32–34). As mentioned earlier, plate types have been reported to affect CAMP MIC values (8). In 2012, the addition of 0.002% polysorbate was reported to improve the MIC results for colistin and polymyxin B, with 4- to 8-fold more potent MICs against over 200 strains with surfactant present (19), with the results confirmed in clinical isolates (17, 18, 20). Greater differences were observed when the initial MIC was lower (<2 µg/ml). As for oritavancin, measurement of colistin concentrations in MHB following incubation in PS, PP, and glass tubes showed substantial time-dependent depletion at lower concentrations, with only 8%, 23%, or 25% of the initial 0.125 µg/ml concentration detected after 24 h, but 84%, 90%, and 80% of an expected 8 µg/ml concentration detected, respectively (21). Dramatic differences were observed between different brands of untreated PS microwell plates, comparing those from Greiner (remarkably, only 2% of 8 µg/ml after 24 h) and Nunc (70% of 8 µg/ml after 24 h). Low-binding PP microtubes showed the least loss at low concentrations (59% of 0.125 µg/ml after 24 h) (21). However, a CLSI-EUCAST working group in 2016 determined that surfactants should not be included in the reference broth microdilution method for colistin, and that untreated PS trays should be employed (7). Two new reports in 2018 described container effects on polymyxin activity. Untreated PS Sensititre GNX2F assay plates (Thermo Fisher) were compared to broth macrodilution in borosilicate glass against 106 carbapenem-resistant strains of Klebsiella pneumoniae, with 97% agreement within one dilution for polymyxin B and 92% for colistin (23). PS and glass-coated plates were compared for broth microdilution assays of colistin and polymyxin against 42 carbapenem-resistant strains of Acinetobacter baumannii (22). For both antibiotics, the PS resulted in greater variability and slightly less potent MICs (glass plate MIC for all 42 strains, 1 or 2 µg/ml; PS plates had 3 isolates with MIC of 2 µg/ml).
Other assay additives can affect MIC values. The importance of plasma protein binding on drug effectiveness is contentious: while it is generally recognized that the free drug concentration correlates with on-target activity, the reduced concentration of free drug caused by higher protein binding is offset by increased overall exposure due to protection from hepatic and nonhepatic clearance (35, 36). Plasma protein binding has been implicated as a major factor limiting the active free concentration of many clinically important antibiotics (37–42). This in turn translates into reduced antibacterial activity and clinical dose escalation that, in certain cases where the antibacterial agent is highly bound, limits its intravenous use (40, 43, 44). Most antibiotics generally have protein binding values of <60% (e.g., aminoglycosides, 0 to 35% for four examples [45]; the carbapenem meropenem, 2% [46]; the oxazolidinone linezolid, 31% [47]; tetracyclines, 24 to 60% for four examples [48]; fluoroquinolones in general, ∼30% [49]; and the glycopeptide vancomycin, 55% [50]). However, the second-generation lipoglycopeptide antibiotics dalbavancin and oritavancin, both approved in 2014, have high human protein binding, estimated at 93 (51) to >95% (52) and 82 to 90% (50, 53), respectively, which leads to exceedingly long half-lives (estimated at 9 to 12 [51, 54] and 10 to 17 [50] days) with once-weekly or even single-injection dosing. Telavancin also has high binding (90 to 93% [55, 61]), though a much shorter half-life (8 h [50]), requiring once-daily dosing. In contrast, the polymyxin lipopeptides colistin and polymyxin B are estimated to have 50 to 60% plasma protein binding (56, 57). Daptomycin is another cationic lipopeptide with high protein binding (92% [58]).
The extent of protein binding of antibiotics is often approximated by a serum reversal MIC instead of standard equilibrium dialysis or ultrafiltration methods. This technique conducts MIC assays without and with added serum proteins, either with broth containing 50 to 95% human or mouse serum, or with added 3 to 4% human serum albumin or bovine serum albumin protein (53, 59, 60). The ratio of retained activity indicates the extent of unbound antibiotic. However, bacteria do not grow as well in human serum as in standard medium, so high concentrations of serum may have a synergistic antimicrobial effect (59). MIC assays of lipoglycopeptides/lipopeptides that bind to both protein and plastic have the potential to be confounded by the opposing effects. High protein binding means that little free antibiotic is available for antimicrobial activity, reducing their MIC potency, but the added protein also reduces nonspecific binding to plastic, resulting in more potent MIC values.
We now demonstrate that plate type can cause large variations in MIC assay results, not only between different types of plate composition/coatings but sometimes in plates of the same polymer from different manufacturers. This supports the previously reported dramatic variation in colistin concentrations when incubated in untreated PS plates from different manufacturers (21). It is evident that to enable an “apples-to-apples” comparison of data from different laboratories, the exact plate type and composition (or vessels used for macrodilution experiments) should be reported when describing MIC assays.
The extent of plate-based variations is highly dependent on the type of antibiotic and appears to correlate with hydrophobic or amphiphilic molecules. We have found that for antibiotics where the addition of Tween 80 leads to more potent activity, the use of NBS plates without additive provides similar results. This suggests that the reduced activity in PS plates is caused by loss of compound due to binding to the plate surface, with even greater loss of compound in TC plates. However, there is a disconnect between the extent of protein binding of antibiotics and their “stickiness” to plastic, based on the observed plate effects. Telavancin (90 to 93% protein binding) showed little alterations in MIC when tested in NBS versus untreated plates, while dalbavancin (93 to >95%) and colistin (60%) both resulted in large variations.
In summary, plate and additive effects are observed across a range of bacteria, but there are subtle variations depending on the antibiotic, plate type, and additives employed. All broth microdilution MIC determinations should clearly specific the plate type and manufacturer and any additives employed. These studies demonstrate that NBS plates can effectively prevent reductions in MIC due to adsorption of compound to the plate surface and allow for assays of lipophilic antibiotics without the need for added surfactant. We are conducting further investigations against a much larger panel of antibiotics to establish the extent of plate-based variations in MIC determinations. It remains to be determined which plate type provides the “true” MIC value that is most relevant to the clinical activity of the antibiotic.
MATERIALS AND METHODS
Materials.
Vancomycin (catalog no. 861987-250MG, lot 087K0694), ciprofloxacin (catalog no. 17850-25G-F, lot 0001396108), oxacillin (catalog no. O1002-1G, lot 018K0610), penicillin G (catalog no. 13752-1G-F, lot WE376306/1), trimethoprim (catalog no. T7883-5G, lot 078K1522), colistin (catalog no. C4461-1G, lot 036K1374, 15,000 units per mg), polymyxin B (catalog no. P0972-10MU, lot 453306, ≥6,000 USP units per mg), rifampin (catalog no. R3501-250MG), polysorbate 80 (Tween 80, catalog no. P8074-500ml), and human serum (catalog no. H4522-20ml) were obtained from Sigma-Aldrich (Sydney, NSW, Australia). Dalbavancin HCl (catalog no. 317136) was purchased from MedKoo Bioscience, MHB (catalog no. 211443) from Bacto Laboratories, beractant (Survanta) from Abbvie Pty Ltd. (catalog no. 1039.008), and lysed horse blood (catalog no. HB100) from Thermo Fisher Scientific (Australia).
Telavancin (61) was synthesised from vancomycin according to procedures in the literature. MCC223, MCC310, and MCC520 were synthesized from vancomycin and purified by high-performance liquid chromatography (HPLC) to >95% purity (24). Their purity was ascertained by analytical liquid chromatography-mass spectrometry (LC-MS) and identity confirmed by high-resolution MS (HRMS) and tandem MS (MS/MS) fragmentation.
The Gram-positive bacteria Staphylococcus aureus (methicillin-sensitive S. aureus [MSSA] ATCC 25923, MRSA ATCC 43300, vancomycin-intermediate S. aureus [VISA] NRS 2/ATCC 700698), Streptococcus pneumoniae (ATCC 33400, multidrug-resistant [MDR] ATCC 700677), Enterococcus faecalis (ATCC 29212) and Streptococcus pyogenes (ATCC 12344), and Gram-negative bacteria Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 13883), Pseudomonas aeruginosa (ATCC 10145), and Acinetobacter baumannii (ATCC 19606) were sourced from the American Type Culture Collection (ATCC) and Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA).
The plate types used were 96-well PS Corning flat-bottom untreated Costar 3370, Corning flat-bottom TC surface COR 3628, Corning flat-bottom NBS surface COR 3641, Thermo Electric flat-bottom untreated PS Nunc-442404, Thermo Electric flat-bottom TC surface Nunc-167008, Thermo Electric U-bottom TC surface Nunc-163320, and Trek Diagnostics untreated PC H511A.
MIC determination via broth microdilution assay.
MIC determinations were done in duplicate (n = 2), with vancomycin, telavancin, dalbavancin, and ciprofloxacin used as positive inhibitor controls for Gram-positive bacteria, and colistin, ciprofloxacin, oxacillin, trimethoprim, polymyxin B, penicillin G, and rifampin for Gram-negative bacteria. A positive control of a row of just the bacteria and a negative control of only the medium were included for every plate tested. The antibiotic standards were prepared to 1.28 mg/ml solution in water. MCC223, MCC310, and MCC520 were prepared to 160 µg/ml solution in water from a stock solution of 1 mM concentration.
The compounds along with standard antibiotics were serially diluted 2-fold across the 96-well plates. Standards ranged from 64 µg/ml to 0.03 µg/ml and compounds from 8 µg/ml to 0.003 µg/ml, with final volumes of 50 µl per well. Gram-positive and Gram-negative bacteria were cultured in Mueller-Hinton broth (catalog no. 211443; Bacto Laboratories) with and without 0.002% Tween at 37°C overnight. A sample of each culture was then diluted 40-fold in fresh MH broth (in presence and absence of Tween) and incubated at 37°C for 2 to 3 h. The resultant mid-log-phase cultures were diluted to 1 × 106 CFU/ml under the same two conditions, and then 50 µl was added to each well of the compound-containing 96-well plates to give a final cell density of 5 × 105 CFU/ml. All the plates were covered and incubated at 37°C for 24 h. MICs were determined visually at 24 h of incubation, with the MIC defined as the lowest concentration at which no growth was visible after incubation.
For experiments in the presence of surfactant, 2% beractant (25 mg/ml) was added to the mid-log-phase cultures and mixed gently and added to all the 96-well plates. For experiments in the presence of serum, a mixture of 50% of human serum along with 50% MHB was prepared and used throughout the experiment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rajaratnam Premraj for the synthesis of telavancin, Rajaratnam Premraj and Tanya Bradford for the synthesis of the vancapticins, and Alysha Elliott for reviewing the manuscript.
This research was supported financially by NHMRC grants APP631632, APP1005350, APP1045326, and AP1063214 and NIH grant R21AI098731/R33AI098731-03. M.A.T.B., S.R., and A.K. were supported by a Wellcome Trust Seeding Drug Discovery Award (094977/Z/10/Z). M.A.C. is a former NHMRC Australian Fellow (grant AF511105) and current NHMRC Principal Research Fellow (grant APP1059354); M.A.C. holds a fractional Professorial Research Fellow appointment at the University of Queensland, with his remaining time as CEO of Inflazome Ltd., a company headquartered in Dublin, Ireland, that is developing drugs to address clinical unmet needs in inflammatory disease by targeting the inflammasome.
A.K., S.R., Y.G., M.A.T.B., and M.A.C. designed the experiments; A.K., S.R., and Y.G. conducted the experiments; and M.A.T.B. drafted the manuscript, with contributions and review from all authors.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01760-18.
REFERENCES
- 1.Felmingham D, Brown DFJ. 2001. Instrumentation in antimicrobial susceptibility testing. J Antimicrob Chemother 48:81–85. doi: 10.1093/jac/48.suppl_1.81. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 11th ed CLSI document M07-A11. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 3.CLSI. 2018. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement, 28th ed CLSI document M100-S28. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 4.Andrews JM. 2006. Determination of minimum inhibitory concentrations. British Society for Antimicrobial Chemotherapy, Birmingham, United Kingdom: http://bsac.org.uk/wp-content/uploads/2012/02/Chapter-2-Determination-of-MICs-2006updated.pdf. [DOI] [PubMed] [Google Scholar]
- 5.Andrews JM. 2001. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 6.International Organization for Standardization. 2006. ISO 20776-1:2006. Clinical laboratory testing and in vitro diagnostic test systems–susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices–part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. International Organization for Standardization, Geneva, Switzerland: https://www.iso.org/standard/41630.html. [Google Scholar]
- 7.CLSI-EUCAST. 2016. Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf. [Google Scholar]
- 8.Wiegand I, Hilpert K, Hancock REW. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 9.Otvos L, Cudic M. 2007. Broth microdilution antibacterial assay of peptides. Methods Mol Biol 386:309–320. doi: 10.1007/978-1-59745-430-8_12. [DOI] [PubMed] [Google Scholar]
- 10.Arhin FF, Sarmiento I, Belley A, McKay GA, Draghi DC, Grover P, Sahm DF, Parr TR Jr, Moeck G. 2008. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob Agents Chemother 52:1597–1603. doi: 10.1128/AAC.01513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arhin FF, Tomfohrde K, Draghi DC, Aranza M, Parr TR Jr, Sahm DF, Moeck G. 2008. Newly defined in vitro quality control ranges for oritavancin broth microdilution testing and impact of variation in testing parameters. Diagn Microbiol Infect Dis 62:92–95. doi: 10.1016/j.diagmicrobio.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Farrell DJ, Mendes RE, Rhomberg PR, Jones RN. 2014. Revised reference broth microdilution method for testing telavancin: effect on MIC results and correlation with other testing methodologies. Antimicrob Agents Chemother 58:5547–5551. doi: 10.1128/AAC.03172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rennie RP, Koeth L, Jones RN, Fritsche TR, Knapp CC, Killian SB, Goldstein BP. 2007. Factors influencing broth microdilution antimicrobial susceptibility test results for dalbavancin, a new glycopeptide agent. J Clin Microbiol 45:3151–3154. doi: 10.1128/JCM.02411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross JE, Mendes RE, Jones RN. 2014. Quality control MIC ranges used for telavancin with application of a revised CLSI reference broth microdilution method. J Clin Microbiol 52:3399–3401. doi: 10.1128/JCM.01210-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scotti R, Dulworth JK, Kenny MT, Goldstein BP. 1993. Effect of protein on ramoplanin broth microdilution minimum inhibitory concentrations. Diagn Microbiol Infect Dis 17:209–211. doi: 10.1016/0732-8893(93)90098-R. [DOI] [PubMed] [Google Scholar]
- 16.Barry AL, Pfaller MA, Fuchs PC. 1993. Ramoplanin susceptibility testing criteria. J Clin Microbiol 31:1932–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dafopoulou K, Zarkotou O, Dimitroulia E, Hadjichristodoulou C, Gennimata V, Pournaras S, Tsakris A. 2015. Comparative evaluation of colistin susceptibility testing methods among carbapenem-nonsusceptible Klebsiella pneumoniae and Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 59:4625–4630. doi: 10.1128/AAC.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hindler JA, Humphries RM. 2013. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol 51:1678–1684. doi: 10.1128/JCM.03385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74:412–414. doi: 10.1016/j.diagmicrobio.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland CA, Nicolau DP. 2014. To add or not to add polysorbate 80: impact on colistin MICs for clinical strains of Enterobacteriaceae and Pseudomonas aeruginosa and quality controls. J Clin Microbiol 52:3810. doi: 10.1128/JCM.01454-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karvanen M, Malmberg C, Lagerbäck P, Friberg LE, Cars O. 2017. Colistin is extensively lost during standard in vitro experimental conditions. Antimicrob Agents Chemother 61:e00857-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipika S, Megha S, Salony V, Ramanpreet K, Basil BX, Malhotra KS, Pallab R, Vikas G. 2018. Comparative evaluation of broth microdilution with polystyrene and glass-coated plates, agar dilution, E-Test, Vitek, and disk diffusion for susceptibility testing of colistin and polymyxin B on carbapenem-resistant clinical isolates of Acinetobacter baumannii. Microb Drug Resist 24:1082–1088. doi: 10.1089/mdr.2017.0251. [DOI] [PubMed] [Google Scholar]
- 23.Richter SS, Karichu J, Otiso J, Van Heule H, Keller G, Cober E, Rojas LJ, Hujer AM, Hujer KM, Marshall S, Perez F, Rudin SD, Domitrovic TN, Kaye KS, Salata R, van Duin D, Bonomo RA. 2018. Evaluation of Sensititre broth microdilution plate for determining the susceptibility of carbapenem-resistant Klebsiella pneumoniae to polymyxins. Diagn Microbiol Infect Dis 91:89–92. doi: 10.1016/j.diagmicrobio.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaskovich MAT, Hansford KA, Gong Y, Butler MS, Muldoon C, Huang JX, Ramu S, Silva AB, Cheng M, Kavanagh AM, Ziora Z, Premraj R, Lindahl F, Bradford TA, Lee JC, Karoli T, Pelingon R, Edwards DJ, Amado M, Elliott AG, Phetsang W, Daud NH, Deecke JE, Sidjabat HE, Ramaologa S, Zuegg J, Betley JR, Beevers APG, Smith RAG, Roberts JA, Paterson DL, Cooper MA. 2018. Protein-inspired antibiotics active against vancomycin- and daptomycin-resistant bacteria. Nat Commun 9:22. doi: 10.1038/s41467-017-02123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehlert FGR, Linde K, Diederich WE. 2017. What are we missing? The detergent Triton X-100 added to avoid compound aggregation can affect assay results in an unpredictable manner. ChemMedChem 12:1419–1423. doi: 10.1002/cmdc.201700329. [DOI] [PubMed] [Google Scholar]
- 26.Wadhwani T, Desai K, Patel D, Lawani D, Bahaley P, Joshi P, Vijay K. 2018. Effect of various solvents on bacterial growth in context of determining MIC of various antimicrobials. Internet J Microbiol 7:1–6. [Google Scholar]
- 27.Li J, Nation RL. 2006. Old polymyxins are back: is resistance close? Clin Infect Dis 43:663–664. doi: 10.1086/506571. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. 2005. Evaluation of colistin as an agent against multi-resistant in Gram-negative bacteria. Int J Antimicrob Agents 25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Nation RL, Owen RJ, Wong S, Spelman D, Franklin C. 2007. Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin Infect Dis 45:594–598. doi: 10.1086/520658. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 31.Nation RL, Li J. 2007. Optimizing use of colistin and polymyxin B in the critically ill. Semin Respir Crit Care Med 28:604–614. doi: 10.1055/s-2007-996407. [DOI] [PubMed] [Google Scholar]
- 32.Hancock RE, Chapple DS. 1999. Peptide antibiotics. Antimicrob Agents Chemother 43:1317–1323. doi: 10.1128/AAC.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaara M, Vaara T. 2010. Structure-activity studies on novel polymyxin derivatives that carry only three positive charges. Peptides 31:2318–2321. doi: 10.1016/j.peptides.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Velkov T, Thompson PE, Nation RL, Li J. 2010. Structure-activity relationships of polymyxin antibiotics. J Med Chem 53:1898–1916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DA, Di L, Kerns EH. 2010. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov 9:929–939. doi: 10.1038/nrd3287. [DOI] [PubMed] [Google Scholar]
- 36.Banker MJ, Clark TH. 2008. Plasma/serum protein binding determinations. Curr Drug Metab 9:854–859. doi: 10.2174/138920008786485065. [DOI] [PubMed] [Google Scholar]
- 37.Dudley MN, Blaser J, Gilbert D, Zinner SH. 1990. Significance of “extravascular” protein binding for antimicrobial pharmacodynamics in an in vitro capillary model of infection. Antimicrob Agents Chemother 34:98–101. doi: 10.1128/AAC.34.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenblatt DJ, Sellers EM, Koch-Weser J. 1982. Importance of protein binding for the interpretation of serum or plasma drug concentrations. J Clin Pharmacol 22:259–263. doi: 10.1002/j.1552-4604.1982.tb02671.x. [DOI] [PubMed] [Google Scholar]
- 39.Merrikin DJ, Briant J, Rolinson GN. 1983. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother 11:233–238. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt S, Rock K, Sahre M, Burkhardt O, Brunner M, Lobmeyer MT, Derendorf H. 2008. Effect of protein binding on the pharmacological activity of highly bound antibiotics. Antimicrob Agents Chemother 52:3994–4000. doi: 10.1128/AAC.00427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wise R. 1985. The relevance of pharmacokinetics to in-vitro models: protein binding–does it matter? J Antimicrob Chemother 15:77–83. doi: 10.1093/jac/15.suppl_A.77. [DOI] [PubMed] [Google Scholar]
- 42.Wise R. 1986. The clinical relevance of protein binding and tissue concentrations in antimicrobial therapy. Clin Pharmacokinet 11:470–482. doi: 10.2165/00003088-198611060-00004. [DOI] [PubMed] [Google Scholar]
- 43.Lee BL, Sachdeva M, Chambers HF. 1991. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob Agents Chemother 35:2505–2508. doi: 10.1128/AAC.35.12.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nix DE, Matthias KR, Ferguson EC. 2004. Effect of ertapenem protein binding on killing of bacteria. Antimicrob Agents Chemother 48:3419–3424. doi: 10.1128/AAC.48.9.3419-3424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon RC, Regamey C, Kirby WM. 1972. Serum protein binding of the aminoglycoside antibiotics. Antimicrob Agents Chemother 2:214–216. doi: 10.1128/AAC.2.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikawa K, Morikawa N, Ohge H, Ikeda K, Sueda T, Taniwaki M, Kurisu K. 2010. Pharmacokinetic-pharmacodynamic target attainment analysis of meropenem in Japanese adult patients. J Infect Chemother 16:25–32. doi: 10.1007/s10156-009-0022-3. [DOI] [PubMed] [Google Scholar]
- 47.Gee T, Ellis R, Marshall G, Andrews J, Ashby J, Wise R. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob Agents Chemother 45:1843–1846. doi: 10.1128/AAC.45.6.1843-1846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green R, Brown JR, Calvert RT. 1976. Disposition of 4 tetracyclines in normal subjeccts. Eur J Clin Pharmacol 10:245–250. doi: 10.1007/BF00558336. [DOI] [Google Scholar]
- 49.Drusano G. 2001. Pharmacokinetics and pharmacodynamics of the fluoroquinolones - Tools for determining dosing strategies and predicting clinical outcomes. J Antimicrob Chemother 47:12. [Google Scholar]
- 50.Cada DJ, Baker DE. 2014. Oritavancin diphosphate. Hosp Pharm 49:1049–1060. doi: 10.1310/hjp4911-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dash RP, Babu RJ, Srinivas NR. 2017. Review of the pharmacokinetics of dalbavancin, a recently approved lipoglycopeptide antibiotic. Infect Dis (Lond) 49:483–492. doi: 10.1080/23744235.2017.1296968. [DOI] [PubMed] [Google Scholar]
- 52.Cavaleri M, Cooper A, Nutley M, Stogniew M. 2002. Protein binding of dalbavancin using isothermal titration microcalorimetry, abstr A-138. Abstr 42nd Intersci Conf Antimicrob Agents Chemother, 27 to 30 September 2002, San Diego, CA. [Google Scholar]
- 53.Arhin FF, Belley A, McKay G, Beaulieu S, Sarmiento I, Parr TR Jr, Moeck G. 2010. Assessment of oritavancin serum protein binding across species. Antimicrob Agents Chemother 54:3481–3483. doi: 10.1128/AAC.00271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seltzer E, Dorr MB, Goldstein BP, Perry M, Dowell JA, Henkel T, Dalbavancin Skin and Soft-Tissue Infection Study Group. 2003. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis 37:1298–1303. doi: 10.1086/379015. [DOI] [PubMed] [Google Scholar]
- 55.Shaw JP, Seroogy J, Kaniga K, Higgins DL, Kitt M, Barriere S. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother 49:195–201. doi: 10.1128/AAC.49.1.195-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 47:1766–1770. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudhani RV, Li J, Nation RL. 2009. Plasma binding of colistin involves multiple proteins and is concentration dependent: potential clinical implications, abstr A1-576:41. Abstr 49th Intersci Conf Antimicrob Agents Chemother, 12 to 15 September 2009, San Francisco, CA. [Google Scholar]
- 58.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother 47:1318–1323. doi: 10.1128/AAC.47.4.1318-1323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeitlinger MA, Derendorf H, Mouton JW, Cars O, Craig WA, Andes D, Theuretzbacher U. 2011. Protein binding: do we ever learn? Antimicrob Agents Chemother 55:3067–3074. doi: 10.1128/AAC.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuji BT, Leonard SN, Rhomberg PR, Jones RN, Rybak MJ. 2008. Evaluation of daptomycin, telavancin, teicoplanin, and vancomycin activity in the presence of albumin or serum. Diagn Microbiol Infect Dis 60:441–444. doi: 10.1016/j.diagmicrobio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Leadbetter MR, Adams SM, Bazzini B, Fatheree PR, Karr DE, Krause KM, Lam BMT, Linsell MS, Nodwell MB, Pace JL, Quast K, Shaw JP, Soriano E, Trapp SG, Villena JD, Wu TX, Christensen BG, Judice JK. 2004. Hydrophobic vancomycin derivatives with improved ADME properties: Discovery of telavancin (TD-6424). J Antibiot 57:326–336. doi: 10.7164/antibiotics.57.326. [DOI] [PubMed] [Google Scholar]
- 62.Baltch AL, Ritz WJ, Bopp LH, Michelsen PB, Smith RP. 2007. Antimicrobial activities of daptomycin, vancomycin, and oxacillin in human monocytes and of daptomycin in combination with gentamicin and/or rifampin in human monocytes and in broth against Staphylococcus aureus. Antimicrob Agents Chemother 51:1559–1562. doi: 10.1128/AAC.00973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrow BJ, Abbanat D, Baum EZ, Crespo-Carbone SM, Davies TA, He WP, Shang WC, Queenan AM, Lynch AS. 2011. Antistaphylococcal activities of the new fluoroquinolone JNJ-Q2. Antimicrob Agents Chemother 55:5512–5521. doi: 10.1128/AAC.00470-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hakanen A, Huovinen P, Kotilainen P, Siitonen A, Jousimies-Somer H. 2002. Quality control strains used in susceptibility testing of Campylobacter spp. J Clin Microbiol 40:2705–2706. doi: 10.1128/JCM.40.7.2705-2706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauernfeind A. 1997. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemoth 40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 66.Sweeney D, Shinabarger DL, Smart JI, Bruss J, Pillar CM. 2017. Evaluation of the bactericidal activity of telavancin against Staphylococcus aureus using revised testing guidelines. Diagn Microbiol Infect Dis 89:83–85. doi: 10.1016/j.diagmicrobio.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 67.Lubenko IY, Strukova EV, Smirnova MV, Vostrov SN, Portnoy YA, Zinner SH, Firsov AA. 2008. Telavancin and vancomycin pharmacodynamics with Staphylococcus aureus in an in vitro dynamic model. J Antimicrob Chemother 62:1065–1069. doi: 10.1093/jac/dkn288. [DOI] [PubMed] [Google Scholar]
- 68.Belley A, Lalonde Seguin D, Arhin F, Moeck G. 2016. Comparative in vitro activities of oritavancin, dalbavancin, and vancomycin against methicillin-resistant Staphylococcus aureus isolates in a nondividing state. Antimicrob Agents Chemother 60:4342–4345. doi: 10.1128/AAC.00169-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aktas G, Derbentli S. 2010. In vitro activity of dalbavancin against staphylococci isolated in Istanbul, Turkey. Chemotherapy 56:444–447. doi: 10.1159/000317763. [DOI] [PubMed] [Google Scholar]
- 70.Lo-Ten-Foe JR, de Smet AMGA, Diederen BMW, Kluytmans JAJW, van Keulen PHJ. 2007. Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother 51:3726–3730. doi: 10.1128/AAC.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fass RJ, Barnishan J. 1979. Minimal inhibitory concentrations of 34 anti-microbial agents for control strains Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853. Antimicrob Agents Chemother 16:622–624. doi: 10.1128/AAC.16.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salmon SA, Watts JL, Case CA, Hoffman LJ, Wegener HC, Yancey RJ. 1995. Comparison of MICs of ceftiofur and other antimicrobial agents against bacterial pathogens of swine from the United-States, Canada and Denmark. J Clin Microbiol 33:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones RN, Anderegg TR, Swenson JM, Grp QCW. 2005. Quality control guidelines for testing Gram-negative control strains with polymyxin B and colistin (polymyxin E) by standardized methods. J Clin Microbiol 43:925–927. doi: 10.1128/JCM.43.2.925-927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker KR, Jana B, Franzyk H, Guardabassi L. 2016. A high-throughput approach to identify compounds that impair envelope integrity in Escherichia coli. Antimicrob Agents Chemother 60:5995–6002. doi: 10.1128/AAC.00537-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaara M, Siikanen O, Apajalahti J, Frimodt MN, Vaara T. 2010. Susceptibility of carbapenemase-producing strains of Klebsiella pneumoniae and Escherichia coli to the direct antibacterial activity of NAB739 and to the synergistic activity of NAB7061 with rifampicin and clarithromycin. J Antimicrob Chemother 65:942–945. doi: 10.1093/jac/dkq040. [DOI] [PubMed] [Google Scholar]
- 76.Ryan JA. 2008. Evolution of cell culture surfaces. BioFiles 3.8:21. [Google Scholar]
- 77.Corning Life Sciences. Corning non binding surface: minimize molecular interactions with a unique, NBS treatment. https://www.corning.com/emea/en/products/life-sciences/products/microplates/assay-surfaces/non-binding-surface.html.
- 78.Bookbinder D, Chow D. 2007. Corning application report: binding comparison of polymer surfaces: introducing Corning nonbinding surface (NBS) microplates. POD ALSP-AN-003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.