This pooled analysis evaluated the relationship of isavuconazole and voriconazole MICs of Aspergillus pathogens at baseline with all-cause mortality and clinical outcomes following treatment with either drug in the SECURE and VITAL trials. Isavuconazole and voriconazole may have had reduced efficacy against pathogens with drug MICs of ≥16 µg/ml, but there was no relationship with clinical outcomes in cases where the MIC was <16 µg/ml for either drug.
KEYWORDS: MIC, clinical trial, isavuconazole, isavuconazonium sulfate, voriconazole
ABSTRACT
This pooled analysis evaluated the relationship of isavuconazole and voriconazole MICs of Aspergillus pathogens at baseline with all-cause mortality and clinical outcomes following treatment with either drug in the SECURE and VITAL trials. Isavuconazole and voriconazole may have had reduced efficacy against pathogens with drug MICs of ≥16 µg/ml, but there was no relationship with clinical outcomes in cases where the MIC was <16 µg/ml for either drug.
INTRODUCTION
Invasive fungal diseases (IFD) are serious medical conditions (1–3). For example, mortality rates for invasive aspergillosis (IA) at 6 weeks are approximately 20% (4). Treatment guidelines from the Infectious Diseases Society of America and the European Conference on Infections in Leukemia recommend voriconazole as a first-line agent for IA (5–7). On the basis of the results of the SECURE and VITAL phase 3 trials (8, 9), isavuconazole was approved by the U.S. Food and Drug Administration for the treatment of adults with IA or invasive mucormycosis and by the European Medicines Agency for treatment of adults with IA or with mucormycosis when amphotericin B is not appropriate. Isavuconazole has also now been included in guidelines as an alternative first-line agent (5–7). Clinical breakpoints for isavuconazole have now been defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for several Aspergillus spp. (10).
This analysis describes the relationships of MICs of isavuconazole and voriconazole against Aspergillus pathogens at baseline in pooled data from the SECURE (8) and VITAL trials (11), which compared isavuconazole (200 mg [administered as 372 mg prodrug dose] orally or intravenously every 8 h for 48 h, then once daily) and voriconazole (6 mg/kg intravenously twice daily on day 1, 4 mg/kg intravenously twice daily on day 2, then intravenously 4 mg/kg twice daily or orally 200 mg twice daily from day 3 onwards) for the primary treatment of IFD caused by Aspergillus spp. and other filamentous fungi, and efficacy of isavuconazole for patients with IA and renal impairment or with other rare IFDs, respectively.
Antifungal susceptibilities were tested (Clinical and Laboratory Standards Institute [CLSI] and EUCAST methods) (12, 13) in 96 Aspergillus sp. isolates (morphologically and molecularly confirmed [14, 15]) obtained at baseline. For all pathogens from patients treated with isavuconazole (n = 55 patients; mean age [standard deviation {SD}] 49.5 [17.5] years; 71 Aspergillus samples), the CLSI and EUCAST MIC values were the same (MIC50, 1 µg/ml; MIC90, 4 µg/ml). Furthermore, the ranges of MICs were comparable (CLSI, 0.25 to 32 µg/ml; EUCAST, 0.12 to 32 µg/ml).
The MIC50 and MIC90 for pathogens from patients treated with voriconazole (n = 23 patients; mean age [SD] 53.1 [16.1] years; 25 Aspergillus samples) were 1 µg/ml and 2 µg/ml, respectively, as determined using either CLSI or EUCAST methods. The ranges of MICs were 0.25 to 2 (CLSI) and 0.25 to 16 (EUCAST) µg/ml. In summary, isavuconazole and voriconazole displayed potent in vitro activity against the majority of Aspergillus spp., with MICs consistent with those recently reported elsewhere (16–20).
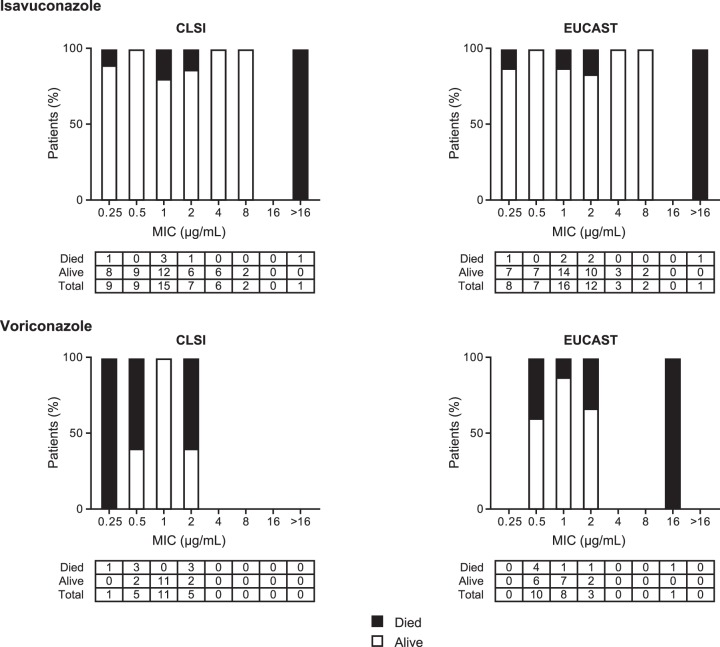
All-cause mortality (ACM) through day 42 was assessed in all patients with proven, probable, or possible IFD who received at least one dose of study drug (Table 1; seen also Table S1 in ths supplemental material). Among isavuconazole-treated patients (Aspergillus spp. only), the ACM rates associated with pathogens having drug MICs of ≤1 µg/ml were 12.1% (4/33; CLSI) and 9.7% (3/31; EUCAST), whereas the ACM rates associated with pathogens having drug MICs of >1 µg/ml were 12.5% (2/16; CLSI) and 16.6% (3/18; EUCAST). Among voriconazole-treated patients (Aspergillus spp. only), the ACM rates associated with pathogens having drug MICs of ≤1 µg/ml were 23.5% (4/17; CLSI) and 27.8% (5/18; EUCAST), whereas the ACM rates associated with pathogens having drug MICs of >1 µg/ml were 60% (3/5; CLSI) and 50% (2/4; EUCAST). With the exception of one patient in the isavuconazole group who was infected with multiple fungal species, all patients in both treatment groups infected with Aspergillus spp. with drug MICs of ≥16 µg/ml (n = 2 for isavuconazole by either CLSI or EUCAST; n = 1 for voriconazole by EUCAST only) died by day 42. For patients with Aspergillus-only infections, ACM at day 42 was generally higher with voriconazole than with isavuconazole (Fig. 1) (Table 1; see also Table S1); however, the study was underpowered to enable a statistical comparison between the groups.
TABLE 1.
All-cause mortality through day 42 classified by baseline drug MICs for Aspergillus sp. isolates alone or with other fungal pathogens: CLSI and EUCAST methodologiesaa
| Methodology, treatment, and target |
No. of isolates with indicated MIC (μg/ml)/total no. of isolates (% of total)b |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | >16 | |
| CLSI | ||||||||
| Isavuconazole | ||||||||
| Aspergillus spp. only | 1/9 (11) | 0/9 | 3/15 (20) | 1/7 (14) | 0/6 | 0/2 | 1/1 (100) | |
| Multiple fungal spp.c | 0/1 | 0/1 | 0/3 | 1/1 (100) | ||||
| Voriconazole | ||||||||
| Aspergillus spp. only | 1/1 (100) | 3/5 (60) | 0/11 | 3/5 (60) | ||||
| Multiple fungal spp.d | 0/1 | |||||||
| EUCAST | ||||||||
| Isavuconazole | ||||||||
| Aspergillus spp. only | 1/8 (13) | 0/7 | 2/16 (17) | 2/12 (17) | 0/3 | 0/2 | 1/1 (100) | |
| Multiple fungal spp.c | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 (100) | 0/1 | ||
| Voriconazole | ||||||||
| Aspergillus spp. only | 4/10 (40) | 1/8 (13) | 1/3 (33) | 1/1 (100) | ||||
| Multiple fungal spp.d | 0/1 | |||||||
See the supplemental materials for ACM data for individual and multiple Aspergillus spp. Only baseline samples are included in this summary. CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration.
The denominator represents the number of patients whose isolates had that drug MIC (where patients had multiple isolates, the isolate with the highest baseline drug MIC was used); the numerator denotes the number of patients who died. The outcome for a patient whose last known survival status was determined before day 42 or was missing and whose last assessment day was before day 42 was treated as representing death.
Data include Lichtheimia corymbifera (n = 2 patients), Fonsecaea monophora, Chaetomium brasiliense, and Rhizopus oryzae.
Data include Penicillium piceum.
FIG 1.
All-cause mortality in patients with Aspergillus spp. only treated with isavuconazole and voriconazole at day 42 using drug MICs for Aspergillus sp. isolates by CLSI and EUCAST methodologies. CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing.
The overall response (composite of clinical, mycological, and radiological [where relevant; see Table S2] responses at end of treatment [EOT] in patients with proven or probable IFD, as assessed by blind-data review committee) (21) was also investigated. Among isavuconazole-treated patients infected with pathogens (Aspergillus spp. only) with drug MICs of ≤1 µg/ml, overall successful responses were observed in 45.5% (15/33; CLSI) and 45.2% (14/31; EUCAST), whereas in cases of pathogens with drug MICs of >1 µg/ml, overall successful responses were observed in 43.8% (7/16; CLSI) and 44.4% (8/18; EUCAST) (Table 2) (see also Fig. S1 and Table S3 in the supplemental material). Among voriconazole-treated patients infected with pathogens (Aspergillus spp. only) with drug MICs of ≤1 µg/ml, overall successful responses were observed in 47.1% (8/17; CLSI) and 44.4% (8/18; EUCAST), whereas the overall successful responses observed for those whose pathogens had drug MICs of >1 µg/ml were 20.0% (1/5; CLSI) and 25.0% (1/4; EUCAST).
TABLE 2.
Overall, clinical, and mycological responses at EOT classified by baseline drug MICs for Aspergillus sp. isolates alone or with other fungal pathogens: CLSI and EUCAST methodologiesaa
| Methodology, treatment, and target |
Outcome | No. of isolates with indicated MIC (μg/ml)/total no. of isolates (% of total)b |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | >16 | ||
| CLSI | |||||||||
| Isavuconazole | |||||||||
| Aspergillus spp. only | Overall success | 4/9 (44) | 5/9 (56) | 6/15 (40) | 3/7 (43) | 3/6 (50) | 1/2 (50) | 0/1 | |
| Clinical success | 6/9 (67) | 9/9 (100) | 9/15 (60) | 5/7 (71) | 4/6 (67) | 2/2 (100) | 0/1 | ||
| Mycological success | 4/9 (44) | 6/9 (44) | 7/15 (47) | 3/7 (43) | 3/6 (50) | 1/2 (50) | 0/1 | ||
| Multiple fungal spp.c | Overall success | 0/1 | 0/1 | 1/3 (33) | 0/1 | ||||
| Clinical success | 1/1 (100) | 0/1 | 2/3 (67) | 0/1 | |||||
| Mycological success | 1/1 (100) | 0/1 | 1/3 (33) | 0/1 | |||||
| Voriconazole | |||||||||
| Aspergillus spp. only | Overall success | 0/1 | 1/5 (20) | 7/11 (64) | 1/5 (20) | ||||
| Clinical success | 0/1 | 2/5 (40) | 10/11 (91) | 2/5 (40) | |||||
| Mycological success | 0/1 | 1/5 (20) | 8/11 (73) | 1/5 (20) | |||||
| Multiple fungal spp.d | Overall success | 1/1 (100) | |||||||
| Clinical success | 1/1 (100) | ||||||||
| Mycological success | 1/1 (100) | ||||||||
| EUCAST | |||||||||
| Isavuconazole | |||||||||
| Aspergillus spp. only | Overall success | 4/8 (50) | 4/7 (57) | 6/16 (38) | 5/12 (42) | 1/3 (33) | 2/2 (100) | 0/1 | |
| Clinical success | 5/8 (63) | 7/7 (100) | 11/16 (69) | 8/12 (67) | 2/3 (67) | 2/2 (100) | 0/1 | ||
| Mycological success | 4/8 (50) | 4/7 (57) | 8/16 (50) | 5/12 (42) | 1/3 (33) | 2/2 (100) | 0/1 | ||
| Multiple fungal spp.c | Overall success | 0/1 | 1/1 (100) | 0/1 | 0/1 | 0/1 | 0/1 | ||
| Clinical success | 1/1 (100) | 1/1 (100) | 0/1 | 1/1 (100) | 0/1 | 0/1 | |||
| Mycological success | 1/1 (100) | 1/1 (100) | 0/1 | 0/1 | 0/1 | 0/1 | |||
| Voriconazole | |||||||||
| Aspergillus spp. only | Overall success | 3/10 (30) | 5/8 (63) | 1/3 (33) | 0/1 | ||||
| Clinical success | 6/10 (60) | 6/8 (75) | 2/3 (67) | 0/1 | |||||
| Mycological success | 4/10 (40) | 5/8 (63) | 1/3 (33) | 0/1 | |||||
| Multiple fungal spp.d | Overall success | 1/1 (100) | |||||||
| Clinical success | 1/1 (100) | ||||||||
| Mycological success | 1/1 (100) | ||||||||
See the supplemental materials for responses for individual and multiple Aspergillus spp. CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; EOT, end of treatment.
The denominator represents the number of patients whose isolates had that drug MIC (where patients had multiple isolates, the isolate with the highest baseline drug MIC was used); the numerator denotes the number of patients who demonstrated a response of success.
Data include Lichtheimia corymbifera (n = 2 patients), Fonsecaea monophora, Chaetomium brasiliense, and Rhizopus oryzae.
Data include Penicillium piceum.
These analyses of primarily wild-type isolates from the SECURE and VITAL trials suggest that a clearly defined relationship between in vitro susceptibility and clinical outcomes may not be detectable below the epidemiological cutoff value of ≤1 µg/ml (CLSI) (22) or ≤2 µg/ml (EUCAST) for most common Aspergillus spp. (23). Moreover, this analysis was limited by the small number of isolates, which prevented statistical analysis of the relationship between susceptibility and outcomes and precluded comments on suboptimal outcomes associated with high MICs. Using clinical data to verify the adequacy of current breakpoints would require much larger studies and enrollment of patients with infections by strains with drug MICs higher than the breakpoint, the latter of which would not be ethically tenable.
Supplementary Material
ACKNOWLEDGMENTS
Isavuconazonium sulfate was codeveloped by Astellas Pharma Global Development, Inc., and Basilea Pharmaceutica International Ltd. Editorial assistance was provided by Barrie J. Anthony and Ed Parr of Envision Scientific Solutions, funded by Astellas Pharma, Inc. We are grateful for the contributions of the investigators and staff who conducted the SECURE and VITAL trials and to the patients who volunteered for this study.
This analysis was funded by Astellas Pharma, Inc.
D.R.A. reports grant support and personal fees for consultancy from Astellas, received during the conduct of the study, and grants from Merck, Cidara, Synexis, Amplyx, Actelion, Theravance, Paratek, Medicines, Geom, Melinta, and Zavante, received outside the submitted work. M.A.G. reports grant support and personal fees for speaking from Astellas, received during the conduct of the study, and grants from Pfizer, grants and personal fees from Meiji Pharmaceuticals, grants from Viamet, and grants and personal fees from Cidara, received outside the submitted work. P.K.M. has nothing to disclose. L.L.K., Q.L., and C.L. are employees of Astellas Pharma Global Development, Inc. M.E.J. is an employee of Basilea Pharmaceutica International Ltd. A.S.H. was an employee of Basilea Pharmaceutica International, Ltd., at the time of the study. W.W.H. reports he holds or has recently held research grants from F2G, AiCuris, Astellas Pharma, Spero Therapeutics, Matinas Biosciences, Antabio, Amplyx, Allecra, Auspherix, and Pfizer. He holds awards from the National Institutes of Health, Medical Research Council, National Institute of Health Research, FDA, and the European Commission (FP7 and IMI). He has received personal fees in his capacity as a consultant for F2G, Amplyx, Ausperix, Spero Therapeutics, Medicines Company, Gilead, and Basilea. He is an Ordinary Council Member for the British Society of Antimicrobial Chemotherapy.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01634-18.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Leventakos K, Lewis RE, Kontoyiannis DP. 2010. Fungal infections in leukemia patients: how do we prevent and treat them? Clin Infect Dis 50:405–415. doi: 10.1086/649879. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 4.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, Oster RA, Anaissie EJ, Walsh TJ, Schuster MG, Wingard JR, Patterson TF, Ito JI, Williams OD, Chiller T, Pappas PG. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis 50:1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, Lass-Florl C, Calandra T, Viscoli C, Herbrecht R. 2017. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 102:433–444. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astellas Pharma US Inc. 2015. CRESEMBA (isavuconazonium sulfate) prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf. Accessed 10 March 2018.
- 8.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee DG, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR III, Lee M, Maher RM, Schmitt-Hoffmann AH, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 9.Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR, Alangaden GJ, Brown JM, Fredricks DN, Heinz WJ, Herbrecht R, Klimko N, Klyasova G, Maertens JA, Melinkeri SR, Oren I, Pappas PG, Ráčil Z, Rahav G, Santos R, Schwartz S, Vehreschild JJ, Young J-AH, Chetchotisakd P, Jaruratanasirikul S, Kanj SS, Engelhardt M, Kaufhold A, Ito M, Lee M, Sasse C, Maher RM, Zeiher B, Vehreschild MJGT. 2016. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 16:828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 10.EUCAST. 2018. Antifungal agents breakpoint tables for interpretation of MICs. http://www.eucast.org/clinical_breakpoints/. Accessed 6 September 2018.
- 11.Perfect JR, Cornely OA, Heep M, Ostrosky-Zeichner L, Mullane KM, Maher RM, Croos-Dabrera R, Lademacher C, Engelhardt M, Chen C, Marty FM. 19 April 2018. Isavuconazole treatment for rare fungal diseases and for invasive aspergillosis in patients with renal impairment: challenges and lessons of the VITAL trial. Mycoses doi: 10.1111/myc.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard: second ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.EUCAST. 2008. Technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 252:982–984. doi: 10.1111/j.1469-0691.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 14.Belmont C. 1996. Identifying filamentous fungi, p 64–77. In St-Germain G, Summerbell R (ed), Aspergillus. Star Publishing, Belmont, CA. [Google Scholar]
- 15.Ghannoum MA, Mukherjee PK, Warshaw EM, Evans S, Korman NJ, Tavakkol A. 2013. Molecular analysis of dermatophytes suggests spread of infection among household members. Cutis 91:237–245. [PubMed] [Google Scholar]
- 16.Astvad KMT, Hare RK, Arendrup MC. 2017. Evaluation of the in vitro activity of isavuconazole and comparator voriconazole against 2635 contemporary clinical Candida and Aspergillus isolates. Clin Microbiol Infect doi: 10.1016/j.cmi.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Florl C, Martin-Mazuelos E, Meis J, Pelaez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob Agents Chemother 57:3823–3828. doi: 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregson L, Goodwin J, Johnson A, McEntee L, Moore CB, Richardson M, Hope WW, Howard SJ. 2013. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: correlation with itraconazole, voriconazole, and posaconazole. Antimicrob Agents Chemother 57:5778–5780. doi: 10.1128/AAC.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2013. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J Clin Microbiol 51:2608–2616. doi: 10.1128/JCM.00863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CLSI. 2018. M59Ed2E: epidemiological cutoff values for antifungal susceptibility testing, 2nd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.EUCAST. 2018. MIC and zone diameter distributions and ECOFFs. http://www.eucast.org/mic_distributions_and_ecoffs/. Accessed 12 September 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.