Tedizolid phosphate, the prodrug of the active antibiotic tedizolid, is an oxazolidinone for the treatment of acute bacterial skin and skin structure infections. Studies in a mouse thigh infection model demonstrated that tedizolid has improved potency and pharmacokinetics/pharmacodynamics (PK/PD) compared with those of linezolid.
KEYWORDS: linezolid, neutropenia, oxazolidinones, pharmacodynamics, pharmacokinetics, tedizolid
ABSTRACT
Tedizolid phosphate, the prodrug of the active antibiotic tedizolid, is an oxazolidinone for the treatment of acute bacterial skin and skin structure infections. Studies in a mouse thigh infection model demonstrated that tedizolid has improved potency and pharmacokinetics/pharmacodynamics (PK/PD) compared with those of linezolid. Subsequent studies showed that the efficacy of tedizolid was enhanced in immunocompetent (IC) mice compared with neutropenic (immunosuppressed [IS]) mice, with stasis at clinically relevant doses being achieved only in the presence of granulocytes. The tedizolid label therefore contains a warning about its use in neutropenic patients. This study reevaluated the PK/PD of tedizolid and linezolid in the mouse thigh infection model in IC and IS mice using a methicillin-resistant Staphylococcus aureus (MRSA) strain (ATCC 33591) and a methicillin-susceptible S. aureus (MSSA) strain (ATCC 29213). The antistaphylococcal effect of doses ranging from 1 to 150 mg/kg of body weight tedizolid (once daily) or linezolid (twice daily) was determined at 24, 48, and 72 h after initiating treatment. In IC mice, stasis was achieved in the absence of antibiotics, and both tedizolid and linezolid reduced the burden further beyond a static effect. In IS mice, tedizolid achieved stasis against MRSA ATCC 33591 and MSSA ATCC 29213 at 72 h at a human clinical dose of 200 mg, severalfold lower than that in earlier studies. Linezolid achieved a static effect against MRSA ATCC 33591 in IS mice at a dose lower than that used clinically. This study demonstrates that, with time, both tedizolid and linezolid at clinically relevant exposures achieve stasis in neutropenic mice with an MRSA or MSSA thigh infection.
INTRODUCTION
Acute bacterial skin and skin structure infections (ABSSSI) are most frequently caused by Gram-positive pathogens, with methicillin-resistant Staphylococcus aureus (MRSA) increasingly being the predominant pathogen in the United States and Europe (1–4). In concert with the growing prevalence of MRSA, rates of hospitalization for ABSSSI have increased in the last decade (5), and surveillance reports have documented the increased isolation of MRSA from hospitalized patients with skin infections (1, 5–7).
Tedizolid phosphate, the prodrug of the novel oxazolidinone tedizolid, is approved for the treatment of ABSSSI (8, 9). Tedizolid phosphate is rapidly and extensively converted by endogenous phosphatases to tedizolid, its microbiologically active moiety, after administration (10, 11). The pharmacokinetics of tedizolid allow for once-daily administration, either orally or intravenously, at equivalent doses (12). In vitro studies indicate that tedizolid is at least 4-fold more potent than linezolid against staphylococci (including MRSA), streptococci, and enterococci (including vancomycin-resistant strains); linezolid is the only other currently approved oxazolidinone (13–15). In 2 phase 3 trials, ESTABLISH-1 and ESTABLISH-2, tedizolid (200 mg once daily for 6 days) was shown to be noninferior to linezolid (600 mg twice daily for 10 days) for treating patients with ABSSSI, with a prespecified endpoint of an early clinical response at from 48 to 72 h, and was well tolerated (16–18).
Translational pharmacokinetic/pharmacodynamic (PK/PD) studies in neutropenic mouse infection models demonstrated the improved efficacy of tedizolid relative to linezolid (19). Dose fractionation studies in the neutropenic mouse thigh infection model showed that the area under the unbound (free) concentration-time curve (fAUC) divided by the MIC (the fAUC/MIC ratio) was the PK/PD index that best correlated with the microbiological effect of both tedizolid and linezolid against MRSA (19). In these studies, the doses of tedizolid phosphate that were examined resulted in greater reductions in methicillin-susceptible S. aureus (MSSA) and MRSA than equivalent doses (in milligrams per kilogram of body weight per day) of linezolid. At matched fAUC/MIC ratios of approximately 44 (linezolid, 44.6; tedizolid phosphate, 44.0), 150 mg/kg of tedizolid resulted in a 2- to 3-log-CFU/g reduction that was approximately 1-log-CFU/g higher than stasis, whereas tedizolid phosphate attained stasis at a dose of 33.8 mg/kg (19). A comparison of the antibacterial efficacy of tedizolid in immunocompetent (IC) and neutropenic (immunosuppressed [IS]) mice showed that the efficacy of tedizolid was greatly enhanced in the presence of granulocytes. In IC mice, stasis was achieved at a daily human-equivalent dose of less than 200 mg tedizolid phosphate after 24, 48, or 72 h. In contrast, in IS animals, stasis was achieved only at daily human-equivalent doses of 2,000, 2,100, or 2,300 mg, which were at least 10-fold higher than the therapeutic dose (20).
Because the tedizolid PK/PD target was based on data from IC mice at exposures 16-fold lower than those in IS mice, the label contains a warning regarding its use in neutropenic patients (8, 20). To reevaluate the earlier observations, we examined the efficacy of tedizolid in the mouse thigh infection model in IC and IS mice using both MRSA and methicillin-susceptible S. aureus (MSSA) strains.
RESULTS
Effect of neutropenia on susceptibility to MRSA and MSSA thigh infection.
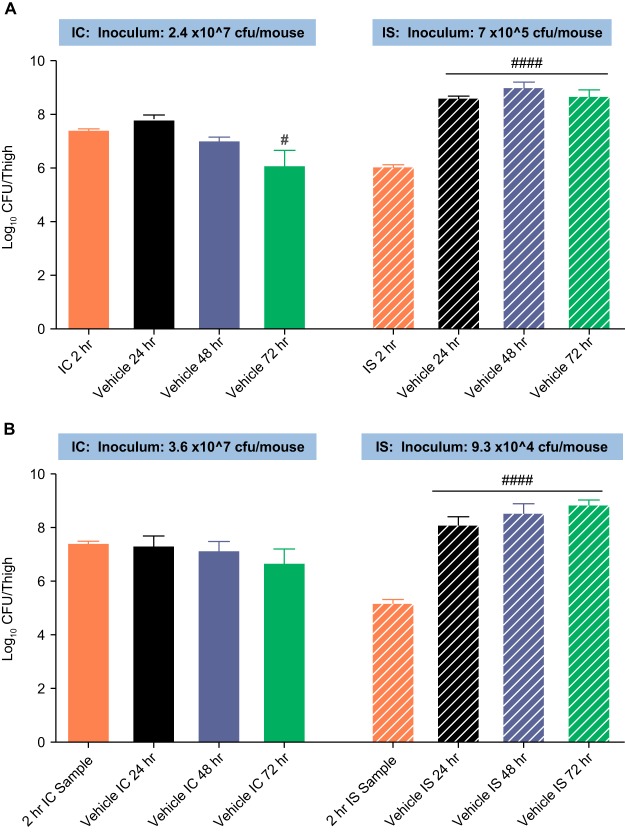
Figure 1 shows the growth of MRSA ATCC 33591 and the MSSA strain over 72 h in IC and IS mice treated with only the vehicle. In IC mice infected with MRSA, there was a significant reduction in the thigh bacterial burden by 72 h (P < 0.0001 compared with the 2-h baseline burden), whereas in IS mice, the bacterial burden showed a significant increase of ∼3 log CFU/thigh at 24, 48, and 72 h relative to that at the 2-h baseline (P < 0.0001). A similar significant and sustained increase in the bacterial burden over 72 h was noted in IS mice infected with the MSSA strain. These results were confirmed in a duplicate study. Thus, immune-mediated bacterial clearance resulted in a reduction in the bacterial burden over time in the absence of drug in IC mice.
FIG 1.
Growth of MRSA ATCC 33591 (A) and MSSA ATCC 29213 (B) in a murine thigh infection model under immunocompetent and neutropenic conditions. P values at each point are based on comparison of the count at each time point compared with the count at 2 h by analysis of variance. #, P < 0.05; ####, P < 0.0001. MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant S. aureus.
Tedizolid pharmacodynamics.
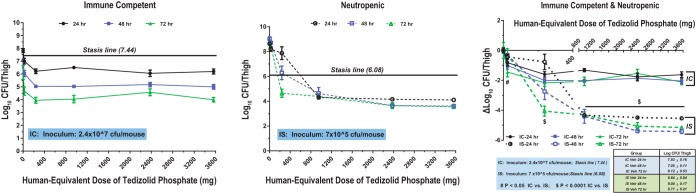
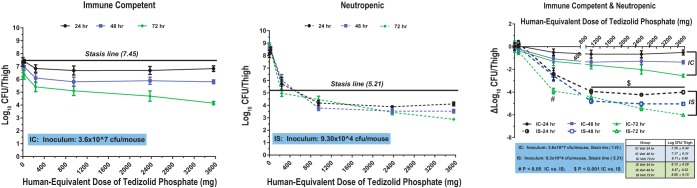
In IC mice, a reduction in the bacterial burden was time dependent but generally independent of the tedizolid dose. However, in IS mice, the reduction in the bacterial burden was dependent on both the dose and the time of evaluation postinoculation. In IC mice infected with MRSA ATCC 33591, the bacterial burden in vehicle-treated mice at 24, 48, and 72 h was 7.83, 7.05, and 6.12 CFU/thigh, respectively. Treatment with tedizolid at doses of ≥1 mg/kg reduced the bacterial burden beyond a static effect for both S. aureus strains (Fig. 2 and 3). At 24 h after bacterial challenge, maximal efficacy (an ∼2-log-CFU reduction) was achieved at tedizolid exposures equivalent to a clinical dose of ≥200 mg daily. For both strains at all doses, the treatment effect was greater at 48 and 72 h in IC mice, after they had received 2 and 3 tedizolid phosphate treatments, respectively. The maximum bacterial load reduction was observed at 72 h. There was no difference among the doses in the burden reduction achieved in mice infected with MRSA, with a dose of 1 or 10 mg/kg providing a similar reduction in bacterial count as the highest dose of 150 mg/kg. For mice infected with MSSA ATCC 29213, there was almost a 2-log difference in the burden at 72 h between the lowest and highest doses.
FIG 2.
Pharmacodynamics of tedizolid against MRSA ATCC 33591 in immunocompetent (IC) and neutropenic (immunosuppressed [IS]) mice. Mice were inoculated with 2.4 × 107 CFU/thigh (IC) or 7 × 105 CFU/thigh (IS). Veh, vehicle.
FIG 3.
Pharmacodynamics of tedizolid against MSSA ATCC 29213 in immunocompetent (IC) and neutropenic (immunosuppressed [IS]) mice. Mice were inoculated with 3.6 × 107 CFU/thigh (IC) or 9.3 × 104 CFU/thigh (IS).
The human-equivalent doses (MEDs) of tedizolid required to achieve stasis in IC and IS mice against the 2 staphylococcal strains are shown in Table 1. In IS mice infected with MRSA ATCC 33591, stasis was observed at 72 h with a MED of 166 mg/day (Table 1; Fig. 2 and 3). Stasis at 24 and 48 h was achieved at MEDs of 594 mg and 333 mg, respectively. In vehicle-treated IS mice, bacterial counts were 8.64, 9.04, and 8.71 log CFU/thigh at 24, 48, and 72 h, respectively. At a dose of 10 mg/kg, tedizolid reduced the bacterial counts by 0.76 log CFU/thigh at 24 h, 2.75 log CFU/thigh at 48 h, and 4.07 log CFU/thigh at 72 h relative to those in the vehicle-treated controls. The maximum bactericidal effect was achieved at higher doses.
TABLE 1.
Estimated tedizolid or linezolid MED required to achieve stasisa
| Agent and strain | Group | Parameter | Value at: |
||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| Tedizolid | |||||
| MRSA ATCC 33591 | IS | MED (mg/day) | 594 | 333 | 166 |
| IS | fAUC/MIC | 32.0 | 17.9 | 8.9 | |
| IC | MED (mg/day) | <23.8 | <23.8 | <23.8 | |
| IC | fAUC/MIC | <1.3 | <1.3 | <1.3 | |
| MSSA ATCC 29213 | IS | MED (mg/day) | 499 | 499 | 214 |
| IS | fAUC/MIC | 13.4 | 13.4 | 5.8 | |
| IC | MED (mg/day) | <23.8 | <23.8 | <23.8 | |
| IC | fAUC/MIC | <1.3 | <1.3 | <1.3 | |
| Linezolid | |||||
| MRSA ATCC 33591 | IS | MED (mg/day) | 720 | 750 | 700 |
| IS | fAUC/MIC | 41.2–82.4 | 42.9–85.8 | 40.0–80.1 | |
| IC | MED (mg/day) | <10 | <10 | <10 | |
| IC | fAUC/MIC | <1.1 | <1.1 | <1.1 | |
| MSSA ATCC 29213 | IS | MED (mg/day) | 560 | 310 | 400 |
| IS | fAUC/MIC | 16.0 | 8.9 | 11.4 | |
| IC | MED (mg/day) | <10 | <10 | <10 | |
| IC | fAUC/MIC | <0.29 | <0.29 | <0.29 | |
Conversions to MED were based on published values equating a mouse dose of 8.42 mg/kg tedizolid to a single human dose of 200 mg and a mouse dose of 120 mg/kg linezolid to a single human dose of 600 mg. IC, immunocompetent; IS, immunosuppressed; MED, human-equivalent dose; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant S. aureus; fAUC/MIC, area under the unbound (free) concentration-time curve (fAUC) divided by the MIC.
Tedizolid also achieved stasis against MSSA ATCC 29213 at 72 h at a MED of 214 mg (Table 1; Fig. 3). Higher doses were required for the maximal bactericidal effect. The MED static dose was 499 mg at both 24 and 48 h.
In humans, the clinical dose of 200 mg achieves an average daily AUC of 25 to 28 µg·h/ml, resulting in an average fAUC/MIC ratio of 5 to 7 for an organism with an MIC of 0.5 µg/ml (8, 20). At 24 and 48 h, the MED required for stasis in IS mice exceeded the clinical dose of 200 mg/day for both strains, but at 72 h, the MED for stasis was 166 mg for MRSA ATCC 33591 and 214 mg for MSSA ATCC 29213. The fAUC/MIC ratio needed for stasis decreased over time for both strains.
Linezolid pharmacodynamics.
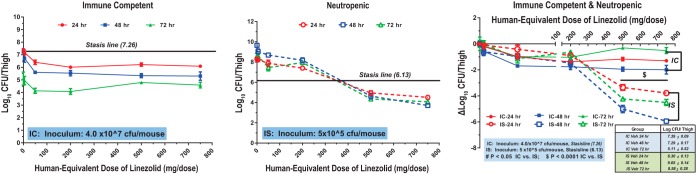
The MEDs of linezolid required to achieve stasis in IC and IS mice against the 2 staphylococcal strains are shown in Table 1. In IC mice, stasis against MRSA ATCC 33591 was achieved without antibacterial treatment (Fig. 4). Maximum efficacy (an ∼2-log reduction) at 24 and 48 h was observed at a MED of 1,500 mg, whereas by 72 h, maximum efficacy was observed at a MED of 100 mg. Maximum efficacy in IC mice was achieved at linezolid doses lower than the clinical dose of 600 mg twice daily. In IS mice, a static effect against MRSA ATCC 33591 was attained at 24, 48, and 72 h with MEDs of 720, 750, and 700 mg/day, respectively (Fig. 4). The static dose did not consistently decrease with time. The reduction in the bacterial burden was significantly greater in IS mice than in IC mice, with a 2- to 4-log greater reduction in the burden in IS mice treated with linezolid MEDs of 1,000 and 1,500 mg/day being achieved (P < 0.0001). The antibacterial efficacy of linezolid at 24, 48, and 72 h after infection with MRSA ATCC 33591 in IC and IS mice is shown in Fig. 4.
FIG 4.
Pharmacodynamics of linezolid against MRSA ATCC 33591 in immunocompetent (IC) and neutropenic (immunosuppressed [IS]) mice. Mice were inoculated with 4.0 × 107 CFU/thigh (IC) or 5 × 105 CFU/thigh (IS).
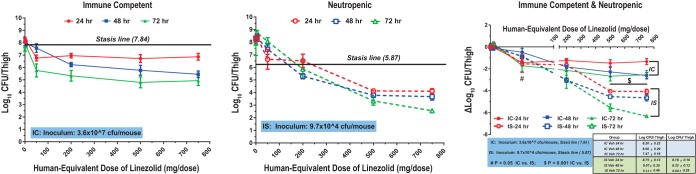
In IS mice, linezolid achieved stasis against MSSA ATCC 29213 at 24, 48, and 72 h at MEDs of 560 mg, 310 mg, and 400 mg, respectively (Fig. 5). Higher doses were required for the maximal bactericidal effect. The static dose did not consistently decrease with time, but MEDs were lower than the linezolid clinical dose.
FIG 5.
Pharmacodynamics of linezolid against MSSA ATCC 29213 in immunocompetent (IC) and neutropenic (immunosuppressed [IS]) mice. Mice were inoculated with 3.6 × 107 CFU/thigh (IC) or 9.7 × 104 CFU/thigh (IS).
DISCUSSION
This study shows that in thigh-infected IS mice, treatment with tedizolid achieved stasis against MRSA (ATCC 33591) and MSSA (ATCC 29213) infection at 72 h at exposures similar to those used in the clinical setting. These findings are in contrast to those from an earlier study in a similar mouse model with MRSA ATCC 33591, which showed that in IS mice tedizolid achieved stasis only at high nonclinical doses (20). Linezolid was also effective against MRSA at or below clinically relevant exposures in both IS and IC mice.
In IS mice, tedizolid achieved stasis against MRSA ATCC 33591 at 72 h at a MED of 166 mg and against MSSA ATCC 29213 at a MED of 214 mg. In humans, a clinical dose of 200 mg daily results in an average fAUC/MIC ratio of 5 to 7, based on an average daily AUC of 25 to 28 μg⋅h/ml (20, 21) and a free fraction of 13.4%. The murine fAUC/MIC ratios needed for stasis against MRSA ATCC 33591 and MSSA ATCC 29213 decreased with time, with the human fAUC/MIC ratio being reached in mice after 3 days. At 24, 48, and 72 h, fAUC/MIC ratios were 32.0, 17.9, and 8.9, respectively, with MRSA ATCC 33591 and 13.4, 13.4, and 5.8, respectively, with MSSA ATCC 29213. Higher clinical doses would be needed for efficacy in the murine system at earlier time points.
Linezolid treatment also decreased the bacterial burden from that at the baseline at all drug concentrations, including doses below the clinical dose of 600 mg twice daily. A static effect against MRSA ATCC 33591 was observed at a dose of approximately 75 mg/kg twice daily, with a further 2-log reduction in the bacterial burden occurring at higher doses. The static dose for MSSA ATCC 29213 was 56 mg/kg twice daily or less. The static doses did not consistently decrease with time, but the MEDs were below the linezolid clinical dose. In humans, the approved dose of 600 mg twice daily achieves an average daily AUC of 199 µg·h/ml with a free fraction of 69%, resulting in an average fAUC/MIC ratio of 137 (MIC, 1 µg/ml) (22). Murine fAUC/MIC ratios at the 24-, 48-, and 72-h murine static doses were 41 to 82, 43 to 86, and 40 to 80, respectively, for MRSA ATCC 33591 (MIC, 1 to 2 µg/ml) and were lower than those following clinical dosing. For MSSA ATCC 29213, fAUC/MIC ratios at the 24-, 48-, and 72-h murine static doses were substantially lower than those for MRSA ATCC 33591 at 16.0, 8.9, and 11.4, respectively. Based on the murine results, the fAUC/MIC achieved clinically with linezolid exceeds the fAUC/MIC needed for efficacy in IS mice.
The current study shows that both tedizolid and linezolid have antibacterial efficacy in IS mice, whereas in a previous murine study, tedizolid achieved stasis at a dose of 33.8 mg/kg at both 24 and 48 h, but a linezolid regimen of 150 mg/kg daily resulted in bacterial densities that were 1 log higher than stasis (19). At doses matched for fAUC/MIC ratios, tedizolid showed efficacy superior to that of linezolid, with a 1.1-log-CFU/g difference (19). However, in an IC murine thigh infection model, human-simulated exposures of tedizolid and linezolid resulted in similar in vivo efficacy against MRSA and MSSA (23).
The antimicrobial effect achieved with both tedizolid and linezolid was more pronounced in IS mice than in IC mice. A decrease from the baseline bacterial burden of up to 6 log CFU/thigh was observed with tedizolid in IS mice, whereas the decrease was ∼1 to 2 log CFU/thigh in IC mice. With linezolid, there was ∼1- to 2-log-CFU/thigh reduction in the colony burden from the baseline in IC mice, whereas the reduction was 4 to 6 log CFU/thigh in IS mice. An inoculum effect in IC mice relative to IS mice, with an approximately 20-fold higher infectious challenge in IC mice, may contribute to the lower ability of both linezolid and tedizolid to reduce the burden in the thighs of IC mice. The improved efficacy of tedizolid in IC mice compared with IS mice has been previously demonstrated; bactericidal activity against MRSA ATCC 33591 in healthy animals was attributed mainly to the effect of tedizolid mediated through granulocytes (20).
The importance of granulocytes in this model is underscored by the results of clearance of the bacterial burden in IC mice in the absence of antibacterial treatment. Neither of the strains showed robust growth in the thighs of IC mice, and stasis was achieved without any antibacterial therapy, which may be ascribed to granulocyte-mediated clearance. The first 2 major families of phagocytic cells are macrophages and neutrophils. Granulocytes are the major component of innate immunity and activate quickly to destroy pathogens through a group of proteins and phagocytic cells. Macrophages and neutrophils display a variety of cell surface receptors that enable them to recognize pathogens (24, 25).
In contrast, both strains showed a 2- to 3-log-CFU increase in IS mice over 72 h due to the loss of phagocytic cells. The static dose of tedizolid in this study was lower than that reported in a previous murine study in IS mice by Drusano et al., in which stasis was achieved by 72 h with a tedizolid MED of approximately 2,000 mg daily (20). The reasons for the disparate findings are unclear, as the current study matched that of Drusano et al. (20) closely, other than slight variations in the inoculum and differences in the mouse strains and growth media used, which are standard in our laboratory and not expected to significantly impact antibiotic efficacy (26). The current study was performed in CD-1 mice, used Trypticase soy broth as the growth medium, and used an inoculum (2.4 × 107/thigh in IC mice and 7 × 105/thigh in IS mice) slightly higher that used by Drusano et al., whereas Drusano et al. used Swiss Webster mice, Mueller-Hinton broth as the growth medium, and an inoculum of 1 × 107/thigh in IC mice and 5 × 105/thigh in IS mice (20). It could be argued that one possible confounding factor is intrinsic interspecies differences in the immune response (i.e., CD-1 mice may have a more robust immune response than Swiss Webster mice); however, our CD-1 mice were rendered neutropenic using a well-established methodology that was tested in the same mouse strain (ICR mice) (27). The same methodology was also used by Drusano et al. to render Swiss Webster mice neutropenic (20); thus, we view the possible impact of any interspecies difference in immune system functioning to be minimal. Of note, in the study by Drusano et al., all untreated infected IS mice were dead by 72 h in the absence of drug therapy, whereas in the current study no mortality was observed and the bacterial burden remained high between 24 and 72 h in untreated IS mice (20). Also, a tedizolid MED of 200 mg had a more pronounced antibacterial effect in IC Swiss Webster mice than in IC CD-1 mice. In the study by Drusano et al., the majority of bacterial killing in IC Swiss Webster mice was attributed to the effect of tedizolid mediated through granulocytes (20).
The 2 phase 3 ESTABLISH trials compared the efficacy of tedizolid at 200 mg once daily for 6 days and linezolid at 600 mg twice daily for 10 days for treating patients with ABSSSI (16, 17). In these studies, the primary efficacy endpoint, an early clinical response at from 48 to 72 h, was achieved by 79.5% and 79.4% of patients treated with tedizolid and linezolid, respectively, in the ESTABLISH-1 trial and 85% and 83% of patients treated with tedizolid and linezolid, respectively, in the ESTABLISH-2 trial (16–18). Although patients with neutropenia were excluded from the studies, the current results in neutropenic mice suggest that, with time, stasis may be achieved in neutropenic patients at the clinical dose. As our findings are inconsistent with those of the studies by Drusano et al. (19, 20), additional nonclinical or clinical studies may be warranted to investigate these differences further. Until efficacy is demonstrated in neutropenic patients in a randomized controlled clinical trial, the current warning and precaution in the tedizolid product labeling are warranted.
In summary, clinically relevant doses of tedizolid can lead to stasis in neutropenic mice with MRSA or MSSA thigh infection and may be predictive of clinical efficacy in neutropenic patients. The antibacterial effect of both tedizolid and linezolid was significantly greater in neutropenic mice than in healthy mice, with an approximately 1- to 4-log additional reduction in the bacterial burden being seen in neutropenic mice, indicating that the efficacies of both tedizolid and linezolid are not affected by neutropenia. The IS mouse model provides a powerful biosystem to study the direct effects between antibiotics and bacteria so that antibacterial potency can be truly evaluated.
MATERIALS AND METHODS
Bacterial strains.
The S. aureus isolates used in these studies were MRSA strain ATCC 33591 (American Type Culture Collection, Manassas, VA) and MSSA strain ATCC 29213. A frozen stock culture of each strain was inoculated into Trypticase soy broth and incubated overnight with shaking. Following centrifugation and reconstitution in fresh culture medium, the turbidity of the broth culture was adjusted by use of a spectrophotometer and then diluted in phosphate-buffered saline to the desired concentrations and used immediately. The bacterial densities in the suspensions were confirmed by quantitative cultures. The tedizolid and linezolid MICs for the 2 strains were as follows: for MRSA ATCC 33591, 0.25 µg/ml and 1 to 2 µg/ml, respectively, and for MSSA ATCC 29213, 0.5 µg/ml and 4 µg/ml, respectively.
Antibiotics.
A 200-mg stock vial of tedizolid phosphate (Sivextro; Merck Sharp & Dohme Corp.) was diluted in 4 ml sterile water to yield 50 mg/ml. This solution was diluted further in phosphate-buffered saline (pH 7.4) to achieve the final weight-based dose in 0.2 ml, based on the average weight of IC or IS mice in the thigh infection studies. Linezolid dosing solutions were prepared by reconstituting linezolid (Zyvox) for oral suspension (Pfizer) per the manufacturer’s instructions and then further diluting it in phosphate-buffered saline (pH 7.4) to achieve the final dose in 0.2 ml, based on the average weight of the mice.
Mice.
Female CD-1 mice (age, 6 to 11 weeks; weight, 21 to 25 g) were used in these studies. Mice were rendered neutropenic by 2 intraperitoneal (i.p.) injections of cyclophosphamide at 4 days (150 mg/kg) and 1 day (100 mg/kg) prior to bacterial challenge. This regimen resulted in severe neutropenia (count, <10/mm3) for at least 3 to 5 days from the time that the second dose of cyclophosphamide was administered (20, 27). Procedures involving the care and use of animals in the study were reviewed and approved by the Institutional Animal Care and Use Committee at Merck Research Laboratories. During the study, the care and use of animals were conducted in accordance with the principles outlined in the guidance of the Association for Assessment and Accreditation of Laboratory Animal Care, the Animal Welfare Act, the American Veterinary Medical Association Euthanasia Panel on Euthanasia, and the Institute for Laboratory Animal Research Guide to the Care and Use of Laboratory Animals.
Thigh infection model.
Mice were injected intramuscularly with approximately 1 × 107 CFU (IC mice) or approximately 5 × 105 CFU (IS mice) of one of the S. aureus strains in the right hind thigh. The actual bacterial inoculum was quantitated by plating the adjusted culture on mannitol salt agar. At 2 h, 5 mice were euthanized and thigh tissue was collected, homogenized, and quantitatively cultured on mannitol salt agar at 37°C. Colonies were enumerated to determine the baseline pretreatment bacterial burden per thigh. Treatment was then initiated with i.p. injections of vehicle or 1, 10, 40, 100, or 150 mg/kg of tedizolid (once daily) or linezolid (twice daily). At 24, 48, and 72 h postchallenge, 5 mice from each dose group were euthanized; infected thigh tissue was collected, homogenized, and quantitatively cultured on mannitol salt agar plates, and colonies were enumerated after a 24-h incubation at 37°C. To show replicability, all studies were performed in duplicate.
Calculation of human-equivalent doses.
Tedizolid human-equivalent doses (MEDs) were calculated based on the previous determination that 8.42 mg/kg of tedizolid in mice is equivalent to 200 mg tedizolid phosphate in humans (total plasma area under the concentration-time curve from time zero to 24 h [AUC0–24] of tedizolid = 20.1 µg·h/ml = 54.3 µM·h) (20). The calculated MEDs of tedizolid for murine doses of 1, 10, 40, 100, and 150 mg/kg were 23.8, 238, 952, 2,380, and 3,570 mg, respectively. For linezolid, murine doses of 10, 40, 100, and 150 mg/kg twice daily were equivalent to MEDs of 50, 200, 500, and 750 mg twice daily, respectively. A murine linezolid dose of 120 mg/kg twice daily was equivalent to the clinical dose of 600 mg twice daily (1,200 mg/day) (19).
Statistical analyses.
The bacterial burden in the thigh was expressed as the number of log CFU per thigh. The burden in the drug-treated groups was compared with that in the vehicle-treated control group. Statistical significance was determined using one-way analysis of variance (ANOVA) in GraphPad Prism software.
ACKNOWLEDGMENTS
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. All authors were employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, when this research was conducted, and may own stock and/or hold stock options in the company.
Medical writing assistance was provided by Meher Dustoor on behalf of Adelphi Communications LLC, New York, NY, and Robert Schupp of The Lockwood Group, Stamford, CT.
Medical writing assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
REFERENCES
- 1.Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. 2007. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998-2004). Diagn Microbiol Infect Dis 57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ. 2011. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis 53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 3.Meddles-Torres C, Hu S, Jurgens C. 2013. Changes in prescriptive practices in skin and soft tissue infections associated with the increased occurrence of community acquired methicillin resistant Staphylococcus aureus. J Infect Public Health 6:423–430. doi: 10.1016/j.jiph.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Sader HS, Farrell DJ, Jones RN. 2010. Antimicrobial susceptibility of Gram-positive cocci isolated from skin and skin-structure infections in European medical centres. Int J Antimicrob Agents 36:28–32. doi: 10.1016/j.ijantimicag.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Edelsberg J, Taneja C, Zervos M, Haque N, Moore C, Reyes K, Spalding J, Jiang J, Oster G. 2009. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 15:1516–1518. doi: 10.3201/eid1509.081228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suaya JA, Mera RM, Cassidy A, O'Hara P, Amrine-Madsen H, Burstin S, Miller LG. 2014. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 14:296. doi: 10.1186/1471-2334-14-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rennie RP, Jones RN, Mutnick AH, SENTRY Program Study Group. 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn Microbiol Infect Dis 45:287–293. doi: 10.1016/S0732-8893(02)00543-6. [DOI] [PubMed] [Google Scholar]
- 8.Merck & Co., Inc. 2015. Sivextro (tedizolid phosphate). Summary of product characteristics. Merck & Co., Inc, Kenilworth, NJ. [Google Scholar]
- 9.Merck & Co., Inc. 2016. Sivextro (tedizolid) tablets. Prescribing information. Merck & Co., Inc, Kenilworth, NJ. [Google Scholar]
- 10.Lodise TP, Drusano GL. 2014. Use of pharmacokinetic/pharmacodynamic systems analyses to inform dose selection of tedizolid phosphate. Clin Infect Dis 58:S28–S34. doi: 10.1093/cid/cit615. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan SD, Bien PA, Munoz KA, Minassian SL, Prokocimer PG. 2014. Pharmacokinetics of tedizolid following oral administration: single and multiple dose, effect of food, and comparison of two solid forms of the prodrug. Pharmacotherapy 34:240–250. doi: 10.1002/phar.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan S, Fang E, Munoz KA, Minassian SL, Prokocimer PG. 2014. Single- and multiple-dose pharmacokinetics and absolute bioavailability of tedizolid. Pharmacotherapy 34:891–900. doi: 10.1002/phar.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaadt R, Sweeney D, Shinabarger D, Zurenko G. 2009. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent. Antimicrob Agents Chemother 53:3236–3239. doi: 10.1128/AAC.00228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown SD, Traczewski MM. 2010. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob Agents Chemother 54:2063–2069. doi: 10.1128/AAC.01569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson KS, Goering RV. 2013. Activity of tedizolid (TR-700) against well-characterized methicillin-resistant Staphylococcus aureus strains of diverse epidemiological origins. Antimicrob Agents Chemother 57:2892–2895. doi: 10.1128/AAC.00274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. 2013. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 309:559–569. doi: 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 17.Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. 2014. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 14:696–705. doi: 10.1016/S1473-3099(14)70737-6. [DOI] [PubMed] [Google Scholar]
- 18.Shorr AF, Lodise TP, Corey GR, De Anda C, Fang E, Das AF, Prokocimer P. 2015. Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 59:864–871. doi: 10.1128/AAC.03688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob Agents Chemother 55:3453–3460. doi: 10.1128/AAC.01565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drusano GL, Liu W, Kulawy R, Louie A. 2011. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob Agents Chemother 55:5300–5305. doi: 10.1128/AAC.00502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong V, Flanagan S, Fang E, Dreskin HJ, Locke JB, Bartizal K, Prokocimer P. 2014. Absorption, distribution, metabolism, and excretion of the novel antibacterial prodrug tedizolid phosphate. Drug Metab Dispos 42:1275–1284. doi: 10.1124/dmd.113.056697. [DOI] [PubMed] [Google Scholar]
- 22.MacGowan AP. 2003. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother 51(Suppl 2):ii17–ii25. [DOI] [PubMed] [Google Scholar]
- 23.Keel RA, Tessier PR, Crandon JL, Nicolau DP. 2012. Comparative efficacies of human simulated exposures of tedizolid and linezolid against Staphylococcus aureus in the murine thigh infection model. Antimicrob Agents Chemother 56:4403–4407. doi: 10.1128/AAC.00122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag JJ. 1991. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob Agents Chemother 35:1953–1959. doi: 10.1128/AAC.35.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tulkens PM. 1991. Intracellular distribution and activity of antibiotics. Eur J Clin Microbiol Infect Dis 10:100–106. doi: 10.1007/BF01964420. [DOI] [PubMed] [Google Scholar]
- 26.Craig WA, Bhavnani SM, Ambrose PG. 2004. The inoculum effect: fact or artifact? Diagn Microbiol Infect Dis 50:229–230. doi: 10.1016/j.diagmicrobio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis 6:55. doi: 10.1186/1471-2334-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]