Echinocandins are front-line agents for treatment of invasive candidiasis. There are no reported agent-specific differences in Candida mutational frequency of resistance or propensity to develop FKS mutations.
KEYWORDS: Candida glabrata, FKS, anidulafungin, caspofungin, echinocandin, micafungin, mutational frequency
ABSTRACT
Echinocandins are front-line agents for treatment of invasive candidiasis. There are no reported agent-specific differences in Candida mutational frequency of resistance or propensity to develop FKS mutations. The objective of this study was to measure spontaneous and FKS mutation rates among Candida glabrata strains. Twenty bloodstream isolates from patients with or without prior echinocandin exposure were included. Minimum inhibitory concentrations (MICs), minimum fungicidal concentrations (MFCs), and mutation prevention concentrations were higher for caspofungin than for anidulafungin (P < 0.0001) and micafungin (P < 0.0001). Mutational frequencies of resistance at 3× the baseline MIC were highest for caspofungin and lowest for micafungin. A total of 247 isolates were recovered at or above the MFC for caspofungin (n = 159), anidulafungin (n = 74), or micafungin (n = 14). Agent-specific MIC increases were noted for anidulafungin and caspofungin, but not micafungin. Thirty-three percent of isolates harbored hot spot mutations in FKS1 (n = 6) or FKS2 (n = 76). Mutations at the Ser629 (Fks1) or Ser663 (Fks2) loci were more common after selection with anidulafungin or micafungin than with caspofungin (P = 0.003). Four isolates demonstrated >4-fold increases in MICs without FKS hot spot mutations; three of these harbored Fks2 mutations upstream of hot spot 1. The final isolate was FKS1 and FKS2 wild-type, but the 50% inhibitory concentrations of caspofungin and micafungin were increased 2.7- and 8-fold, respectively. In conclusion, micafungin may be superior in vitro to the other agents in limiting the emergence of resistance among C. glabrata. Caspofungin exposure may be most likely to promote resistance development. These data provide a foundation for future investigations of newly developed echinocandin agents.
INTRODUCTION
Echinocandins are the agents of choice for the treatment of invasive candidiasis (1). There are some pharmacokinetic (PK) differences between echinocandins (2), but no conclusive therapeutic differences in efficacy have been reported (3). Accordingly, anidulafungin, caspofungin, and micafungin are considered interchangeable by consensus guidelines (1). Widespread echinocandin usage has been accompanied by reports of emerging drug resistance among clinical isolates, particularly those of the haploid species Candida glabrata (4). Resistance is mediated by point mutations in FKS1 (all Candida species) or FKS2 (C. glabrata) hot spots that result in attenuated echinocandin activity (5). Most FKS mutations confer resistance to the entire echinocandin class, but some agent-specific mutations have been reported (6). Non-FKS mediated echinocandin resistance is uncommon but has been reported (7, 8).
Caspofungin resistance rates among C. glabrata are higher than anidulafungin or micafungin resistance rates (9, 10). At least in part, this reflects methodological issues with caspofungin susceptibility testing (11), and recently revised susceptibility breakpoints (4). It is unknown whether there are agent-specific differences in Candida mutational frequency rates or propensity to develop FKS mutations. As new echinocandin agents with unique PK characteristics and novel mechanisms of action enter late stages of clinical development, it is important to understand the limitations of currently available therapies. The objective of this study was to measure mutational frequency and FKS mutation rates among C. glabrata clinical isolates exposed to each echinocandin agent in vitro.
RESULTS
MICs, MFCs, MPCs, and spontaneous mutation frequencies for different echinocandins.
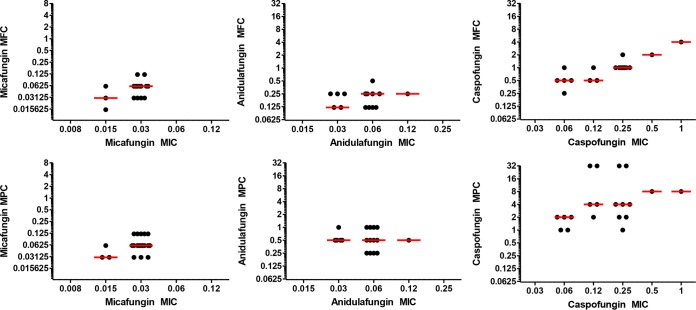
Echinocandin minimal inhibitory concentrations (MICs), minimum fungicidal concentrations (MFCs), and mutation prevention concentrations (MPCs) against parental C. glabrata isolates are listed in Table 1 and Table S1 in the supplemental material. The geometric mean MICs, MFCs, and MPCs were higher for caspofungin than for anidulafungin (P < 0.0001 for each) or micafungin (P < 0.0001 for each). The median fold differences between the MICs and MFCs or MPCs were significantly lower for micafungin (2-fold for both) than they were for anidulafungin (4.17- and 16.67-fold, respectively; P < 0.0001 for each) or caspofungin (4.17- and 16.67-fold, respectively; P < 0.0001 for each, Fig. 1).
TABLE 1.
MICs, MFCs, and MPCs by echinocandin for 20 C. glabrata clinical isolates
| Echinocandin | MIC (µg/ml) |
MFC (µg/ml) |
MPC (µg/ml) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | MFC50 | MFC90 | Range | MPC50 | MPC90 | Range | |
| Anidulafungin | 0.06 | 0.06 | 0.03–0.12 | 0.25 | 0.25 | 0.12–0.5 | 0.5 | 1 | 0.25–1 |
| Caspofungin | 0.185 | 0.275 | 0.06–1 | 1 | 2 | 0.25–4 | 4 | 32 | 1–32 |
| Micafungin | 0.03 | 0.03 | 0.015–0.03 | 0.06 | 0.06 | 0.015–0.12 | 0.06 | 0.12 | 0.03–0.12 |
FIG 1.
Comparison of minimum fungicidal (MFC, top row) and mutation prevention (MPC, bottom row) concentrations for each echinocandin agent stratified by baseline MIC. Horizontal lines indicate the median value for each group.
In rank order, the median spontaneous mutation frequency of resistance of C. glabrata isolates at 3× the baseline MIC was 3.08 × 10−9 for micafungin, 3.54 × 10−7 for anidulafungin, and 1.02 × 10−5 for caspofungin. Twenty-five percent (5/20) of the isolates did not yield colonies on Sabouraud dextrose agar (SDA) plates containing 3× the MIC of micafungin. The mutation frequency rates did not differ among isolates from patients with or without prior echinocandin exposure or among isolates susceptible or not susceptible to caspofungin (Table 2).
TABLE 2.
Rates of spontaneous mutation frequency by echinocandin agenta
| Echinocandin agent | Median mutation frequency rate |
P | Median mutation frequency rate |
P | ||
|---|---|---|---|---|---|---|
| Prior EC exposure (n = 10) | No prior EC exposure (n = 10) | CAS susceptible (n = 10) | CAS nonsusceptible (n = 10) | |||
| Anidulafungin | 3.002 × 10−7 | 5.869 × 10−7 | 0.85 | 2.875 × 10−7 | 3.952 × 10−7 | 0.60 |
| Caspofungin | 1.096 × 10−5 | 1.022 × 10−5 | 0.91 | 1.787 × 10−5 | 3.038 × 10−5 | 0.28 |
| Micafungin | 5.777 × 10−9 | 2.465 × 10−9 | 0.79 | 5.777 × 10−9 | 2.465 × 10−9 | 0.91 |
EC, echinocandin; CAS, caspofungin.
Characterization of spontaneous mutants.
A total of 247 C. glabrata isolates were recovered from echinocandin-containing agar plates at concentrations at or above the MFC (see Table S2 in the supplemental material). Echinocandin MICs were not increased (≤2-fold change) against 20% (50/247) of the corresponding isolates compared to parent isolates. For the remaining 80% (197/247) of isolates, >2-fold increases in the MIC of at least one agent were observed. By agent, >2-fold increases in caspofungin, anidulafungin, and micafungin MICs were noted against 72% (178/247), 48% (119/247), and 35% (87/247) of isolates, respectively. Isolates from anidulafungin-containing plates were more likely to exhibit >2-fold increases in anidulafungin MIC than >2-fold increases in caspofungin (P = 0.002) or micafungin (P = 0.0001) MICs. Similar results were noted for caspofungin MICs against isolates recovered from caspofungin-containing plates (P < 0.0001 versus anidulafungin or micafungin) (Table 3). In contrast, all isolates from micafungin-containing plates demonstrated a >2-fold increase in MIC of each agent. Using CLSI interpretive criteria, 68% (168/247), 40% (100/247), and 31% (76/247) of the isolates were resistant to caspofungin, anidulafungin, and micafungin, respectively.
TABLE 3.
Characteristics of selected isolates by echinocandin agent
| Factor | Echinocandin selection plate |
||
|---|---|---|---|
| Anidulafungin (n = 74) | Caspofungin (n = 159) | Micafungin (n = 14) | |
| Median drug concn (range) for selection | 0.5 (0.25–8) | 2 (0.5–8) | 0.25 (0.25–4) |
| No. (%) | |||
| >2-Fold anidulafungin MIC increase | 62 (84)a | 43 (27) | 14 (100) |
| >2-Fold caspofungin MIC increase | 48 (65) | 116 (73)b | 14 (100) |
| >2-Fold micafungin MIC increase | 30 (41) | 43 (27) | 14 (100) |
| FKS hot spot mutation | 23 (31)c | 47 (30) | 12 (86)d |
Colonies selected from anidulafungin-containing agar were more likely to have >2-fold MIC increases to anidulafungin than >2-fold MIC increases to caspofungin (P = 0.002) or micafungin (P = 0.0001).
Colonies selected from caspofungin-containing agar were more likely to have >2-fold MIC increases to caspofungin than >2-fold MIC increases to anidulafungin or micafungin (P < 0.0001 for both).
One additional isolate harbored a non-hot spot mutation in FKS2 (E655K).
All 14 isolates harbored mutations in FKS; two isolates harbored mutations outside the hot spot region (E655G and E655K) in FKS2.
Thirty-three percent (82/247) of breakthrough isolates harbored FKS hot spot mutations in FKS1 (n = 6) or FKS2 (n = 76) (Tables 3 and 4). All FKS1 mutations were within hot spot 1; 82% (62/76) and 18% (14/76) of the FKS2 mutations were identified in hot spots 1 and 2, respectively. The most common amino acid mutation was a deletion of Phe659 within the HS1 region of Fks2 (F659del). Eighty-three percent (5/6) of the FKS1 mutant isolates were selected from anidulafungin-containing agar plates compared to 23% (18/76) of the FKS2 mutant isolates (P = 0.006). Substitutions at amino acids Ser629 (Fks1; serine to phenylalanine or proline) or Ser663 (Fks2; serine to proline) were more frequent following selection with anidulafungin or micafungin (29% [10/35] of mutants) than with caspofungin (4% [2/47]; P = 0.003). Median echinocandin MICs were higher against FKS2 mutant isolates than against FKS1 mutants (P < 0.01 for each agent; Table 4).
TABLE 4.
FKS mutations in C. glabrata recovered from in vitro selection
| FKS mutation (gene) | No. of FKS mutant isolates | No. of FKS mutant isolates selected from each echinocandin plate |
Median (range) echinocandin MIC (µg/ml) |
||||
|---|---|---|---|---|---|---|---|
| Anidulafungin | Caspofungin | Micafungin | Anidulafungin | Caspofungin | Micafungin | ||
| F659del (FKS2) | 54 | 12 | 36 | 7 | 2 (0.015– 32) | 16 (0.25–32) | 4 (0.015–32) |
| R1378S or R1378G (FKS2) | 14 | 3 | 9 | 2 | 0.75 (0.06–4) | 16 (2–32) | 1.5 (0.015–32) |
| S663P or S663F (FKS2) | 6 | 2 | 1 | 3 | 1.5 (1–4) | 2 (1–32) | 1 (0.5–8) |
| S629P (FKS1) | 6 | 5 | 1 | 0 | 0.75 (0.12–1) | 2 (0.25–2) | 0.25 (0.06–1) |
| P667H or P667T (FKS2) | 2 | 1 | 1 | 0 | 0.19 (0.12–0.25) | 2.5 (1–4) | 0.06 (0.06) |
| E655G or E655K (FKS2)a | 3 | 1 | 0 | 2 | 8 (4–32) | 8 (4–32) | 32 (2–32) |
Noted for being outside hot spot regions in FKS2.
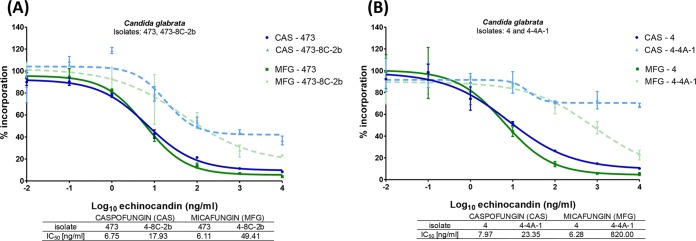
Two percent (4/247) of isolates demonstrated >4-fold increases in MICs of each echinocandin but did not exhibit FKS hot spot mutations. Interestingly, three of these isolates harbored Fks2 mutations at Glu655 (glutamic acid to glycine [Gly; n = 1] or lysine [Lys; n = 2]), which is upstream of hot spot 1. The final isolate exhibited increased MICs of all three agents but lacked a mutation in either FKS1 or FKS2. The 50% inhibitory concentrations (IC50s) of caspofungin and micafungin with β-(1,3)-d-glucan synthase from this isolate were increased 2.7- and 8-fold, respectively, relative to the enzyme isolated from the parental isolate (Fig. 2A). Moreover, the caspofungin and micafungin IC50s with the enzyme from a non-HS FKS2 mutant isolate (Glu655Lys) were 2.9- and 131-fold higher, respectively, in comparison to its parent isolate (Fig. 2B).
FIG 2.
(A) IC50 values for caspofungin (CAS) and micafungin (MFG) against parental (473) and passage (473-8C-2b) isolates. (B) IC50 values for CAS and MFG against parental (4) and passage (4-4A-1) isolates.
The FKS mutational frequency rates at concentrations greater than or equal to the MFC were 3.36 × 10−9 for micafungin, 9.72 × 10−9 for anidulafungin, and 2.04 × 10−8 for caspofungin. The sensitivities of caspofungin, anidulafungin, and micafungin MICs in identifying a FKS mutant isolates as resistant were 98% (83/85), 87% (74/85), and 87% (74/85), respectively. The corresponding specificities were 48% (77/162), 84% (136/162), and 99% (160/162), respectively.
DISCUSSION
To our knowledge, this is the first study to compare the spontaneous mutational frequency and emergence of in vitro resistance for the three commercially available echinocandin agents. Our data provide new insights into the phenotypic and molecular characteristics of echinocandin resistance among C. glabrata, the Candida species most often responsible for resistance in the clinic (4, 12). Micafungin MICs, MFCs, and MPCs were lowest among the three agents, followed in escalating order by those of anidulafungin and caspofungin. The rates of spontaneous and FKS mutational frequency followed the same order and did not differ for isolates collected from patients with prior echinocandin exposure or by the baseline echinocandin MIC. Likewise, fewer spontaneous and FKS mutants arose following micafungin selection compared to anidulafungin or caspofungin selection. Taken together, the data suggest that micafungin may be superior in vitro to the other agents in preventing the emergence of resistance among C. glabrata and that caspofungin may be most prone to induce resistance and FKS mutations. It is unclear whether these findings hold clinical relevance and worth noting that in the presence of human serum the heightened potency of anidulafungin and micafungin, relative to caspofungin, is largely mitigated (13). Nevertheless, they provide a foundation for further investigations, particularly as points of reference for the newly developed β-(1,3)-d-glucan synthase inhibitors rezafungin (Cidara, San Diego, CA) and ibrexafungerp (Scynexis, Jersey City, NJ).
Our data support the hypothesis that different FKS mutations are selected for after exposure to specific echinocandin agents. Some FKS variants differentially affect anidulafungin and caspofungin efficacy in vivo relative to micafungin (5, 6). In the present study, few breakthrough isolates were selected after micafungin exposure, but each harbored an FKS mutation, including two isolates with mutations upstream of FKS2 hot spot 1. By comparison, 32 and 30% of isolates selected following anidulafungin and caspofungin exposure, respectively, harbored FKS mutations. FKS mutations at hot spot loci Ser629 (Fks1) or Ser663 (Fks2) were more common following exposure to anidulafungin or micafungin than caspofungin. Mutations at these loci are associated with the highest echinocandin IC50s (14). It is important to note that we only assessed the presence of FKS mutations among isolates with >2-fold increases in MIC; thus, other mutations could have developed that were not associated with MIC changes. Future studies may indicate whether our laboratory observations are recapitulated in clinical practice. To this end, the most frequent mutation following caspofungin exposure in vitro was an amino acid deletion at Phe659, which is analogous to our clinical experience with this agent (12, 15). In contrast, Fks2 mutations at Ser663 were the most common mutations encountered at two centers using micafungin (16, 17).
Among C. glabrata clinical isolates, resistance to all three echinocandin agents is rare in the absence of FKS mutations. In this study, only four breakthrough isolates demonstrated >4-fold increases in MICs of all echinocandins without developing an FKS hot spot mutation. Sensitivity to the echinocandin class of the 1,3-β-d-glucan synthase enzyme was decreased in one of these isolates but not as substantially as in an E655K FKS2 mutant isolate; the latter mutation is upstream of previously defined hot spots (Fig. 2A and B). There are few studies that have investigated echinocandin resistance mechanisms other than FKS mutations. Caspofungin reduced susceptibility due to dysregulation of membrane sphingolipid biosynthesis was described in C. glabrata following in vitro exposure to the agent (8, 18). In addition to exhibiting 4- to 32-fold reductions in caspofungin susceptibility, these isolates had heightened susceptibility to micafungin (CRS-MIS phenotype) (8). Of note, the majority (55%) of breakthrough isolates with >2-fold increases in caspofungin MICs in the present study had micafungin MICs of ≤0.015 µg/ml. A mutator genotype in C. glabrata mediated by mutations in the mismatch repair gene MSH2, which is associated with multidrug resistance in vitro, has been described previously (19). Among clinical isolates, MSH2 polymorphisms did not correlate with echinocandin resistance in low-resistance settings (20). MSH2 polymorphisms were not evaluated in the present study; however, a prior report did not find a correlation with selection of micafungin resistance and MSH2 genotype (21). In patients, resistance is most commonly encountered following echinocandin exposure, which can serve as a useful Bayesian indicator for clinical decision making before susceptibility test results are available (4).
Anidulafungin and micafungin antifungal susceptibility testing has been advocated as surrogate markers of echinocandin resistance against C. glabrata given the interlaboratory and methodological variability associated with caspofungin susceptibility testing (4, 9–11, 22). The data presented here support this approach. Using the current CLSI interpretive criteria, caspofungin resistance was highly sensitive (98%) for identifying laboratory-generated FKS mutant isolates but poorly specific (48%). The corresponding sensitivities/specificities for anidulafungin and micafungin resistance were 87%/84% and 87%/99%, respectively. Moreover, changes in caspofungin MICs were often nonspecific. Indeed, relative to parent isolates, >2-fold MIC increases for caspofungin were associated with FKS hot spot mutations in 46% of isolates compared to 69 and 94% of isolates showing the same MIC increases for anidulafungin and micafungin, respectively (Table 3). Spontaneous and FKS mutation rates did not vary by baseline caspofungin MIC, including isolates below, at, or above the CLSI caspofungin susceptibility breakpoint. Taking these data together with our previous clinical findings (4, 9, 10, 12, 22), it is important to recognize that the use of CLSI breakpoints results in disproportionately high rates of caspofungin resistance among C. glabrata clinical and laboratory isolates. These data support the CLSI recommendation to perform confirmatory testing with anidulafungin or micafungin, or FKS genotyping when caspofungin nonsusceptible isolates are identified (23). On balance, caspofungin resistance (or nonsusceptibility if intermediate criteria are considered) is likely to be the most sensitive marker of FKS mutations, particularly for mutations that confer minimal changes to anidulafungin or micafungin MICs (6, 8). Ongoing challenges in interpreting caspofungin susceptibility testing results speak to the importance of optimizing testing methods and breakpoints for newly developed echinocandin agents.
Echinocandins are now endorsed broadly as the front-line agents for treatment of invasive candidiasis (1), which places increased importance on understanding of resistance mechanisms and potential differences between agents. As evidenced by a prior randomized clinical trial, differences between agents are not likely to be evident upon initial treatment courses (3). Rather, differential treatment success and resistance rates are more likely to become unmasked following prolonged courses of therapy for deep-seated infections. Indeed, infections such as intra-abdominal candidiasis (IAC) may represent hidden reservoirs for echinocandin resistance (24). Echinocandin pharmacokinetics-pharmacodynamics have not been evaluated systematically at deep-seated sites of infection, such as within intra-abdominal abscesses or tissues. Our data suggest that caspofungin MPCs exceed clinically achievable levels at these sites, whereas anidulafungin and micafungin MPCs are lower. New technology such as matrix-assisted laser desorption/ionization mass spectrometry imaging now allow the visualization of drug disposition within target organs (25). Using a murine model of IAC, we demonstrated that micafungin is quickly distributed into liver and kidney tissue, but the drug concentrates around the outer rim, rather than inside, fungal lesions. In contrast, a new second-generation echinocandin, rezafungin, is distributed at high concentrations throughout lesions (25). As agents like rezafungin and ibrexafungerp enter the clinic, it will be imperative to identify therapeutic advantages of each. An understanding of site-specific PKs and the propensity of various agents to induce resistance could be incorporated into novel treatment paradigms that improve clinical response rates and limit the emergence of echinocandin resistance.
In conclusion, the present study indicates that currently available echinocandins have differences in in vitro propensity for the emergence of resistance in C. glabrata and may select for agent-specific FKS mutations. Anidulafungin and caspofungin MICs can be selectively increased following exposure to the respective agents. Future studies are needed to determine whether these in vitro findings are relevant clinically and to devise echinocandin dosing regimens that durably suppress the emergence of resistance. It is possible that investigational agents with improved pharmacokinetics (such as rezafungin) or stability against some FKS variants (such as ibrexafungerp) will prove to be valuable additions to the antifungal armamentarium. As echinocandin utilization continues to grow, active surveillance for FKS and non-FKS mechanisms of resistance is needed.
MATERIALS AND METHODS
Isolates.
Twenty C. glabrata bloodstream isolates from unique patients at the University of Pittsburgh Medical Center were included in the study. Isolates were selected from patients with and without prior echinocandin exposure (n = 10 each), and they demonstrated a range of caspofungin MICs (n = 10 each demonstrated MICs above and at or below the Clinical and Laboratory Standards Institute [CLSI] susceptibility breakpoint). All isolates harbored wild-type FKS genes at baseline. Prior to testing, isolates were retrieved from −80°C stock, subcultured onto SDA plates, grown at 35°C for 24 to 48 h, and subcultured again for 24 h.
Echinocandin susceptibility testing.
Anidulafungin, caspofungin, and micafungin MICs were determined in triplicate according to CLSI document M27-A3 using a 50% turbidity endpoint at 24 h. Standard powders of anidulafungin (Pfizer, New York, NY), caspofungin (Merck, Rahway, NJ), and micafungin (Astellas Pharma, Japan) were obtained from the manufacturers. When insufficient growth was identified at 24 h, the endpoint was determined at 48 h. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality controls. The quality control strains were incorporated into each set of experiments, and MICs were within the expected range (23).
Spontaneous mutant frequency.
C. glabrata isolates were grown overnight in 15 ml of yeast extract-peptone-dextrose (YPD) broth. One hundred microliters of overnight cultures (5 × 108 to 1 × 109 CFU/ml) were streaked in duplicate onto SDA plates with or without echinocandins. Each agent was added to SDA plates at a concentration of 3× the MIC of each isolate. Plates were incubated at 35°C for 5 days. The spontaneous mutation frequency rate was calculated as the ratio of viable colonies growing on drug-containing plates over the starting inoculum. Experiments were performed in triplicate, and the average mutation frequency rate was used for further analysis. Consistent with prior reports in bacteria (26, 27) and yeasts (28), we have referred to the spontaneous mutational frequency rate as a resistance rate of colonies selected by drug-containing agar; however, genomic mutations were only assessed for isolates exhibiting >2-fold MIC increases.
Minimum fungicidal and mutation prevention concentrations.
Echinocandin MFCs and MPCs were determined in duplicate by streaking 200 µl of overnight culture onto SDA plates containing echinocandin agents at concentrations ranging from 0.015 to 16 µg/ml. Plates were incubated at 35°C for 5 days and inspected daily. Echinocandin MFCs and MPCs were defined as the lowest concentrations to inhibit >99.9 and 100% of fungal growth, respectively (28–31). Colonies growing at or above the MFC were saved at –80°C for subsequent analysis.
Determination of FKS mutations.
Echinocandin MICs were determined against colonies growing at or above the MFC of any agent. FKS hot spots were sequenced for colonies demonstrating a >2-fold MIC increase (relative to the parent isolate). FKS genes were sequenced outside hot spot regions for any isolate with elevated MICs not harboring FKS hot spot mutations. Briefly, C. glabrata genomic DNA was extracted from yeast cells grown overnight in YPD broth and purified using an ExoSAP-IT genomic DNA purification kit (Affymetrix, Santa Clara, CA). Hot spots 1 and 2 of FKS1 and FKS2 were amplified using PCR as previously described (4, 32). Standard Sanger DNA sequencing of purified PCR amplicons was performed with a 3130 DNA analyzer (Applied Biosystems, Carlsbad, CA). DNA sequences were analyzed with a sequence scanner (Applied Biosystems), and the corresponding amino acid sequences were compared to sequences in C. glabrata databases (https://www.ncbi.nlm.nih.gov/blast/).
Glucan synthase assay.
Candida glabrata isolates were grown with vigorous shaking at 37°C to early stationary phase in YPD broth and then cells were collected by centrifugation. Cell disruption, membrane protein extraction and partial 1,3-β-d-glucan synthase purification by product entrapment were performed as previously described (14). Reactions were initiated by the addition of product-entrapped glucan synthase. Sensitivity to caspofungin and micafungin was measured in a polymerization assay using a 96-well 0.65-μm-pore size multiscreen HTS filtration system (Millipore Corporation, Bedford, MA) in a final volume of 100 µl, as previously described (32). Serial dilutions of the drugs (0.01 to 10,000 ng/ml) were used as calibration standards. Caspofungin and micafungin were dissolved in water. Inhibition profiles and half-maximal inhibitory concentration (IC50) values were determined using a normalized response (variable-slope) curve-fitting algorithm with Prism software (version 6.05; GraphPad, Irvine, CA). The kinetic data are the result of experiments performed in triplicate (14).
Statistical analysis.
Categorical and continuous variables were compared using chi-square or Mann-Whitney U tests, respectively. McNemar’s chi-square test was used to compare echinocandin-specific MIC differences between agents. All tests were two tailed, and a P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by an investigator initiated grant from Merck awarded to R.K.S. and in part by the National Institutes of Health (NIH) awards K08 AI114883 to R.K.S., R01 AI109025 to D.S.P., and R21 AI121555 to C.J.C, as well as by an Arnold O. Beckman postdoctoral fellowship from the Arnold and Mabel Beckman Foundation to K.R.H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01692-18.
REFERENCES
- 1.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eschenauer G, Depestel DD, Carver PL. 2007. Comparison of echinocandin antifungals. Ther Clin Risk Manag 3:71–97. doi: 10.2147/tcrm.2007.3.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL, Ostrosky-Zeichner L, Reboli AC, Suh B, Digumarti R, Wu C, Kovanda LL, Arnold LJ, Buell DN. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 4.Shields RK, Nguyen MH, Clancy CJ. 2015. Clinical perspectives on echinocandin resistance among Candida species. Curr Opin Infect Dis 28:514–522. doi: 10.1097/QCO.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, Hope W. 2012. Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FKS resistance mutations. Antimicrob Agents Chemother 56:2435–2442. doi: 10.1128/AAC.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KK, Maccallum DM, Jacobsen MD, Walker LA, Odds FC, Gow NA, Munro CA. 2012. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother 56:208–217. doi: 10.1128/AAC.00683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Healey KR, Katiyar SK, Castanheira M, Pfaller MA, Edlind TD. 2011. Candida glabrata mutants demonstrating paradoxical reduced caspofungin susceptibility but increased micafungin susceptibility. Antimicrob Agents Chemother 55:3947–3949. doi: 10.1128/AAC.00044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields RK, Nguyen MH, Press EG, Updike CA, Clancy CJ. 2013. Caspofungin MICs correlate with treatment outcomes among patients with Candida glabrata invasive candidiasis and prior echinocandin exposure. Antimicrob Agents Chemother 57:3528–3535. doi: 10.1128/AAC.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. 2013. Anidulafungin and micafungin minimum inhibitory concentration breakpoints are superior to caspofungin for identifying FKS mutant Candida glabrata and echinocandin resistance. Antimicrob Agents Chemother 57:6361–6365. doi: 10.1128/AAC.01451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields RK, Nguyen MH, Press EG, Cumbie R, Driscoll E, Pasculle AW, Clancy CJ. 2015. Rate of FKS mutations among consecutive candida isolates causing bloodstream infection. Antimicrob Agents Chemother 59:7465–7470. doi: 10.1128/AAC.01973-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paderu P, Garcia-Effron G, Balashov S, Delmas G, Park S, Perlin DS. 2007. Serum differently alters the antifungal properties of echinocandin drugs. Antimicrob Agents Chemother 51:2253–2256. doi: 10.1128/AAC.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690–3699. doi: 10.1128/AAC.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, Du C, Clancy CJ. 2012. Presence of an FKS mutation rather than minimum inhibitory concentration is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother 56:4862–4869. doi: 10.1128/AAC.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, Garey KW. 2014. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis doi: 10.1093/cid/ciu407. [DOI] [PubMed] [Google Scholar]
- 17.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healey KR, Katiyar SK, Raj S, Edlind TD. 2012. CRS-MIS in Candida glabrata: sphingolipids modulate echinocandin-Fks interaction. Mol Microbiol 86:303–313. doi: 10.1111/j.1365-2958.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healey KR, Zhao Y, Perez WB, Lockhart SR, Sobel JD, Farmakiotis D, Kontoyiannis DP, Sanglard D, Taj-Aldeen SJ, Alexander BD, Jimenez-Ortigosa C, Shor E, Perlin DS. 2016. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat Commun 7:11128. doi: 10.1038/ncomms11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delliere S, Healey K, Gits-Muselli M, Carrara B, Barbaro A, Guigue N, Lecefel C, Touratier S, Desnos-Ollivier M, Perlin DS, Bretagne S, Alanio A. 2016. Fluconazole and echinocandin resistance of Candida glabrata correlates better with antifungal drug exposure rather than with MSH2 mutator genotype in a French cohort of patients harboring low rates of resistance. Front Microbiol 7:2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bordallo-Cardona MA, Escribano P, Marcos-Zambrano LJ, Diaz-Garcia J, de la Pedrosa EG, Canton R, Bouza E, Guinea J. 2017. Low and constant micafungin concentrations may be sufficient to lead to resistance mutations in FKS2 gene of Candida glabrata. Med Mycol 56:903–906. doi: 10.1093/mmy/myx124. [DOI] [PubMed] [Google Scholar]
- 22.Eschenauer GA, Nguyen MH, Shoham S, Vazquez JA, Morris AJ, Pasculle WA, Kubin CJ, Klinker KP, Carver PL, Hanson KE, Chen S, Lam SW, Potoski BA, Clarke LG, Shields RK, Clancy CJ. 2014. Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrob Agents Chemother 58:1897–1906. doi: 10.1128/AAC.02163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2017. Performance standards for antifungal susceptibility testing of yeasts. Approved standard M60. CLSI, Wayne, PA. [Google Scholar]
- 24.Shields RK, Nguyen MH, Press EG, Clancy CJ. 2014. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother 58:7601–7605. doi: 10.1128/AAC.04134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Prideaux B, Nagasaki Y, Lee MH, Chen PY, Blanc L, Ho H, Clancy CJ, Nguyen MH, Dartois V, Perlin DS. 2017. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother 61:e01009-17. doi: 10.1128/AAC.01009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drusano GL, Lodise TP, Melnick D, Liu W, Oliver A, Mena A, VanScoy B, Louie A. 2011. Meropenem penetration into epithelial lining fluid in mice and humans and delineation of exposure targets. Antimicrob Agents Chemother 55:3406–3412. doi: 10.1128/AAC.01559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louie A, Castanheira M, Liu W, Grasso C, Jones RN, Williams G, Critchley I, Thye D, Brown D, Vanscoy B, Kulawy R, Drusano GL. 2012. Pharmacodynamics of beta-lactamase inhibition by NXL104 in combination with ceftaroline: examining organisms with multiple types of beta-lactamases. Antimicrob Agents Chemother 56:258–270. doi: 10.1128/AAC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bordallo-Cardona MA, Marcos-Zambrano LJ, Sanchez-Carrillo C, de la Pedrosa EGG, Canton R, Bouza E, Escribano P, Guinea J. 2018. Mutant prevention concentration and mutant selection window of micafungin and anidulafungin in clinical Candida glabrata Isolates. Antimicrob Agents Chemother 62:e01982-17. doi: 10.1128/AAC.01982-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X, Drlica K. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin Infect Dis 33:S147–S156. doi: 10.1086/321841. [DOI] [PubMed] [Google Scholar]
- 30.Drlica K. 2003. The mutant selection window and antimicrobial resistance. J Antimicrob Chemother 52:11–17. doi: 10.1093/jac/dkg269. [DOI] [PubMed] [Google Scholar]
- 31.Canton E, Peman J, Viudes A, Quindos G, Gobernado M, Espinel-Ingroff A. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn Microbiol Infect Dis 45:203–206. doi: 10.1016/S0732-8893(02)00525-4. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Kelly R, Kahn JN, Robles J, Hsu MJ, Register E, Li W, Vyas V, Fan H, Abruzzo G, Flattery A, Gill C, Chrebet G, Parent SA, Kurtz M, Teppler H, Douglas CM, Perlin DS. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother 49:3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.