Increasing bacterial resistance and poor patient adherence rates limit the effectiveness of conventional antibiotic therapies for urinary tract infection (UTI). The objective of this study was to investigate whether a single aminoglycoside dose adequately treated UTI.
KEYWORDS: cystitis, pyelonephritis, antibiotic resistance, antimicrobial stewardship, gentamicin, amikacin, plazomicin, aminoglycosides, netilmicin
ABSTRACT
Increasing bacterial resistance and poor patient adherence rates limit the effectiveness of conventional antibiotic therapies for urinary tract infection (UTI). The objective of this study was to investigate whether a single aminoglycoside dose adequately treated UTI. A systematic search of PubMed/MEDLINE and Google Scholar databases was performed through September 2018 for English language original research articles assessing the efficacy of one-time parenteral aminoglycoside as UTI monotherapy. Of 252 potentially relevant studies, 13 studies met the inclusion criteria, representing 13,804 patients. Patient ages ranged from 2 weeks to >70 years; both inpatient and outpatient settings were represented. Cystitis was more common than pyelonephritis, and more females were represented than males. Escherichia coli was the most commonly isolated uropathogen. The pooled microbiologic cure rate with single-dose aminoglycoside therapy was 94.5% ± 4.3%. Cure was sustained (no recurrence) for 73.4% ± 9.6% of patients at day 30. Lower cure rates were observed among patients with radiographic urinary tract abnormality (chi-square P < 0.01). Across all studies, 63/13,804 (0.5%) cases of nephrotoxicity, vestibular toxicity, or injection site reaction were reported; no hearing loss was observed. Single-dose aminoglycoside therapy appears to be an effective treatment option for lower UTI in nonseptic patients, with minimal toxicity. Additional studies would be beneficial to confirm efficacy for pyelonephritis. When resistance to first-line UTI agents is endemic, aminoglycosides may serve as β-lactam- and fluoroquinolone-sparing options.
INTRODUCTION
Antibiotic resistance is a significant and growing issue both within the United States and globally. A common indication for antibiotic prescribing across both inpatient and outpatient settings is treatment of urinary tract infection (UTI). UTIs are most commonly caused by Enterobacteriaceae and other Gram-negative organisms; however, antibiotic resistance rates among uropathogens to guideline-recommended first-line agents (1) have been increasing, with statistically significant increases in Escherichia coli resistance to nitrofurantoin and trimethoprim-sulfamethoxazole from 2003 to 2012 (2). While fluoroquinolones and β-lactams have been utilized as alternative therapies for UTI, decreasing uropathogen susceptibility, particularly to the fluoroquinolones (2), remains a concern. Additionally, from an antibiotic stewardship perspective, restricting the use of broad-spectrum agents recommended as first-line therapies for common inpatient conditions (e.g., pneumonia and intra-abdominal infection) is ideal in order to best preserve their antimicrobial activity. Thus, there exists a need for effective alternative agents for UTI.
The aminoglycosides were an early addition to the clinician’s antibiotic arsenal, starting in the 1940s with streptomycin and expanding through the 1970s. Over the past several decades, aminoglycosides have maintained excellent clinical activity against the majority of uropathogens, including drug-resistant Enterobacteriaceae (3). As aminoglycosides are eliminated in their active form nearly exclusively by the renal route, they emerged quickly as excellent therapeutic options for the treatment of UTI; however, they fell out of favor as first-line agents due to toxicities associated with multiday administration, with aminoglycoside use declining by 41% from 2002 to 2009 (4). However, with increasing rates of antibiotic resistance and recent reinvigoration of the aminoglycoside class with the introduction of plazomicin (5), clinicians have recently regarded the aminoglycosides with renewed interest.
A particularly attractive option is the use of a one-time dose of aminoglycoside for UTI treatment. Single-dose therapy is advantageous, as it eliminates the need for patient adherence, a particular concern for genitourinary infections, where nonadherence rates approach 60% (6), and may avert the need for inpatient admission due to the lack of a susceptible oral antibiotic option. Aminoglycosides are an ideal drug class for single-dose treatment of UTI, as they are excreted in high concentrations in the urine, exceeding plasma concentrations by up to 100-fold within an hour after parenteral administration (7). As an example, with only 1 mg/kg gentamicin (well below the doses typically used for Gram-negative infections), peak urine concentrations may exceed 400 µg/ml (8), 100 times the 2018 Clinical and Laboratory Standards Institute (CLSI) breakpoint for Enterobacteriaceae (9) and far exceeding the peak-to-MIC ratio of 10 to 12 that is recommended for efficacy. Additionally, after a single dose, concentrations remain above therapeutic levels for most uropathogens for 72 h or longer (10). Thus, the objective of this study was to systematically review the efficacy of single-dose aminoglycoside therapy for the treatment of UTI.
RESULTS
A total of 252 potential articles were screened (Fig. 1), with 13 articles (11–23) representing 13,804 patients (median, 37 patients) meeting the inclusion criteria (Table 1). All studies were published between the years of 1978 and 1991. Both inpatient (6/13) and outpatient (10/13) populations were represented; however, no studies were conducted in emergency department (ED) settings or skilled nursing facilities. Four studies (30.8%) were multicenter. The duration of patient follow-up ranged from 48 h to 3 years. Seven studies (53.8%) included a comparator arm, either a single oral dose of fosfomycin (3 studies) or 5 to 10 days of oral conventional therapy (trimethoprim-sulfamethoxazole, amoxicillin, or oral cephalosporin). For the nonrandomized studies, a mean bias index of 0.65 was identified reflecting acceptable internal validity. The randomized controlled trials were assessed as having low or unclear bias risk; notably, no studies were blinded.
FIG 1.
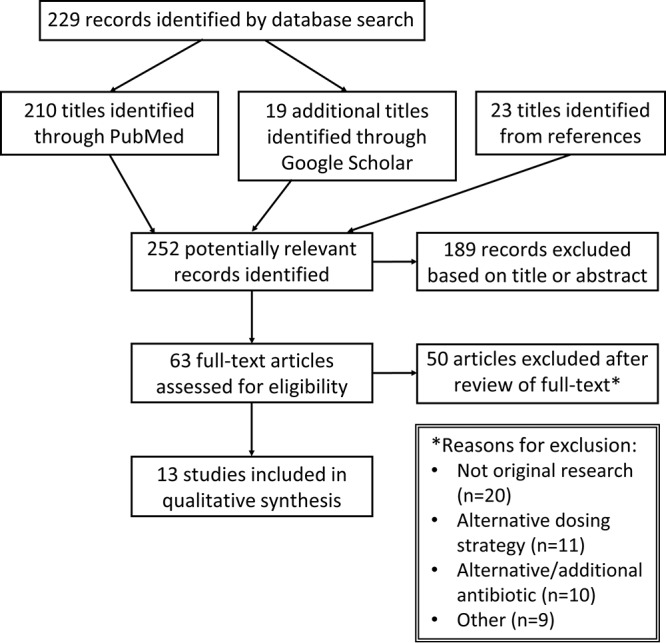
Study selection flowchart.
TABLE 1.
Included studies of single-dose aminoglycoside therapy for urinary tract infectiona
| Reference | Setting | Population |
UTI details |
Aminoglycoside regimen (name [dose])c | Efficacy (no. [%]) |
Safetyf |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group | Subject/sex distributionb | No. (%) with urinary malformation | Upper or Lower | Initial or Recurrent | Microbiologic cure | Clinical cure | Relapse | Recurrenced | Sustained microbiologic curee | No. (%) of ADE | No. of ADE type | |||
| Varese et al. (11) | Inpatient | Children | 24 F/8 M | 10 (31) | NR | Mixed | Gentamicin (5 mg/kg) | 31 (97) | NR | 2 (6) | 6 (19) | 25 (78) | 0 | |
| Vigano et al. (12) | Inpatient | Children | 25 F/5 M | 12 (40) | Lower | Mixed | Netilmicin (4.5 mg/kg) | 29 (97) | NR | 2 (7) | 4 (13) | 25 (83) | 0 | |
| Varese (13) | Mixed | Children | 23 F/12 M | 7 (20) | NR | NR | Netilmicin (5 mg/kg) | 35 (100) | NR | 1 (3) | 5 (14) | 30 (86) | 0 | |
| Fairley et al. (14) | Outpatient | NR | 35 F/2 M | 16 (43) | Mixed | Recurrent | Kanamycin (500 mg) | 37 (89) | NR | 11 (30) | 16 (43) | NA | 0 | |
| Bailey et al. (15) | Outpatient | Adults | 22 F | 0 | Lower | NR | Netilmicin (150 mg) | 22 (95) | NR | 0 | 0 | NA | 0 | |
| Rocca Rossetti (16) | Outpatient | Mixed | 13,258 NR | 0 | Lower | NR | Amikacin (adults, 500 mg; children, 7.5 mg/kg) | NR | 10,983 (83) | NR | NR | NA | 60 (0.5) | 7 NT, 53 VT |
| Caramalli et al. (17) | Inpatient | Adults | 38 F/38 M | NR | NR | Mixed | Amikacin (15 mg/kg), netilmicin (5 mg/kg) | 73 (96) | 72 (95) | NR | 25 (33) | 48 (63) | 0 | |
| Principi et al. (18) | Mixed | Children | 40 F/24 M | 10 (16) | Lower | Mixed | Netilmicin (5 mg/kg) | 62 (97) | NR | NR | 10 (16) | 52 (81) | 3 (5) | 3 ISP |
| Prát et al. (19) | Outpatient | Adults | 36 F/8 M | 21 (48) | NR | Mixed | Netilmicin (300 mg) | 39 (89) | NR | NR | 14 (32) | NA | 1 (2) | 1 PAR |
| Grimwood et al. (20) | Mixed | Children | 29 F/10 M | 26 (67) | Mixed | Mixed | Gentamicin (3 mg/kg) | 34 (87) | NR | 5 (13) | 7 (18) | 27 (69) | 0 | |
| Khan et al. (21) | Outpatient | Children | 21 F | 0 | Lower | Recurrent | Gentamicin (5 mg/kg, maximum 300 mg) | 21 (100) | NR | 0 | 9 (43) | 12 (57) | 0 | |
| Wallen et al. (22) | Outpatient | Children | 26 F | 0 | Lower | Initial | Amikacin (7.5 mg/kg, maximum 240 mg) | 24 (92) | NR | 4 (15) | 6 (23) | 18 (69) | 0 | |
| Krzeska (23) | Outpatient | Children | 92 F/28 M | NR | NR | Mixed | Amikacin (10 mg/kg) | NR | NR | NR | 12 (10)g | NA | 0 | |
NR, not reported.
Numbers represent number of females (F) and males (M) or number of isolates.
All doses administered intramuscularly.
dRelapses plus reinfections.
eFor studies with minimum 30 days follow-up. NA, not applicable.
fADE, adverse drug event; NT, nephrotoxicity; VT, vestibular toxicity; ISP, injection site pain; PAR, paresthesia.
gWithin 3 years.
Patient population.
Across all studies, patient ages ranged from 2 weeks to >70 years. The majority of studies included children only (53.8%), 3 adults only (including 1 exclusively in elderly patients [mean age, 74 years]), 1 including both children and adults, and 1 not reporting age. Females represented 79.5% of all patients. Nine studies included patients with urinary malformations (e.g., vesicoureteral reflux; range, 0 to 50% of patients). Only one study reported the inclusion of patients with moderate and severe renal impairment (8/44 patients and 2/44 patients, respectively) (19).
Most UTIs were lower tract infections (cystitis); 2 studies also included patients with upper tract infections (e.g., symptoms associated with pyelonephritis), and 5 studies did not explicitly specify UTI type. No cases of sepsis or bacteremia were reported. Both initial UTIs and recurrent episodes were represented (58.0% versus 42.0%, respectively). Ten studies reported on UTI symptoms, including four studies (12, 15–17) requiring symptoms as an inclusion criterion. The most commonly reported symptoms were dysuria, frequency, and urgency. Five studies (12, 15, 18, 21, 22) excluded patients with fever or elevated inflammatory markers. Urinalysis results (e.g., pyuria) were not reported, with the exception that some studies listed a positive nitrite test as an inclusion criterion. Eleven studies reported bacteriuria counts; virtually all were >105 CFU/ml.
Intervention.
Netilmicin was the most frequently represented aminoglycoside, followed by amikacin and gentamicin. Dosing was most commonly weight based; however, fixed-dosing schemes were utilized as well, particularly among adult patients. The doses used were at or below those used for large-dose extended-interval regimens. All doses were administered intramuscularly.
Microbiology.
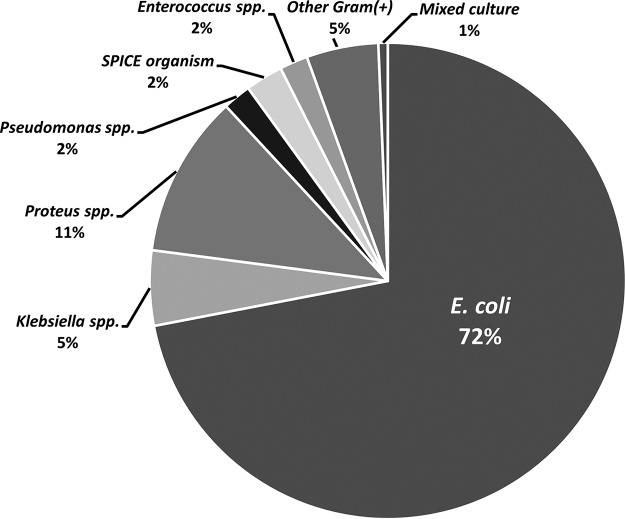
Isolated bacteria were identified for 471 patients. E. coli represented the vast majority of isolates (72.0%), followed by Proteus spp. and Klebsiella spp. (Fig. 2). Among the 306 isolates for which susceptibility data were available, only 3 organisms were reported as aminoglycoside resistant (2 Proteus spp. and 1 Klebsiella sp.); however, several studies excluded patients with resistant organisms (n not quantified). Only 1 study of netilmicin included MIC data (12), with 0.5 μg/ml as the highest reported MIC for E. coli (n = 21) and Klebsiella pneumoniae (n = 2) and 2 μg/ml for Proteus mirabilis (n = 6), all of which are below the 2018 CLSI breakpoint of ≤8 μg/ml (9).
FIG 2.
Distribution of bacteria from urine cultures. SPICE organism, any of the following: Serratia spp., Providencia spp., Morganella spp., Citrobacter spp., or Enterobacter spp.
Efficacy of single dose.
Microbiologic cure rates were able to be determined for 11/13 studies. All were in excess of 85%, with an overall cure rate of 94.5% ± 4.3%. Only 2 studies reported clinical cure, with rates of 82.8% (16) and 94.7% (17). Among the studies with a minimum of 30 days follow-up, an overall 19.0% (84/443) 30-day recurrence rate was reported, with roughly equal numbers of relapses and reinfections. Among the studies with adequate data, the percentage of patients with sustained microbiologic cure at 30 days was 73.4% ± 9.6%. Among the studies providing comparisons of cure rates for patients with and without urinary tract abnormalities (11, 12, 14, 19, 20), patients with anatomical abnormalities were less likely to have initial microbiologic cure (86.3% versus 96.9%, P < 0.01), and, among studies with adequate data, were less likely to have sustained microbiologic cure at 30 days (57.3% versus 87.5%, P < 0.001). There was no significant difference in microbiologic cure rate in a comparison of pediatric-only studies (11–13, 18, 20, 22) and adult-only studies (15, 17, 19) (95% microbiologic cure rate and 94% microbiologic cure rate, respectively; P > 0.05). There were inadequate data to compare sustained cure rates. Only one study (23) of children age 5 to 15 years stratified efficacy data by sex, with a 9.8% 3-year recurrence rate among 92 female patients and 10.7% 3-year recurrence rate among 28 male patients; this difference was not statistically significant. Among studies including a comparator arm, pooled initial microbiologic cure rates were 95.4% ± 4.2% for the aminoglycosides and 95.6% ± 3.9% for the comparators (P = 0.71, nonsignificant), and pooled sustained microbiologic cure rates were 71.0% ± 9.8% and 72.8% ± 11.5% for the aminoglycosides and comparators, respectively (P = 0.76, nonsignificant).
Safety of single-dose therapy.
Across all studies, 64 adverse effects attributed to single-dose aminoglycoside therapy were reported (64/13,804 [0.5%]), with 7 nephrotoxicity, 53 vestibular toxicity (e.g., tinnitus and equilibrium disorders), 3 cases of discomfort at the injection site, and 1 case of transient paresthesia around the mouth. Ten studies reported no adverse events. In one report (16), where patients received either single-dose aminoglycoside therapy or conventional (7-day) aminoglycoside therapy, a pooled 4.5% incidence of skin reaction or gastrointestinal disturbance was observed, though the rate among patients receiving only the single dose was not reported. However, the same study reported a lower rate of nephro- or vestibular toxicity in the single-dose group than was observed in the 7-day group (0.37% versus 2.63%, respectively). The adverse event rate across all nonaminoglycoside comparator groups was 3.5% (8/226).
DISCUSSION
Across 13 studies evaluating single-dose aminoglycoside therapy for UTI, high (87 to 100%) microbiologic cure rates were observed, with the majority of patients experiencing no recurrence of infection within 30 days, supporting the feasibility of single-dose therapy as a therapeutic strategy. These results were observed for both adult and pediatric patients and in both inpatient and outpatient settings. While dosing strategies varied across the studies, at this time, to ensure high urinary peaks, doses at the upper end of those used in the included studies (5 mg/kg of body weight for gentamicin or tobramycin and 15 mg/kg for amikacin) are recommended. Given their 4-fold increased potency compared to amikacin (24), lower doses of tobramycin or gentamicin may also feasibly be considered given success rates of amikacin with 10 to 15 mg/kg dosing. Use of adjusted body weight (AdjBW) in place of actual body weight (ABW) for patients at >120% of their ideal body weight (IBW) is recommended when dosing aminoglycosides [AdjBW = IBW + 0.4(ABW − IBW)], with the calculated dose rounded for adults to the nearest 20 mg for gentamicin and tobramycin and 25 mg for amikacin, given the available vial sizes and concentrations.
Combating antibiotic resistance is a multifaceted issue, requiring innovative strategies to best optimize and preserve the current antibiotic armamentarium. Optimization of cystitis treatment represents an excellent target for stewardship programs. In addition to facilitating improved UTI screening and diagnosis and advocating for the shortest effective treatment courses, promoting narrower-spectrum antibiotics with a niche exclusive or near-exclusive to UTI (e.g., nitrofurantoin) aids in the preservation of the antimicrobial activity of fluoroquinolones and parenteral β-lactams by reserving their use for patients who truly require these agents. Although aminoglycosides do have a role in expanding empirical Gram-negative coverage for select inpatient conditions, use as monotherapy is predominantly limited to genitourinary conditions. Thus, increased incorporation of aminoglycosides into UTI treatment algorithms may be an effective fluoroquinolone- and β-lactam-sparing strategy.
Single-dose intramuscular therapy has multiple potential benefits, including increased patient adherence and convenience in the outpatient setting. Additionally, with over 75% of UTI antibiotic prescriptions written for nonrecommended treatment durations (25), the incorporation of single-dose aminoglycoside therapy into facility treatment guidelines may reduce the collateral damage from prolonged prescription courses and patient reuse of leftover antibiotics. Of note, in one study (20), it was suggested that aminoglycoside therapy was less apt to alter commensal bowel or vaginal flora, which may reduce the likelihood of colonization with resistant bacterial strains and Clostridium difficile. Although none of the included studies in this review were conducted in the ED, it is arguably the most promising setting for single-dose UTI therapy in regions where uropathogen resistance to first-line agents is endemic. Among patients with resistant organisms, many lack identifiable risk factors, leading to high rates of discordant empirical antibiotic therapy (26). Increased utilization of single-dose aminoglycoside therapy may lead to enhanced activity against these resistant isolates and potentially avert inpatient admission for intravenous antibiotic therapy. Although not studied for cystitis as single-dose therapy, plazomicin has demonstrated excellent activity in vitro against carbapenem-resistant Enterobacteriaceae, including KPC-producing strains (27), and may be a viable alternative to the few KPC-active multiday intravenous antibiotics currently available.
Although patients with upper tract disease were represented in several of the included studies, data specific to pyelonephritis are lacking at this time. Additionally, there is no evidence to support the use of single-dose aminoglycoside therapy for septic or bacteremic patients. Further considerations regarding the appropriateness of single-dose therapy are outlined in Table 2. Of note, while patients (predominantly children) with anatomic abnormalities had lower response rates, this is commonly observed with standard antibiotic regimens as well (28) and should not be taken as a definite indication that single-dose therapy is inappropriate. Indeed, multiple authors (22, 23) have noted single-dose therapy to be of increased benefit for this population, as it allows treatment failure to be detected sooner, prompting earlier radiographic investigation or urologic consultation for patients suspected to have undiagnosed urogenital abnormalities.
TABLE 2.
Provider considerations for evaluating the appropriateness of single-dose AG therapy for UTIa
| Single-dose AG therapy may be appropriate |
| Lower tract infection (cystitis) |
| Local endemicity of organisms resistant to first-line UTI agents |
| Inpatient admission may be averted |
| Questionable patient adherence to oral therapy |
| Patient preference over oral therapy |
| Otherwise healthy individual |
| Alternative therapy recommended |
| Urosepsis/bacteremia |
| Previous infection with AG-resistant organism |
| High risk of Enterococcus sp. infection |
| Chronic renal insufficiency |
| Patient history of significant AG-mediated adverse drug event |
aAG, aminoglycoside; UTI, urinary tract infection.
One of the greatest worries when administering aminoglycoside therapy is concern for adverse drug events, including nephrotoxicity, hearing loss, vestibular toxicity, and rare neuromuscular blockade. Although the potential for these events should be recognized, nephrotoxicity rarely develops with short aminoglycoside courses of 3 days or fewer, even with high doses, and is almost always reversible (29). Common non-drug-related causes of acute kidney injury (e.g., intravascular volume depletion) should also be considered. In a systematic review of 24,107 patients receiving a single gentamicin dose, only 1.6% experienced transient serum creatinine elevations, with no rises in serum creatinine reported for studies where all patients were <75 years of age (30). No cases of ototoxicity were reported. In the largest included study of over 10,000 patients receiving single-dose amikacin for UTI, nephrotoxicity was reported even less frequently, at 0.04% (16). Thus, routine laboratory or auditory monitoring following single-dose therapy does not appear to be indicated in the absence of significant patient risk factors, particularly in younger, otherwise-healthy patients. However, selection of an alternate regimen for patients with significant renal impairment may be advisable due to a lack of data for this population.
Limitations.
Limitations of the present study should be acknowledged. First, all of the studies identified are older, with none conducted within the past decade. The revival of older treatments is not unprecedented; fosfomycin similarly experienced a resurgence of clinician interest since its introduction over four decades ago (31). Nevertheless, new studies would be welcomed to evaluate the performance of single-dose aminoglycoside therapy against modern uropathogens, which may have elevated aminoglycoside MICs compared to isolates from the 1980s, particularly Pseudomonas aeruginosa, which represented only 2% of isolates. Encouragingly, in one study of gentamicin monotherapy for acute pyelonephritis, two-thirds of inpatients with infection caused by gentamicin-resistant Enterobacteriaceae had early clinical success at 72 h (32), suggesting that aminoglycoside therapy may be effective for many UTI patients even with MICs above the breakpoint, either due to enhanced drug concentrations even within the upper urinary tract or due to spontaneous immune-mediated clearance of infection. An additional limitation is that symptom data were lacking, and clinical cure was infrequently evaluated; thus, some patients may not have had a true UTI. Third, no studies compared single-dose aminoglycoside therapy to intravenous cephalosporins or to nitrofurantoin, which has demonstrated superior sustained clinical resolution rates for lower UTI versus single-dose oral fosfomycin (33). Several of the comparator antibiotics are also not frequently used in modern practice due to resistance (e.g., oral amoxicillin), and these comparisons were unblinded and may have been underpowered.
Conclusions.
Existing evidence provides support for single-dose aminoglycoside therapy as a plausible treatment for cystitis in adults and children. Modern studies would help confirm efficacy for pyelonephritis and against contemporary uropathogens. Single-dose aminoglycoside therapy is a promising strategy deserving of enhanced consideration in the current era of multidrug resistance and patient nonadherence.
MATERIALS AND METHODS
Search strategy and selection criteria.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (34). The PubMed/MEDLINE and Google Scholar databases were searched from inception through September 2018, using the following search strategy: (single dose OR one time) AND (aminoglycoside OR amikacin OR gentamicin OR kanamycin OR netilmicin OR plazomicin OR sisomicin OR tobramycin) AND (urinary tract infection OR cystitis OR pyelonephritis OR urosepsis). The references of the full-text retrieved articles and previous reviews were manually perused to identify additional relevant studies. Unpublished studies, conference abstracts, and gray literature were not considered.
Articles were limited to original research studies published in the English language, with the following inclusion criteria: (i) use of an antibiotic of the aminoglycoside class as single-dose parenteral therapy (exclusively or within a numerically separable cohort), (ii) no other active antibiotic administered concomitantly, (iii) indication of urinary tract infection (documentation of symptoms was not required; however, articles solely evaluating eradication of asymptomatic bacteriuria were excluded), and (iv) evaluation of efficacy (i.e., microbiologic and/or clinical cure).
Data collection process and bias assessment.
Identification of potential studies was performed by two independent reviewers using the outlined search strategy, and any study that met the inclusion criteria was retrieved in full. Relevant information was extracted manually using a standardized data collection form. Any discrepancies were resolved by a third independent reviewer. Risk of bias was assessed for all included studies using the Cochrane Collaboration’s tool for randomized controlled trials (35) or the Reisch tool for nonrandomized interventional studies (36). Potential biases affecting the cumulative evidence were to be addressed in the “Limitations” paragraph above.
Outcome definitions.
Microbiologic cure was defined as the documented eradication of bacteria from the urine within 7 days of antibiotic administration. Clinical cure was defined as the resolution of UTI signs and symptoms (e.g., dysuria and frequency). Reinfection was defined as UTI recurrence on day 15 to 30 after therapy and/or documentation that the infecting organism differed from the organism associated with the index infection. Relapse was defined as UTI recurrence on day 1 to 14 posttherapy not meeting the definition for reinfection. Recurrences represent any new UTI within 30 days of the index infection, i.e., the sum of relapses and reinfections. Recurrence data beyond 30 days were not extracted (unless representing the only measure of recurrence), as recurrence beyond this point was considered unlikely to be significantly influenced by the antibiotic selected for primary treatment. Sustained microbiologic cure rates were reported for studies with a minimum 30-day follow-up duration and represented the proportion of patients with microbiologic cure and free from UTI recurrence through day 30.
Synthesis of results.
Because of variability in patient population, aminoglycoside regimens, and reporting of outcome measures, results are summarized descriptively, with the exception of select between-group comparisons of dichotomous variables analyzed via the chi-square or Fisher exact test.
ACKNOWLEDGMENTS
We declare no relevant conflicts of interests.
This study was conducted at Midwestern University with no internal or external sources of funding.
REFERENCES
- 1.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, Bordon J. 2016. Antibiotic resistance among urinary isolates from female outpatients in the United States in 2003 and 2012. Antimicrob Agents Chemother 60:2680–2683. doi: 10.1128/AAC.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sader HS, Castanheira M, Shortridge D, Mendes RE, Flamm RK. 2017. Antimicrobial activity of ceftazidime-avibactam tested against multidrug-resistant Enterobacteriaceae and Pseudomonas aeruginosa isolates from U.S. medical centers, 2013 to 2016. Antimicrob Agents Chemother 61:e01045-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ababneh M, Harpe S, Oinonen M, Polk RE. 2012. Trends in aminoglycoside use and gentamicin-resistant gram-negative clinical isolates in US academic medical centers: implications for antimicrobial stewardship. Infect Control Hosp Epidemiol 33:594–601. doi: 10.1086/665724. [DOI] [PubMed] [Google Scholar]
- 5.Connolly LE, Riddle V, Cebrik D, Armstrong ES, Miller LG. 2018. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother 62:e01989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kardas P, Devine S, Golembesky A, Roberts C. 2005. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents 26:106–113. doi: 10.1016/j.ijantimicag.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Wood MJ, Farrell W. 1976. Comparison of urinary excretion of tobramycin and gentamicin in adults. J Infect Dis 134:S133–S136. doi: 10.1093/infdis/134.Supplement_1.S133. [DOI] [PubMed] [Google Scholar]
- 8.Bennett JE, Dolin R, Blaser MJ. 2015. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 9.CLSI. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed. CLSI document M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Kahlmeter G, Kamme G. 1975. Prolonged excretion of gentamicin in a patient with unimpaired renal function. Lancet i:286. [DOI] [PubMed] [Google Scholar]
- 11.Varese LA, Grazioli F, Viretto A, Antoniola P. 1980. Single-dose (bolus) therapy with gentamicin in management of urinary tract infections. Int J Pediatr Nephrol 1:104–105. [Google Scholar]
- 12.Vigano A, Dalla Villa A, Bianchi C, Gandini G, Gaboardi F, Principi N. 1985. Single-dose netilmicin therapy of complicated and uncomplicated lower urinary tract infections in children. Acta Paediatr Scand 74:584–588. doi: 10.1111/j.1651-2227.1985.tb11034.x. [DOI] [PubMed] [Google Scholar]
- 13.Varese AL. 1987. Trometamol salt of fosfomycin versus netilmicin: randomized multicenter study in children’s lower urinary tract infections. Eur Urol 13:119–121. doi: 10.1159/000472876. [DOI] [PubMed] [Google Scholar]
- 14.Fairley K, Whitworth J, Kincaid-Smith P, Durman O. 1978. Single-dose therapy in management of urinary tract infection. Med J Aust 2:75–76. [DOI] [PubMed] [Google Scholar]
- 15.Bailey RR, Blake E, Peddie BA. 1984. Comparison of single dose netilmicin with a five-day course of co-trimoxazole for uncomplicated urinary tract infections. N Z Med J 97:262–264. [PubMed] [Google Scholar]
- 16.Rocca Rossetti S. 1986. Single-shot vs conventional therapy with amikacin for treatment of uncomplicated urinary tract infections: a multicenter study. Chemioterapia 5:394–399. [PubMed] [Google Scholar]
- 17.Caramalli S, Amprimo MC, Cavalli G, Mantelli M, Pollastrelli E, Raiteri F, Varese LA, Franchino L. 1991. Effect and pharmacokinetics of netilmicin given as bolus intramuscular administration: an open comparative trial versus amikacin and fosfomycin in elderly patients affected by urinary tract infections. Int J Clin Pharmacol Res 11:55–65. [PubMed] [Google Scholar]
- 18.Principi N, Corda R, Bassetti D, Varese LA, Peratoner L. 1990. Fosfomycin trometamol versus netilmicin in children's lower urinary tract infections. Chemotherapy 36:41–45. doi: 10.1159/000238816. [DOI] [PubMed] [Google Scholar]
- 19.Prát V, Horcicková M, Hatala M, Matousovic K, Liska M, Hnátek J, Milotová Z. 1984. Single-dose treatment with netilmicin for different clinical forms of urinary tract infections. Infection 12:99–101. doi: 10.1007/BF01641681. [DOI] [PubMed] [Google Scholar]
- 20.Grimwood K, Abbott GD, Fergusson DM. 1988. Single dose gentamicin treatment of urinary infections in children. N Z Med J 101:539–541. [PubMed] [Google Scholar]
- 21.Khan AJ, Kumar K, Evans HE. 1987. Single-dose gentamicin therapy of recurrent urinary tract infection in patients with normal urinary tracts. J Pediatr 110:131–135. doi: 10.1016/S0022-3476(87)80308-6. [DOI] [PubMed] [Google Scholar]
- 22.Wallen L, Zeller WP, Goessler M, Connor E, Yogev R. 1983. Single-dose amikacin treatment of first childhood E. coli lower urinary tract infections. J Pediatr 103:316–319. doi: 10.1016/S0022-3476(83)80376-X. [DOI] [PubMed] [Google Scholar]
- 23.Krzeska I. 1989. Amikacin single dose treatment for uncomplicated urinary tract infections in children. J Chemother 1:862–863. [PubMed] [Google Scholar]
- 24.Sutherland CA, Verastegui JE, Nicolau DP. 2016. In vitro potency of amikacin and comparators against E. coli, K. pneumoniae and P. aeruginosa respiratory and blood isolates. Ann Clin Microbiol Antimicrob 15:39. doi: 10.1186/s12941-016-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durkin MJ, Keller M, Butler AM, Kwon JH, Dubberke ER, Miller AC, Polgreen PM, Olsen MA. 2018. An assessment of inappropriate antibiotic use and guideline adherence for uncomplicated urinary tract infections. Open Forum Infect Dis 5:ofy198. doi: 10.1093/ofid/ofy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frazee BW, Trivedi T, Montgomery M, Petrovic DF, Yamaji R, Riley L. 2018. Emergency department urinary tract infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae: many patients have no identifiable risk factor and discordant empiric therapy is common. Ann Emerg Med 72:449–456. doi: 10.1016/j.annemergmed.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. 2018. In vitro activity of plazomicin against gram-negative and gram-positive isolates collected from U.S. hospitals and comparative activities of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother 62:e00313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts KB. 2011. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128:596–610. [DOI] [PubMed] [Google Scholar]
- 29.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 39:650–655. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayward RS, Harding J, Molloy R, Land L, Longcroft-Neal K, Moore D, Ross JDC. 2018. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br J Clin Pharmacol 84:223–238. doi: 10.1111/bcp.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sastry S, Doi Y. 2016. Fosfomycin: resurgence of an old companion. J Infect Chemother 22:273–280. doi: 10.1016/j.jiac.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wie SH, Kim HW, Chang UI. 2014. Effects of gentamicin monotherapy for the initial treatment of community-onset complicated non-obstructive acute pyelonephritis due to Enterobacteriaceae in elderly and non-elderly women. Clin Microbiol Infect 20:1211–1218. doi: 10.1111/1469-0691.12711. [DOI] [PubMed] [Google Scholar]
- 33.Huttner A, Kowalczyk A, Turjeman A, Babich T, Brossier C, Eliakim-Raz N, Kosiek K, Martinez de Tejada B, Roux X, Shiber S, Theuretzbacher U, von Dach E, Yahav D, Leibovici L, Godycki-Cwirko M, Mouton JW, Harbarth S. 2018. Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women: a randomized clinical trial. JAMA 319:1781–1789. doi: 10.1001/jama.2018.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. 2011. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reisch JS, Tyson JE, Mize SG. 1989. Aid to the evaluation of therapeutic studies. Pediatrics 84:815–827. [PubMed] [Google Scholar]