WCK 5222 is a novel β-lactam–β-lactam-enhancer combination of cefepime (FEP) and zidebactam (ZID). ZID is a novel β-lactam enhancer with a dual action of binding to Gram-negative penicillin-binding protein 2 (PBP2) and β-lactamase inhibition.
KEYWORDS: antibacterial agents, Gram-negative bacterial infections, drug resistance, Acinetobacter baumannii, Pseudomonas aeruginosa, beta-lactamases, Enterobacteriaceae, bacterial drug resistance, chronic kidney disease, multiple drug resistance, pharmacokinetics, pharmacology
ABSTRACT
WCK 5222 is a novel β-lactam–β-lactam-enhancer combination of cefepime (FEP) and zidebactam (ZID). ZID is a novel β-lactam enhancer with a dual action of binding to Gram-negative penicillin-binding protein 2 (PBP2) and β-lactamase inhibition. WCK 5222 is being developed as a new therapeutic option for the treatment of complicated multidrug-resistant Gram-negative pathogen infections. We investigated the effect of renal impairment on the pharmacokinetics (PK) and safety of WCK 5222 in 48 subjects based on Cockcroft-Gault-estimated creatinine clearance (CLCR). We enrolled mild (n = 6; CLCR, 60 to <90 ml/min), moderate (n = 6; CLCR, 30 to <60 ml/min), and severe (n = 6; CLCR, <30 ml/min; not on dialysis) impairment, end-stage renal disease (ESRD) on hemodialysis (HD) (n = 6), and matched normal controls (n = 24; CLCR, ≥90 ml/min). Healthy control subjects and mild and moderate renal impairment subjects received a single 60-min intravenous (i.v.) infusion of 3 g WCK 5222 (2 g FEP/1 g ZID); severe renal impairment and HD subjects received a single 60-min i.v. infusion of 1.5 g WCK 5222 (1 g FEP plus 0.5 g ZID). Body and renal clearance decreased, and plasma half-life (t1/2) and the area under the concentration-time curve from time zero extrapolated to infinity (AUC0–∞ [h µg/ml]) increased in a graded relationship with severity of renal impairment for both FEP and ZID. Our findings suggest that dose adjustments for WCK 5222 will be required according to the degree of renal impairment. Overall, WCK 5222 (FEP-ZID) was found to be safe and well tolerated in subjects with normal and impaired renal function. (This study has been registered at ClinicalTrials.gov under identifier NCT02942810.)
INTRODUCTION
Although β-lactamases constitute the primary mediators of resistance for many Gram-negative bacterial pathogens, the successful discovery and development of effective β-lactamase inhibitors have been an enormous challenge (1). Wockhardt is developing WCK 5222, a combination of cefepime (FEP), a fourth-generation cephalosporin, and WCK 5107 (international nonproprietary name [INN], zidebactam), a novel β-lactam enhancer. WCK 5222 is a new antibacterial agent targeted at multidrug-resistant Gram-negative pathogens, including metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa and OXA-carbapenemase-expressing Acinetobacter baumannii (2–5).
Zidebactam (ZID) is the first ever pan-β-lactamase-stable β-lactam-enhancer antibiotic. ZID is endowed with high-affinity penicillin-binding protein 2 (PBP2) binding across Gram negatives, including P. aeruginosa and A. baumannii (2). FEP has been in clinical use since 1996, following its approval for the treatment of multiple indications, such as moderate-to-severe pneumonia, complicated and uncomplicated urinary tract infections, complicated intra-abdominal infections, and uncomplicated skin and skin structure infections, and as empirical therapy for febrile neutropenia (6).
WCK 5222 is a novel β-lactam–β-lactam-enhancer combination that elicits rapid bactericidal activity at sub-MICs through simultaneous inactivation of PBP2 (ZID) and PBP3 (FEP). The high-affinity PBP2 binding action of ZID enhances the antibacterial activity of FEP; thus, the combination is distinguished from β-lactam–β-lactamase inhibitor combinations, which merely restore the activity of partner β-lactam antibiotics. This augmentation in FEP action results regardless of β-lactamase expression. Moreover, ZID is reported to inhibit several class A and class C β-lactamases (7, 8).
Sponsor-conducted in vitro and in vivo studies have shown that the β-lactam-enhancing mechanism of action, unlike the β-lactamase inhibitor mechanism, obviates the need to inhibit β-lactamases, including metallo-β-lactamases or OXA carbapenemase. This novel mechanistic approach enables WCK 5222 to demonstrate in vitro activity against multidrug-resistant Gram-negative pathogens (2, 3, 9–11).
Principal elimination of FEP occurs via renal excretion, with an average half-life (t1/2) of 2 h and total body clearance (CL) of 120 ml/min (6, 12, 13). FEP pharmacokinetics (PK) is linear over the dosage range of 250 mg to 2 g, and urinary recovery of unchanged FEP accounts for approximately 85% of the administered dose. It was demonstrated that FEP clearance is reduced, and t1/2 and area under the plasma concentration-time curve from time 0 extrapolated to infinity (AUC0–∞) are increased with increasing severity of renal impairment. Therefore, FEP dose reduction is required when prescribed in patients with significant renal impairment (13).
ZID clearance (5.02 to 6.57 liters/h) occurs primarily via renal excretion, and the volume of distribution (15.3 to 19.7 liters) and renal clearance (4.85 liters/h to 6.77 liters/h) appeared to be consistent and dose independent across the dose range of 250 mg to 3 g. ZID elimination half-life ranged from 1.84 to 2.39 h and appeared to be independent of dose. Overall, the AUC0–∞ and maximum plasma concentration observed (Cmax) of ZID increased in a dose-proportional manner (9).
When coadministered in healthy normal subjects, the majority (>80%) of the administered doses of ZID and FEP were excreted as unchanged drug within the first (0 to 6 h) collection interval, with complete dose recovery within 24 h postdose. No significant PK drug interaction was observed with coadministered FEP and ZID (9). It is anticipated that FEP and ZID elimination may be prolonged in patients with compromised renal function. Accordingly, this study has been designed to evaluate the effect of renal impairment on the pharmacokinetics and safety of FEP and ZID.
RESULTS
Forty-eight subjects were enrolled into the study, and each subject received a single 60-min i.v. infusion of WCK 5222. A summary of demographic and baseline characteristics for all enrolled subjects is provided in Table 1. Forty-two subjects were male, and 6 subjects were female. The mean (standard deviation [SD]) age of the study population was 60.5 (8.74) years. Thirty-nine subjects were White (Hispanic or Latino), and 9 subjects were Black or African-American. The mean (SD) body mass index (BMI) was 27.7 (4.3) kg/m2. All the renal impairment groups were comparable to their corresponding control groups with respect to age, BMI, and the proportion of males to females. The ESRD subjects were all non-Hispanics, whereas their controls were mainly Hispanics. The baseline CLCR results, estimated by Cockcroft and Gault, were consistent with study entry criteria and were comparable within treatment groups.
TABLE 1.
Demographic and baseline characteristics
| Characteristica | Mild |
Moderate |
Severe |
ESRD on HDb |
Total (n = 48) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Impairment (n = 6) |
Control (n = 6) |
Impairment (n = 6) |
Control (n = 6) |
Impairment (n = 6) |
Control (n = 6) |
Impairment (n = 6) |
Control (n = 6) |
||
| Age (yr) | |||||||||
| Mean (SD) | 69.5 (6.63) | 62.3 (6.12) | 62.7 (7.47) | 58.8 (6.97) | 60.2 (10.68) | 58.2 (12.78) | 57.8 (5.49) | 54.2 (7.14) | 60.5 (8.74) |
| Sex (no. [%]) | |||||||||
| Male | 6 (100.0) | 6 (100.0) | 4 (66.7) | 4 (66.7) | 5 (83.3) | 5 (83.3) | 6 (100.0) | 6 (100.0) | 42 (87.5) |
| Female | 0 | 0 | 2 (33.3) | 2 (33.3) | 1 (16.7) | 1 (16.7) | 0 | 0 | 6 (12.5) |
| Ethnicity (no. [%]) | |||||||||
| Hispanic or Latino | 5 (83.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 5 (83.3) | 6 (100.0) | 0 | 5 (83.3) | 39 (81.3) |
| Not Hispanic or Latino | 1 (16.7) | 0 | 0 | 0 | 1 (16.7) | 0 | 6 (100.0) | 1 (16.7) | 9 (18.8) |
| Race (no. [%]) | |||||||||
| Black or African-American | 1 (16.7) | 0 | 0 | 0 | 1 (16.7) | 0 | 6 (100.0) | 1 (16.7) | 9 (18.8) |
| White | 5 (83.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 5 (83.3) | 6 (100.0) | 0 | 5 (83.3) | 39 (81.3) |
| Ht (mean [SD]) (cm) | 169.0 (6.6) | 175.3 (5.0) | 166.0 (12.7) | 173.9 (7.2) | 164.8 (3.8) | 172.6 (8.1) | 170.7 (5.8) | 173.6 (5.1) | 170.7 (7.6) |
| Wt (mean [SD]) (kg) | 79.9 (12.1) | 88.3 (8.9) | 82.7 (18.9) | 85.7 (10.4) | 73.3 (19.6) | 78.8 (9.6) | 75.3 (12.9) | 82.2 (10.3) | 80.8 (13.3) |
| BMI (mean [SD]) (kg/m2) | 28.1 (4.7) | 28.7 (2.7) | 30.0 (6.3) | 28.4 (3.4) | 26.9 (6.7) | 26.6 (3.7) | 25.7 (3.1) | 27.3 (3.4) | 27.7 (4.3) |
| Creatinine clearance (mean [SD])b | 75.8 (6.3) | 138.6 (17.3) | 42.1 (7.7) | 163.8 (58.8) | 18.0 (6.5) | 136.9 (40.3) | 91.8 (67.1) | ||
BMI, body mass index.
ESRD, end-stage renal disease; HD, hemodialysis. Creatinine clearance (in milliliters per minute) estimated using the Cockcroft-Gault formula. Subjects with ESRD on HD were anuric.
Effect of renal impairment on FEP and ZID PK parameters.
Forty-six of 48 subjects (95.8%) were included in the plasma PK analysis, and 42 subjects (87.5%) were included in the urine PK population. None of the 6 subjects undergoing HD were able to produce urine and were therefore excluded from the urine PK analysis. PK parameters were evaluated across the spectrum of mild, moderate, and severe renal impairment and in patients with ESRD on HD. Both FEP and ZID demonstrated a graded reduction in clearance and corresponding increases in t1/2, AUC0–t, and AUC0–∞ with decreasing renal function. The PK parameters for FEP and ZID are summarized in Table 2, respectively. A statistical comparison of primary parameters across renal impairment cohorts is provided in Table 3. The plasma concentration-versus-time curve for FEP is displayed in Fig. 1A, and the plasma concentration-versus-time curve for ZID is displayed in Fig. 1B.
TABLE 2.
Summary of plasma PK parameters of FEP and ZIDa
| Parameter by drug | Data by renal impairment vs controls |
ESRD on HD |
||||||
|---|---|---|---|---|---|---|---|---|
| Mild |
Moderate |
Severe |
||||||
| Impairment (n = 6) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | Impairment (n = 4)b | Control (n = 6) | |
| FEP | ||||||||
| AUC0–t (h µg/ml) | ||||||||
| Mean | 479.0 | 351.9 | 922.9 | 307.0 | 698.3 | 331.1 | 1,247.8 | 293.2 |
| SD | 92.4 | 62.6 | 228.8 | 57.3 | 255.0 | 63.2 | 304.3 | 69.5 |
| AUC0–∞ (h µg/ml) | ||||||||
| Mean | 483.6 | 355.1 | 932.0 | 310.8 | 749.1 | 340.5 | 1,581.6 | 298.4 |
| SD | 92.8 | 63.5 | 231.8 | 57.8 | 305.1 | 71.0 | 585.9 | 69.2 |
| Cmax (µg/ml) | ||||||||
| Mean | 132.1 | 126.2 | 134.2 | 116.8 | 64.0 | 125.3 | 499.1 | 113.2 |
| SD | 25.3 | 32.6 | 18.1 | 9.5 | 13.5 | 22.8 | 584.6 | 29.5 |
| Tmax (h) | ||||||||
| Mean | 1.0 | 1.0 | 0.9 | 1.0 | 1.4 | 1.0 | 0.8 | 0.9 |
| SD | 0.1 | 0.2 | 0.2 | 0.2 | 1.3 | 0.1 | 0.5 | 0.2 |
| t1/2 (h) | ||||||||
| Mean | 3.6 | 2.9 | 6.5 | 2.5 | 10.4 | 2.6 | 21.0 | 2.6 |
| SD | 0.7 | 1.1 | 1.3 | 0.7 | 4.8 | 0.6 | 7.2 | 0.3 |
| λz (1/h) | ||||||||
| Mean | 0.2 | 0.3 | 0.1 | 0.3 | 0.1 | 0.3 | 0.0 | 0.3 |
| SD | 0.0 | 0.3 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| CL (ml/min) | ||||||||
| Mean | 71.2 | 96.7 | 37.8 | 110.3 | 25.7 | 102.3 | 11.7 | 117.2 |
| SD | 13.6 | 19.0 | 9.6 | 19.4 | 10.6 | 20.9 | 4.1 | 28.8 |
| Vz (liters) | ||||||||
| Mean | 22.0 | 23.4 | 20.8 | 23.1 | 20.7 | 21.9 | 19.4 | 25.8 |
| SD | 3.7 | 9.7 | 3.7 | 4.2 | 5.2 | 3.0 | 2.2 | 4.2 |
| Vss (liters) | ||||||||
| Mean | 17.6 | 19.2 | 17.6 | 17.3 | 19.0 | 17.1 | 17.7 | 18.4 |
| SD | 3.1 | 3.5 | 2.8 | 2.0 | 4.7 | 3.6 | 3.3 | 4.0 |
| Rresidence time (h) | ||||||||
| Mean | 4.2 | 3.3 | 8.2 | 2.7 | 13.9 | 2.9 | 27.7 | 2.6 |
| SD | 1.0 | 0.5 | 2.2 | 0.5 | 6.5 | 0.8 | 10.2 | 0.3 |
| ZID | ||||||||
| AUC0–t (h µg/ml) | ||||||||
| Mean | 202.7 | 151.2 | 431.6 | 129.3 | 368.6 | 139.1 | 694.2 | 127.4 |
| SD | 42.0 | 26.8 | 107.5 | 26.0 | 145.8 | 28.7 | 188.5 | 32.1 |
| AUC0–∞ (h µg/ml) | ||||||||
| Mean | 205.1 | 153.2 | 438.5 | 130.9 | 416.6 | 142.5 | 1,184.8 | 129.6 |
| SD | 42.6 | 26.9 | 112.3 | 26.3 | 204.1 | 30.1 | 657.9 | 32.0 |
| Cmax (µg/ml) | ||||||||
| Mean | 55.1 | 54.4 | 57.8 | 49.5 | 29.0 | 53.1 | 219.9 | 48.9 |
| SD | 11.1 | 13.6 | 7.0 | 4.9 | 6.3 | 9.6 | 267.7 | 13.1 |
| Tmax (h) | ||||||||
| Mean | 1.0 | 1.0 | 0.9 | 0.9 | 1.6 | 1.0 | 0.9 | 0.9 |
| SD | 0.1 | 0.2 | 0.2 | 0.2 | 1.2 | 0.1 | 0.8 | 0.2 |
| t1/2 (h) | ||||||||
| Mean | 3.7 | 2.7 | 7.1 | 2.6 | 12.9 | 2.5 | 35.4 | 2.6 |
| SD | 0.8 | 1.1 | 2.2 | 0.8 | 6.5 | 0.6 | 19.0 | 0.4 |
| λz (1/h) | ||||||||
| Mean | 0.2 | 0.3 | 0.1 | 0.3 | 0.1 | 0.3 | 0.0 | 0.3 |
| SD | 0.0 | 0.3 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| CL (ml/min) | ||||||||
| Mean | 84.3 | 111.9 | 40.3 | 131.5 | 24.4 | 121.5 | 9.0 | 135.8 |
| SD | 17.4 | 22.0 | 10.7 | 25.3 | 11.6 | 26.4 | 4.7 | 36.1 |
| Vz (liters) | ||||||||
| Mean | 26.5 | 26.0 | 23.4 | 28.0 | 23.4 | 25.4 | 21.8 | 29.8 |
| SD | 4.1 | 10.5 | 4.5 | 6.5 | 6.5 | 4.1 | 0.5 | 6.4 |
| Vss (liters) | ||||||||
| Mean | 21.2 | 21.9 | 20.4 | 20.5 | 21.2 | 19.9 | 20.6 | 21.0 |
| SD | 3.8 | 3.6 | 3.5 | 2.7 | 5.2 | 4.3 | 2.4 | 5.1 |
| Residence time (h) | ||||||||
| Mean | 4.3 | 3.3 | 9.0 | 2.7 | 17.4 | 2.8 | 48.7 | 2.6 |
| SD | 1.2 | 0.5 | 3.0 | 0.5 | 9.7 | 0.8 | 27.2 | 0.4 |
n = 46. Mean indicates arithmetic mean.
Two subjects in the ESRD on HD group were excluded from the plasma PK population due to spuriously high concentrations.
TABLE 3.
Comparison of primary parameters across renal impairment cohorts for FEP and ZIDa
| Parameter | Renal impairment category |
Geometric mean by group |
Geometric mean ratio impairment/control |
95% CI for ratiob | Correlation coefficientc |
|
|---|---|---|---|---|---|---|
| Impairment | Control | |||||
| FEP | ||||||
| AUC0–t (h µg/ml) | Mild | 471.5 | 347.0 | 1.4 | 0.885–2.087 | −0.922 |
| Moderate | 898.6 | 302.7 | 3.0 | 1.933–4.561 | ||
| Severe | 1,317.1 | 325.8 | 4.0 | 2.632–6.210 | ||
| ESRD on HD | 2,439.9 | 286.1 | 8.2 | 4.848–13.876 | ||
| AUC0–∞ (h µg/ml) | Mild | 476.1 | 350.1 | 1.4 | 0.864–2.142 | −0.902 |
| Moderate | 907.4 | 306.5 | 3.0 | 1.880–4.662 | ||
| Severe | 1,395.5 | 334.3 | 4.2 | 2.651–6.574 | ||
| ESRD on HD | 3,005.6 | 291.5 | 9.9 | 5.702–17.35 | ||
| Cmax (µg/ml) | Mild | 130.1 | 122.9 | 1.1 | 0.480–2.336 | −0.317 |
| Moderate | 133.1 | 116.5 | 1.1 | 0.518–2.522 | ||
| Severe | 125.9 | 123.2 | 1.0 | 0.463–2.254 | ||
| ESRD on HD | 459.2 | 110.1 | 4.1 | 1.555–10.800 | ||
| ZID | ||||||
| AUC0–t (h µg/ml) | Mild | 199.1 | 149.1 | 1.3 | 0.8391–2.1249 | −0.933 |
| Moderate | 420.1 | 127.2 | 3.3 | 2.0752–5.2548 | ||
| Severe | 687.8 | 136.6 | 5.0 | 3.1634–8.0104 | ||
| ESRD on HD | 1,349.3 | 124.0 | 10.4 | 5.8927–18.3868 | ||
| AUC0–∞ (h µg/ml) | Mild | 201.4 | 151.1 | 1.3 | 0.7923–2.2404 | −0.888 |
| Moderate | 426.1 | 128.8 | 3.3 | 1.9676–5.5639 | ||
| Severe | 754.1 | 139.9 | 5.4 | 3.2065–9.0672 | ||
| ESRD on HD | 2,096.0 | 126.2 | 16.0 | 8.4401–30.1467 | ||
| Cmax (µg/ml) | Mild | 54.3 | 53.1 | 1.0 | 0.4596–2.2728 | −0.353 |
| Moderate | 57.4 | 49.3 | 1.2 | 0.5233–2.5874 | ||
| Severe | 57.0 | 52.2 | 1.1 | 0.4908–2.4268 | ||
| ESRD on HD | 200.4 | 47.4 | 4.2 | 1.5746–11.1512 | ||
PK parameters of subjects in the severe and ESRD on HD impairment groups were dose-normalized to a 3-g dose by multiplying by 2 prior to analysis.
Simultaneous 95% confidence intervals across all renal impairment categories using Bonferroni adjustment.
Pearson correlation coefficient for log-transformed PK parameter versus creatinine clearance (Cockcroft-Gault formula) across all renal impairment subjects.
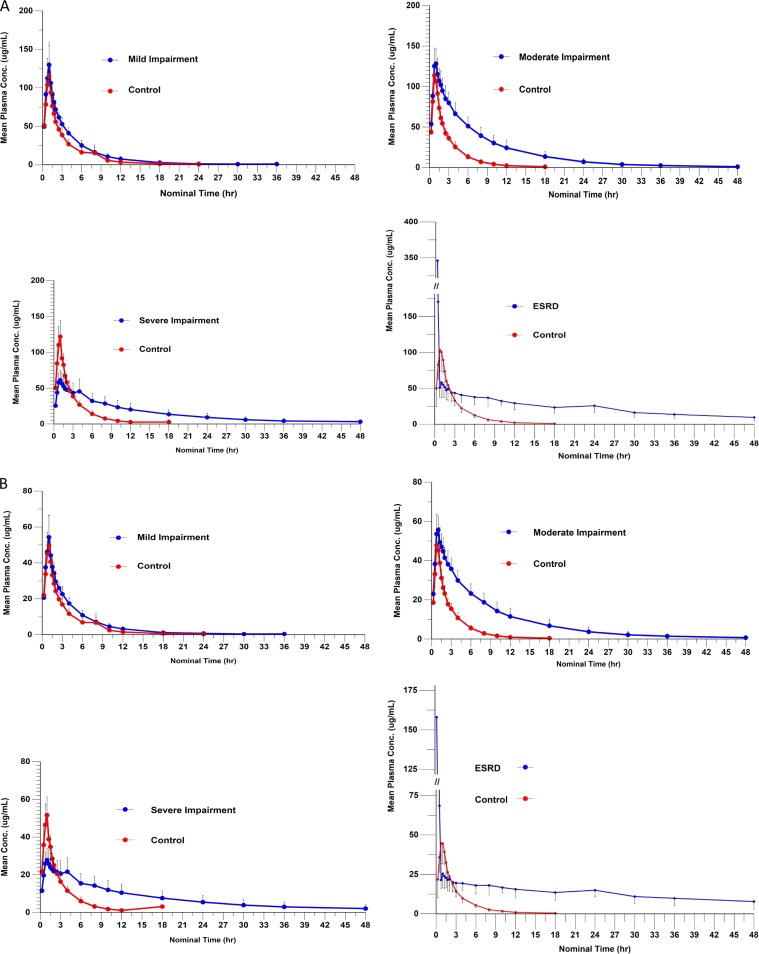
FIG 1.
(A) Plasma FEP concentration (conc) versus time. (B) Plasma ZID concentration versus time.
Effect of renal impairment on FEP PK parameters.
In control subjects, mean peak FEP concentrations were consistent among the control groups and reached a maximum of 113 to 126 µg/ml shortly before or just after the end of the 60-min infusion. Concentrations declined in a roughly monophasic manner, with a mean t1/2 of 2.6 to 2.9 h. The plasma clearances were consistent (97 to 117 ml/min). Drug concentrations in subjects with mild, moderate, and severe renal insufficiency also reached a maximum shortly before or just after the end of the 60-min infusion. A decrease in clearance was seen even in the subjects with mild renal dysfunction and became much more apparent with the moderate impairment group, with the concentration-versus-time plots becoming flatter as the severity of the renal disease increased. The variability between AUC0–∞, Cmax, t1/2, and CL parameters increased with degree of renal insufficiency.
Mild renal impairment.
Following a single 3-g dose of WCK 5222 (2 g FEP plus 1 g ZID), the mean Cmax was reached within the 60-min infusion time. The mean Cmax (SD) value was 132 (25.3) µg/ml versus 126 (32.6) µg/ml in healthy control subjects. Plasma clearance was modestly reduced to 71.2 (13.6) ml/min in mild renal impairment compared to 96.7 (19.0) ml/min in healthy control subjects, and the AUC0–∞ was 484 (92.8) h µg/ml in subjects with mild impairment versus 355 (63.5) h µg/ml in control subjects.
Moderate renal impairment.
Following a single 3-g dose of WCK 5222 (2 g FEP plus 1 g ZID), the mean Cmax was reached within the 60-min infusion time. The Cmax was 134 (18.1) µg/ml compared to 117 (9.53) µg/ml in healthy control subjects. Plasma clearance was reduced to 37.8 (9.62) ml/min in subjects with moderate renal impairment compared to 110 (19.5) ml/min in healthy control subjects. The AUC0–∞ was markedly increased to 932 (232) h µg/ml in subjects with moderate renal impairment compared to 311 (57.8) h µg/ml in healthy control subjects.
Severe renal impairment.
Following a single 1.5-g dose of WCK 5222 (1 g FEP plus 0.5 g ZID), the mean Cmax was reached within the 60-min infusion period, similar to healthy control subjects. The Cmax was 64 (13.5) µg/ml compared to 125 (22.8) µg/ml in healthy control subjects, which could be accounted for by half the dose being received by the severe renal impairment group. Plasma clearance was reduced to 25.7 (10.6) ml/min in the severe renal impairment group, roughly one-fourth that of 102 (21) ml/min in the healthy control subjects. The AUC0–∞ was increased to 749 (305) h µg/ml in subjects with severe renal impairment compared to 340 (71) h µg/ml in healthy control subjects.
ESRD on HD.
The time to Cmax (Tmax) was reached within the 60-min infusion period. Plasma clearance was 8.98 (5.25) ml/min in ESRD patients versus 117 (28.8) for healthy controls. The Cmax values for ESRD subjects were anomalous. For two of the subjects on dialysis, Cmax values were roughly half that of controls as was expected. Two subjects on HD had very high (up to 50-fold higher than controls) Cmax values during infusion and were excluded from the PK analysis due to such high concentrations.
Effect of renal impairment on ZID PK parameters.
In control subjects, mean peak ZID concentrations were consistent among the control groups and reached a maximum of 49 to 54 µg/ml shortly before or just after the end of the 60-min infusion. Concentrations declined in a roughly monophasic manner, with a t1/2 of 2.6 to 2.7 h. The plasma clearances were consistent (112 to 136 ml/min). Drug concentrations in subjects with renal insufficiency also reached a maximum shortly before or just after the end of the 60-min infusion and were roughly equivalent to those of the controls, except for those subjects on dialysis. In subjects with renal impairment, the departure from control profiles was as abrupt as that seen with FEP, with the mild renal dysfunction profiles becoming distinct from the control profiles in the moderate impairment group. The variability of AUC0–∞, Cmax, t1/2, and CL parameters increased with degree of renal insufficiency.
Mild renal impairment.
Following a single 3-g dose of WCK 5222, the mean Cmax was reached within the 60-min infusion time. The Cmax was 55.1 (11.1) µg/ml and was roughly equivalent to that observed in healthy control subjects, at 54.4 (13.6) µg/ml. Plasma clearance was reduced to 84.3 (17.3) ml/min in subjects with mild renal impairment compared to 112 (22.0) ml/min in healthy control subjects, and the AUC0–∞ values were 205 (42.6) h µg/ml versus 153 (26.9) h µg/ml, respectively.
Moderate renal impairment.
Following a single 3-g dose of WCK 5222, the mean Cmax was reached within the 60-min infusion time. The Cmax was 57.8 (7.03) µg/ml, compared to 49.5 (4.91) µg/ml in the healthy control subjects. Plasma clearance was sharply reduced to 40.3 (10.7) ml/min in subjects with moderate renal impairment compared to 132 (25.3) ml/min in the healthy control subjects. The AUC0–∞ was increased to 438 (112) h µg/ml in subjects with moderate renal impairment compared to 131 (26.3) h µg/ml in healthy control subjects.
Severe renal impairment.
Following a single 1.5-g dose of WCK 5222, the mean Cmax was reached within a 60-min infusion. The Cmax was 29 (6.25) µg/ml, compared to 53.1 (9.62) µg/ml in healthy control subjects, which could be accounted for by the severe renal impairment group receiving half the dose of drug. Plasma clearance was reduced to 24.3 (11.6) ml/min in subjects with severe renal impairment, roughly one-third that of the122 (26.3) ml/min found in healthy control subjects. The AUC0–∞ was increased to 417 (204) h µg/ml in subjects with severe renal impairment, compared to 143 (30.1) h µg/ml in healthy control subjects.
ESRD requiring HD.
Cmax values for ESRD subjects on HD were as anomalous as the values for ZID in the same two subjects. The Cmax values in these same subjects were up to 130 times those of the control, and these two subjects undergoing HD were excluded from the PK analysis due to highly anomalous plasma concentrations during infusion that may have been the result of venous shunting. The Tmax was reached within the 60-min infusion period. Plasma clearance was 7.0 (4.77) ml/min in ESRD patients versus 136 (36) ml/min for healthy controls, and the AUC0–∞ was 1,715 (1,006) h µg/ml in ESRD patients versus 130 (32) h µg/ml in the healthy controls.
Urine excretion and renal clearance.
The amount of FEP excreted from time zero to the end of each collection time is summarized in Table 4. Renal elimination was accounted for almost all of intact drug in healthy normal subjects. More than 95% of intact drug was excreted in the urine after 8 h.
TABLE 4.
Amount of FEP and ZID excreted in urinea
| Time postdose (mean [SD]) (h) |
Amt excreted in urine (mg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mild |
Moderate |
Severe |
ESRD on HD |
|||||
| Impairment (n = 6) |
Control (n = 6) |
Impairment (n = 6) |
Control (n = 6) |
Impairment (n = 6) |
Control (n = 6) |
Impairment (n = 0)b |
Control (n = 6) |
|
| FEP | ||||||||
| 0–2 | 807.3 (281.3) | 851.7 (408.3) | 278.45 (108.4) | 951.1 (190.6) | ||||
| 0–4 | 1,328.6 (344.3) | 1,512.9 (439.2) | 648.0 (216.9) | 1,632.7 (403.0) | ||||
| 0–8 | 1,819.3 (466.7) | 1,976.4 (376.8) | 1,001.5 (243.9) | 2,069.5 (429.6) | ||||
| 0–12 | 2,026.1 (450.6) | 2,107.1 (367.3) | 1,292.9 (235.3) | 2,244.0 (486.4) | ||||
| 0–24 | 2,144.3 (446.2) | 2,193.1 (358.7) | 1,610.6 (194.4) | 2,306.3 (499.7) | 587.1 (232.0) | 2,586.8 (987.3) | 2,403.8 (472.1) | |
| 0–48 | 2,150.8 (443.6) | 2,193.1 (358.7) | 1,701.0 (136.3) | 2,306.3 (499.7) | 646.9 (213.8) | 2,586.8 (987.3) | 2,403.8 (472.1) | |
| ZID | ||||||||
| 0–2 | 351.8 (104.2) | 365.6 (163.9) | 123.0 (49.6) | 447.1 (95.7) | ||||
| 0–4 | 572.0 (106.1) | 666.6 (179.2) | 292.3 (101.3) | 718.3 (170.2) | ||||
| 0–8 | 782.0 (153.3) | 868.6 (156.1) | 452.3 (114.9) | 900.3 (179.9) | ||||
| 0–12 | 871.0 (137.0) | 916.5 (158.6) | 583.0 (103.9) | 969.6 (203.6) | ||||
| 0–24 | 922.6 (133.5) | 946.4 (164.5) | 743.5 (70.5) | 992.5 (209.6) | 286.6 (108.3) | 1,081.5 (371.4) | 923.1 (214.8) | |
| 0–48 | 922.6 (133.5) | 946.4 (164.5) | 793.6 (35.1) | 992.5 (209.6) | 326.1 (98.8) | 1,081.5 (371.4) | 923.1 (214.8) | |
n = 42.
Subjects with ESRD were unable to produce urine and were not included in the urine PK analysis.
In the mild renal impairment group, 91% of the FEP dose was eliminated within 8 h. In the moderate renal impairment group, 50% of FEP was recovered up to 8 h. In subjects with severe renal impairment, the mean urinary recovery was 65% up to 48 h. Renal clearances declined with increasing impairment, falling from ∼120 ml/min for control subjects to ∼18 ml/min for severely impaired subjects.
The amounts of ZID excreted from time zero to the end of each collection time are summarized in Table 4. In healthy normal subjects, 90% of intact drug was excreted by 72 h. In the mild renal impairment group, urine recovery of ZID was 92% up to 48 h. In the moderate renal impairment group, urine recovery of ZID was 79% up to 48 h. In the severe renal impairment group, urine recovery of ZID was 65% up to 48 h.
Renal clearances declined with increasing level of impairment, falling from ∼120 ml/min for control subjects to 17 ml/min for severely impaired subjects. Dialysis subjects were anuric and were excluded from the urine PK analysis for both compounds.
Parallel changes in FEP and ZID clearances in renal impairment subjects.
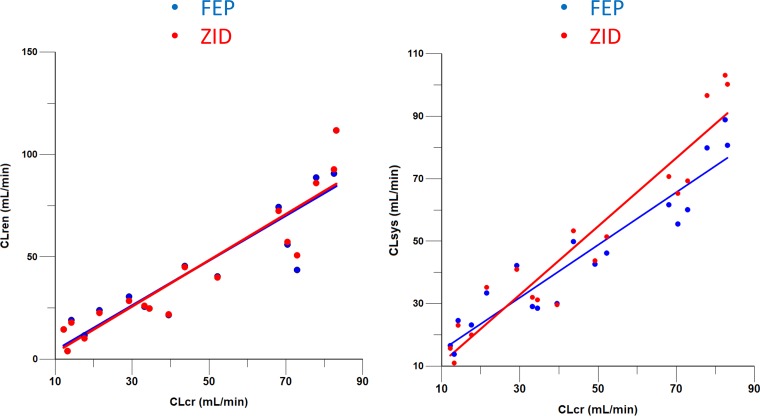
To assess the relative match of renal clearance (CLR) and systemic clearance (CLsys) for FEP and ZID, we employed the methods developed by Derendorf et al. (14, 15). The plots are displayed in Fig. 2. The results of regression analysis applied to determine mean slope and confidence intervals (CIs) for impaired subject data are displayed in Table 5. The CLR and CLsys versus CLCR plots are virtually superimposable for FEP and ZID. These results suggest that FEP and ZID are closely matched with similar clearances, and the FEP/ZID concentration ratios should be relatively independent of the degree of renal impairment.
FIG 2.
Renal clearance (CLren) and systemic clearance (CLsys) versus CLCR for FEP and ZID.
TABLE 5.
Linear mixed-effects analysis of CLR and CLsys versus CLCR for renal impairment subjects
| Compound | Parameter | Mixed-effect model | Mean slope | Lower CI | Upper CI | P value |
|---|---|---|---|---|---|---|
| FEP | CLR | Intercept + CLCR | 1.10 | 0.82 | 1.37 | <0.0001 |
| ZID | 1.13 | 0.88 | 1.37 | <0.0001 | ||
| FEP | CLsys | Intercept + CLCR | 0.84 | 0.69 | 0.99 | <0.0001 |
| ZID | 1.09 | 0.90 | 1.28 | <0.0001 |
Safety evaluation.
All 48 subjects were included in the evaluation of the safety of the single intravenous dose of WCK 5222. A summary of adverse events (AEs) is provided in Table 6. Of 24 subjects with renal impairment, two experienced a total of three AEs. Two AEs in one subject (injection site erythema and injection site swelling) were considered to be certainly related to the study drug administration. One AE (atrial fibrillation noted at safety follow-up) was considered to be not related to the study drug. One healthy control subject experienced two AEs (pruritus and rash) that were considered probably/likely related to the study drug. There were no severe adverse events (SAEs) or deaths reported during the study. No increasing or decreasing trends from baseline were observed in clinical laboratory test parameters, vital signs, electrocardiogram (ECG) results, or physical examination findings. There were no clinically significant abnormal findings noted for any of these safety parameters.
TABLE 6.
Summary of treatment-emergent adverse eventsa
| Subject group | Specific adverse event | Relatedness to FEP-ZIDb | Severity |
|---|---|---|---|
| Renal impairment (mild) | Injection site erythema | Certainly | Mild |
| Injection site swelling | Certainly | Moderate | |
| Renal impairment (moderate) | Atrial fibrillation | Unrelated | Moderate |
| Control | Pruritus | Probably/likely | Mild |
| Rash | Probably/likely | Mild |
Adverse events following a single intravenous dose of WCK 5222. n = 48.
Relationship to FEP-ZID rated as certainly related, probably/likely related, possibly related, unlikely related, not related, or nonassessable.
DISCUSSION
This phase 1 open-label single-dose study was conducted to evaluate the PK and safety of FEP and ZID following a single 60-min i.v. infusion of WCK 5222 (FEP plus ZID) in male and female subjects with normal and impaired renal function.
The PK findings for both FEP and ZID are consistent with their high percentage of elimination by renal excretion. Urinary recovery of intact drug was high for both compounds in subjects with normal renal function. Although recovery remained high in subjects with mild renal impairment, it decreased sharply in subjects with moderate and severe renal impairment.
The PK results for FEP in renal impairment are consistent with findings from a previous investigation (13) of the effect of renal impairment on the PK of 1 g FEP delivered over 30 min. For example, the previous study reported a mean (SD) plasma t1/2 of 2.29 (0.55) h and CL of 131 (24.6) ml/min in healthy normal control subjects, and a t1/2 of 10.5 (2.96) h and CL of 25.9 (6.73) ml/min in subjects with severe renal impairment, with mean creatinine clearance of 17 (4.64) ml/min. Consistent with this investigation, we observed a t1/2 of 2.56 (0.612) h and CL of 102 (21) ml/min in healthy normal subjects, and a t1/2 of 10.4 (4.77) h and CL of 25.7 (10.6) ml/min in subjects with severe renal impairment, with a mean creatinine clearance of 17.98 (6.46) ml/min.
ZID renal elimination was markedly prolonged in renal impairment. Half-life was prolonged from 2.48 (0.59) h in healthy normal volunteers versus 12.9 (6.53) h in patients with severe impairment. Plasma clearance was reduced from 122 (26.3) ml/min to 24.3 (11.6) ml/min in patients with severe renal impairment.
A marked reduction in clearance and corresponding increases in t1/2 and AUC0–∞ were observed starting with the moderate renal impairment group. These changes became more pronounced in the severe impairment and ESRD on HD groups. These findings suggest that the dose and/or duration of infusion of WCK 5222 should be adjusted in subjects with moderate or severe renal impairment and subjects on HD. In addition, the variability of PK parameters was increased with increasing renal impairment.
We used a lower dose of FEP-ZID for the severe and ESRD groups for safety reasons. To allow a comparison of PK across impairment groups, we normalized the PK across the severe and ESRD groups by multiplying by 2. PK parameters that are a function of exposure (Cmax and AUC) need to be normalized if they are going to be compared if different doses were used. This is not a perfectly exact approach, as lowering the dose across groups may give a lower exposure that may not be proportional to that with a full dose, potentially biasing the data and thereby making normalization by 2 imperfect. On the other hand, we wanted to have a quantitative analysis of differences in PK across groups, and PK parameters that are a function of exposure (Cmax and AUC) need to be normalized if they are going to be compared if different doses were used.
In the ESRD population, spurious and unexpectedly high drug concentrations were observed in 2 out of 6 subjects. The Cmax and concentration values during infusion for these two subjects were many-fold higher for both FEP and ZID than the values for other subjects with ESRD. Although the mechanisms for this observation is not entirely clear, we hypothesize the high concentrations of both FEP and ZID were the result of abnormal venous anatomy producing venous shunting from the infusion site to the sampling site. Dialysis subjects had vascular access for HD in one arm. This precluded blood drawing or i.v. catheter placement in this access arm for safety reasons. Therefore, neither dosing nor sampling could be conducted from the access arm, leaving only the opposite nonaccess arm for both the i.v. infusion of WCK 5222 and PK sampling. To attempt to avoid runoff and anomalous high PK concentrations during the infusion of WCK 5222, the i.v. dose was infused proximally in a large antecubital vein, and sampling was done from a distal hand vein in the same arm. Subjects on HD may occasionally have a rearrangement of the venous anatomy due to damage to veins that occurs over time with multiple phlebotomies, i.v. catheters, and previous access from surgeries. We consider that it is likely that distortion of the normal venous anatomy may have resulted in some runoff from the infusion site directly into the sampling site, resulting in the high PK concentrations. Supporting this hypothesis is the observation for both FEP and ZID that the plasma concentration fell to the anticipated range promptly upon termination of the i.v. infusion. Even a small runoff of a 1-g dose could have a major effect on sample concentrations and therefore the Cmax. The spurious Cmax values were observed for both FEP and ZID in the same two patients. Given the number of samples involved and the fact that both FEP and ZID were affected, contamination by phlebotomists was unlikely. Therefore, the samples from these two subjects were excluded from the PK summary analysis.
Another complication in interpreting the Cmax data is that the severely impaired group had obviously lower concentrations than those of all other groups (including the ESRD group). There are a number of factors involved. The doses for severe and ESRD were half those for the mild and moderate subjects, resulting in a lowering of plasma concentrations which, for the case of the severely impaired subjects, was not compensated by a decrease in clearance, which would cause higher concentrations. The reason that the ESRD subjects had higher concentrations than the severely impaired subjects within the first hour (during infusion and when Tmax was observed) is described above. After infusion, the low clearance compensated for the lower dose, and concentrations were high.
The ESRD subjects were all non-Hispanics, whereas their controls were mainly Hispanics. According to the study protocol, patients with renal impairment were matched with healthy control subjects based upon sex, age, and BMI, as is customary for a renal impairment study. The study participants were not specifically matched according the race or ethnicity. There are very little data on whether ethnicity can effect PK to a significant degree. The differences in renal elimination between ESRD patients and their matches was quite marked. Given that the ESRD and healthy subjects were well matched according to the known key factors of age, sex, and BMI, we consider that any potential effects of ethnicity on pharmacokinetics were small.
Another complication in interpreting the Cmax data is that the severely impaired group had obviously lower concentrations than all other groups (including ESRD). There are a number of factors involved. The doses for severe and ESRD were half those for the mild and moderate subjects, resulting in a lowering of plasma concentrations which, for the case of the severely impaired subjects, was not compensated by a decrease in clearance, which would cause higher concentrations. The reason that the ESRD subjects had higher concentrations than the severely impaired subjects within the first hour (during infusion and when Tmax was observed) is described above. After infusion, the low clearance compensated for the lower dose, and concentrations were high.
All 48 subjects were included in the evaluation of safety. Following a single intravenous infusion, a total of 5 AEs, 3 mild and 2 moderate, were observed in 3 subjects. There were no SAEs or deaths reported during the study. No clinically significant findings or increasing or decreasing trends from baseline were observed for any of the safety parameters.
In summary, the clearance of both FEP and ZID given as a 60-min infusion was reduced, and the corresponding t1/2 and AUC0–∞ increased sharply beginning with the moderate renal impairment group; these changes were further amplified in the severe and ESRD cohorts. These findings are not surprising given the high percentages of both drugs eliminated by renal excretion of intact drug. Accordingly, adjustments in dose and/or duration of infusion will be required depending on the degree of renal impairment. The dispositions of ZID and FEP appear to be compatible with parallel elimination patterns and a stable FEP/ZID concentration ratio.
A single i.v. infusion of WCK 5222 was found to be generally safe and well tolerated in subjects with both normal and impaired renal function.
MATERIALS AND METHODS
The primary objective of the study is to assess the effect of renal impairment on the PK of WCK 5222. The secondary objective of the study is to assess the safety and tolerability of WCK 5222 following a single intravenous dose in subjects with normal and impaired renal function. This investigation (ClinicalTrials.gov identifier NCT02942810) was approved by the Western Institutional Review Board (IRB) and the Human Subjects Research Office of the University of Miami and was performed in accordance with U.S. FDA regulations, ICH E6(R1) Good Clinical Practice (16), and the Declaration of Helsinki. Written informed consent was obtained directly from all participants prior to entry into the study and prior to any study procedures.
Overall study design.
This investigation was a phase 1 open-label parallel-group study conducted at a single academic phase 1 research center to evaluate the pharmacokinetics (PK) and tolerability of WCK 5222 in subjects with renal impairment and healthy control subjects. The U.S. FDA recommends conducting a pharmacokinetic study in patients with impaired renal function when the drug is likely to be used in such patients and when renal impairment is likely to alter the pharmacokinetics of the drug and/or its active metabolites (17–20). The design and conduct of this study follow recommendations described in an FDA guidance document dated March 2010 (17, 18).
The study cohorts comprised 48 male and female subjects 18 to 80 years of age with mild renal impairment (n = 6), moderate renal impairment (n = 6), severe renal impairment (n = 6), and stable end-stage renal disease (ESRD) requiring hemodialysis (HD; n = 6), and 24 healthy control subjects matched for sex, age (±10 years), and BMI (±15%) enrolled in a 1:1 ratio. The enrollment of all 48 study participants was conducted over a 3- to 4-month period in 2016 to 2017.
A 60-min infusion of 3 g WCK 5222 (2 g FEP plus 1 g ZID) was administered to mild and moderate renal impairment subjects and matched control subjects, and a 60-min infusion of 1.5 g WCK 5222 (1 g FEP plus 0.5 g ZID) was administered to severe renal impairment subjects and those with ESRD on HD (Table 7). Subjects with renal impairment were stratified into impairment and dosage groups based upon the Cockcroft-Gault-estimated creatinine clearance (in milliliters per minute) (21).
TABLE 7.
Renal impairment group and WCK 5222 dosage
| Group | Category | CLCR (ml/min) | n | WCK 5222 dose (g) |
|---|---|---|---|---|
| 1 | Normal | ≥90 | 24 | 3 (2 FEP and 1 ZID) |
| 2 | Mild | 60–89 | 6 | 3 (2 FEP and 1 ZID) |
| 3 | Moderate | 30–59 | 6 | 3 (2 FEP and 1 ZID) |
| 4 | Severe | <30 (not on dialysis) | 6 | 1.5 (1 FEP and 0.5 ZID) |
| 5 | ESRD on HD | 6 | 1.5 (1 FEP and 0.5 ZID) |
The study consisted of a screening period (days −28 to −1), check-in (day −1), 4 total days of confinement (days −1 to 3), and a safety follow-up visit within 7 to 14 days after discharge. Subjects remained confined in the Clinical Pharmacology Research Unit from day −1 (1 day prior to study drug administration) through the completion of the scheduled study procedures on the end of confinement day (day 3). Subjects received a standardized meal during confinement days. On day 1, each enrolled participant received a single i.v. infusion of WCK 5222 over 60 min. Blood and urine samples for determination of FEP and ZID concentrations were obtained at predose and at time points up to 48 h postinitiation of WCK 5222 infusion.
Subjects requiring HD were admitted to the Clinical Pharmacology Research Unit after their routine dialysis and administered the drug on a nondialysis day. Safety was assessed throughout the study by evaluation of local tolerability at the injection site, adverse event (AE) monitoring, clinical laboratory tests, electrocardiogram (ECG), physical examination, and monitoring of vital signs.
Study participants.
To be eligible for study participation, men or nonpregnant women were required to be 18 to 80 years of age and have a body mass index between 18 and 40 kg/m2. Patients with renal impairment were required to have stable renal function for at least 1 month prior to screening, as determined by the investigator. Subjects receiving hemodialysis were required to have a stable dialysis prescription, stable clinical status (no significant changes in symptomatology, physical findings, or medical regimen), and stable laboratory profile for 2 months in the estimation of the investigator.
Healthy normal control subjects were required to have no evidence of any disease or condition that could affect the pharmacokinetics of FEP or ZID, normal or nonclinically significant findings at physical examination, clinical laboratory evaluations, and electrocardiogram. Subjects with renal impairment were required to have a resting blood pressure of 90 to 155/50 to 100 mm Hg, whereas healthy volunteers were required to have a resting blood pressure of 90 to 145/60 to 95 mm Hg; other requirements were a resting heart rate of 40 to 100/min, and a 12-lead ECG with no clinically significant findings. The QT interval corrected by Fredericia (QTcF) was required to be <500 ms in patients with renal impairment and <470 ms in healthy volunteers. Males could not have donated sperm until 6 weeks after the dose of study drug. Female subjects must have been of nonchildbearing potential with at least 12 months of spontaneous amenorrhea, have undergone sterilization at least 6 months prior to screening, or practiced 2 highly effective methods of birth control (determined by the investigator; one of the methods was required to be a barrier technique) until 6 weeks following the administration of study drug.
Potential subjects were excluded if they were participating in another investigational drug or device study or treated with an investigational drug within 30 days or 5 half-lives, whichever was longer before dosing. Potential subjects were excluded if there was evidence of uncontrolled cardiovascular, central nervous, respiratory, endocrine, immune, or hematologic system disorder, malignancy, or any other disorder that in the opinion of the investigator would confound the subject’s participation and follow-up in the clinical trial. Other exclusion criteria were history of clinically significant drug or food allergy, history of significant drug abuse during past 1 year, or any positive test result for drug abuse at screening or day −1 unless the positive drug screen was due to documented prescription drug use. Potential subjects were excluded if they smoked more than 10 cigarettes daily for 60 days prior to drug administration and through the follow-up visit, had a positive screen for HIV 1/2 at the time of screening, were unwilling to stay in the clinical unit for the required duration as per the protocol, or were unwilling to consume the standard meal to be provided as per the protocol. Medications essential for the management of renal impairment and concomitant stable medical conditions for the renal impairment patients were allowed as per the discretion of the investigator. Potential subjects were excluded if they had received FEP or ZID within 2 months of the study drug administration or were allergic or intolerant to either FEP or ZID.
Healthy volunteers were excluded for use of any concomitant medication within 7 days from the screening, except those deemed safe for the study by the investigator and medical monitor. Healthy volunteers were excluded if there was a history of any clinically significant chronic and/or active hepatic disease or elevations in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels more than 1.5 times the upper limit of normal (ULN), hepatitis (hepatitis A, B, or C) infection, biliary tract disease, or history of any significant gastrointestinal surgery.
Sample collection for pharmacokinetic analysis.
Blood samples for PK analysis were collected at predose and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 8, 10, 12, 18, 24, 30, 36, and 48 h postinitiation of WCK 5222 infusion for all cohorts. At each time point, 6-ml blood samples were collected into tubes containing sodium heparin and centrifuged to harvest the plasma at about 4°C for 10 min at 2,000 × g. Urine was collected into prelabeled bulk collection containers at predose and at 0 to 2, 2- to 4-, 4- to 8-, 8- to 12-, 12- to 24-, and 24- to 48-h intervals in subjects with mild and moderate renal impairment and healthy controls, and at 0- to 24- and 24- to 48-h intervals in subjects with severe renal impairment and ESRD on HD. Subjects with ESRD on HD were unable to produce urine and were not included in the urine drug recovery analysis. Both plasma and urine samples were stored at approximately −70°C until analysis.
Bioanalytical methods.
The concentrations of FEP and ZID were analyzed using validated liquid chromatography-tandem mass spectrometry (LC-MS/MS). Chromatographic separation was carried out on an Imtakt Unison UK-C18 column (50 mm by 3 mm; inner diameter [i.d.], 3 μm). Mass spectrometric detection was performed on an API 5500 QTrap triple quadrupole tandem mass spectrometer (Applied Biosystems/MDS Sciex, Canada) equipped with a turbo ion spray source operated in the positive- or negative-ionization mode. Cefepime-d3 and avibactam were used as internal standards (IS) for the determination of FEP and ZID, respectively. Plasma samples were treated with 0.1% formic acid in methanol for protein precipitation, and urine samples were diluted with 0.1% formic acid in water. After vortexing and centrifugation, the supernatants were transferred into injection vials containing 10 mM ammonium acetate in water, and the aliquots from reconstituted samples were injected into the LC-MS/MS system for FEP and ZID concentration analysis.
The MS detection was performed in two periods. ZID and avibactam were detected in the negative mode in period 1 from 0 to 1.10 min. The ion spray voltage was set at −3 kV, the source temperature was maintained at 600°C, and the collision energy for ZID and avibactam was set at −29 and −32, respectively. Quantification was determined by using multiple-reaction monitoring (MRM) mode of the transitions at m/z 390.1 → 96.9 for ZID and at m/z 264.0 → 95.9 for avibactam. FEP and cefepime-d3 were detected in the positive mode in period 2 from 1.10 to 2.1 min. The ion spray voltage was set at 2 kV, the source temperature was maintained at 600°C, and the collision energy for FEP and cefepime-d3 was set at 15. Quantification was obtained by using MRM mode of the transitions at m/z 481.3 → 396.0 for FEP and at m/z 484.2 → 396.0 for cefepime-d3.
Data acquisition and integration of MRM chromatograms were performed using the software package Analyst 1.5.2 (AB Sciex, Framingham, MA). For further data processing, including the calculation of concentration data, LIMS version 7.4.1 (Thermo Scientific) was used. A linear regression (weighted 1/x2) was applied to a plot of the peak area ratio versus concentration for the standards to obtain the calibration curve. The lower limit of quantitation (LLOQ) was 0.5 µg/ml for FEP and 0.25 µg/ml for ZID in human plasma and 16 µg/ml for FEP and ZID in urine samples. The quantitation range in plasma was 0.5 to 200 µg/ml for FEP, 0.25 to 100 µg/ml for ZID, and 16 to 1,200 µg/ml for both compounds in urine.
Quality control (QC) samples were used to demonstrate adequate precision and accuracy of the assay. Acceptable sample stability and sensitivity were also demonstrated.
For plasma QC samples, precision intraday variability ranged from 2.61% to 11.51% and 0.60% to 3.87% for FEP and ZID, respectively, and accuracy for intraday variability ranged from −7.27% to 3.10% and −4.09% to 4.09% for FEP and ZID, respectively. Precision for interday variability ranged from 1.64% to 10.40% and 1.77% to 5.31% for FEP and ZID, respectively, and accuracy for interday variability ranged from −5.23% to 4.10% and −4.12% to 2.43% for FEP and ZID, respectively. Recovery was 100.08% and 101.76% for FEP and ZID, respectively. Reinjection stability confirmed for up to 49.4 h at 15°C. Postpreparative stability was confirmed for up to 49.6 h refrigerated. Benchtop stability was confirmed for up to 6 h in ice water. Freeze-thaw stability was confirmed for up to four cycles at −20°C and −70°C. Long-term stability confirmed for up to 178 days at −70°C. No hemolysis effects were observed in 1.5/0.75 and 150/75 µg/ml FEP/ZID QCs prepared in hemolyzed human sodium heparin plasma. No hyperlipidemic impact was observed in 1.5/0.75 and 150/75 µg/ml FEP/ZID hyperlipidemic QC samples. No matrix effect impact was observed in six different lots of human plasma.
For urine QC samples, precision intraday variability ranged from 1.97% to 7.46% and 1.70% to 5.25% for FEP and ZID, respectively, and accuracy for intraday variability ranged from −2.23% to 13.32% and −4.10% to 3.66% for FEP and ZID, respectively. Precision for interday variability ranged from 1.93% to 16.40% and 1.58% to 11.76% for FEP and ZID, respectively, and accuracy for interday variability ranged from −2.17% to 10.37% and −1.70% to 7.57% for FEP and ZID, respectively. Recovery in urine was 96.69% and 98.05% for FEP and ZID, respectively. Reinjection stability was confirmed for up to 48.1 h at 15°C. Postpreparative stability was confirmed for up to 48.1 h refrigerated. Benchtop stability was confirmed for up to 24 h at room temperature. Freeze-thaw stability was confirmed for up to four cycles at −20°C and −70°C. Long-term stability was confirmed for up to 172 days at −70°C. No matrix effect impact was observed in six different lots of human urine.
Statistical methods and pharmacokinetic analysis.
The sample size selected for this study was selected in accordance with an FDA guidance document dated March 2010 (17, 18) and other pharmacokinetic studies conducted in subjects with renal impairment of a similar nature. The primary endpoint was to compare the PK of FEP and ZID after a single i.v. dose of WCK 5222 in patients with renal impairment versus healthy volunteers with normal renal function. Noncompartmental analysis (NCA) was used to calculate the pharmacokinetic parameters for FEP and ZID using WinNonlin version 6.4 (Pharsight Corporation, Mountain View, CA, USA). All statistical evaluations were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA).
The area under the concentration-time curve from time zero to the time of the last measured concentration (AUC0–t) and to infinity (AUC0–∞) for both FEP and ZID were determined for the comparison of systemic exposure between subjects with renal impairment and healthy volunteers. The plasma PK parameters maximum plasma concentration observed (Cmax), time to the maximum observed plasma concentration (Tmax), terminal elimination rate constant (λz), elimination half-life (t1/2), systemic clearance (CL), apparent volume of distribution (Vz), and volume of distribution at steady state (Vss) based on plasma FEP and ZID concentrations were also determined and compared between subjects with renal impairment and healthy subjects. Cumulative urinary excretion to the end of each postdose collection interval (Ae0–48) was determined along with the renal clearance (CLR) for comparison between renal impairment and healthy subjects.
Plasma concentration levels and PK parameters were listed and summarized by renal impairment group using descriptive statistics. The PK parameters from each group of renal impairment subjects were compared to those of their matched control healthy volunteers by analysis of variance (ANOVA) using the general linear models (GLM) procedure in SAS version 9.3. The Cmax, AUC0–t, and AUC0–∞ values were natural log transformed prior to statistical analysis, with the other PK parameters being analyzed without any transformations. The differences between the mean PK parameters for each renal impairment group and their matched control group were determined. The 95% confidence intervals on the ratios of the impaired-to-control means were calculated. Both the evaluation of statistical significance for differences between impaired and control subjects and construction of the 95% confidence intervals were adjusted for multiple comparisons.
Because both FEP and ZID are eliminated primarily by the kidneys, a question arises as to whether subject with mild, moderate, and severe renal impairment will have the same relative ratios of FEP/ZID concentrations. An imbalance of the FEP/ZID ratio could happen if renal clearance (CLR) and systemic clearance (CLsys) do not change in approximately the same direction and magnitude for both drugs. Derendorf et al. (14, 15) previously approached this potential problem for the combination piperacillin-tazobactam by regression of the values of CLR, and CLsys, with CLCR for piperacillin and for tazobactam and plotted the results for both drugs. The plots were essentially parallel, indicating that the drugs were appropriately matched for a combination product. We employed a similar regression approach to the combination FEP-ZID.
Safety monitoring and adverse events.
Adverse events (AEs) were recorded from the time the informed consent form was signed until completion of the follow-up visit. The safety of a single intravenous dose of WCK 5222 was assessed by the incidence of treatment-emergent adverse events, clinical laboratory tests, physical examinations, vital signs, and 12-lead ECGs. Subjects were asked nonleading questions to determine the occurrence of AEs at regular intervals during the study. Severity, seriousness, and expectedness were assessed for each AE observed. The relationship to study drug was rated as certainly related, probably/likely related, possibly related, unlikely related, not related or nonassessable.
ACKNOWLEDGMENTS
We acknowledge Rolando Rodco for study coordination, Olga Acosta for regulatory and IRB coordination, Michelle Monteagudo for administrative support, and Ana Meza for data management. We also acknowledge the University of Miami HSRO and the JHS Research Office for their reviews of this protocol and The Office of Research Administration for administrative support.
The University of Miami Miller School of Medicine received a clinical research grant from Wockhardt Limited, Mumbai, India on behalf of Richard A. Preston for work on the design and conduct of this clinical trial.
Richard A. Preston was the study principal investigator. He is professor of medicine and chief of the Clinical Pharmacology Research Unit, Division of Clinical Pharmacology, Department of Medicine, Miller School of Medicine, University of Miami, Miami, FL. Grigor Mamikonyan is vice president for clinical operations for Clinartis LLC. Clinartis LLC received a clinical research grant for conduct of parts of this clinical research work. Stephane DeGraff and James Chiou are clinical research associates of the Clinical Pharmacology Research Unit, Division of Clinical Pharmacology Department of Medicine, Miller School of Medicine. Christopher J. Kemper and Allan Xu were contracted directly by Clinartis for biostatistical and bioanalytical support, respectively. Mushtaque Mastim, Ravindra Yeole, Rajesh Chavan, Anusuya Patel, and Ashima Bhatia are employees of Wockhardt Limited, Mumbai, India. H. David Friedland is an employee of Wockhardt’s Morton Grove Pharmaceutical, Inc., Morton Grove, IL.
REFERENCES
- 1.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Olivera A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “beta-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 4.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Olivera A. 2017. Potent β-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother 61:e01238-17. doi: 10.1128/AAC.01238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moya B, Barcelo I, Udaykar A, Bhagwat S, Patel M, Bou G, Oliver A. 2016. WCK 5222 [cefepime-zidebactam, FEP-ZID]: mechanistic basis behind novel β-lactam–β-lactam enhancer combination against metallo-β-lactamase (MBL)-producing E. coli (EC), K. pneumoniae (KP), P. aeruginosa (PA) and its impact on therapeutically relevant bactericidal exposures assessed through in vitro pharmacodynamic modelling (IVPM) and mouse lung eradication studies, abstr 1982. IDWeek 2016, 26 to 30 October 2016, New Orleans, LA. [Google Scholar]
- 6.Hospira. 2012. Maxipime (cefepime hydrochloride, USP) for injection for intravenous or intramuscular use. Hospira, Inc, Lake Forest, IL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050679s036lbl.pdf. [Google Scholar]
- 7.Palwe S, Joshi PR, Khande HN, Biniwale SS, Bhagwat SS, Patel MV. 2016. WCK 5107 (zidebactam, ZID): a pan Gram-negative β-lactam enhancer augmenting β-lactam pharmacodynamics in wild type and carbapenemase producers (CP), poster 438. ASM Microbe, 16 to 20 June 2016, Boston, MA. [Google Scholar]
- 8.Khande HN, Joshi PR, Palwe S, Bhagwat SS, Patel MV. 2016. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: activity against ESBL, class C, and KPC-expressing enterics and Pseudomonas (PA) expressing AmpC (PS AmpC) or OXA β-lactamases (PS OXA). ASM Microbe, 16 to 20 June 2016, Boston, MA. [Google Scholar]
- 9.Wockhardt. 2016. Investigator’s brochure, cefepime-zidebactam: a novel β-lactam–β-lactam enhancer based combination for Gram negative infections. Wockhardt, Mumbai, India. [Google Scholar]
- 10.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity tested Gram-negative organisms producing clinically-relevant beta-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 12.Endimiani A, Perez F, Bonomo RA. 2008. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther 6:805–824. doi: 10.1586/14787210.6.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DRP, Pittman KA. 1990. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther 48:268–276. doi: 10.1038/clpt.1990.149. [DOI] [PubMed] [Google Scholar]
- 14.de la Pena A, Derendorf H. 1999. Pharmacokinetic properties of beta-lactamase inhibitors. Int J Clin Pharmacol Ther 37:63–75. [PubMed] [Google Scholar]
- 15.Derendorf H, Costa TD. 1996. Pharmacokinetics of piperacillin, tazobactam and its metabolite in renal impairment. Int J Clin Pharmacol Ther 34:482–488. [PubMed] [Google Scholar]
- 16.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use. 1996. ICH harmonized tripartite guideline. Guideline for good clinical practice E6(R1). International Council for Harmonisation, Geneva, Switzerland: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. [PubMed] [Google Scholar]
- 17.Center for Drug Evaluation and Research (CDER). 2010. Guidance for industry. Pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Silver Spring, MD: https://www.fda.gov/downloads/drugs/guidances/ucm204959.pdf. [Google Scholar]
- 18.Food and Drug Administration. 2010. Summary minutes of the Advisory Committee for Pharmaceutical Science and Clinical Pharmacology, March 17, 2010. https://wayback.archive-it.org/7993/20170113113150/http:/www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AdvisoryCommitteeforPharmaceuticalScienceandClinicalPharmacology/UCM210807.pdf.
- 19.Huang SM, Temple R, Xiao S, Zhang L, Lesko LJ. 2009. When to conduct a renal impairment study during drug development: US Food and Drug Administration perspective. Clin Pharmacol Ther 86:475–479. doi: 10.1038/clpt.2009.190. [DOI] [PubMed] [Google Scholar]
- 20.Lalonde RL, Wagner JA. 2009. Drug development perspective on pharmacokinetic studies of new drugs in patients with renal impairment. Clin Pharmacol Ther 86:557–561. doi: 10.1038/clpt.2009.182. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]