Mycobacterium abscessus is emerging as an important pathogen in chronic lung diseases, with concern regarding patient-to-patient transmission. The recent introduction of routine whole-genome sequencing (WGS) as a replacement for existing reference techniques in England provides an opportunity to characterize the genetic determinants of resistance.
KEYWORDS: macrolides, nontuberculous mycobacteria, whole-genome sequencing
ABSTRACT
Mycobacterium abscessus is emerging as an important pathogen in chronic lung diseases, with concern regarding patient-to-patient transmission. The recent introduction of routine whole-genome sequencing (WGS) as a replacement for existing reference techniques in England provides an opportunity to characterize the genetic determinants of resistance. We conducted a systematic review to catalogue all known resistance-determining mutations. This knowledge was used to construct a predictive algorithm based on mutations in the erm(41) and rrl genes which was tested on a collection of 203 sequentially acquired clinical isolates for which there were paired genotype/phenotype data. A search for novel resistance-determining mutations was conducted using a heuristic algorithm. The sensitivity of existing knowledge for predicting resistance in clarithromycin was 95% (95% confidence interval [CI], 89 to 98%), and the specificity was 66% (95% CI, 54 to 76%). The subspecies alone was a poor predictor of resistance to clarithromycin. Eight potential new resistance-conferring single nucleotide polymorphisms (SNPs) were identified. WGS demonstrated probable resistance-determining SNPs in regions that the NTM-DR line probe cannot detect. These mutations are potentially clinically important, as they all occurred in samples that were predicted to be inducibly resistant and for which a macrolide would therefore currently be indicated. We were unable to explain all resistance, raising the possibility of the involvement of other as yet unidentified genes.
INTRODUCTION
Members of the Mycobacterium abscessus complex (M. abscessus) are rapidly growing nontuberculous mycobacteria (NTM) of increasing clinical concern because of a rising burden of associated pulmonary disease (1). M. abscessus poses a significant problem, particularly in patients with cystic fibrosis (CF), where infection is associated with a more rapid decline in lung function and can be a barrier to transplantation (2). Of particular concern are the findings from recent work that have suggested person-to-person transmission of virulent clones among the CF population within a health care setting (3, 4), although not all studies have supported this (5, 6).
The taxonomy of M. abscessus is contentious. It is currently divided into three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii (7). The organism has intrinsic resistance to multiple antibiotics, including β-lactams, rifampin, and aminoglycosides, due to the synergistic action of the cell envelope and genetic factors (8). Treatment requires prolonged courses of multiple antibiotics, but outcomes are thought to vary across the different subspecies. M. abscessus subsp. massiliense has been associated with clarithromycin susceptibility and favorable treatment outcomes, whereas M. abscessus subsp. abscessus has been associated with inducible macrolide resistance and poorer treatment outcomes (9).
Whole-genome sequencing (WGS) has been implemented in stages across England since December 2016, replacing existing reference techniques for mycobacterial identification. As a consequence, there is now the opportunity to explore the molecular determinants of drug resistance for all clinical NTM isolates. Macrolides are important agents in the management of NTM infection. The American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) and British Thoracic Society (BTS) guidelines recommend including a macrolide in treatment regimens when samples either are susceptible or demonstrate inducible resistance (10, 11). They act by binding to the 50S ribosomal subunit, and resistance in mycobacteria primarily occurs through target site modification, for example, by erm methylases and point mutations (12). As there is a particularly strong correlation between in vitro susceptibility and the clinical response to macrolide treatment of M. abscessus infections (13, 14), we have undertaken a study to assess the feasibility of predicting clarithromycin susceptibility from whole-genome sequencing data for all three subspecies of M. abscessus.
RESULTS
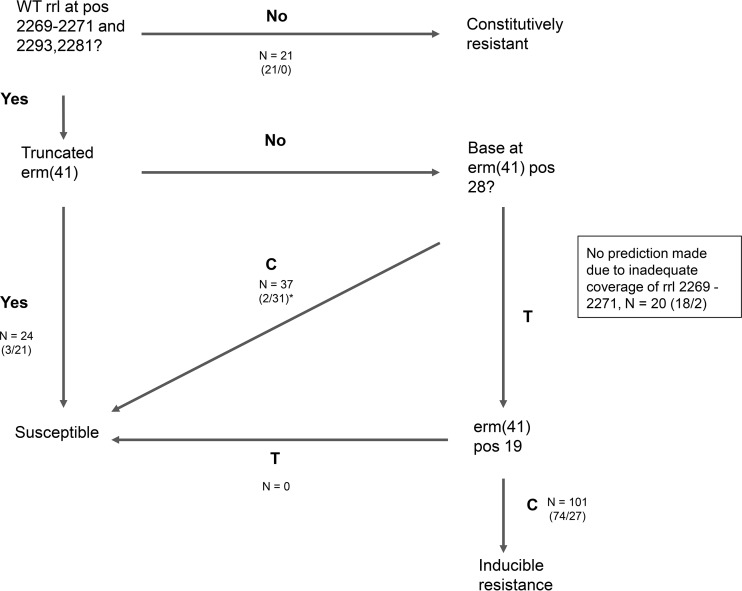
We studied 143 M. abscessus subsp. abscessus, 20 M. abscessus subsp. bolletii, and 40 M. abscessus subsp. massiliense genomes. Genotypic predictions were made on the basis of mutations identified by the literature search. All relevant mutations identified were contained in the genes rrl and erm(41) (Fig. 1 and Table 1). The genes rplV, whiB7, and rpld were also considered of potential interest and were additionally searched for variants.
FIG 1.
Decision algorithm for predicting drug resistance in M. abscessus based on the literature search, with the numbers of isolates meeting each predictive criterion shown. Numbers in parentheses represent the number resistant/the number sensitive. *, 4 isolates had intermediate susceptibility; pos, position.
TABLE 1.
Resistance-determining mutations for clarithromycin identified in the literature searcha
| erm(41) length | Nucleotide at: |
Phenotype [reference(s)] | |||
|---|---|---|---|---|---|
| erm(41) position 28 | rrl position 2270 (2058) | rrl position 2271 (2059) | Other | ||
| Full | T | A | A | Inducible resistance (3, 15, 16, 20, 21, 27–37), sensitive (33) | |
| Truncated | A | A | Sensitive (9, 15, 20, 21, 29, 32–38) | ||
| Full | C | A | A | Sensitive (15, 16, 20, 21, 28–33, 35–37, 39) | |
| Full or truncated | C or T | G | A | Resistant (3, 15, 16, 18, 20, 21, 27, 28, 36–41) | |
| Full or truncated | C or T | C | A | Resistant (3, 15, 20, 21, 28, 38, 41) | |
| Full or truncated | T | T | A | Resistant (15, 20, 27) | |
| Full | A | C | Resistant (20, 27, 40, 41) | ||
| Full or truncated | T or C | A | G | Resistant (15, 16, 18, 20, 21, 28, 37, 40) | |
| Truncated | A | T | Resistant (27) | ||
| Full | T | A | A | C19T in erm(41) | Sensitive (42) |
| Truncated | A | A | A2269G in rrl (2057) | Resistant (16) | |
| Full | Unknown | Unknown | Unknown | A2293C in rrl (2082) + G2281C in rrl (2069) | Resistant (41) |
M. abscessus numbering is used, with E. coli numbering provided in parentheses.
Genotypic predictions.
Inducible resistance was predicted in 101 isolates, of which 74 (73%) were reported to be phenotypically resistant. After excluding isolates for which no prediction could be made due to missing data in key genomic loci (n = 20) as well as those with an intermediate phenotype (n = 4), the sensitivity was 95/100 (95%; 95% confidence interval [CI], 89 to 98%) and the specificity was 52/79 (66%; 95% CI, 54 to 76%). The very major error rate (phenotype resistant, WGS prediction sensitive) was 5/100 (5%; 95% CI, 1 to 9%), and the major error rate (phenotype susceptible, WGS prediction resistant) was 27/79 (34%; 95% CI, 24 to 44%). The positive predictive value was 95/122 (78%; 95% CI, 69 to 85%), and the negative predictive value was 52/57 (91%; 95% CI, 81 to 97%) (Table 2). The F score for WGS predictions was 0.86. When isolates with a prediction of inducible resistance were further excluded, the specificity of a resistance prediction was 21/21 (100%; 95% CI, 93 to 100%) and the sensitivity was 21/26 (81%; 95% CI, 61 to 93%).
TABLE 2.
WGS predictions versus DST phenotype for clarithromycina
| Genomic prediction | No. of isolates with the following in vitro phenotype: |
||
|---|---|---|---|
| Sensitive | Resistant | Intermediate | |
| No prediction | 2 | 18 | 0 |
| Inducible resistance | 27 | 74 | 0 |
| Resistant | 0 | 21 | 0 |
| Sensitive | 52 | 5 | 4 |
The sensitivity (95%; 95% CI, 89 to 98%), specificity (66%; 95% CI, 54 to 76%), positive predictive value (78%; 95% CI, 69 to 85%), and negative predictive value (91%; 95% CI, 81.0 to 97%) were calculated by excluding isolates with an intermediate phenotype and those for which no prediction was made due to inadequate coverage at key positions.
Clarithromycin resistance in the subspecies.
Of 143 M. abscessus subsp. abscessus isolates, 81 were resistant, 58 were sensitive, and 4 were intermediate. For M. abscessus subsp. bolletii, 18/20 were resistant, and for M. abscessus subsp. massiliense, 19/40 were resistant (Table 3). There was one M. abscessus subsp. massiliense isolate carrying a full-length erm(41) gene which was phenotypically resistant to clarithromycin. This was not unexpected from a genotypic perspective, as it harbored a wild-type thymine nucleotide at position 28 of erm(41), associated with inducible resistance.
TABLE 3.
Summary of genotypes and corresponding clarithromycin phenotypes for the 203 isolatesa
| Organism | Nucleotide at erm(41) pos. 28 | erm(41) length | Nucleotide at erm(41) pos. 19 | Nucleotide at rrl pos.: |
N | Phenotypeb | Prediction | ||
|---|---|---|---|---|---|---|---|---|---|
| 2269 | 2270 | 2271 | |||||||
| M. abscessus subsp. abscessus | T | Full | C | A | C | A | 5 | 5R | R |
| T | Full | C | A | T | A | 2 | 2R | R | |
| C | Full | C | A | A | A | 37 | 4I, 2R, 31S | S | |
| T | Full | C | A | A | A | 87 | 62R, 25S | R | |
| 12 | 10R, 2Sc | ||||||||
| M. abscessus subsp. bolletii | T | Full | C | G | A | A | 1 | 1R | R |
| T | Full | C | A | G | A | 2 | 2R | R | |
| T | Full | C | A | A | A | 13 | 11R, 2S | R | |
| 4 | 4Rc | ||||||||
| M. abscessus subsp. massiliense | T | Truncated | C | A | C | A | 3 | 3R | R |
| T | Truncated | C | A | G | A | 3 | 3R | R | |
| T | Truncated | C | A | A | G | 5 | 5R | R | |
| T | Truncated | C | A | A | A | 24 | 21S, 3R | S | |
| T | Full | C | A | A | A | 1 | 1R | R | |
| 4 | 4Rc | ||||||||
pos., M. abscessus numbering position in the indicated gene; prediction, genotypic prediction using the algorithm shown in Fig. 1; N, total number of isolates with the genotype.
The phenotype indicates the number of isolates that are sensitive (S), resistant (R), or inducibly resistant (I).
These isolates were excluded due to inadequate coverage over rrl positions 2270 and 2271.
Mechanisms of resistance.
The negative predictive value of a truncated erm(41) gene for clarithromycin susceptibility was 53% [21/39; there was one M. abscessus subsp. massiliense isolate with a full-length erm(41)]. In 11/18 instances, resistance in the presence of a truncated erm(41) could be explained by a mutation in position 2270 or 2271 in rrl. No coverage at all was seen at these positions for 4/18 isolates. No genomic explanation could be identified for the remaining three discordant isolates (Table 3).
All isolates which had any mutation of position 2269, 2270, or 2271 (Escherichia coli numbering, positions 2057, 2058, and 2059, respectively) in rrl were resistant to clarithromycin (21/203 [10%]). Such a mutation was found in 3 M. abscessus subsp. bolletii, 11 M. abscessus subsp. massiliense, and 7 M. abscessus subsp. abscessus isolates. We did not observe any isolates with an rrl mutation which also harbored a T28C mutation in erm(41). Where this occurred in isolates reported in the literature, they were always resistant (15, 16).
Of 37 isolates with a T28C mutation in erm(41) and no other relevant mutations, 84% (31/37) were susceptible to clarithromycin, 11% (4/31) had intermediate susceptibility, and 5% (2/31) were resistant. This mutation was exclusively found in M. abscessus subsp. abscessus isolates. We did not identify any drug resistance-associated mutations in any of these intermediate or resistant isolates. Across all three subspecies, of 101 isolates with the erm(41)_T28 call associated with inducible resistance (and no other relevant mutation), 73% (74/101) were resistant and 27% (27/101) were susceptible at the final day 21 reading.
De novo search for resistance-determining mutations.
The search for potential novel resistance-determining mutations for clarithromycin revealed 13 single nucleotide polymorphisms (SNPs) of interest (Table 4). Of these, five have previously been described in the literature. There were, additionally, four SNPs (rrl_A2746T, rrl_G836A, rrl_T2674G, and rrl_T636C) which were only ever seen in resistant isolates but which always co-occurred with known resistance-determining SNPs. There was one phenotypically resistant isolate which harbored 18 novel SNPs. On performing a BLAST analysis of the nucleotide sequence of a 120-base region encompassing all of these SNPs, there was a 99% match (E value, 2 × 10−53) with Streptococcus species. This therefore likely represents sample contamination with flora from the nasopharynx. No new resistance-associated variants were discovered in rplV, rpld, or whiB7.
TABLE 4.
Mutations (both novel and previously described) detected during the de novo search for resistance-determining SNPsc
| Position | Nucleotide/amino acid change | Rule metd |
|---|---|---|
| rrl 2039 | A > G | 1 |
| rrl 1401 | T > C | 2 |
| rrl 371 | T > C | 2 |
| rrl 795 | G > A | 1 |
| rrl 2270a | A > C | 1 |
| rrl 2270a | A > G | 2 |
| rrl 2271a | A > G | 2 |
| rrl 2270a | A > T | 2 |
| erm(41) 131 | A > V | 2 |
| rrl 2279 | G > A | 2 |
| rrl 2269a | A > G | 2 |
| erm(41) −31b | A > T | 2 |
| rrl 1932 | A > G | 2 |
The mutation is already described in the literature. M. abscessus rrl numbering 2270 and 2271 is E. coli numbering 2058 and 2059, respectively.
Mutation in the erm(41) promoter region 31 bases upstream of start of the coding region.
All numbering is relative to that for M. abscessus.
Rule 1, occurs as the only SNP in relevant regions in resistant isolates; rule 2, all samples are resistant when SNP occurs, and the SNP is never seen in sensitive isolates.
DISCUSSION
We conducted a systematic review of drug resistance-determining mutations for clarithromycin in M. abscessus and used the results to make genotypic predictions. The sensitivity of this approach was 95% (95% CI, 89 to 98%), and the positive predictive value was 78% (95% CI, 69 to 85%). The prevalence of resistance among our collection of isolates was high compared to that which has been reported elsewhere (9, 17–19).
These results show that for clarithromycin, drug resistance can be predicted from WGS data as it has been previously through targeted PCR and line probe assays, such as the Hain GenoType NTM-DR assay. Assessment of the genotype of erm(41) with molecular diagnostics allows prediction of its functional status, which has been thought to correlate with the treatment outcome (10). Similarly, as the absence of a functional erm(41) gene has been associated with good therapeutic outcomes, its molecular detection ought to be beneficial to patients (9), although in our study this alone was not an adequate predictor of in vitro resistance. A genotypic prediction of inducible resistance produced a variable phenotype in our study (27/101 sensitive isolates). Discriminating such isolates that are predicted to be inducibly resistant but that are unexpectedly sensitive after prolonged incubation with clarithromycin or that show high-level resistance at early time points may help to identify additional genotypic markers to better identify patients more likely to benefit from the use of macrolides.
In addition to the mutations identified in the literature search, we also managed to identify variants that may plausibly be new resistance-determining mutations. However, these will require validation against an independent data set. The use of routinely collected diagnostic data to improve our understanding of the molecular determinants of drug resistance is a key advantage that WGS has over line probe assays or PCR. The eight previously undescribed mutations that we report on in this work could be of clinical importance because they all occurred in samples which the existing literature predicts to be inducibly resistant. As BTS guidelines recommend that patients with such isolates be given a macrolide, it is important to determine further whether these SNPs are true resistance determinants and whether macrolide therapy should be avoided in their presence.
Previous authors have suggested that it is clinically useful to discriminate between subspecies (9), as M. abscessus subsp. massiliense is typically associated with durable susceptibility to clarithromycin and M. abscessus subsp. bolletii and M. abscessus subsp. abscessus are typically associated with inducible resistance (unless the T28C mutation is present). We found identifying subspecies alone to be an inadequate predictor of the in vitro clarithromycin susceptibility phenotype. There were three M. abscessus subsp. massiliense isolates in our collection that were resistant to clarithromycin and had no mutations known to be relevant. Mougari and colleagues found that in 39/40 M. abscessus subsp. massiliense isolates selected for clarithromycin resistance, this could be explained by an rrl mutation at positions 2270 and 2271, with a further sample containing an rplV insertion (20). All of our isolates contained this insertion (which was also present in the reference sequence with GenBank accession number NC_010397.1), which was associated with susceptibility to clarithromycin, except in the presence of a relevant rrl mutation.
In keeping with previous reports, we identified an isolate of M. abscessus subsp. massiliense with a full-length erm(41) and a thymine nucleotide at position 28 (21). This likely represents recombination between the subspecies. A recent study showed that the Hain GenoType NTM-DR line probe assay incorrectly predicted subspecies in 8% of samples, presumably because it lacks the whole-genome resolution provided by sequencing and is vulnerable to between-species recombination (15).
Despite analyzing all mutations occurring in erm(41) and rrl for the full collection of genomes, we were unable to predict all clarithromycin resistance. This may be because there are other genes implicated or due to unreliable drug susceptibility testing (DST) results. Future work should aim to select discordant genotypes and identify additional infrequently occurring genetic loci implicated in clarithromycin resistance, for example, by using genome-wide association (GWAS) approaches. All of the new clarithromycin resistance-conferring mutations that we discovered occurred in isolates which we originally predicted to be inducibly resistant. Although M. abscessus is primarily thought to be an environmental organism, these patients may be colonized for long periods with subsequent potential exposure to multiple courses of macrolides. An alternative hypothesis may therefore be that some or all of these SNPs are compensatory mutations which act to reduce a fitness cost of the expression of erm, which has been experimentally demonstrated in other bacteria (22). There were four SNPs which occurred only in resistant samples but which were always seen with a known drug resistance-causing SNP; these four SNPs possibly also represent compensatory mutations.
Key weaknesses of our study include the fact that we were unable to establish a temporal relationship between antibiotic prescribing and inducible phenotypic resistance, as we did not have the relevant ethics approval to link to patient records. If, for example, any SNPs on our list of novel mutations were observed in isolates from patients who had never previously had macrolide therapy, it would be much more likely that they were genuine resistance-conferring mutations rather than compensatory mutations. In addition, it is possible that some of the genomes were the same patient replicates over a number of months or years, although this may have also diversified the range of mutations observed. We chose to include all available samples to maximize the detection of low-frequency resistance-determining SNPs, meaning that there was no validation set available. Our list of novel resistance-determining SNPs will therefore require validation on an independent data set before being applied to the clinical setting. We chose to target a select list of genes with known SNPs identified in the literature search; other approaches, such as GWAS, will likely be additive to the knowledge base that we present here.
In summary, WGS allows identification of known resistance-conferring mutations as well as demonstration of probable novel resistance-determining SNPs in regions that the Hain NTM-DR line probe assay cannot detect and that, if further validated, may change management. Identification of subspecies alone inadequately predicts macrolide resistance in M. abscessus. Our data do not support the replacement of phenotypic tests at this time; as more paired genome/DST data become available in the near future and we learn more about the molecular determinants of drug resistance, it is likely that the sensitivity and specificity of WGS resistance prediction will improve. Given that WGS data are already being produced in the United Kingdom for the purposes of molecular epidemiology, it would now be possible to phase out existing molecular tests and replicate their results in silico at no additional cost.
MATERIALS AND METHODS
Literature search.
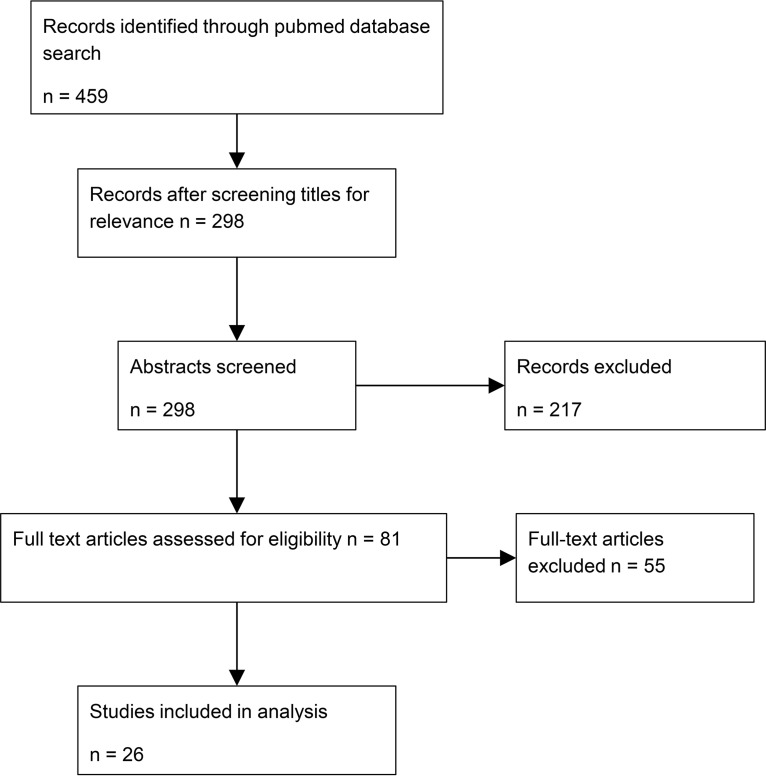
We first conducted a systematic review of the literature to search for known drug resistance-conferring mutations in M. abscessus. PubMed was searched with the terms “Mycobacterium abscessus” AND “clarithromycin” OR “macrolide” OR “drug resistance” OR “antibiotic resistance,” looking for English-language articles published up to April 2018. To be included in the final list, articles had to contain the genotypes of coding regions relevant to clarithromycin resistance in M. abscessus, in addition to paired drug susceptibility data. Studies looking at both clinical and nonclinical samples were included. A total of 298 abstracts were screened for relevance, and 81 full-text articles were obtained; of these, 26 met the inclusion criteria (Fig. 2).
FIG 2.
Flow diagram showing the stages of the systematic literature search.
Sample selection and sequencing.
We next sought all available clinical isolates (n = 180) which had undergone whole-genome sequencing by the Public Health England (PHE) laboratory in Birmingham, UK, as part of the routine diagnostic work flow and for which paired phenotypic data were also available. We supplemented this with 23 isolates for which the same data were available from a WGS archive at the University of Oxford. Isolates were collected between May 2014 and January 2017, and no prior selection according to the site of isolation or whether M. abscessus complex disease confirmed by guidelines was made. Clinical samples were cultured in BD Bactec MGIT liquid mycobacterial growth indicator tubes, from which an aliquot was removed to be prepared for WGS as previously described (23).
Libraries for Illumina MiSeq sequencing were prepared using the Illumina Nextera XT protocol with manual library normalization. Samples were batched at 12 to 16 per flow cell, and paired-end sequencing was performed with a MiSeq reagent kit (v2). Bioinformatics was performed using the PHE bioinformatics pipeline as previously described (23, 24). Briefly, reads were mapped to the M. abscessus subsp. abscessus reference genome (GenBank accession number NC_010397.1) with Stampy (v1.22), and variants were called using Samtools (v0.1.18) (only variants with ≥5 high-quality reads, a mean quality per base of ≥25, and >90% high-quality bases were retained as variants; heterozygous variants with >10% minor variants were not retained). A self-self BLAST approach was used to mask repetitive regions. Subspecies were identified by computing maximum likelihood (ML) phylogenetic trees incorporating published representative isolates from each subspecies. A whole-genome SNP alignment was used as input to IQ-TREE OMP (v1.5.5) using a generalized time-reversible model. The erm(41) and rplV genes were manually inspected for insertions/deletions from aligned FASTA files using the Seaview (v4.6.2) program.
Drug susceptibility testing.
Phenotypic drug susceptibility testing (DST) was performed at the PHE National Mycobacterial Reference Service in London, UK. DST was performed using the broth microdilution method with 96-well Rapmyco microtiter plates (Mueller-Hinton medium with TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffer; Thermo Fisher). The plates were read at day 3 postinoculation, and if growth was poor, they were read again at day 5, according to Clinical and Laboratory Standards Institute (CLSI) guidelines (25). Isolates deemed susceptible or intermediate were reincubated, and the plates were read again at days 7, 14, and 21. Those found to be resistant (R; MIC ≧ 8 µg/ml) at any of these time points were described as phenotypically resistant. A call of phenotypically sensitive (S; MIC ≦ 2 µg/ml) or intermediate (I; MIC >2 to <8 µg/ml) was made only after the full 21 days of incubation. This study was an opportunistic retrospective analysis of routinely collected clinical data, and as such, phenotypic testing was not repeated on discordant isolates.
Genotypic prediction of clarithromycin susceptibility.
We used BioPython software to extract base calls from whole-genome sequence FASTA files, comparing these to a list of genomic loci which our literature search indicated were associated with clarithromycin resistance (Table 1). We then predicted phenotypes using a hierarchical algorithm (Fig. 1). A resistant phenotype was predicted where any mutations were present at rrl position 2270 or 2271 (E. coli numbering, positions 2058 and 2059, respectively) or where the less well characterized rrl_A2269G, rrl_A2293C, or rrl_G2281A mutation was seen. In the absence of these mutations, susceptibility was predicted where an isolate had a truncated erm(41) gene or a C nucleotide at position 28 in erm(41). Inducible resistance was predicted where a wild-type call (T) was present at position 28 in erm(41). However, if an erm(41)_C19T mutation was also present, susceptibility instead of inducible resistance was predicted. In cases where there was a null call at rrl 2270/2271, we subsequently attempted local assembly of the rrl gene using the Ariba tool (26), followed by comparison by alignment against the reference sequence. Where this was not possible due to low coverage in this region, no prediction was made. The statistics quoted were calculated using R Studio (v1.1.383).
Search for novel resistance-conferring mutations.
We attempted to characterize new resistance-conferring mutations within genes linked to drug resistance from the literature search. To maximize the power for discovering new potential resistance-conferring mutations, we included all genomes available to us. All variants in these genes or their promoter regions were extracted from variant call files using Python software. Phylogenetic SNPs, assumed to be benign, were identified by considering each subspecies in turn and excluded from further analysis.
We considered variants at the level of SNPs in promoter regions or rRNA and amino acid changes in coding regions. A mutation (a variant in an isolate with an observable phenotype) was characterized as causing resistance if it occurred as the only variant in the relevant region in a resistant isolate or if it was always associated with resistance when it was seen and did not co-occur with any other mutations known to cause resistance. Variants were characterized as consistent with susceptibility (benign) if all isolates were susceptible when a variant occurred alone or if it occurred only in susceptible isolates. We assumed no prior knowledge in this section of the analysis, and the identification of known resistance-conferring SNPs was used as an internal validation of our approach.
Accession number(s).
All newly sequenced data have been uploaded to NCBI under BioProject accession number PRJNA420644.
ACKNOWLEDGMENTS
We have no conflicts of interest to declare.
No specific funding was acquired for this work. All sequencing data were created as part of the routine PHE clinical diagnostic service.
The research was supported by the National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at University of Oxford in partnership with Public Health England (PHE) and by Oxford NIHR Biomedical Research Centre. T. Peto is a NIHR Senior Investigator. The report presents independent research funded by NIHR. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, the Department of Health, or Public Health England.
REFERENCES
- 1.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN. 2010. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esther CR Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler IC, Chapman SJ, Clayton A, Cullen M, Daniels T, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Humphrey H, Isalska B, Jensen-Fangel S, Jönsson B, Jones AM, et al. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris KA, Underwood A, Kenna DTD, Brooks A, Kavaliunaite E, Kapatai G, Tewolde R, Aurora P, Dixon G. 2014. Whole-genome sequencing and epidemiological analysis do not provide evidence for cross-transmission of Mycobacterium abscessus in a cohort of pediatric cystic fibrosis patients. Clin Infect Dis 60:1007–1016. doi: 10.1093/cid/ciu967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tortoli E, Kohl TA, Trovato A, Baldan R, Campana S, Cariani L, Colombo C, Costa D, Cristadoro S, Di Serio MC, Manca A, Pizzamiglio G, Rancoita PMV, Rossolini GM, Taccetti G, Teri A, Niemann S, Cirillo DM. 2017. Mycobacterium abscessus in patients with cystic fibrosis: low impact of inter-human transmission in Italy. Eur Respir J 50:1602525. doi: 10.1183/13993003.02525-2016. [DOI] [PubMed] [Google Scholar]
- 7.Adekambi T, Sassi M, van Ingen J, Drancourt M. 2017. Reinstating Mycobacterium massiliense and Mycobacterium bolletii as species of the Mycobacterium abscessus complex. Int J Syst Evol Microbiol 67:2726–2730. doi: 10.1099/ijsem.0.002011. [DOI] [PubMed] [Google Scholar]
- 8.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 9.Koh W-J, Jeon K, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 10.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Andres Floto R. 2017. British Thoracic Society guideline for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). BMJ Open Respir Res 4:e000242. doi: 10.1136/bmjresp-2017-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 12.Nash KA, Brown-Elliott BA, Wallace RJ. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon K, Kwon OJ, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park YK, Kim CK, Koh W-J. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 14.Choi H, Kim S-Y, Kim DH, Huh HJ, Ki C-S, Lee NY, Lee S-H, Shin S, Shin SJ, Daley CL, Koh W-J. 2017. Clinical characteristics and treatment outcomes of patients with acquired macrolide-resistant Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 61:e01146-17. doi: 10.1128/AAC.01146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehrmann J, Kurt N, Rueger K, Bange F-C, Buer J. 2016. GenoType NTM-DR for identifying Mycobacterium abscessus subspecies and determining molecular resistance. J Clin Microbiol 54:1653–1655. doi: 10.1128/JCM.00147-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio M, March F, Garrigó M, Moreno C, Español M, Coll P. 2015. Inducible and acquired clarithromycin resistance in the Mycobacterium abscessus complex. PLoS One 10:e0140166. doi: 10.1371/journal.pone.0140166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatakeyama S, Ohama Y, Okazaki M, Nukui Y, Moriya K. 2017. Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infect Dis 17:197. doi: 10.1186/s12879-017-2298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YM, Tong XL, Xu HT, Ju Y, Cai M, Wang C. 2016. Prevalence and antimicrobial susceptibility of Mycobacterium abscessus in a general hospital, China. Biomed Environ Sci 29:85–90. doi: 10.3967/bes2016.009. [DOI] [PubMed] [Google Scholar]
- 19.Cowman S, Burns K, Benson S, Wilson R, Loebinger MR. 2016. The antimicrobial susceptibility of non-tuberculous mycobacteria. J Infect 72:324–331. doi: 10.1016/j.jinf.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Mougari F, Bouziane F, Crockett F, Nessar R, Chau F, Veziris N, Sapriel G, Raskine L, Cambau E. 2017. Selection of resistance to clarithromycin in Mycobacterium abscessus subspecies. Antimicrob Agents Chemother 61:e00943-16. doi: 10.1128/AAC.00943-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shallom SJ, Moura NS, Olivier KN, Sampaio EP, Holland SM, Zelazny AM. 2015. New real-time PCR assays for detection of inducible and acquired clarithromycin resistance in the Mycobacterium abscessus group. J Clin Microbiol 53:3430–3437. doi: 10.1128/JCM.01714-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta P, Sothiselvam S, Vázquez-Laslop N, Mankin AS. 2013. Deregulation of translation due to post-transcriptional modification of rRNA explains why erm genes are inducible. Nat Commun 4:1984. doi: 10.1038/ncomms2984. [DOI] [PubMed] [Google Scholar]
- 23.Votintseva AA, Pankhurst LJ, Anson LW, Morgan MR, Gascoyne-Binzi D, Walker TM, Quan TP, Wyllie DH, Del Ojo Elias C, Wilcox M, Walker AS, Peto TEA, Crook DW. 2015. Mycobacterial DNA extraction for whole-genome sequencing from early positive liquid (MGIT) cultures. J Clin Microbiol 53:1137–1143. doi: 10.1128/JCM.03073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker TM, Kohl TA, Omar SV, Hedge J, Del Ojo Elias C, Bradley P, Iqbal Z, Feuerriegel S, Niehaus KE, Wilson DJ, Clifton DA, Kapatai G, Ip CLC, Bowden R, Drobniewski FA, Allix-Béguec C, Gaudin C, Parkhill J, Diel R, Supply P, Crook DW, Smith EG, Walker AS, Ismail N, Niemann S, Peto TEA. Modernizing Medical Microbiology (MMM) Informatics Group. 2015. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect Dis 15:1193–1202. doi: 10.1016/S1473-3099(15)00062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2000. Susceptibility testing mycobacteria, nocardia, and other aerobic actinomycetes: tentative standard. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 26.Hunt M, Mather AE, Sánchez-Busó L, Page AJ, Parkhill J, Keane JA, Harris SR. 2017. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom 3:e000131. doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastian S, Veziris N, Roux A-L, Brossier F, Gaillard J-L, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer FP, Castelberg C, Quiblier C, Böttger EC, Somoskövi A. 2014. Erm(41)-dependent inducible resistance to azithromycin and clarithromycin in clinical isolates of Mycobacterium abscessus. J Antimicrob Chemother 69:1559–1563. doi: 10.1093/jac/dku007. [DOI] [PubMed] [Google Scholar]
- 29.Hanson KE, Slechta ES, Muir H, Barker AP. 2014. Rapid molecular detection of inducible macrolide resistance in Mycobacterium chelonae and M. abscessus strains: a replacement for 14-day susceptibility testing? J Clin Microbiol 52:1705–1707. doi: 10.1128/JCM.03464-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown-Elliott BA, Vasireddy S, Vasireddy R, Iakhiaeva E, Howard ST, Nash K, Parodi N, Strong A, Gee M, Smith T, Wallace RJ. Jr. 2015. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol 53:1211–1215. doi: 10.1128/JCM.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie W, Duan H, Huang H, Lu Y, Chu N. 2015. Species identification and clarithromycin susceptibility testing of 278 clinical nontuberculosis mycobacteria isolates. Biomed Res Int 2015:506598. doi: 10.1155/2015/506598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramírez A, de Waard JH, Araque M. 2015. Molecular mechanisms of clarithromycin resistance in Mycobacterium abscessus complex clinical isolates from Venezuela. J Glob Antimicrob Resist 3:205–209. doi: 10.1016/j.jgar.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Chua KYL, Bustamante A, Jelfs P, Chen SC-A, Sintchenko V. 2015. Antibiotic susceptibility of diverse Mycobacterium abscessus complex strains in New South Wales, Australia. Pathology 47:678–682. doi: 10.1097/PAT.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Sung H, Park J-S, Choi S-H, Shim T-S, Kim M-N. 2016. Subspecies distribution and macrolide and fluoroquinolone resistance genetics of Mycobacterium abscessus in Korea. Int J Tuberc Lung Dis 20:109–114. doi: 10.5588/ijtld.15.0068. [DOI] [PubMed] [Google Scholar]
- 35.Jeong SH, Kim S-Y, Huh HJ, Ki C-S, Lee NY, Kang C-I, Chung DR, Peck KR, Shin SJ, Koh W-J. 2017. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis 60:49–56. doi: 10.1016/j.ijid.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Carvalho NFD, Pavan F, Sato DN, Leite CQF, Arbeit RD, Chimara E. 2018. Genetic correlates of clarithromycin susceptibility among isolates of the Mycobacterium abscessus group and the potential clinical applicability of a PCR-based analysis of erm(41). J Antimicrob Chemother 73:862–866. doi: 10.1093/jac/dkx476. [DOI] [PubMed] [Google Scholar]
- 37.Chew KL, Cheng JWS, Hudaa Osman N, Lin RTP, Teo JWP. 2017. Predominance of clarithromycin-susceptible Mycobacterium massiliense subspecies: characterization of the Mycobacterium abscessus complex at a tertiary acute care hospital. J Med Microbiol 66:1443–1447. doi: 10.1099/jmm.0.000576. [DOI] [PubMed] [Google Scholar]
- 38.Koh W-J, Jeong B-H, Kim S-Y, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, Ki C-S, Lee NY, Kim HK, Choi YS, Kim J, Lee S-H, Kim CK, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis 64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 39.Zhu YC, Mitchell KK, Nazarian EJ, Escuyer VE, Musser KA. 2015. Rapid prediction of inducible clarithromycin resistance in Mycobacterium abscessus. Mol Cell Probes 29:514–516. doi: 10.1016/j.mcp.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Wallace RJ Jr, Meier A, Brown BA, Zhang Y, Sander P, Onyi GO, Böttger EC. 1996. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob Agents Chemother 40:1676–1681. doi: 10.1128/AAC.40.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Li B, Chu H, Zhang Z, Luo L, Ma W, Yang S, Guo Q. 2017. Rapid detection of mutations in erm(41) and rrl associated with clarithromycin resistance in Mycobacterium abscessus complex by denaturing gradient gel electrophoresis. J Microbiol Methods 143:87–93. doi: 10.1016/j.mimet.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Kim S-Y, Shin SJ, Jeong B-H, Koh W-J. 2016. Successful antibiotic treatment of pulmonary disease caused by Mycobacterium abscessus subsp. abscessus with C-to-T mutation at position 19 in erm(41) gene: case report. BMC Infect Dis 16:207. doi: 10.1186/s12879-016-1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]