Candidiasis is a potentially lethal condition that is caused by systemic dissemination of Candida albicans, a common fungal commensal residing mostly on mucosal surfaces. The transition of C. albicans from an innocuous commensal to an opportunistic pathogen goes hand in hand with its morphological transformation from a fungus to a hyphal appearance. On the one hand, the latter manifestation enables C. albicans to penetrate tissues, while on the other hand, the expression of many hypha-specific genes also endows it with the capacity to trigger particular cytokine responses. The Nlrp3 inflammasome is a crucial component of the innate immune system that provokes release of the IL-1β cytokine from myeloid cells upon encountering C. albicans hyphae. Our study reveals the peptide candidalysin as one of the hypha-derived drivers of Nlrp3 inflammasome responses in primary macrophages and, thus, contributes to better understanding the fungal mechanisms that determine the pathogenicity of C. albicans.
KEYWORDS: Candida albicans, NLRP3, candidalysin, inflammasome, primary macrophages
ABSTRACT
Candida albicans is an opportunistic fungal pathogen that can cause life-threatening infections, particularly in immunocompromised patients. C. albicans induced activation of the Nlrp3 inflammasome, leading to secretion of bioactive interleukin 1β (IL-1β) is a crucial myeloid cell immune response needed for antifungal host defense. Being a pleiomorphic fungus, C. albicans can provoke Nlrp3 inflammasome responses only upon morphological transformation to its hyphal appearance. However, the specific hyphal factors that enable C. albicans to activate the Nlrp3 inflammasome in primary macrophages remain to be revealed. Here, we identify candidalysin, a peptide derived from the hypha-specific ECE1 gene, as a fungal trigger for Nlrp3 inflammasome-mediated maturation and secretion of IL-1β from primary macrophages. Direct peptide administration experiments showed that candidalysin was sufficient for inducing secretion of mature IL-1β from macrophages in an Nlrp3 inflammasome-dependent manner. Conversely, infection experiments using candidalysin-deficient C. albicans showed that candidalysin crucially contributed to the capacity of this fungus to induce maturation and secretion of IL-1β from primary macrophages. These complementary observations identify the expression of candidalysin as one of the molecular mechanisms by which hyphal transformation equips C. albicans with its proinflammatory capacity to elicit the release of bioactive IL-1β from macrophages.
OBSERVATION
Candida albicans is a commensal fungus that can transform to a highly pathogenic organism capable of establishing severe mycoses in immunocompromised patients (1, 2). Many factors discriminating between the harmless versus potentially damaging states of C. albicans relate to its appearance as a pleiomorphic fungus. Although its yeast-to-hypha morphological transition boosts the expression levels of many C. albicans proteins, such as adhesins and secreted enzymes (3–6), the individual impacts of these hypha-specific fungal factors on host innate immunity are not always clear.
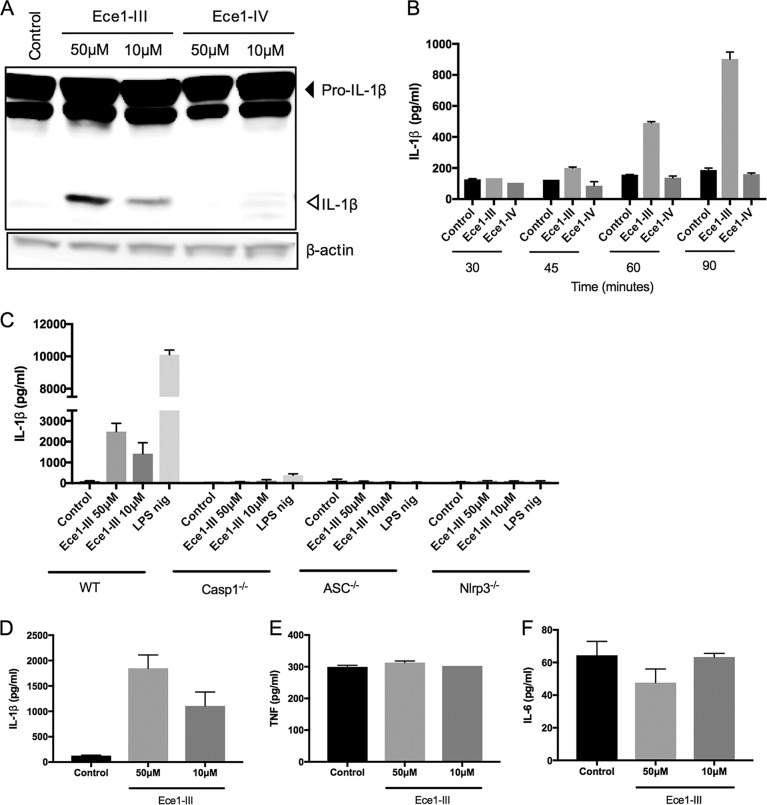
The Nlrp3 inflammasome is a signaling complex that mediates maturation and release of the proinflammatory cytokine interleukin 1β (IL-1β), crucial for protecting the host against systemic C. albicans infection (7–10). While several studies showed that C. albicans transition from yeast cells to hyphae was necessary to activate Nlrp3 inflammasomes, additional hypha-derived factors are needed to activate Nlrp3 (10–13). Based on reports that several bacteria utilize toxins forming pores in the cellular membrane to cause the efflux of intracellular K+ for triggering Nlrp3-driven inflammasome activation (14), we hypothesized that the cellular membrane-damaging potential of the Ece1III toxin, a C. albicans peptide also termed candidalysin that is encoded by the hypha-specific ECE1 gene (15), might initiate inflammasome responses. To investigate this possibility, we performed experiments in which we mimicked fungal β-glucan-mediated inflammasome priming by treating primary bone marrow-derived macrophages (BMDMs) with curdlan and then administered Ece1III peptide to these curdlan-primed cells (see the supplemental material). Interestingly, while the ECE1-derived control Ece1IV peptide did not induce IL-1β processing, the Ece1III peptide had a dose-dependent capacity to induce IL-1β maturation in curdlan-primed macrophages (Fig. 1A), leading to increased secretion of IL-1β, starting from 60 min after stimulation (Fig. 1B). In addition, the Ece1III peptide induced IL-1β secretion from wild-type (WT) macrophages but not from macrophages lacking either caspase-1, ASC, or Nlrp3 (Fig. 1C). While these findings identify candidalysin as a canonical Nlrp3 inflammasome activator, we next verified the specificity of this peptide-induced cytokine response. Because curdlan by itself provokes secretion of NF-κB-dependent cytokines, we performed experiments in which we washed away this priming agent prior to candidalysin treatment for specifically assessing cytokine induction by the latter. These experiments demonstrated that candidalysin-treated cells specifically secreted IL-1β without releasing the inflammasome-independent cytokines tumor necrosis factor (TNF) and IL-6 (Fig. 1D to F). Together, these results showed that candidalysin was sufficient to specifically provoke secretion of IL-1β from primary macrophages by activating the Nlrp3 inflammasome.
FIG 1.
Candidalysin induces Nlrp3 inflammasome-mediated IL-1β secretion and maturation in primary macrophages. (A) Wild-type BMDMs were primed with 100 µg/ml curdlan for 3 h and then left untreated (control) or incubated with the indicated concentrations of either the Ece1-III or the Ece1-IV peptide. At 2 h posttreatment, cell lysates were immunoblotted for IL-1β maturation. (B) Wild-type BMDMs were primed with 100 µg/ml curdlan for 3 h and then left untreated (control) or incubated with 50 µM Ece1-III peptide. Supernatants were collected at the indicated time points after peptide administration and analyzed for secreted IL-1β by multiplex Luminex. (C) BMDMs of the indicated genotypes were primed with 100 µg/ml curdlan for 3 h and then left untreated (control) or were incubated with the indicated concentrations of Ece1-III. At 90 min posttreatment, culture supernatants were analyzed for secreted IL-1β by the multiplex Luminex assay. As a positive control, BMDMs were primed with LPS (500 ng/ml) for 3 h and incubated with nigericin (nig) for 45 min. (D to F) Wild-type BMDMs were primed with 100 µg/ml curdlan for 3 h, after which the culture medium was aspirated and replaced with either control medium or medium containing the indicated concentrations of Ece1-III. At 90 min posttreatment, the culture supernatants were analyzed for secreted IL-1β (D), TNF (E), and IL-6 (F) by the multiplex Luminex assay. Data shown in panels B to F are the means ± standard deviations (SD) of results from triplicate wells from a representative experiment out of two independent experiments. Data shown in panel A are representative of two independent experiments.
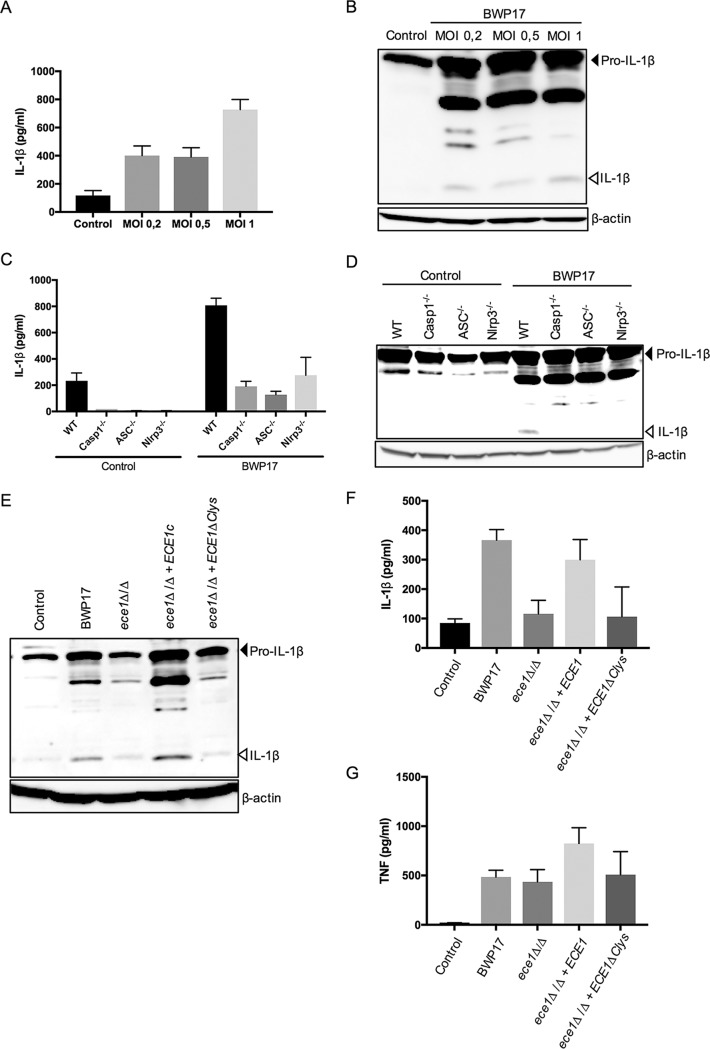
Our next experiments aimed to address the endogenous fungal contribution of candidalysin to Nlrp3 activation in primary macrophages. In accordance with a prior report showing that C. albicans by itself can perform the priming as well as the activation step for eliciting Nlrp3 inflammasome responses from naive BMDMs (10), incubating unprimed macrophages with C. albicans at a multiplicity of infection (MOI) of 0.2 for 24 h sufficed to provoke IL-1β secretion (Fig. 2A). Moreover, Western blotting showed that infecting naive macrophages with these low numbers of C. albicans was sufficient for detecting IL-1β maturation (Fig. 2B). In contrast to WT BMDMs, macrophages lacking either caspase-1 or ASC failed to mature or secrete IL-1β upon C. albicans infection (Fig. 2C and D). Upstream of ASC and caspase-1, both Nlrp3 and Nlrc4 have been suggested as inflammasome receptors upon C. albicans infection in mice (8, 10, 16–18). However, deleting Nlrp3 fully abrogated C. albicans-induced IL-1β maturation and secretion to the same extent as deleting caspase-1 or ASC did (Fig. 1C and D). These observations showed that overnight infection of unprimed BMDMs with C. albicans specifically activated the Nlrp3 inflammasome and, thus, validated this experimental setting for assessing the physiological contribution of candidalysin to whole-fungus-induced Nlrp3 inflammasome responses. For this purpose, we used the parental wild-type BWP17 strain and an ece1Δ/Δ strain that lacks the entire ECE1 gene product, from which candidalysin is produced (see the supplemental material). In addition, we also used ece1Δ/Δ strains that were reconstituted either with the complete ECE1 gene (ece1Δ/Δ+ECE1) or with an ECE1 gene from which the candidalysin-encoding sequence was deleted (ece1Δ/Δ+ECE1ΔClys). While WT C. albicans induced both maturation and secretion of IL-1β in unprimed macrophages, both of these inflammasome responses were diminished upon infection with the ece1Δ/Δ strain, demonstrating that an ECE1-derived fungal factor was crucial for C. albicans-induced Nlrp3 inflammasome activation (Fig. 2E and F). As expected, reconstituting the ece1Δ/Δ strain with the complete ECE1 gene restored its capacity to induce IL-1β maturation and secretion to levels similar those of WT fungi (Fig. 2E and F). In contrast, reconstituting the ece1Δ/Δ strain with a candidalysin-deficient ECE1 gene did not elicit higher IL-1β secretion levels from macrophages than those elicited by the ece1Δ/Δ strain (Fig. 2E). In addition, the inability of inducing IL-1β maturation by the ece1Δ/Δ strain could not be corrected by expressing a candidalysin-deficient ECE1 gene (Fig. 2F). In contrast to what occurred with IL-1β, levels of release of the inflammasome-independent cytokine TNF did not differ when infections with the various C. albicans strains were compared (Fig. 2G), showing that candidalysin specifically controlled inflammasome-dependent cytokine secretion.
FIG 2.
Candidalysin crucially contributes to C. albicans-induced IL-1β secretion and maturation in unprimed primary macrophages. (A, B) Naïve wild-type BMDMs were left untreated (control) or were incubated at the indicated MOIs of live C. albicans. At 24 h postinfection, culture supernatants were analyzed for secreted IL-1β by enzyme-linked immunosorbent assay (ELISA) (A) and cell lysates were immunoblotted for IL-1β maturation (B). (C, D) Naïve BMDMs of the indicated genotypes were left untreated (control) or were incubated at an MOI of 0.5 with live C. albicans cells. At 24 h postinfection, culture supernatants were analyzed for secreted IL-1β by ELISA (C) and cell lysates were immunoblotted for IL-1β maturation (D). (E to G) Naïve wild-type BMDMs were left untreated (control) or were incubated at an MOI of 0.5 with the indicated C. albicans strains. At 24 h postinfection, cell lysates were immunoblotted for IL-1β maturation (E) and culture supernatants were analyzed for secreted IL-1β (F) and TNF (G) by the Luminex assay. Data shown in panels A, C, F, and G are the means ± SD of results from triplicate wells from a representative experiment out of two independent experiments. Data shown in panels B, D, and E are representative for two independent experiments.
In summary, we showed that the Nlrp3 inflammasome-activating potential of C. albicans at least partially relies on candidalysin. In fact, it was known that candidalysin administration provokes IL-1β release in human TR146 epithelial cells (19). However, while it is not clear whether inflammasome activation takes part in this epithelial candidalysin effect, we show that IL-1β secretion upon candidalysin administration to macrophages depends entirely on the Nlrp3 inflammasome. As various bacterial virulence factors activate the Nlrp3 inflammasome due to pore-forming capacities (14), it is conceivable that the Nlrp3 inflammasome-activating potential of candidalysin derives from an ability to damage cellular membranes. Indeed, a recent study showed that candidalysin-induced Nlrp3 inflammasome activation in macrophages was associated with membrane permeabilization and decreased cytosolic K+ levels (20). While this study, thus, confirmed our observations and identified K+ efflux as the candidalysin-induced mechanism triggering Nlrp3 inflammasome activation, it is striking that a candidalysin-deficient C. albicans strain used in this study was not defective in IL-1β secretion at 5 h postinfection in lipopolysaccharide (LPS)-primed murine macrophages (20). This seems in contrast with our observation showing that naive BMDMs infected with the ece1Δ/Δ+ECE1ΔClys strain for 24 h displayed less IL-1β maturation and secretion. However, as the ece1Δ/Δ+ECE1ΔClys strain still provoked residual amounts of IL-1β processing and secretion from unprimed macrophages, both our experiments and those of Kasper et al. (20) indicate that C. albicans harbors multiple redundant factors capable of activating the Nlrp3 inflammasome. In this respect, recent genome-wide screening studies performed with immortalized macrophages identified a myriad of fungal factors as potential contributors to C. albicans-induced inflammasome activation (21, 22). Given the discrepancy between the crucial contributing role for candidalysin in naive macrophages observed in our study versus its dispensable role in LPS-primed macrophages used in the Kasper et al. study (20), it is conceivable that the various C. albicans Nlrp3 activators act with different kinetics and that their activities depend on specific host cell factors. Along these lines, it is possible that activated murine BMDMs are prone to rapid candidalysin-independent inflammasome activation, while naive macrophages may undergo a slower candidalysin-dependent Nlrp3 inflammasome activation. In conclusion, while additional mechanisms certainly exist, we identified candidalysin as a hyphal C. albicans factor that crucially contributes to Nlrp3 inflammasome activation in naive murine macrophages.
Supplemental materials and methods. Download Text S1, DOCX file, 0.05 MB (56.7KB, docx) .
Copyright © 2019 Rogiers et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
C. albicans strains used in this study. Download Table S1, DOCX file, 0.01 MB (13.9KB, docx) .
Copyright © 2019 Rogiers et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We are grateful for excellent technical support by Amelie Fossoul and Maarten Verdonckt.
O.R. is supported by Strategic Basic Research (SB) grant 1S55817N from the Research Foundation—Flanders (FWO). Research in the A.W. lab is supported by Odysseus grant G.0C49.13N and the research grants 3G.0447.18 and 3G.0448.18 from the Fund for Scientific Research—Flanders. Research in the P.V.D. lab is supported by the Fund for Scientific Research—Flanders (Infect-ERA grant G0H7816N). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
O.R. and U.C.F. performed experiments; all authors designed experiments and analyzed data; S.K., G.V.L., M.A.J.-R., P.V.D., and A.W. supervised the project. O.R. and A.W. wrote the manuscript with input from all other authors.
We have no conflicts of interest or financial disclosures to report.
Footnotes
Citation Rogiers O, Frising UC, Kucharíková S, Jabra-Rizk MA, van Loo G, Van Dijck P, Wullaert A. 2019. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. mBio 10:e02221-18. https://doi.org/10.1128/mBio.02221-18.
REFERENCES
- 1.Underhill DM, Iliev ID. 2014. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Richardson JP, Willems HME, Moyes DL, Shoaie S, Barker KS, Tan SL, Palmer GE, Hube B, Naglik JR, Peters BM. 2017. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect Immun 86:e00645-17. doi: 10.1128/IAI.00645-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassone A, Vecchiarelli A, Hube B. 2016. Aspartyl proteinases of eukaryotic microbial pathogens: from eating to heating. PLoS Pathog 12:e1005992. doi: 10.1371/journal.ppat.1005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. 2012. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 10:85–93. doi: 10.1586/eri.11.152. [DOI] [PubMed] [Google Scholar]
- 6.de Groot PW, Bader O, de Boer AD, Weig M, Chauhan N. 2013. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell 12:470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ. 2006. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis 193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 8.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. 2009. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joly S, Sutterwala FS. 2010. Fungal pathogen recognition by the NLRP3 inflammasome. Virulence 1:276–280. doi: 10.4161/viru.1.4.11482. [DOI] [PubMed] [Google Scholar]
- 10.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 12.Wellington M, Koselny K, Sutterwala FS, Krysan DJ. 2014. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 13:329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellington M, Koselny K, Krysan DJ. 2012. Candida albicans morphogenesis is not required for macrophage interleukin 1beta production. mBio 4:e00433-12. doi: 10.1128/mBio.00433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. 2013. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Hofs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Forster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Hader A, Kurzai O, Luo T, Kruger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR. 2016. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghi M, De Luca A, Puccetti M, Jaeger M, Mencacci A, Oikonomou V, Pariano M, Garlanda C, Moretti S, Bartoli A, Sobel J, van de Veerdonk FL, Dinarello CA, Netea MG, Romani L. 2015. Pathogenic NLRP3 inflammasome activity during Candida infection is negatively regulated by IL-22 via activation of NLRC4 and IL-1Ra. Cell Host Microbe 18:198–209. doi: 10.1016/j.chom.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, Bistoni F, Cassone A, Hube B, Vecchiarelli A. 2013. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur J Immunol 43:679–692. doi: 10.1002/eji.201242691. [DOI] [PubMed] [Google Scholar]
- 18.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. 2011. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL, Ho J, Huppler AR, Ramani K, McGeachy MJ, Mufazalov IA, Waisman A, Kane LP, Biswas PS, Hube B, Naglik JR, Gaffen SL. 2017. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol 2:eaam8834. doi: 10.1126/sciimmunol.aam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasper L, König A, Koenig P-A, Gresnigt MS, Westman J, Drummond RA, Lionakis MS, Groß O, Ruland J, Naglik JR, Hube B. 2018. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun 9:4260. doi: 10.1038/s41467-018-06607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koselny K, Mutlu N, Minard AY, Kumar A, Krysan DJ, Wellington M. 2018. A genome-wide screen of deletion mutants in the filamentous Saccharomyces cerevisiae background identifies ergosterol as a direct trigger of macrophage pyroptosis. mBio 9:e01204-18. doi: 10.1128/mBio.01204-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Meara TR, Duah K, Guo CX, Maxson ME, Gaudet RG, Koselny K, Wellington M, Powers ME, MacAlpine J, O'Meara MJ, Veri AO, Grinstein S, Noble SM, Krysan D, Gray-Owen SD, Cowen LE. 2018. High-throughput screening identifies genes required for Candida albicans induction of macrophage pyroptosis. mBio 9:e01581-18. doi: 10.1128/mBio.01581-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental materials and methods. Download Text S1, DOCX file, 0.05 MB (56.7KB, docx) .
Copyright © 2019 Rogiers et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
C. albicans strains used in this study. Download Table S1, DOCX file, 0.01 MB (13.9KB, docx) .
Copyright © 2019 Rogiers et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.