Key Points
Transplant-related geriatric syndromes of delirium or fall are associated with potentially modifiable pretransplant geriatric risk factors.
Transplant-related delirium and fall are associated with increased long-term nonrelapse mortality and reduced overall survival.
Abstract
Multifactorial geriatric syndromes are highly prevalent in older patients with cancer. Because an increasing number of older patients undergo allogeneic hematopoietic stem cell transplantation (allo-HCT), we examined the incidence and impact of transplant-related geriatric syndromes using our institutional database and electronic medical records. We identified 527 patients age 60 years or older who had undergone first allo-HCT from 2001 to 2016 for hematologic malignancies. From the initiation of conditioning to 100 days posttransplant, new geriatric syndromes were predominantly delirium with a cumulative incidence of 21% (95% confidence interval [CI], 18%-25%) at day 100 followed by fall at 7% (95% CI, 5%-9%). In multivariable analyses of available pretransplant variables, fall within the last year, potentially inappropriate use of medication, thrombocytopenia, and reduced creatinine clearance were significantly associated with delirium; age older than 70 years and impaired activities of daily living were significantly associated with fall. In the 100-day landmark analysis, both delirium (hazard ratio [HR], 1.66; 95% CI, 1.09-2.52; P = .023) and fall (HR, 2.14; 95% CI, 1.16-3.95; P = .026) were significantly associated with increased nonrelapse mortality; moreover, fall (HR, 1.93; 95% CI, 1.18-3.14; P = .016), but not delirium, was significantly associated with reduced overall survival. Here, we establish baseline incidences and risk factors of common transplant-related geriatric syndromes. Importantly, we demonstrate significant associations of delirium and fall with inferior transplant outcomes. The burden and impact of transplant-related geriatric syndromes warrant the institution of patient-centered, preemptive, longitudinal, and multidisciplinary interventions to improve outcomes for older allo-HCT patients.
Visual Abstract
Introduction
As a therapeutic modality with curative intent, allogeneic hematopoietic stem cell transplantation (allo-HCT) has been increasingly used in older adults with hematologic malignancies because of increasing incidence and poor prognosis.1-3 An analysis by the Center for International Blood and Marrow Transplant Research revealed that the recent increase is mostly driven by the older patient population, with recipients age 60 years or older accounting for more than 30% of allo-HCTs in 2016.4 Several possibilities could explain this recent increase. First, the development of reduced-intensity conditioning regimens has allowed for patients who are older and more frail to undergo allo-HCT, which has traditionally been limited to younger patients because of significant regimen-related toxicities and intense immunosuppression.5 Second, the selection of appropriate older candidates and donors for allo-HCTs has improved, and the biological age based on a comprehensive geriatric assessment rather than the chronological age is now increasingly used to determine eligibility for allo-HCT.6,7 Improved supportive care such as newer antimicrobials, expert management of adverse effects, and better treatments for acute and chronic graft-versus-host disease have all contributed to improved outcomes in older adults.8,9 Despite these advances, the mortality and morbidity of allo-HCT has remained high in older patients, with long-term overall survival (OS) of 30% to 40% and a 2-year nonrelapse mortality (NRM) rate of ∼30%.4,10-13 Although hematologic malignancies in older patients tend to have a higher risk for relapse,14 these suboptimal outcomes suggest that there are additional age-related aspects of allo-HCT that need to be investigated and improved.
Geriatric syndromes are defined as multifactorial clinical conditions that occur when accumulated effects of impairments in multiple organ systems render an older person vulnerable to situational challenges.15 These conditions include cognitive impairment such as delirium and dementia, mechanical falls, osteoporosis, malnutrition, sarcopenia, urinary incontinence, decubitus ulcer, and failure to thrive, among others.15 Although these conditions are clinically heterogeneous, they often have multiple risk factors, involve multiple organ systems, and contribute to the frailty phenotype.16 Geriatric syndromes are highly prevalent in older adults and are associated with increased disability, reduced survival, and poor quality of life.17 Two landmark studies published in 2006 revealed a high prevalence of geriatric syndromes among patients with solid tumors in both inpatient and outpatient settings.18,19 This strong association was subsequently validated by using the Medicare administrative claims database.20 Multifactorial geriatric syndromes can complicate cancer therapy and increase the use of health care resources; conversely, cancer diagnosis and treatment may also lead to new geriatric syndromes.21,22
In allo-HCT, the incidence and risk factors for the development of geriatric syndromes have not been examined in a large cohort of patients. Several small prospective studies have reported an incidence of delirium ranging from 35% to 50% after allo-HCT, with diverse clinical presentations, time courses, and risk factors.23-26 Moreover, delirium has been negatively associated with long-term cognition, quality of life, and even survival.24,26,27 We hypothesize that common geriatric syndromes associated with allo-HCT could be identified in a large cohort of older patients, and they could have a negative impact on survival outcomes. We report here the identification, characterization, and impact analyses of transplant-related geriatric syndromes.
Methods
Patients and transplant care
Patients age 60 years or older who underwent a first allo-HCT for hematologic malignancies between 2001 and 2016 at our institution were included in this analysis. A waiver of authorization for this retrospective review was obtained from the Institutional Review and Privacy Board. Pathologic diagnosis for each patient was confirmed at our institution. Routine eligibility criteria for allo-HCT included availability of an HLA-identical or 1- or 2-allele mismatched adult related or unrelated donor, a haploidentical donor, or umbilical cord blood units; absence of active fulminant infection; and lack of serious coexisting comorbidities that would preclude administration of cytoreductive regimens. Posttransplant care and disease monitoring followed standard institutional guidelines. Patient demographic data, disease and transplant characteristics, NRM, relapse or disease progression, cause of death, and survival data were retrieved from our institutional transplant database. All data were anonymized to protect the identities of participants involved in the research. The HCT–Comorbidity Index (HCT-CI) and revised Disease Risk Index (DRI) were assigned according to the published criteria.28,29
Geriatric and laboratory characteristics
Geriatric syndrome case identification was performed through chart review by 2 investigators (R.J.L. and T.A.E.), and differences were reconciled through discussion with the primary treating physician. We systematically reviewed diagnostic codes and structured clinical notes from the physicians, advanced practice providers, nursing staff, consultants, and ancillary clinical staff such as physical therapists, occupational therapists, dietitians, and social workers. A similar approach for geriatric syndrome case findings has been described previously, although we did not use a software-based natural language processing algorithm.30,31 Geriatric syndromes examined included delirium defined by the established diagnostic criteria32 and mechanical fall. The time frame for case identification was from the initiation of conditioning to 100 days after stem cell infusion (supplemental Table 1). After case identification, each occurrence of delirium and fall received an in-depth review, and likely causes, workup, and treatment by the primary team were summarized. For risk factor analyses, all pretransplant geriatric variables such as impairments in basic activities of daily living (ADL), nutrition, prior falls, and medication use were defined and extracted as described in supplemental Table 1. Use of potentially inappropriate medication (PIM) was defined by the American Geriatrics Society 2015 Updated Beers Criteria.33 Pretransplant laboratory variables were taken from the last clinic visit before admission or on the day of admission for transplantation as described in supplemental Table 1.
Statistical methods
OS and progression-free survival (PFS) were estimated by using the Kaplan-Meier method, whereas delirium, fall, relapse, and NRM were estimated using the cumulative incidence method for competing risks. Death was considered a competing risk for delirium, fall, and relapse, with relapse considered a competing risk for NRM. Given that delirium and fall were measured from the start of conditioning, day −10 was used as time zero for these outcomes, whereas transplant date was used as time zero for OS, PFS, relapse, and NRM. All estimates presented here reflect the time posttransplant. For delirium and fall, all patients without prior events were censored at day 100. The association of clinical, transplant, and geriatric characteristics with outcomes was evaluated by using Cox proportional hazards models, with cause-specific models used for delirium, fall, and NRM. Variables included age, sex, transplant period, Karnofsky performance score (KPS), graft source, conditioning intensity, recipient cytomegalovirus (CMV) status, HCT-CI, revised DRI, geriatric variables of impairment in ADL, nutrition, mobility, medications, and laboratory variables as summarized in supplemental Table 1. In assessing the effects of delirium and fall on OS and NRM, a landmark analysis at day 100 was used. Multivariable models were built using a forward selection procedure; all univariable covariates with P < .1 were considered candidates for multivariable analysis. To meet the proportional hazards assumption, the final multivariable model for delirium was stratified by graft source. All statistical analyses were performed using R 3.5.0 (R development core team, Vienna, Austria).
Results
Patients characteristics and transplantation outcomes
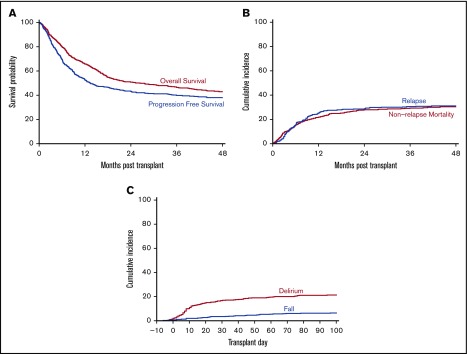
Baseline characteristics of the study cohort are listed in Table 1. The median age was 65 years (range, 60-78.7 years) with 39% of patients being female. The study cohort was heterogeneous regarding disease diagnosis and transplant approaches. Almost two-thirds of the patients (62%) received a myeloablative conditioning regimen. Ninety-five percent of patients received a fludarabine-containing regimen. The comorbidity burden was high with 56% of patients having an HCT-CI of ≥3. Baseline geriatric deficits were common in the geriatric domains of function, mobility, nutrition, and medications. Ten percent of patients had abnormal creatinine clearance and 13% had platelet counts of <50 × 109/L on admission. With a median follow-up of 46 months for survivors, the 3-year probability for OS was 46% (95% confidence interval [CI, 42%-51%), and for PFS, it was 40% (95% CI, 35%-44%) (Figure 1A). The 2-year cumulative incidence of NRM was 28% (95% CI, 24%-32%), and of relapse/disease progression, it was 29% (95% CI, 25%-33%) (Figure 1B).
Table 1.
Demographic, transplant, geriatric, and laboratory characteristics
| Characteristic | No. | % |
|---|---|---|
| No. of patients | 527 | |
| Median follow-up of survivors (range), mo | 46 (8-143) | |
| Median age (range), y | 65 (60-78.7) | |
| Female sex | 203 | 39 |
| Transplant diagnosis | ||
| AML | 210 | 40 |
| ALL | 28 | 5 |
| MDS | 120 | 23 |
| Lymphoma | 94 | 18 |
| Other hematologic malignancies | 75 | 14 |
| KPS ≥90 | 261 | 50 |
| HCT-CI | ||
| 0-2 | 233 | 44 |
| ≥3 | 294 | 56 |
| DRI revised | ||
| Low/intermediate | 386 | 73 |
| High/very high | 141 | 27 |
| Graft type | ||
| Bone marrow | 40 | 8 |
| Peripheral blood | 453 | 86 |
| Cord blood | 34 | 6 |
| Donor type | ||
| Match related | 156 | 30 |
| Match unrelated | 242 | 46 |
| Cord blood | 34 | 6 |
| Mismatched (≤7/8) unrelated | 81 | 15 |
| Haploidentical | 14 | 3 |
| Conditioning intensity | ||
| Myeloablative | 327 | 62 |
| Nonmyeloablative/reduced intensity | 200 | 38 |
| Recipient CMV status | ||
| Negative | 208 | 39 |
| Positive | 319 | 61 |
| GVHD prophylaxis | ||
| Ex vivo CD34+ selected | 241 | 46 |
| Unmodified | 265 | 50 |
| Posttransplant cyclophosphamide | 21 | 4 |
| ADL | ||
| Normal | 507 | 96 |
| Impaired | 20 | 4 |
| Potentially inappropriate use of medication | ||
| No | 284 | 54 |
| Yes | 243 | 46 |
| Falls within last year | ||
| No | 427 | 81 |
| Yes | 99 | 19 |
| Weight loss of 10 pounds in the last 3 mo | ||
| No | 435 | 83 |
| Yes | 92 | 17 |
| Albumin level, g/dL | ||
| <3.5 | 31 | 6 |
| ≥3.5 | 496 | 94 |
| Platelet count on admission, ×109/L | ||
| ≥50 | 459 | 87 |
| <50 | 68 | 13 |
| Creatinine clearance, mL/min | ||
| ≥60 | 472 | 90 |
| <60 | 55 | 10 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; GVHD, graft-versus-host disease; MDS, myelodysplastic syndrome.
Figure 1.
Outcomes of allo-HCT in older adults and the prevalence of transplant-related geriatric syndromes. (A) Estimates of OS and PFS of the cohort using the Kaplan-Meier method. (B) Estimates of cumulative incidences of NRM and relapse/disease progression using the cumulative incidence method. (C) Estimates of cumulative incidences of delirium and fall using the cumulative incidence method.
Incidence of geriatric syndromes
From the start of conditioning to 100 days after stem cell infusion, we identified 112 patients who experienced at least 1 episode of delirium for a cumulative incidence of 21% (95% CI, 18%-25%) (Figure 1C). The median time to delirium onset was 18 days after the start of conditioning. We also identified 34 patients who had fallen at least once during the same period for a cumulative incidence of 7% (95% CI, 5%-9%) (Figure 1C). The median time to fall was 31.5 days after the start of conditioning. We found similar incidence of delirium or fall among the subgroup of patients who received transplantation on the platform using CD34+ selected graft (supplemental Table 2).
Risk factors for delirium and fall
We next examined pretransplant variables for their association with the development of delirium and fall. On univariable analysis, we found that sex, age, graft source, HCT-CI, transplantation time period, falls within the last year, PIM use, platelet count, and creatinine clearance were associated with the development of delirium (P < .1; supplemental Table 2). Similarly, age, conditioning intensity, ADL, revised DRI, and falls within the last year were associated with new fall (P < .1; supplemental Table 2). On multivariable analysis, falls within the last year, PIM use, platelet count <50 × 109/L, and creatinine clearance <60 mL/min were significantly associated with an increased risk of delirium (Table 2; model stratified by graft source). Similarly, age 70 years or older and impairment in ADL remained significantly associated with an increased risk of new fall, with falls within the last year approaching statistical significance (P = .056; Table 2).
Table 2.
Multivariable analyses of delirium and falls
| HR | 95% CI | P | |
|---|---|---|---|
| Delirium* | |||
| Prior falls | <.001 | ||
| No | Reference | ||
| Yes | 2.09 | 1.39-3.15 | |
| PIM user | .003 | ||
| No | Reference | ||
| Yes | 1.79 | 1.22-2.65 | |
| Platelets, ×109/L | <.001 | ||
| ≥50 | Reference | ||
| <50 | 2.57 | 1.63-4.06 | |
| Creatinine clearance, mL/min | .001 | ||
| ≥60 | Reference | ||
| <60 | 2.36 | 1.43-3.9 | |
| Fall | |||
| Prior falls | .056 | ||
| No | Reference | ||
| Yes | 2.14 | 1.02-4.52 | |
| Age, y | .011 | ||
| <70 | Reference | ||
| ≥70 | 3.03 | 1.39-6.62 | |
| ADL | .011 | ||
| Normal | Reference | ||
| Impaired | 4.36 | 1.66-11.4 |
Stratified by graft source to ensure proportional hazards.
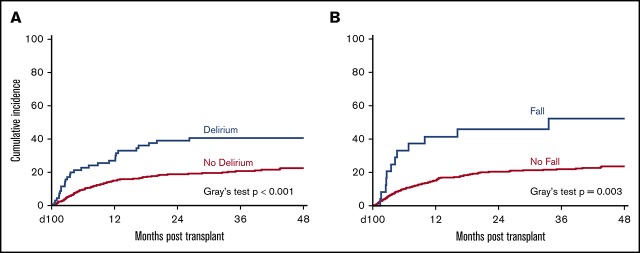
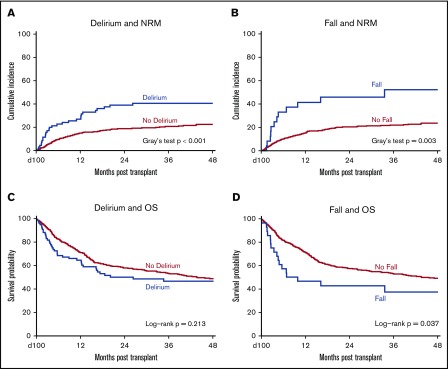
Impact of delirium and fall
We next investigated the impact of transplant-related delirium and fall on transplant outcomes. In the univariable analysis of NRM, significant covariates included delirium within 100 days after transplant, fall within 100 days after transplant, KPS, transplantation time period, HCT-CI, graft source, conditioning intensity, recipient CMV status, ADL impairment, PIM use, and creatinine clearance (P < .1; Figure 2A-B; supplemental Table 3). On multivariable analysis, delirium within 100 days (hazard ratio [HR], 1.66; 95% CI, 1.09-2.52; P = .023), fall within 100 days (HR, 2.14; 95% CI, 1.16-3.95; P = .026), and HCT-CI ≥3 (HR, 2.04; 95% CI, 1.37-3.04; P < .001) remained significantly associated with NRM (Table 3). For the univariable analysis of OS, significant covariates included fall within 100 days after transplantation, KPS, HCT-CI, revised DRI, graft source, recipient CMV status, ADL, PIM use, and creatinine clearance (P < .1; Figure 2C-D; supplemental Table 3). On multivariable analysis, fall within 100 days (HR, 1.93; 95% CI, 1.18-3.14; P = .016), high or very high revised DRI (HR, 1.79; 95% CI, 1.35-2.39; P < .001), and positive recipient CMV status (HR, 1.48; 95% CI, 1.13-1.93; P = .004) remained significantly associated with reduced OS (Table 3). Delirium within 100 days was not significantly associated with OS (Table 3).
Figure 2.
Impacts of delirium and fall on transplant outcomes. (A) The significant association of delirium with increased incidence of NRM using a landmark analysis at 100 days. (B) The significant association of fall with increased incidence of NRM using a landmark analysis at 100 days. (C) The nonsignificant association of delirium with reduced OS using a landmark analysis at 100 days. (D) The significant association of fall with reduced OS using a landmark analysis at 100 days.
Table 3.
Multivariable analyses of NRM and OS
| HR | 95% CI | P | |
|---|---|---|---|
| NRM | |||
| Delirium (day 100) | .023 | ||
| No | Reference | ||
| Yes | 1.66 | 1.09-2.52 | |
| Fall (day 100) | .026 | ||
| No | Reference | ||
| Yes | 2.14 | 1.16-3.95 | |
| HCT-CI | <.001 | ||
| 0-2 | Reference | ||
| ≥3 | 2.04 | 1.37-3.04 | |
| KPS | .054 | ||
| ≥90 | Reference | ||
| <90 | 1.45 | 0.99-2.12 | |
| OS | |||
| Fall (day 100) | .016 | ||
| No | Reference | ||
| Yes | 1.93 | 1.18-3.14 | |
| DRI, revised | <.001 | ||
| Low/intermediate | Reference | ||
| High/very high | 1.79 | 1.35-2.39 | |
| Recipient CMV | .004 | ||
| Negative | Reference | ||
| Positive | 1.48 | 1.13-1.93 |
Landmark analyses at day 100.
Etiologies of delirium and fall
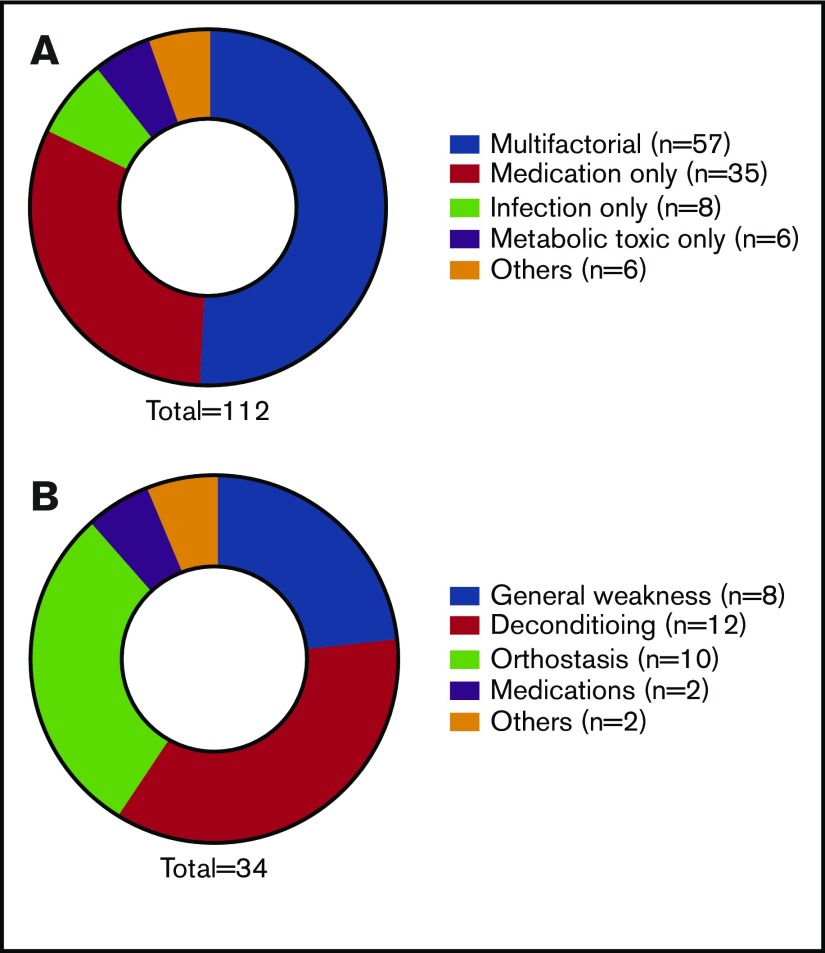
Finally, we examined the possible etiology and treatment of delirium and fall. The cause of delirium seemed to be most commonly multifactorial from a combination of medication, metabolic and toxic encephalopathy, infection, and/or others (Figure 3A). The median duration of delirium was 6 days, with mostly multimodal treatment, including 1:1 observation, frequent reorientation, adjustment of medication, treatment of underlying causes, administration of antipsychotics, or a combination of treatments. Likewise, the cause of fall was multifactorial, including general weakness, deconditioning, orthostasis, medications, and other causes (Figure 3B). The treatment most commonly consisted of intensified physical therapy and rehabilitation.
Figure 3.
Possible etiologies of delirium and fall. (A) Causes of delirium (%) identified by the primary transplantation team. (B) Causes of fall (%) identified by the primary transplantation team.
Discussion
In this study, we examined the baseline incidence of common geriatric syndromes in the peritransplantation period and demonstrated their significant negative impacts on allo-HCT outcomes in a large cohort of older patients. We found that cumulative incidences of transplant-related delirium and fall were 21% and 7%, respectively. The incidence of delirium in our study was lower than that in smaller prospective cohort studies of HCT patients.24-27 However, it is comparable with the reported incidence of postoperative delirium in older adults at our institution and that from a large registry of patients with solid tumors after surgery.22,34 These differences may be explained by diverse patient populations, various study designs, and improvements in detection methods.32 By contrast, the incidence of fall in allo-HCT patients has not been studied extensively, with a single small study reporting a 45% incidence rate.35
We have identified several predictors of transplant-related delirium and fall, including falls within the last year, advanced age, PIM use, impaired functional status, thrombocytopenia, and decreased creatinine clearance. Previous studies in general medical and surgical patients have identified prior cognitive impairment, severity of illness, high-risk medications, visual impairment, and malnutrition as significant risk factors for delirium,32,36 as well as age, female sex, decreased mobility, visual impairment, cognitive impairment, and the use of psychotropic medication as significant risk factors for mechanical fall.37 In the transplantation population, poor pretransplant executive and cognitive function, renal dysfunction, and greater use of opioids after transplant were identified as delirium risk factors, but prior geriatric deficits were not assessed.25,26 We have shown here for the first time that falls within the last year and PIM use before admission, 2 common and highly prevalent geriatric conditions in both the general population and oncology patients,33,38-42 were significantly associated with peritransplant delirium. Importantly, since these 2 geriatric conditions are potentially modifiable through intensive rehabilitation and drug deprescribing practice, we should consider using universal screening and referral guidelines for these patients before allo-HCT. In addition, we have found that thrombocytopenia (platelet count <50 × 109/L) was associated with the development of delirium along with renal dysfunction on admission, although they may not be easily modified. Taken together, our results suggest that strategies for preventing potential delirium and falls based on the patient’s risk factors during allo-HCT may warrant further investigation.32,37,43
Delirium and fall have profound negative impacts on the function, survival, and quality of life for general medical, surgical, and cancer patients,17-22 although their impact on older allo-HCT patients is unknown. We have demonstrated that transplant-related delirium or fall was associated with significantly increased NRM. In fact, HCT-CI, the only other significant risk factor for mortality in our analysis, is a surrogate of geriatric comorbidity.10,28 We acknowledge that the mechanism by which delirium and fall directly contribute to mortality has remained unclear, and that these syndromes likely constitute the geriatric frailty phenotype which could have an impact on survival. Nevertheless, in our large cohort of older patients with heterogeneous hematologic malignancies, disease diagnosis and risks, transplant approaches, and geriatric deficits, major predictors of NRM are geriatric comorbidities and the development of geriatric syndromes of delirium and fall. Interestingly, transplant-related fall, but not delirium, is associated with reduced OS, suggesting differential effects of individual geriatric syndromes on patient outcomes. Our results yield significant insights on geriatric contributing factors to inferior outcomes in older allo-HCT recipients,1,44 namely, pretransplant geriatric comorbidities and the development of geriatric syndromes. There are likely other potential posttransplant contributors to OS and NRM in older patients, including organ toxicities, infections, and graft-versus-host disease, and we are actively examining these factors in relation to comorbidities and geriatric syndromes.
Our study has some limitations. First, given its retrospective design and despite our attempt to systematically review medical charts, the incidence of individual geriatric syndromes may be very difficult to capture and is thus underestimated and less well-characterized than in prospective studies. Second, the heterogeneity in disease diagnosis, transplant approaches, and posttransplant care may introduce biases. Third, in addition to pretransplant risk factors, allo-HCT itself may introduce additional risk factors for delirium and fall such as new medications, frequent infections, and prolonged hospitalization. These factors were not addressed in this study. Fourth, most patients did not receive official geriatrics consultation for these events. We also lack information on pretransplant cognitive impairment or the contribution of other potentially important etiologies such as human herpesvirus 6 to delirium.45 Finally, this is a single-institution study with many patients on the CD34+ cell selection platform; thus, these findings may not be applicable to other settings depending on institutional resources. We expect that the upcoming Blood and Marrow Transplant Clinical Trials Network CHARM prospective study will help address many of these issues.
These limitations notwithstanding, we have established a baseline incidence of common peritransplant geriatric syndromes in a large cohort of older allo-HCT patients. We have identified novel, potentially modifiable risk factors of falls within the last year and use of PIM for delirium. Most importantly, we have demonstrated significantly negative associations of delirium and fall with transplant outcomes. Our results have far-reaching implications in allo-HCT for older patients. First, our results address an underinvestigated area that contributes to mortality in older allo-HCT patients. Second, the identification of potentially modifiable risk factors suggests that all older patients should benefit from pretransplant geriatric assessment and targeted interventions such as a pharmacist-led medication review,46,47 pre-habilitation, and peritransplant delirium and fall prevention strategies.32,37,43,48,49 We summarize potential prevention and intervention strategies in supplemental Table 4. Finally, given the negative consequences of geriatric syndromes, focused geriatric interventions such as intensified neurocognitive, physical, and occupational rehabilitation should be strongly considered for patients who developed these complications peritransplant. The burden and impact of peritransplant geriatric syndromes warrant the institution of patient-centered, preemptive, longitudinal, and multidisciplinary interventions to improve allo-HCT outcomes for older patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health, National Cancer Institute (P01 CA23766) and Cancer Center Support (P30 CA008748) (to Memorial Sloan Kettering Cancer Center). R.J.L. was supported by the New York State Empire Clinical Research Investigator Program and the Elsa U. Pardee Foundation for Cancer Research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented in part at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
Authorship
Contribution: R.J.L., P.D.H., T.A.E., B.K.-G., and S.A.G. designed the research, analyzed the data, and wrote the paper; R.J.L., T.A.E., P.B.D., A.A.J., M.-A.P., C.S.S., H.R.C.-M., J.N.B., B.C.S., R.T., E.B.P., M.A.M., A.S., and S.A.G. collected and interpreted data; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sergio A. Giralt, Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: giralts@mskcc.org; and Richard J. Lin, Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: linr@mskcc.org.
References
- 1.Giralt SA. Hematopoietic cell transplantation for older adults. In: Korc-Grodzicki B, Tew WP, eds. Handbooks of Geriatrics Oncology – Practical Guide to Caring for the Older Cancer Patient New York, NY: Demos Medical Publishing; 2017:241-254. [Google Scholar]
- 2.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER Stat Databases: November 2017 submission. https://seer.cancer.gov/data-software/documentation/seerstat. Accessed 15 July 2018.
- 3.Pulte D, Jansen L, Castro FA, Brenner H. Changes in the survival of older patients with hematologic malignancies in the early 21st century. Cancer. 2016;122(13):2031-2040. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2017. http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx. Accessed 15 July 2018.
- 5.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artz AS. Biologic vs physiologic age in the transplant candidate. Hematology Am Soc Hematol Educ Program. 2016;2016:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn T, McCarthy PL Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31(19):2437-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majhail NS, Chitphakdithai P, Logan B, et al. Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant. 2015;21(1):142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevallier P, Szydlo RM, Blaise D, et al. Reduced-intensity conditioning before allogeneic hematopoietic stem cell transplantation in patients over 60 years: A report from the SFGM-TC. Biol Blood Marrow Transplant. 2012;18(2):289-294. [DOI] [PubMed] [Google Scholar]
- 12.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of allogeneic stem cell transplantation in elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Biol Blood Marrow Transplant. 2016;22(4):651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NN, Ahn KW, Litovich C, et al. Outcomes of Medicare-age eligible NHL patients receiving RIC allogeneic transplantation: a CIBMTR analysis. Blood Adv. 2018;2(8):933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appelbaum FR. Impact of allogeneic hematopoietic cell transplantation on the outcome of older patients with acute myeloid leukemia. Best Pract Res Clin Haematol. 2017;30(4):320-326. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood. 2018;131(5):515-524. [DOI] [PubMed] [Google Scholar]
- 17.Kane RL, Shamliyan T, Talley K, Pacala J. The association between geriatric syndromes and survival. J Am Geriatr Soc. 2012;60(5):896-904. [DOI] [PubMed] [Google Scholar]
- 18.Flood KL, Carroll MB, Le CV, Ball L, Esker DA, Carr DB. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24(15):2298-2303. [DOI] [PubMed] [Google Scholar]
- 19.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006;24(15):2304-2310. [DOI] [PubMed] [Google Scholar]
- 20.Mohile SG, Fan L, Reeve E, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29(11):1458-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naeim A, Reuben D. Geriatric syndromes and assessment in older cancer patients. Oncology (Williston Park). 2001;15(12):1567-1577. [PubMed] [Google Scholar]
- 22.Tan HJ, Saliba D, Kwan L, Moore AA, Litwin MS. Burden of geriatric events among older adults undergoing major cancer surgery. J Clin Oncol. 2016;34(11):1231-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fann JR, Alfano CM, Burington BE, Roth-Roemer S, Katon WJ, Syrjala KL. Clinical presentation of delirium in patients undergoing hematopoietic stem cell transplantation. Cancer. 2005;103(4):810-820. [DOI] [PubMed] [Google Scholar]
- 24.Beglinger LJ, Duff K, Van Der Heiden S, Parrott K, Langbehn D, Gingrich R. Incidence of delirium and associated mortality in hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant. 2006;12(9):928-935. [DOI] [PubMed] [Google Scholar]
- 25.Weckmann MT, Gingrich R, Mills JA, Hook L, Beglinger LJ. Risk factors for delirium in patients undergoing hematopoietic stem cell transplantation. Ann Clin Psychiatry. 2012;24(3):204-214. [PMC free article] [PubMed] [Google Scholar]
- 26.Fann JR, Hubbard RA, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Pre- and post-transplantation risk factors for delirium onset and severity in patients undergoing hematopoietic stem-cell transplantation. J Clin Oncol. 2011;29(7):895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J Clin Oncol. 2007;25(10):1223-1231. [DOI] [PubMed] [Google Scholar]
- 28.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anzaldi LJ, Davison A, Boyd CM, Leff B, Kharrazi H. Comparing clinician descriptions of frailty and geriatric syndromes using electronic health records: a retrospective cohort study. BMC Geriatr. 2017;17(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharrazi H, Anzaldi LJ, Hernandez L, et al. The value of unstructured electronic health record data in geriatric syndrome case identification. J Am Geriatr Soc. 2018;66(8):1499-1507. [DOI] [PubMed] [Google Scholar]
- 32.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: Advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246. [DOI] [PubMed] [Google Scholar]
- 34.Korc-Grodzicki B, Sun SW, Zhou Q, et al. Geriatric assessment as a predictor of delirium and other outcomes in elderly patients with cancer. Ann Surg. 2015;261(16):1085-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueki S, Ikegame K, Kozawa M, Miyamoto J, Mori R, Ogawa H. Risk analysis of falls in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin J Oncol Nurs. 2014;18(4):396-399. [DOI] [PubMed]
- 36.Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297(1):77-86. [DOI] [PubMed] [Google Scholar]
- 38.Lin RJ, Ma H, Guo R, Troxel AB, Diefenbach CS. Potentially inappropriate medication use in elderly non-Hodgkin lymphoma patients is associated with reduced survival and increased toxicities. Br J Haematol. 2018;180(2):267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karuturi MS, Holmes HM, Lei X, et al. Potentially inappropriate medication use in older patients with breast and colorectal cancer. Cancer. 2018;124(14):3000-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(37):993-998. [DOI] [PubMed] [Google Scholar]
- 41.Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerard EJ, Deal AM, Williams GR, Jolly TA, Nyrop KA, Muss HB. Falls in older adults with cancer: Evaluation by oncology providers. J Oncol Pract. 2015;11(6):470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adeola M, Azad R, Kassie GM, et al. Multicomponent interventions reduce high-risk medications for delirium in hospitalized older adults. J Am Geriatr Soc. 2018;66(8):1638-1645. [DOI] [PubMed] [Google Scholar]
- 44.Rosko A, Artz A. Aging: Treating the older patient. Biol Blood Marrow Transplant. 2017;23(2):193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zerr DM, Fann JR, Breiger D, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117(19):5243-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitman A, DeGregory K, Morris A, Mohile S, Ramsdale E. Pharmacist-led medication assessment and deprescribing intervention for older adults with cancer and polypharmacy: a pilot study. Support Care Cancer. 2018;26(12):4105-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nightingale G, Hajjar E, Swartz K, Andrei-Sendecki J, Chapman A. Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol. 2015;33(13):1453-1459. [DOI] [PubMed] [Google Scholar]
- 48.Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dykes PC, Carroll DL, Hurley A, et al. : Fall prevention in acute care hospitals: a randomized trial. JAMA. 2010;304(17):1912-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.