Abstract
Objective
To investigate the effect of upper limb rehabilitation combining robot with low-frequency repetitive transcranial magnetic stimulation (rTMS) on unilateral spatial neglect in stroke patients.
Methods
Patients who had hemispatial neglect after right hemisphere stroke were randomly divided into rTMS only group, robot only group, and combined group. All groups received conventional neglect therapy and additional treatment for each group. rTMS group received rTMS therapy. Robot group received robot therapy, while combined group received both therapies. The effect of therapy was assessed with Motor-Free Visual Perception Test-3 (MVPT-3), line bisection test, star cancellation test, Catherine Bergego Scale (CBS), Mini-Mental State Examination (MMSE), and the Korean version of Modified Barthel Index (K-MBI). These measurements were evaluated before and after treatment.
Results
For each group, 10 patients were recruited. There were no significant differences in baseline characteristics or initial values among the three groups. Two weeks after the therapy, all groups showed significant improvement in MVPT-3, line bisection test, star cancellation test, CBS, MMSE, and K-MBI. However, changes in measurements showed no significant differences among groups.
Conclusion
Treatment effect of the combined therapy of robotic therapy and low-frequency rTMS therapy for hemispatial neglect was not statistically different from that of each single treatment.
Results
of this study did not prove the superiority of any of the three treatments. Further study with large number of patients is needed to evaluate the superiority of these treatments.
Keywords: Stroke, Perceptual disorders, Rehabilitation, Robotics, Transcranial magnetic stimulation
INTRODUCTION
Hemispatial neglect denotes impaired or lost ability to react to or process sensory stimuli (visual, auditory, tactile, and olfactory) presented in the hemispace contralateral to a lesion of the right or left cerebral hemisphere of human [1]. Hemispatial neglect commonly occurs when there are lesions in the right inferior parietal lobe, thalamus, and basal ganglia [2-5]. As the right hemisphere plays a major role in spatial recognition, hemispatial neglect symptoms are known to be more severe and persist longer when the lesion is located in the right hemisphere than that in the left hemisphere [6]. These patients are limited in their ability to carry out activities of daily living such as getting dressed and practicing personal hygiene. In addition, unilateral spatial neglect hampers effects of rehabilitation treatments and slows down functional recovery [7-9]. Therefore, rehabilitation of hemispatial neglect is essential to improve the quality of life (QOL) of stroke patients.
The most widely used and proven treatments for hemispatial neglect are visual scanning training, limb activating treatment, and prism adaptation treatment [10-13]. Optokinetic stimulation, neck muscle vibration, trunk rotation, eye patching, and music therapy have also been used to treat hemispatial neglect [14].
In recent years, robotic therapy has been considered as a potential tool to achieve muscle strength recovery in stroke patients through rehabilitation. Its clinical application has also been increasing [15]. Recent studies have also demonstrated that robot-assisted hemispatial neglect therapy is effective for the recovery of hemispatial neglect [16]. By modifying the existing robot therapy in another study, patient was placed on the right side of the robot monitor [17]. The patient obtained visual scanning effect in addition to the effect of robot therapy [17].
A previous study has reported that the combination of low-frequency repetitive transcranial magnetic stimulation (rTMS) therapy with a conventional therapy is more effective in treating hemispatial neglect than the conventional therapy alone [18]. Recently, it has been reported that rTMS therapy has a synergic effect on the recovery of hemispatial neglect when it is combined with other therapies [19].
We hypothesized that a combination of rTMS therapy and robotic therapy would be effective for hemispatial neglect like other proven rTMS combined therapies due to synergic effects. To test our hypothesis, the objective of this study was to investigate the effect of combination therapy of rTMS treatment with robot therapy on rehabilitation of hemispatial neglect in stroke patients.
MATERIALS AND METHODS
Subjects
This study was conducted on patients with left hemispatial neglect due to right-hemisphere stroke from January 2017 to December 2017. Hemispatial neglect was defined as a mean deviation of 5 mm or more to the right side determined by the line bisection test [20]. Among these patients, those with the following conditions were excluded from this study: (1) patients with a previous history of brain injury, stroke, or other neurological or neuropsychiatric disorder, (2) patients who could not perform robotic therapy or hemispatial neglect test due to cognitive decline, (3) patients whose muscle strength on the left upper limb was below 2, (4) patients with visual field defect, and (5) patients whose sitting balance was severely impaired to the extent that they could not receive robot therapy.
All patients who met the eligibility criteria were randomly assigned consecutively to the rTMS only group, the robot only group, or the combined group using to a computer-generated randomization table according to random permuted blocks of three.
Intervention
All three groups received conventional rehabilitation treatment for hemispatial neglect consisting of visual scanning therapy and passive range of movement of joints by occupational therapists. All three groups received the conventional therapy 30 minutes a day, 5 days a week for 2 weeks (10 times total). Each group received separate treatments in addition to the conventional treatment.
The rTMS group was further treated by rTMS therapy with a coil stimulator shaped like a figure-8 at diameter of 70 mm using MagPro (MagVenture Inc., Farum, Denmark). The coil was held with the handle posterior and oriented sagittally and positioned on the scalp according to the 10-20 system, an internationally recognized method to describe the relation between the location of scalp electrodes and underlying areas of the cerebral cortex. The rTMS stimulation site corresponded with position P5 localized over the left posterior parietal cortex (Fig. 1A). Session included 900 stimuli applied over contralesional posterior parietal cortex at an intensity of 95% motor thresholds and a frequency of 0.9 Hz [21].
Fig. 1.

All groups received additional treatment. (A) The repetitive transcranial magnetic stimulation treatment was applied to the contralesional posterior parietal cortex at an intensity 95% motor thresholds and a frequency of 0.9 Hz. (B) During robot therapy, monitor was settled on the left side of patients to keep their attention to the left side.
Patients in the robot group received additional treatment for hemispatial neglect using a rehabilitation robot (Neuro-X; Apsun Inc., Seoul, Korea) for upper limbs. During robot therapy, patient sat on the right side of the robot with the robot’s monitor on the left side of the patient (Fig. 1B). Robot therapy program was conducted through games that induced passive and active assistive range of motion of the wrist, elbow, and shoulder joints. These games consisted of two isometric exercises and two range of motion exercises. The two isometric exercises used wrist extension and wrist flexion, in which the default muscle strength for wrist extension and wrist flexion in the patient were measured quantitatively before the start of the game so that the game was continued only when a force exceeding a certain level of strength was applied. These isometric exercises consisted of an archery game, in which the patient shot apples randomly coming from the top left and right of the monitor, and a goalkeeper game, in which the patient blocked balls randomly flying from the bottom left and right of the monitor (Fig. 2A, 2B). The range of motion exercises were performed in a passive or active assistive mode using shoulder abduction, shoulder adduction, and elbow extension and flexion. They consisted of a dolphin & circus game and a skateboard game (Fig. 2C, 2D). All game programs included sound effects to help participants focus.
Fig. 2.
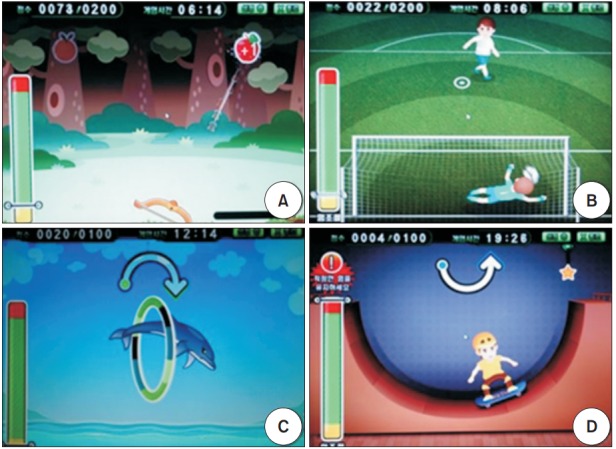
Four game programs on the Neuro-X system (Apsun Inc., Seoul, Korea). (A) Archery game, (B) goalkeeper game, (C) dolphin & circus game, and (D) skateboard game.
Patients in the combined group were treated with both rTMS therapy and robot therapy. To maximize the therapeutic effect in the combined group, the rTMS therapy was performed first and then the robot therapy was performed within 2 hours after the first therapy was completed which was the aftereffect period [22].
Individual therapies were performed in the rTMS group and the robot group for 20 minutes per day, 5 days a week for 2 weeks (10 times total). The combined group received both treatments. Thus, they received treatment for 40 minutes per day, 5 days a week for 2 weeks (10 times total).
Evaluation
Treatment effect was assessed using Motor-Free Visual Perception Test 3 (MVPT-3), line bisection test, star cancellation test, Albert’s test, Catherine Bergego Scale (CBS), Mini-Mental State Examination (MMSE), and the Korean version of the Modified Barthel Index (K-MBI). These indices were measured before and after the 2-week treatment by an occupational therapist who did not directly participate in the treatment process. CBS was created with the assistance of a primary caregiver.
MVPT-3 is a tool for evaluating visual perception of a patient. MVPT-3 is composed of 6 categories and 65 items, including visual discrimination, form consistency, visual short-term memory, visual closure, spatial orientation, and figure ground, with higher scores indicating better starting perception ability. During MVPT-3 evaluation, specific directions and demonstrations were provided to the patient for accurate assessment. The occupational therapist performed the test for 20–30 minutes depending on the patient’s ability. As all patients were 18 years of age or older, the maximum MVPT-3 score was 54. The total score of each patient was used for data analysis [23].
In the line bisection test, test paper was placed at the center of the patient’s midline. The examiner demonstrated how to divide the line into two halves using the uppermost line of 20 lines and then let the patient divide the remaining 19 lines into two halves. Missing lines were not included in the calculation. The deviation between the position of the line that the patient thought was the center of the line and the actual midpoint of the line was divided by half the length of the bisector. The average value of all percentages was used as the result of the line bisection test [24].
The star cancellation test is an index used to evaluate the scan ability of the examinee. It consists of 56 small stars, 52 large stars, words, and letters. The total number of small stars marked by the examinee was evaluated [25].
In Albert’s test, the test paper was placed at the center of the patient. The examiner demonstrated how to mark four lines displayed at the center and then let the patient mark all lines visible on the remaining 36 oblique lines. Of 18 lines displayed on the left side, the number of lines marked by the patient was used as the result of Albert’s test [26].
CBS is the only evaluation tool available that can assess not only hemispatial neglect in personal (body parts or on the body surface), peri-personal (within arm’s reach), and extra-personal (beyond arm’s reach) spaces, but also perceptions, spherical attentiveness, and motor area. In addition, CBS can directly assess decrease in daily life performance and restriction of participation related to hemispatial neglect through 10 items. Each item is classified into four levels from 0 to 3 points according to the severity of hemispatial neglect. Total score ranges from 0 to 30 points, with higher score indicating more severe hemispatial neglect [27].
Patient’s cognitive function was assessed by MMSE and patient’s ability of daily performance was assessed by KMBI. Approval for this study was obtained from the Institutional Review Board of Dong-A University Hospital (No. 18-134). Written consent was received from all patients.
Statistical analysis
SPSS version 21.0 (IBM, Armonk, NY, USA) for Windows was used for all statistical analyses. Comparisons among the rTMS group, the robot group, and the combined group were statistically processed through Kruskal-Wallis test. Evaluation of all indicators, including various indicators to determine the degree of hemispatial neglect, was conducted using Wilcoxon signed-rank test. Statistical significance was considered when p-value was less than 0.05.
RESULTS
During the study period, 86 right hemisphere stroke patients had been admitted to our department. A total of 47 patients were excluded from this study. The remaining 39 patients who had left hemispatial neglect after right hemisphere stroke were recruited. They were then were assigned at random to the rTMS group, the robot group, or the combined group (13 patients in each group). Nine patients dropped out due to early discharge from the hospital or declines of their medical condition. Finally, 10 patients per each group completed all treatment sessions (Fig. 3). Among rTMS group patients, 7 suffered from cerebral infarction and 3 suffered from cerebral hemorrhage. The mean duration after the onset of stroke was 19.2±13.4 days (range, 7–45 days). Among robot group patients, 4 suffered from cerebral infarction and 6 suffered from cerebral hemorrhage. The mean duration after the onset of stroke was 24.5±22.4 days (range, 7–55 days). Among combined group patients, 5 suffered from cerebral infarction and 5 suffered from cerebral hemorrhage. The mean duration after the onset of stroke was 15.3±9.8 daya (range, 6–35 days). No significant differences in baseline characteristics or neglect indices (MVPT, line bisection, star cancellation, Albert’s test, and CBS) were found among the three groups (Table 1). After the 2-week-treatment for hemispatial neglect, statistically significant improvements (p<0.05) were observed for MVPT, line bisection, star cancellation, Albert’s test, CBS, MMSE, and K-MBI scores compared to those at baseline (before treatment) (Table 2). However, no statistically significant differences were found in changes of scores of each index after treatment among the three groups (Table 3).
Fig. 3.
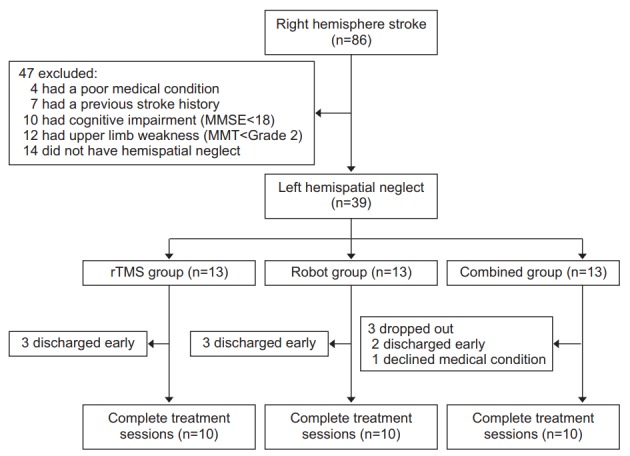
The algorithm used for enrollment of subjects. MMSE, Mini-Mental State Examination; MMT, Manual Muscle Test; rTMS, repetitive transcranial magnetic stimulation.
Table 1.
Baseline characteristics of patients in three groups
| Characteristic | rTMS group (n=10) | Robot group (n=10) | Combined group (n=10) | p-value |
|---|---|---|---|---|
| Age (yr) | 70.3±9.6 | 66.6±12.2 | 62.5±16.5 | 0.606 |
| Sex | ||||
| Male | 5 (50.0) | 5 (50.0) | 5 (50.0) | |
| Female | 5 (50.0) | 5 (50.0) | 5 (50.0) | |
| Lesion | ||||
| Ischemic:Hemorrhagic | 7:3 | 4:6 | 5:5 | |
| Cortical:Subcortical | 4:6 | 4:6 | 7:3 | |
| Duration (day) | 19.2±13.4 | 24.5±22.4 | 15.3±9.8 | 0.632 |
| MVPT (14–65) | 20.9±8.0 | 24.4±6.5 | 24.0±5.5 | 0.458 |
| Line bisection (%) | 31.7±6.9 | 26.7±6.9 | 27.1±4.8 | 0.103 |
| Star cancellation (0–56) | 7.3±6.0 | 8.0±2.5 | 9.7±4.2 | 0.307 |
| Albert’s test (0–18) | 6.0±3.0 | 5.9±2.1 | 7.8±1.9 | 0.174 |
| CBS (0–30) | 22.7±4.3 | 24.6±3.0 | 21.3±3.1 | 0.081 |
| MMSE (0–30) | 20.1±4.8 | 16.8±5.0 | 21.5±3.5 | 0.093 |
| K-MBI (0–100) | 32.3±15.2 | 35.7±18.2 | 42.2±20.7 | 0.547 |
Values are presented as mean±standard deviation.
rTMS, repetitive transcranial magnetic stimulation; MVPT, Motor-Free Visual Perception Test; CBS, Catherine Bergego Scale; MMSE, Mini-Mental State Examination; K-MBI, Korean version of Modified Barthel Index.
Table 2.
Changes of measurements by treatment for hemispatial neglect
| rTMS group (n=10) |
Robot group (n=10) |
Combined group (n=10) |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| MVPT (14–65) | 20.9±8.0 | 35.4±5.6* | 24.4±6.5 | 36.1±5.7* | 24.0±5.5 | 39.1±6.0* |
| Line bisection (%) | 31.7±6.9 | 22.1±5.1* | 26.7±6.9 | 20.4±7.7* | 27.1±4.8 | 18.3±5.7* |
| Star cancellation (0–56) | 7.3±6.0 | 15.2±4.9* | 8.0±2.5 | 14.5±3.5* | 9.7±4.2 | 18.0±3.9* |
| Albert’s test (0–18) | 6.0±3.0 | 12.7±3.6* | 5.9±2.1 | 11.2±2.9* | 7.8±1.9 | 15.0±2.5* |
| CBS (0–30) | 22.7±4.3 | 15.2±3.7* | 24.6±3.0 | 17.5±4.1* | 21.3±3.1 | 12.4±3.1* |
| MMSE (0–30) | 20.1±4.8 | 21.9±3.9* | 16.8±5.0 | 19.7±3.0* | 21.5±3.5 | 24.6±2.6* |
| K-MBI (0–100) | 32.3±15.2 | 47.3±11.1* | 35.7±18.2 | 50.2±14.7* | 42.2±20.7 | 57.6±18.5* |
Values are presented as mean ± standard deviation.
rTMS, repetitive transcranial magnetic stimulation; MVPT, Motor-Free Visual Perception Test; CBS, Catherine Bergego Scale; MMSE, Mini-Mental State Examination; K-MBI, Korean version of Modified Barthel Index.
p<0.05 by Wilcoxon signed-rank test.
Table 3.
Changes of measurements in three groups
| rTMS group (n=10) | Robot group (n=10) | Combined group (n=10) | p-value | |
|---|---|---|---|---|
| ΔMVPT | 13.8±4.6 | 11.7±4.1 | 15.1±3.07 | 0.085 |
| ΔLine bisection | -9.6±2.6 | -6.5±4.1 | -10.8±3.52 | 0.098 |
| ΔStar cancellation | 7.9±4.2 | 6.5±2.2 | 9.3±2.1 | 0.125 |
| ΔAlbert’s test | 6.7±1.8 | 5.4±2.5 | 8.2±1.2 | 0.077 |
| ΔCBS | -7.5±2.3 | -7.1±3.0 | -9.2±1.4 | 0.152 |
| ΔMMSE | 1.8±1.1 | 2.9±2.5 | 3.1±1.5 | 0.455 |
| ΔK-MBI | 15.0±6.7 | 14.5±5.1 | 15.4±5.4 | 0.870 |
Values are presented as mean ± standard deviation.
rTMS, repetitive transcranial magnetic stimulation; MVPT, Motor-Free Visual Perception Test; CBS, Catherine Bergego Scale; MMSE, Mini-Mental State Examination; K-MBI, Korean version of Modified Barthel Index.
DISCUSSION
Hemispatial neglect is a symptom that occurs in patients after a stroke. It is characterized by the inability to detect, respond, or orient to contralesional stimuli. Hemispatial neglect usually appears more often, more severely, and longer in right hemispheric stroke compared to left one. Reported incidence of hemispatial neglect in stroke patients ranges from 13% to 82% [28]. Hemispatial neglect affects patients’ activity of daily living, QOL, ability to participate in rehabilitation, depression, anxiety, and social isolation [29]. It can also increase the risk of falling and other injuries, thus putting a heavy burden on the caregiver [30]. Hemispatial neglect in stroke patients greatly affects the QOL and prognosis of patients. For this reason, various therapeutic approaches are currently being used for its treatment.
Rehabilitation efficacy of visual scanning training, prism therapy, mirror therapy, sensory stimulation, and caloric test for hemispatial neglect has been proven. Consequently, these therapies are currently applied in clinical practice. In addition, new therapies for neglect treatment have been applied through several recent studies. Among these new therapies, treatment using noninvasive brain stimulation and treatment assisted with computer programs and robots have been reported to be effective in rehabilitation of hemispatial neglect [17,18].
Robot therapy has recently attracted attention in the stroke rehabilitation field. Several studies have demonstrated that robot therapy is effective in neglect rehabilitation by helping stroke patients perform repetitive and intensive task-specific movements with appropriate assistance and guidance of the therapist, thereby increasing the activity of the contralesional limb [31]. A study of Varalta et al. [16] in 2014 also reported that information displayed on the monitor during the rehabilitation robot therapy provided visual feedback to patients, thus facilitating goal-directed movements and inducing persistent visual stimulation to the hemispatial neglect site. In a study of Choi et al. [17] in 2017, a robot computer monitor was placed at the hemispatial neglect site to maximize the effect of visual stimulation of the existing robot therapy. This robot therapy was found to be as effective as the conventional neglect rehabilitation therapy.
Several studies have reported that low-frequency rTMS therapy is effective in rehabilitation of hemispatial neglect [18]. According to a study of Yang et al. [32] in 2015, low-frequency rTMS treatment of the contralateral posterior parietal cortex in stroke patients with hemispatial neglect symptoms is more effective in treating neglect than that in the sham group. In their study, structural changes of the brain as well as functional improvement of hemispatial neglect were monitored using diffusion tensor imaging technique. Results of their study revealed that signals from the left superior longitudinal fasciculus, superior occipitofrontal fascicle, inferior frontooccipital fasciculus, and the right side were all increased. On the basis of these findings, the authors reported that low-frequency rTMS therapy could improve symptoms of hemispatial neglect by restoring the network of intrahemispheric and inter-hemispheric connectivity [32].
In recent years, a combination of this low-frequency rTMS with various other therapies has attracted attention. This kind of combination therapy has been reported to be an ideal rehabilitation therapy for stroke patients because it suppresses inter-hemisphere inhibition of the contra-lesion through low-frequency rTMS and increases ipsilesional excitability through other therapeutic tools at the same time [33]. In 2018, Johnson et al. [34] reported that the application of a combined contralesional lowfrequency rTMS therapy and computer-robot-based visual reality therapy was more effective for rehabilitation of hemispatial neglect than each single therapy alone. In their study, functional MRI data showed that the ipsilesional activation was actually higher in the combined group than that in each single treatment group [34]. In a study of Yang et al. [19] in 2017, combined treatment of sensory cueing by applying a vibration wristwatch to the contralesional limb and low-frequency rTMS therapy showed a synergic effect in comparison with the conventional rehabilitation therapy, thus achieving a better therapeutic effect on hemispatial neglect. Taking findings of these studies together, when low-frequency rTMS therapy suppressing the contralesional inter-hemisphere inhibition was applied in combination with visual, vibration, and tactile stimulation to the hemispatial neglect site or with robot-assisted activation of the contralesional limb that led to an increase in excitability of the ipsilesion, the combination therapy was more effective than each individual treatment.
Similar to the abovementioned combination therapy, both visual sensory stimulation to the hemispatial neglect site and activation of the contralesional limb were applied simultaneously in the present study by using low-frequency rTMS and robot therapy in combination. Results of the present study showed that this combination therapy was effective in treating hemispatial neglect when the treatment effect was compared between before and after treatment. However, when treatment effects were compared among the three groups, no significant difference was found among the three groups. Although no statistically significant difference was observed in the treatment effect among the three groups, changes in treatment scores were greater in the combined treatment group than those in the other two single treatment groups. Therefore, significant differences might appear if the study is conducted with a larger sample size for a longer treatment period.
The present study is different from other low-frequency rTMS combined therapies for hemispatial neglect reported previously. This study aimed to maximize the effect of hemispatial neglect therapy by making the most of results from previous studies focusing on therapeutic effect. Modeling of this study was designed as such. First, in addition to increased ipsilesional excitability of the robotic contralesional limb activation therapy which has been demonstrated in previous studies, the effect of increasing the visual stimulation of the hemispatial neglect site by moving the position of the computer monitor to the hemispatial neglect side was further applied. Thus, a higher ipsilesional excitability than the elevated ipsilesional excitability of the brain obtained through a single existing tool was induced. Second, the present study took advantage of the finding of a recent study which indicated that the second therapy of the combination therapy should be performed within 2 hours following the rTMS therapy to enhance the aftereffect and ultimately the efficacy of rTMS combined therapy by performing the robot therapy within 2 hours after the low-frequency rTMS therapy was completed [22]. Thus, the present study was conducted in a condition in which the increase in ipsilesional excitability of the brain and the suppression of the contralesional inter-hemisphere inhibition were maximized in comparison with other rTMS combined therapies targeting hemispatial neglect, through which the maximum treatment effect of the hemispatial neglect therapy was attempted to be achieved.
To the best of our knowledge, this study is the first attempt to modify previously proven treatment among other combined therapies for hemispatial neglect to maximize the therapeutic effect and perform combined treatment according to the aftereffect period to improve the effect of rTMS. A higher efficiency in the treatment of hemispatial neglect can be achieved by applying combination therapies consisting of several therapeutic tools and by modifying timing of the treatment in the same way as in this study.
This study has some limitations. First, the sample size was too small and the long-term effect of treatment on hemispatial neglect could not be assessed. Second, no true control group receiving no conventional treatment for hemispatial neglect was established because of ethical reasons. Thus, the improvement of hemispatial neglect could be part of the natural progression. Third, except for CBS, all other assessment tools for hemispatial neglect were limited to evaluation of hemispatial neglect in the peri-personal space, thus failing to fully exclude selection bias.
Therefore, if specific and long-term studies on each hemispatial neglect subtype are conducted in the future with the addition of larger-scale evaluation indices capable of equal assessments of all hemispatial neglect subtypes, a statistical difference in therapeutic effect of the combination therapy might appear.
In conclusion, this study showed that treatment effect of the combined therapy of robotic therapy and lowfrequency rTMS therapy for hemispatial neglect was not statistically different from that of each single treatment. In this study, it was impossible to prove the superiority of any of the three treatments. Further well-designed studies with a larger population are required to better elucidate differential roles of each treatment.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Luaute J, Halligan P, Rode G, Rossetti Y, Boisson D. Visuo-spatial neglect: a systematic review of current interventions and their effectiveness. Neurosci Biobehav Rev. 2006;30:961–82. doi: 10.1016/j.neubiorev.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Pierce SR, Buxbaum LJ. Treatments of unilateral neglect: a review. Arch Phys Med Rehabil. 2002;83:256–68. doi: 10.1053/apmr.2002.27333. [DOI] [PubMed] [Google Scholar]
- 3.Heilman KM, Valenstein E. Frontal lobe neglect in man. Neurology. 1972;22:660–4. doi: 10.1212/wnl.22.6.660. [DOI] [PubMed] [Google Scholar]
- 4.Watson RT, Heilman KM. Thalamic neglect. Neurology. 1979;29:690–4. doi: 10.1212/wnl.29.5.690. [DOI] [PubMed] [Google Scholar]
- 5.Damasio AR, Damasio H, Chui HC. Neglect following damage to the frontal lobe or basal ganglia. Neuropsychologia. 1980;18:123–32. doi: 10.1016/0028-3932(80)90058-5. [DOI] [PubMed] [Google Scholar]
- 6.Kerkhoff G. Spatial hemineglect in humans. Prog Neurobiol. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 7.Paolucci S, Antonucci G, Guariglia C, Magnotti L, Pizzamiglio L, Zoccolotti P. Facilitatory effect of neglect rehabilitation on the recovery of left hemiplegic stroke patients: a cross-over study. J Neurol. 1996;243:308–14. doi: 10.1007/BF00868403. [DOI] [PubMed] [Google Scholar]
- 8.Paolucci S, Traballesi M, Gialloreti LE, Pratesi L, Lubich S, Antonucci G, et al. Changes in functional outcome in inpatient stroke rehabilitation resulting from new health policy regulations in Italy. Eur J Neurol. 1998;5:17–22. doi: 10.1046/j.1468-1331.1998.510017.x. [DOI] [PubMed] [Google Scholar]
- 9.Rode G, Tiliket C, Boisson D. Predominance of postural imbalance in left hemiparetic patients. Scand J Rehabil Med. 1997;29:11–6. [PubMed] [Google Scholar]
- 10.Weinberg J, Diller L, Gordon WA, Gerstman LJ, Lieberman A, Lakin P, et al. Visual scanning training effect on reading-related tasks in acquired right brain damage. Arch Phys Med Rehabil. 1977;58:479–86. [PubMed] [Google Scholar]
- 11.Antonucci G, Guariglia C, Judica A, Magnotti L, Paolucci S, Pizzamiglio L, et al. Effectiveness of neglect rehabilitation in a randomized group study. J Clin Exp Neuropsychol. 1995;17:383–9. doi: 10.1080/01688639508405131. [DOI] [PubMed] [Google Scholar]
- 12.Robertson IH, North N. Spatio-motor cueing in unilateral left neglect: the role of hemispace, hand and motor activation. Neuropsychologia. 1992;30:553–63. doi: 10.1016/0028-3932(92)90058-t. [DOI] [PubMed] [Google Scholar]
- 13.Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395:166–9. doi: 10.1038/25988. [DOI] [PubMed] [Google Scholar]
- 14.Frassinetti F, Angeli V, Meneghello F, Avanzi S, Ladavas E. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain. 2002;125(Pt 3):608–23. doi: 10.1093/brain/awf056. [DOI] [PubMed] [Google Scholar]
- 15.Lo AC. Clinical designs of recent robot rehabilitation trials. Am J Phys Med Rehabil. 2012;91(11 Suppl 3):S204–16. doi: 10.1097/PHM.0b013e31826bcfa3. [DOI] [PubMed] [Google Scholar]
- 16.Varalta V, Picelli A, Fonte C, Montemezzi G, La Marchina E, Smania N. Effects of contralesional robot-assisted hand training in patients with unilateral spatial neglect following stroke: a case series study. J Neuroeng Rehabil. 2014;11:160. doi: 10.1186/1743-0003-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YS, Lee KW, Lee JH, Kim SB, Park GT, Lee SJ. The effect of an upper limb rehabilitation robot on hemispatial neglect in stroke patients. Ann Rehabil Med. 2016;40:611–9. doi: 10.5535/arm.2016.40.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BR, Chun MH, Kim DY, Lee SJ. Effect of high- and low-frequency repetitive transcranial magnetic stimulation on visuospatial neglect in patients with acute stroke: a double-blind, sham-controlled trial. Arch Phys Med Rehabil. 2013;94:803–7. doi: 10.1016/j.apmr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Yang NY, Fong KN, Li-Tsang CW, Zhou D. Effects of repetitive transcranial magnetic stimulation combined with sensory cueing on unilateral neglect in subacute patients with right hemispheric stroke: a randomized controlled study. Clin Rehabil. 2017;31:1154–63. doi: 10.1177/0269215516679712. [DOI] [PubMed] [Google Scholar]
- 20.Kerkhoff G, Bucher L, Brasse M, Leonhart E, Holzgraefe M, Volzke V, et al. Smooth pursuit “bedside” training reduces disability and unawareness during the activities of daily living in neglect: a randomized controlled trial. Neurorehabil Neural Repair. 2014;28:554–63. doi: 10.1177/1545968313517757. [DOI] [PubMed] [Google Scholar]
- 21.Shindo K, Sugiyama K, Huabao L, Nishijima K, Kondo T, Izumi S. Long-term effect of low-frequency repetitive transcranial magnetic stimulation over the unaffected posterior parietal cortex in patients with unilateral spatial neglect. J Rehabil Med. 2006;38:65–7. doi: 10.1080/16501970500441807. [DOI] [PubMed] [Google Scholar]
- 22.Park JW, Kim SB, Lee KW, Lee JH, Park JG, Lee SJ. Effects of hand training during the aftereffect period of low-frequency rTMS in subacute stroke patients. Ann Rehabil Med. 2018;42:521–7. doi: 10.5535/arm.2018.42.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercier L, Desrosiers J, Hebert R, Rochette A, Dubois MF. Normative data for the motor-free visual perception test-vertical. Phys Occup Ther Geriatr. 2001;19:39–50. [Google Scholar]
- 24.Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–17. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- 25.Agrell BM, Dehlin OI, Dahlgren CJ. Neglect in elderly stroke patients: a comparison of five tests. Psychiatry Clin Neurosci. 1997;51:295–300. doi: 10.1111/j.1440-1819.1997.tb03201.x. [DOI] [PubMed] [Google Scholar]
- 26.Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–64. doi: 10.1212/wnl.23.6.658. [DOI] [PubMed] [Google Scholar]
- 27.Chen P, Hreha K, Fortis P, Goedert KM, Barrett AM. Functional assessment of spatial neglect: a review of the Catherine Bergego scale and an introduction of the Kessler foundation neglect assessment process. Top Stroke Rehabil. 2012;19:423–35. doi: 10.1310/tsr1905-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar APS, Vaz PG, Marchese RR, Stein C, Pinto C, Pagnussat AS. noninvasive brain stimulation improves hemispatial neglect after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99:355–366. doi: 10.1016/j.apmr.2017.07.009. e1. [DOI] [PubMed] [Google Scholar]
- 29.Dai CY, Liu WM, Chen SW, Yang CA, Tung YC, Chou LW, et al. Anosognosia, neglect and quality of life of right hemisphere stroke survivors. Eur J Neurol. 2014;21:797–801. doi: 10.1111/ene.12413. [DOI] [PubMed] [Google Scholar]
- 30.Semrau JA, Wang JC, Herter TM, Scott SH, Dukelow SP. Relationship between visuospatial neglect and kinesthetic deficits after stroke. Neurorehabil Neural Repair. 2015;29:318–28. doi: 10.1177/1545968314545173. [DOI] [PubMed] [Google Scholar]
- 31.Masiero S, Carraro E, Ferraro C, Gallina P, Rossi A, Rosati G. Upper limb rehabilitation robotics after stroke: a perspective from the University of Padua, Italy. J Rehabil Med. 2009;41:981–5. doi: 10.2340/16501977-0404. [DOI] [PubMed] [Google Scholar]
- 32.Yang W, Liu TT, Song XB, Zhang Y, Li ZH, Cui ZH, et al. Comparison of different stimulation parameters of repetitive transcranial magnetic stimulation for unilateral spatial neglect in stroke patients. J Neurol Sci. 2015;359:219–25. doi: 10.1016/j.jns.2015.08.1541. [DOI] [PubMed] [Google Scholar]
- 33.Zhang RG, Liu SX, Wang FY, Ma XC, Yang YH. Treatment of unilateral neglect using repetitive transcranial magnetic stimulation (rTMS) and sensory cueing (SC) in stroke patients. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:309–13. [PubMed] [Google Scholar]
- 34.Johnson NN, Carey J, Edelman BJ, Doud A, Grande A, Lakshminarayan K, et al. Combined rTMS and virtual reality brain-computer interface training for motor recovery after stroke. J Neural Eng. 2018;15:016009. doi: 10.1088/1741-2552/aa8ce3. [DOI] [PMC free article] [PubMed] [Google Scholar]


