Abstract
Background
Zika virus (ZIKV) infection has been associated with prolonged viral excretion in human semen and causes testicular atrophy and infertility in 10-week-old immunodeficient mice.
Methods
Male IFNAR−/− mice, knockout for type I interferon receptor, were immunized with GLS-5700, a deoxyribonucleic acid-based vaccine, before a subcutaneous ZIKV challenge with 6 × 105 plaque-forming units at 13 weeks of age. On day 28 postinfection, testes and epididymides were collected in some mice for histological and functional analyses, whereas others were mated with naive female wild-type C57BL/6J.
Results
Although all mice challenged with ZIKV developed viremia, most of them were asymptomatic, showed no weight loss, and survived infection. On day 28 postinfection, none of the unvaccinated, infected mice (9 of 9) exhibited abnormal spermatozoa counts or motility. However, 33% (3 of 9) and 36% (4 of 11) of mated males from this group were infertile, from 2 independent studies. Contrarily, males from the noninfected and the vaccinated, infected groups were all fertile. On days 75 and 207 postinfection, partial recovery of fertility was observed in 66% (2 of 3) of the previously infertile males.
Conclusions
This study reports the effects of ZIKV infection on male fertility in a sublethal, immunodeficient mouse model and the efficacy of GLS-5700 vaccination in preventing male infertility.
Keywords: animal model, DNA vaccine, infertility, sublethal, Zika virus
Male IFNAR−/− mice infected with ZIKV at an older age were shown to be infertile without overt signs of disease. This outcome was prevented by immunization with a DNA vaccine. A partial recovery of fertility was observed later after infection.
Zika virus (ZIKV) was first identified in 1947 from a sentinel monkey in Uganda [1]. After an extended epidemiological lull, ZIKV was reported in Pacific Islands and then confirmed to be actively circulating in Brazil in 2015 before spreading throughout the Americas [2]. After the reports of neurological disorders such as Guillain-Barré syndrome and microcephaly that were temporally associated to ZIKV infection [3], the World Health Organization (WHO) stated on February 1, 2016, that the epidemic was a Public Health Emergency of International Concern [4], a status subsequently lifted on November 18, 2016. These events led to a huge research effort being conducted on this re-emerging pathogen. Although vector-based transmission is predominant, it was rapidly reported that ZIKV transmission in humans could occur via sexual contact and that virus excretion in semen could last for weeks [5–7]. Sexual transmission was reported in male-to-female and male-to-male relationships and also suspected in a female-to-male case [3, 7–16]. These clinical observations were further studied in animal models, and ZIKV-induced histological damage was found in the genital tract of infected mice of both sexes [17–21]. It was also established that exposure of both immunocompetent and immunodeficient mice to ZIKV through the vaginal route led to virus replication in the genital mucosa and to fetal transmission [22]. Further studies in male mice revealed that the genital damage resulting from ZIKV challenge correlated with reduced fertility [23, 24]. Finally, it was shown that vasectomized immunodeficient mice shed reduced amounts of infectious ZIKV in semen [25].
In an effort to reduce ZIKV propagation and associated disease burden, several experimental vaccines have been developed (approximately 40 as of March 2018), with 3 deoxyribonucleic acid (DNA) vaccines, 3 inactivated ZIKV vaccines, 1 messenger ribonucleic acid (RNA) vaccine, 1 peptide vaccine, and 1 recombinant viral vector vaccine having entered into Phase I or II clinical trials [26, 27]. GLS-5700, a DNA-based vaccine that encodes the premembrane and envelope antigenic regions of ZIKV, is currently being assessed in 2 Phase I clinical studies (Clinical Trials Gov NCT02809443 and NCT02887482). GLS-5700 was shown to confer complete protection against death and infection-associated weight loss, as well as virus-induced pathology in the brain and testes of IFNAR−/− mice [19, 28].
In the current study, we sought to investigate ZIKV-induced fertility loss in male IFNAR−/− mice that developed viremia without clinical signs of disease after infection at 13 weeks of age. GLS-5700-vaccinated and infected mice were compared with uninfected and ZIKV-infected counterparts. This study demonstrates the loss and partial recovery of fertility in a sublethal model of male IFNAR−/− mice, as well as its prevention by vaccination with GLS-5700.
METHODS
Ethics Statement
Animal experiments were performed at the animal facility of the Research Center of the CHU de Québec - Université Laval. All animals were used in accordance with the Canadian Council on Animal Care guidelines, and all protocols were approved by the Animal Care Ethics Committee of the Université Laval under protocols nos. 2016011 and 2017054.
Animal Experiments
Male B6.129S2-Ifnar1tm1Agt/Mmjax (referred to as IFNAR−/−), deficient for type I interferon receptor, were purchased from The Jackson Laboratories (Mutant Mouse Resource and Research Center) at 8 weeks of age. Female wild-type C57BL/6J were purchased from The Jackson Laboratories and were used for mating with males. Male IFNAR−/− mice were given intramuscular (IM) injections at 9 (day 28 before infection) and 11 (day 14 before infection) weeks of age with 50 µg (in 30 µL of injectable sterile water) of GLS-5700, an optimized synthetic DNA-based vaccine (GeneOne Life Science), or with 30 µL sterile 0.9% saline for nonvaccinated animals. After IM injection, the CELLECTRA adaptive constant current electroporation device (Inovio Pharmaceuticals, Plymouth Meeting, PA) was used at the site of injection to enhance efficacy of DNA vaccination, as described previously [28]. Mice were infected subcutaneously, 2 weeks after the last immunization, with 150 µL ZIKV (strain ZIKV/Homo sapiens/PRI/PRVABC59/2015, GenBank: KX087101.2) in each leg (total dose of 6 × 105 plaque-forming units [PFU]). The mock group was composed of 12 nonvaccinated, noninfected mice (labeled 1-1 to 1-12), the ZIKV group consisted of 18 nonvaccinated and infected mice (labeled 2-1 to 2-18), and the GLS-5700 group consisted of 12 vaccinated and infected mice (labeled 3-1 to 3-12). Blood samples were collected from male IFNAR−/− mice on days 2, 4, 6, 8, 10, 14, 21, 28, and 91 postinfection. Because the early timepoints were close, mice were divided into 2 groups of equal number, which were bled alternately every 2 days. Half of the IFNAR−/− mice (mock, 1-7 to 1-12; ZIKV, 2-10 to 2-18; GLS-5700, 3-7 to 3-12) were sacrificed on day 28 postinfection to collect epididymides and testes, whereas the remaining animals were used for mating studies. Mating studies were validated in a second experiment consisting of 12 male IFNAR−/− mice (labeled R-1 to R-12) infected with 6 × 105 PFU of a new virus stock at 13 weeks of age.
Determination of Viral Ribonucleic Acid Load by Reverse-Transcriptase Droplet Digital Polymerase Chain Reaction
For serum, RNA was extracted from 70 µL of sample with the MagNA Pure LC total nucleic acid isolation kit (Roche Molecular System, Laval, Quebec, Canada) and eluted in 100 µL. Some samples were adjusted to 70 µL with sterile H2O, and the dilution factor was taken into account. The right testis was weighed and homogenized using the OMNI International TH homogenizer (OMNI International, Ottawa, Ontario, Canada) in a 15-mL Falcon tube containing 1 mL phosphate-buffered saline (PBS) with phosphatase (PhosSTOP, Roche) and protease (cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail; Roche) inhibitors. Homogenized samples were centrifuged for 10 minutes at 1455 ×g at 4°C. The RNA extraction was performed on 200 µL supernatant with the same isolation kit and protocol, but elution was done in 50 µL elution buffer. The digital droplet PCR (ddPCR) workflow and data analyses were performed with the One-Step RT-ddPCR Advanced Supermix per the manufacturer’s instructions. In brief, 20 µL reaction mix were used for producing droplets with the QX200 droplet generator (Bio-Rad, Mississauga, Ontario, Canada). Primers and probes (ie, ZIKV 1086 [1086–1102], 5’-CCGCTGCCCAACACAAG-3’; ZIKV 1162c [1162–1139], 5’-CCACTAACGTTCTTTTGCAGACAT-3’; ZIKV 1107-FAM [1107–1137], 5’-6 FAM/AGCCTAC CTTGACAAGCAGTCAGACACTCAA/ZEN-3’IBFQ-3’) targeting the membrane and envelope genes of ZIKV were previously described [29]. The ddPCR allows for absolute quantification of nucleic acids without the need for a standard curve. Droplet-partitioned samples were then transferred to a 96-well plate, sealed, and cycled in a C1000 deep-well thermocycler (Bio-Rad) under the following cycling protocol: 50°C for 60 minutes (Reverse transcription) and 95°C for 10 minutes (DNA polymerase activation), followed by 45 cycles of 95°C for 30 seconds and 56°C for 1 minute, followed by postcycling steps of 98°C for 10 minutes (enzyme inactivation) and an infinite 10°C hold. The cycled plate was then transferred and read in the FAM channel using the QX200 droplet reader (Bio-Rad), and data analysis was performed using the QuantaSoft software (version 1.7.4; Bio-Rad).
Evaluation of Sperm Counts and Motility
Mature sperm was collected from the right caudal epididymis obtained from IFNAR−/− mice. Each epididymis was placed into 1 well of a 24-well plate containing 1 mL sterile PBS and dissected using 23-gauge needles. Sperm was allowed to swim out for 30 minutes at 37°C. Counts and motility were evaluated as described in the fifth edition of the ‘‘WHO Laboratory Manual for the Examination and Processing of Human Semen’’ [30]. Motility was evaluated after a wet preparation of 20.1 µm in depth as per the protocol established by WHO [30].
Histology and Immunohistochemistry Techniques and Evaluation
The left testis and epididymis were collected and kept in formalin. Four-µm-thick histologic sections were obtained from formalin-fixed, paraffin-embedded testicular and epididymal tissues and stained with hematoxylin-eosin for conventional histology. For immunohistochemistry (IHC), the paraffin sections were quenched for 10 minutes in aqueous 3% H2O2 and rinsed in MilliQ water. Epitopes were retrieved using Dako Target Retrieval Solution pH high (Dako, Glostrup, Danemark) in a Pre-Treatment Link module (Dako). The staining was carried out using a Dako autostainer Link 48. Tissues were blocked with Flex peroxidase block (Dako). The primary antibody was an α-Flavivirus (D1-4G2-4-15) rabbit monoclonal antibody (Absolute Antibody Ltd, Oxford, UK) used at a dilution of 1:400 at room temperature for 20 minutes. Tissues were then visualized using a horseradish peroxidase-labeled polymer, FLEX (Dako), for 20 minutes and reacted with the chromogen diaminobenzidine (DAB). The sections were then counterstained with Mayer’s hematoxylin (Dako). Scoring of histology and IHC was performed by a pathologist (C.C.), blind to experimental groups. Testicular histology was assessed for spermatogenesis and evaluated as complete or absent/atrophic, whereas epididymal histology was assessed for the presence of spermatozoids or azoospermia. For IHC, a semiqualitative scale was used (0, no staining; 1+, weak staining; 2+, moderate staining; 3+, intense staining) in epididymides, seminiferous tubules, and Leydig cells.
Mating Studies
Mating studies were performed in 2 independent experiments. In the first experiment, female C57BL/6J mice at 16 weeks of age were placed in contact with the IFNAR−/− males (mock, 1-1 to 1-6; ZIKV, 2-1 to 2-9; GLS-5700, 3-1 to 3-6) at a ratio of 2 females per male on days 28 and 75 postinfection (males were 17 weeks and 23 weeks old, respectively), whereas 9-week-old female C57BL/6J mice were placed in contact with IFNAR−/− males on day 207 postinfection (males were 42 weeks old). In the second experiment, mating of ZIKV-infected males (R-1 to R-12) with 9-week-old C57BL/6J female mice was performed on days 14 and 28 postinfection (males were 15 weeks and 17 weeks old). Animals were kept together for 2 weeks, and then females were removed to follow up on gestation for another 3 weeks, starting on the day of separation from the males. Females were sacrificed before giving birth when possible to assess the number of fetuses. Male infertility was defined as a lack of pregnancies of both females in each cage, whereas a male was considered fertile when at least 1 female became pregnant in each cage. Fertility of all males was confirmed either on day 14 postinfection of the first experiment or on day 14 before infection of the second experiment.
Data Analysis
Generated results were analyzed using Prism 7 (version 7.02; GraphPad Software Inc.). Statistical analyses were performed using one-way analysis of variance with Tukey’s multiple comparison posttests. A P value ≤0.05 was considered as statistically significant. Grubb’s test to identify outliers in the data recorded for size and weight of testes was performed using the QuickCalcs on www.graphpad.com (accessed March 8, 2018), by selecting a level of significance alpha = 0.01.
RESULTS
Induction of a Sublethal Zika Virus Infection in IFNAR−/− Mice
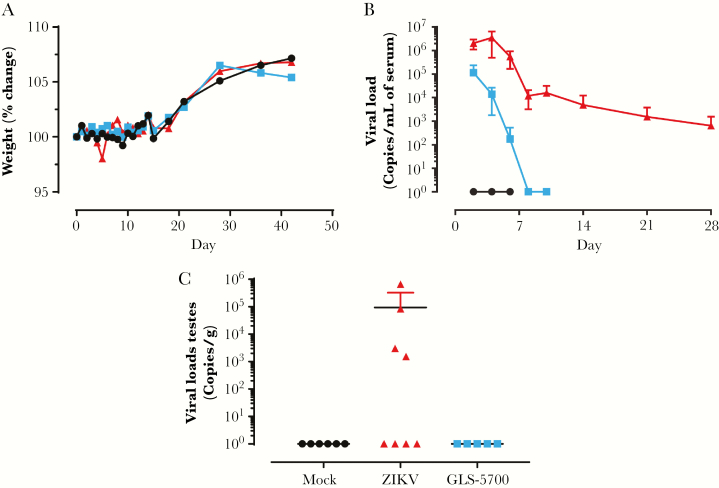
To establish a sublethal animal model, male IFNAR−/− mice were infected with 6 × 105 PFU of ZIKV at 13 weeks of age. The other 2 groups consisted of uninfected animals as well as mice vaccinated with GLS-5700 2 weeks before ZIKV challenge using a prime-boost regimen with a 2-week interval between injections (days 28 and 14 before infection). In the first experiment, all challenged and control male IFNAR−/− mice had no significant weight loss, nor clinical signs of the disease, and all animals survived to infection (Figure 1a). In the second study, 17% of ZIKV-infected mice (2 of 12) exhibited a transient weight loss by day 8 postinfection, and 1 animal was found dead on the same day (Supplementary Figure S1). In the first experiment, viral load was detected in both infected groups on day 2 postinfection, with a maximal value at 1.1 × 105 genome copies/mL serum in vaccinated mice (Figure 1b). It is interesting to note that the viral RNA load in serum was controlled in all vaccinated animals and was below the detection limit of the assay by day 8 postinfection. The nonvaccinated group exhibited an increasing viral load until day 4 postinfection, peaking at 3.5 × 106 genome copies/mL serum, and maintained a low viral RNA load up to day 28. On day 91 postinfection, the viral RNA load in serum had reached undetectable levels in the ZIKV group (data not shown). The time evolution of viral RNA load in serum of ZIKV-infected mice was comparable in both independent experiments (Figure S1). Viral loads were also measured in the testes of animals on day 28 postinfection in the first experiment. Viral RNA load, ranging from 1.5 × 103 to 6.6 × 105 genome copies/g, was detected in the testes of 44% (4 of 9; 2-13, 2-15, 2-17, and 2-18) of the ZIKV mice, whereas none of the GLS-5700 counterparts had detectable RNA levels (Figure 1c, Table 1).
Figure 1.
Clinical parameters of male IFNAR−/− mice. (a) Percentage of weight change, (b) viral load in serum, and (c) viral load in the testes on day 28 postinfection of mock ( ), Zika virus ([ZIKV]
), Zika virus ([ZIKV]  ), and GLS-5700 (
), and GLS-5700 ( ) groups of male IFNAR−/− mice.
) groups of male IFNAR−/− mice.
Table 1.
Histological and Immunohistochemical Findings and Viral Load in Testis of Male IFNAR−/− Micea
| Group | Male ID | Spermatogenesis | Spermatozoa in Epididymides | Viral Load, Testes (Genome Copies/g) | IHC, Epididymides | IHC, Seminiferous Tubules | IHC, Leydig Cells |
|---|---|---|---|---|---|---|---|
| Mock | 1–7 | Complete | + | - | - | - | 0 |
| 1–8 | Complete | + | - | - | - | 0 | |
| 1–9 | Complete | + | - | - | - | 0 | |
| 1–10 | Complete | + | - | - | - | 0 | |
| 1–11 | Complete | + | - | - | - | 0 | |
| 1–12 | Complete | + | - | - | - | 0 | |
| ZIKV | 2–10 | Complete | + | - | - | - | 2+ |
| 2–11 | Absent (atrophied testis) | - | - | - | 3+ | 2+ | |
| 2–12 | Complete | + | - | - | - | 2+ | |
| 2–13 | Complete | + | 6.63E+05 | - | - | 3+ | |
| 2–14 | Complete | + | - | - | - | 2+ | |
| 2–15 | Complete | + | 3.01E+03 | - | - | 3+ | |
| 2–16 | Complete | + | - | - | - | 3+ | |
| 2–17 | Complete | + | 1.54E+03 | - | - | 3+ | |
| 2–18 | Complete | + | 8.44E+04 | - | - | 2+ | |
| GLS-5700 | 3–7 | Complete | + | - | - | - | 2+ |
| 3–8 | Complete | + | - | - | - | 3+ | |
| 3–9 | Complete | + | - | - | - | 2+ | |
| 3–10 | Complete | + | - | - | - | 3+ | |
| 3–11 | Complete | + | - | - | - | 1+ | |
| 3–12 | Complete | + | - | - | - | 1+ |
Abbreviations: ID, identification; IHC, immunohistochemistry; ZIKV, Zika virus.
aFor IHC, a semiqualitative scale was used (0 = no staining; 1+ = weak staining; 2+ = moderate staining; 3+ = intense staining) in epididymis, seminiferous tubules, and Leydig cells. ‘‘+’’ = Presence; ‘‘-’’ = Absence.
Sublethal Challenge With Zika Virus Leads to Limited Gross Testicular Damage
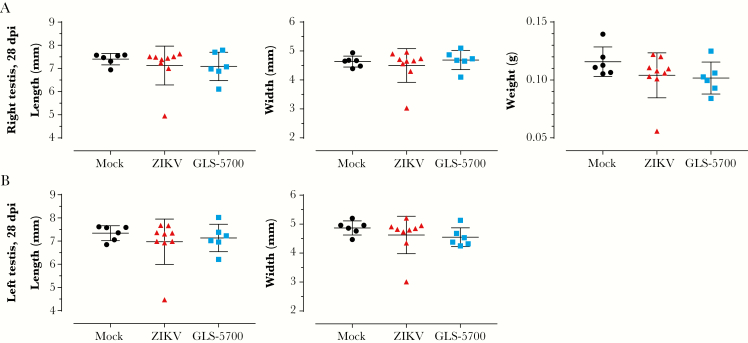
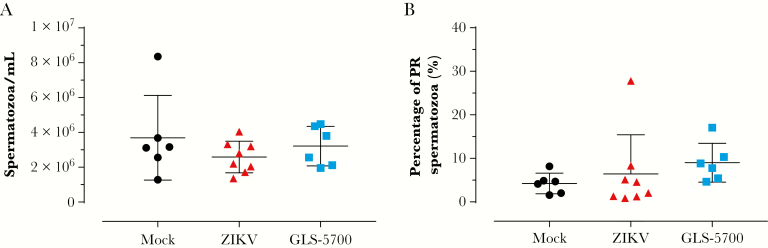
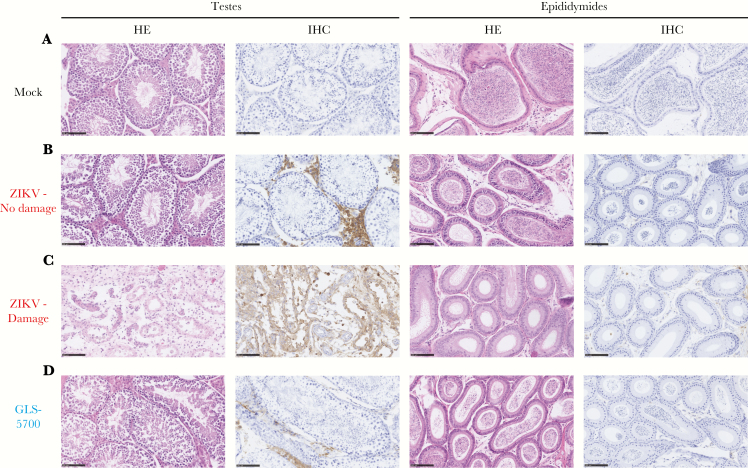
In the first experiment, half of the animals in each group were sacrificed for collection of the testes and epididymides on day 28 postinfection. Macroscopic evaluation of the testes did not reveal any statistically significant differences in the mean values for length, width, and weight for both vaccinated and nonvaccinated animals, when compared with the noninfected group (Figure 2), except the left testis of mouse 2-11 and the right testis of mouse 2-13, which belong to the ZIKV group, that were significantly smaller in length and width, and lighter than their counterparts (Supplementary Table S1). Spermatogenesis was complete, and the epididymides contained spermatozoa in all mock (6 of 6) and GLS-5700 animals (6 of 6). Regarding the ZIKV group, 89% (8 of 9) had completed spermatogenesis, whereas 1 animal (2-11) exhibited azoospermia (Table 1). No statistically significant differences were observed between the sperm counts and progressively motile spermatozoa of the 3 groups on day 28 postinfection (Figure 3). Likewise, histological analyses of the testes and epididymides showed no apparent microscopic differences between the 3 groups, including for Leydig and Sertoli cells (data not shown). An absence of inflammatory cells was also noted, except in the testis of mouse 2-11. It is interesting to note that IHC analyses confirmed the presence of ZIKV antigens in Leydig cells of the testes from all ZIKV-infected mice (9 of 9) and GLS-5700 animals (6 of 6), but the staining for viral antigens seemed to be reduced in the vaccine group (Figure 4, Table 1). No viral antigens were detectable in the epididymides or the seminiferous tubules of all infected groups, except for the azoospermic animal (2-11) where viral antigens were identified in the seminiferous tubules (Figure 4c).
Figure 2.
Macroscopic parameters of the testes in male IFNAR−/− mice. (a) Length, width, and weight of the right testis; (b) length and width of the left testis collected from mock ( ), Zika virus ([ZIKV]
), Zika virus ([ZIKV]  ), and GLS-5700 (
), and GLS-5700 ( ) groups of IFNAR−/− mice on day 28 postinfection. Abbreviation: dpi, days postinfection.
) groups of IFNAR−/− mice on day 28 postinfection. Abbreviation: dpi, days postinfection.
Figure 3.
Spermatozoa characterization in male IFNAR−/− mice. (a) Sperm count and (b) percentage of progressively motile (PR) spermatozoa of mock ( ), Zika virus ([ZIKV]
), Zika virus ([ZIKV]  ), and GLS-5700 (
), and GLS-5700 ( ) groups of male IFNAR−/− mice on day 28 postinfection.
) groups of male IFNAR−/− mice on day 28 postinfection.
Figure 4.
Histopathological and immunohistochemical analysis of the testes and epididymides in male IFNAR−/− mice. (a) Histology and immunohistochemistry (IHC) of a representative testicle and epididymis of mock IFNAR−/− mice (mouse 1–7). (b) Histology and IHC of a representative testicle and epididymis of Zika virus (ZIKV)-infected IFNAR−/− mice that did not experience any damage to reproductive organs (mouse 2–13). (c) Histology and IHC of a representative testicle and epididymis of ZIKV-infected IFNAR−/− mice with damage to reproductive organs (mouse 2–11). (d) Histology and IHC of a representative testicle and epididymis of GLS-5700-vaccinated IFNAR−/− mice (mouse 3–7). All photographs were obtained from the left testis of each animal collected on day 28 postinfection and taken at a magnification of ×200; Scale bar = 100 µm. Abbreviation: HE, hematoxylin and eosin staining.
Partial Recovery of Fertility in IFNAR−/− Mice After Sublethal Zika Virus Infection
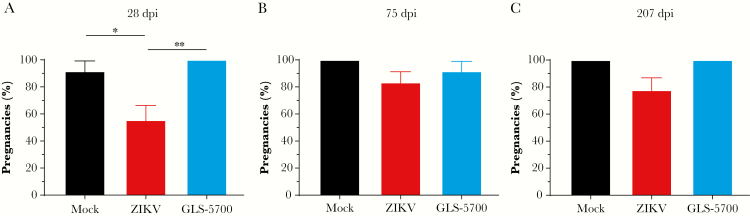
In the first experiment, each remaining IFNAR−/− male mouse from the 3 groups was placed in contact with 2 naive C57BL/6J female mice on days 28, 75, and 207 postinfection to evaluate their fertility. On day 28 postinfection, a significant 36% (P = .033) decrease in fertility was observed for the nonvaccinated and infected animals, when compared with the mock group, whereas a 44% decrease was observed when compared with vaccinated animals (P = .007) (Figure 5a). Fertility of vaccinated animals post-ZIKV challenge was similar to uninfected mice. More specifically, on day 28 postinfection, 3 of 9 males (33%; 2-2, 2-6, and 2-8) from the ZIKV group were unable to induce pregnancy in both females. After mating on day 75 postinfection, a partial recovery of fertility was observed in 2 of 3 males (2-6 and 2-8) previously identified as infertile, leaving 1 infertile male (2-2) (Figure 5b, Table 2). This infertile male still had not recovered by day 207 postinfection (Figure 5c, Table 2). At sacrifice on day 267 postinfection, mouse 2-2 exhibited 2 atrophied testes, whereas both 2-6 and 2-8 had their left testis atrophied (data not shown). In the second experiment, 2 of 11 males (18%; R-3 and R-6) were infertile on day 14, whereas 4 of 11 males (36%; R-2, R-3, R-6, and R-7) were infertile on day 28 postinfection, confirming the percentage of fertility loss observed in the first experiment. It is important to note that all male IFNAR−/− mice used in this study were fertile as assessed on day 14 postinfection (first experiment; data not shown) or on day 14 before infection (second experiment; Supplementary Table S2). In the first experiment, mean litter sizes for fertile males, defined here by at least 1 pregnant female in each cage, were of 7.7, 7.9, and 7.6 pups on days 28, 75, and 207 postinfection, respectively, with no statistically significant differences between the mating periods (Table 2). Similar mean litter sizes for fertile males were determined in the second experiment, which were of 7.1, 6.9, and 7.9 pups on days 14 before infection and days 14 and 28 postinfection, respectively, with no statistically significant differences between the mating periods (Supplementary Table S2).
Figure 5.
Fertility of male IFNAR−/− mice with C57BL/6J females. Percentage of naive C57BL/6J female mice that became pregnant after mating with mock ( ), Zika virus ([ZIKV]
), Zika virus ([ZIKV]  ), and GLS-5700 (
), and GLS-5700 ( ) groups of IFNAR−/− male mice on days (a) 28, (b) 75, and (c) 207 postinfection. *, P < .05; **, P < .01. Abbreviation: dpi, days postinfection.
) groups of IFNAR−/− male mice on days (a) 28, (b) 75, and (c) 207 postinfection. *, P < .05; **, P < .01. Abbreviation: dpi, days postinfection.
Table 2.
Number of Fetuses Collected in Naive C57BL/6J Female Mice Mated With Male IFNAR−/− Micea
| Mating 1 (28 dpi) | Mating 2 (75 dpi) | Mating 3 (207 dpi) | |||||
|---|---|---|---|---|---|---|---|
| Female 1 | Female 2 | Female 3 | Female 4 | Female 5 | Female 6 | ||
| Group | Male ID | Number of Fetuses | |||||
| Mock | 1-1 | 8 | 7 | 10 | 8 | 2 | 7 |
| 1–2 | 8 | 9 | 8 | 9 | N/A | N/A | |
| 1–3 | 9 | - | 8 | 5 | 9 | 10 | |
| 1–4 | 6 | 8 | 9 | 6 | 8 | 8 | |
| 1–5 | 5 | 8 | 7 | 9 | 7 | 6 | |
| 1–6 | 9 | 9 | 11 | 8 | 7 | 7 | |
| Average | 7.5 | 8.2 | 8.8 | 7.5 | 6.6 | 7.6 | |
| ZIKV | 2-1 | 7 | 9 | 9 | 11 | 9 | 7 |
| 2-2 | -a | -a | -a | -a | -a | -a | |
| 2–3 | 11 | 7 | 9 | 9 | 7 | 9 | |
| 2–4 | 9 | 1 | 10 | 9 | 9 | 6 | |
| 2–5 | - | 11 | 6 | 9 | - | 9 | |
| 2–6 | -a | -a | - | 9 | 8 | 6 | |
| 2–7 | 2 | - | 6 | 9 | 11 | - | |
| 2–8 | -a | -a | 5 | 6 | 8 | 6 | |
| 2–9 | 9 | 10 | 9 | 8 | 7 | 5 | |
| Average | 7.6 | 7.6 | 7.7 | 8.8 | 8.4 | 6.9 | |
| GLS-5700 | 3-1 | 10 | 9 | 7 | 8 | 8 | 8 |
| 3-2 | 6 | 6 | 6 | 7 | 7 | 9 | |
| 3-3 | 7 | 10 | 5 | 6 | 8 | 8 | |
| 3–4 | 10 | 9 | - | 7 | 5 | 7 | |
| 3–5 | 2 | 8 | 8 | 7 | 10 | 9 | |
| 3–6 | 9 | 7 | 8 | 10 | 8 | 7 | |
| Average | 7.3 | 8.2 | 6.8 | 7.5 | 7.7 | 8.0 | |
Abbreviations: dpi, days postinfection; ID, identification; NA, data not available due to natural death of the animal.
aThe males that were considered infertile, defined by the lack of pregnancies of both females in each cage. -, no pregnancy.
DISCUSSION
The current study reports the deleterious effects of ZIKV on fertility of male IFNAR−/− mice infected at 13 weeks of age in a sublethal model of infection in the absence of clinical manifestations. This study also reports an efficacious prophylactic countermeasure to prevent these effects and the partial recovery of fertility in male mice previously unable to induce pregnancy. Previous studies reported that, in a lethal model of ZIKV infection in IFNAR−/− mice, surviving males exhibited alterations to reproductive organs (ie, testicular atrophy, reduced sperm count, and motility), which could lead to infertility but be prevented by GLS-5700, a DNA-based vaccine [19, 23]. The IFNAR−/− mouse has been previously established as a lethal model for ZIKV infection when challenge is performed at a young age (under 8 weeks), whereas older individuals remained susceptible to infection in the absence of mortality [31]. Therefore, we sought to establish a sublethal model of ZIKV infection in older IFNAR−/− mice that did not develop clinical signs of disease but that nevertheless led to overall fertility loss.
In our model, only 1 nonvaccinated mouse succumbed to infection, despite elevated viremia in all animals. Mice infected with ZIKV did not demonstrate any observable signs of disease, except for 2 animals from the second experiment that exhibited a transient weight loss. Furthermore, gross pathology of the reproductive organs did not reveal any macroscopic alterations in terms of length, width, or weight of the testes, except for 5 of 18 (28%) nonvaccinated, infected animals. All other infected animals did not exhibit tissue or cellular damage to the testes, even though IHC did reveal the presence of ZIKV antigens in Leydig cells. Although reproductive organs seemed unaffected and sperm counts and motility were similar between each experimental group, fertility was nonetheless significantly reduced on day 28 postinfection in the ZIKV group. In our study, male infertility was defined as a lack of pregnancy of both females in each cage during a 2-week mating period that allowed for 3 estrus cycles to occur. Because we did not check for the presence of a copulatory plug, we cannot exclude the possibility that there was no mating. However, the 3 animals that were unable to induce pregnancy in their female cagemates were exhibiting 1 or both atrophic testes at the time of euthanasia. It is interesting to note that male fertility loss was prevented by immunization with GLS-5700. Although this ZIKV-induced infertility has been documented before in IFNAR−/− mice [23, 24], this is the first time, to our knowledge, that a study evaluates the long-term effects on fertility and reports partial recovery.
In the current study, the male that remained infertile had most likely irreversible damage to reproductive organs. However, males that recovered could shed light on the pathogenicity of ZIKV. A recent study used electron microscopy to highlight not only the physical attachment of ZIKV virions to mature sperm, but also to epididymal residual bodies. Sperm of infected mice was also found to contain more spermatozoa with residual bodies than control animals [32]. These bodies, or cytoplasmic droplets, have been associated with reduced fertility in multiple animal species [33]. Another article suggested that dysregulation of the hypothalamic-pituitary-gonadal axis could be an explanation for infertility [34]. Although this region has not been described as a target yet, the tropism of ZIKV for cells of the nervous system has been described before [35] and could lead to a disruption of the production or transport of gonadotropin-releasing, luteinizing, or follicle-stimulating hormones resulting in infertility [36]. Therefore, complete virus clearance from these organs over an extended period could explain why fertility was recovered at a later time point.
CONCLUSIONS
Even if it is sublethal, this mouse model is immunodeficient regarding type I interferon receptor function, and thus these observations do not necessarily reflect the situation prevailing in humans. Indeed, no reports have been published so far regarding damage to the testes or infertility in infected humans. Therefore, one can only speculate whether spermatozoa-related parameters such as counts or motility are affected in male individuals. However, this remains a preoccupying concern because infectious ZIKV was found to linger in human sperm up to 69 days in 1 recorded case, whereas viral RNA was detectable from 3 to 188 days after the onset of symptoms [14, 37]. Identification of the mechanism(s) responsible for the reduction in fertility remains paramount in our understanding of ZIKV infection, because more sensitive methods might need to be developed to detect potential signs of infertility in humans. Altogether, our understanding of the pathogenesis of ZIKV infection is still incomplete, and surveillance of the long-term effects on human fertility should be carefully monitored in individuals identified as infected, whether they are symptomatic or not. Finally, this study also highlights the role of the synthetic DNA GLS-5700 vaccine as a prophylactic option in a scenario in which male fertility could be compromised after ZIKV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M.-A. d., C. R., M.-C. V., J. C., and C. C. performed the experiments. M.-A. d., B. D. G., J. P., C. C., R. R. T., G. P. K., and G. B. analyzed the data and wrote the manuscript. R. d. N. A., K. G. M., Y. K. P., C. C. R., J. N. M., N. Y. S., J. J. K., and D. B. W. edited the manuscript. M.-A. d., J. P., G. P. K., and G. B. designed the experiments. All the authors have read the manuscript, provided comments, and agreed to the submission of its final version.
Acknowledgments. G. B. is the holder of the Canada research chair on emerging viruses and antiviral resistance. We thank the staff at the animal facility of the CHU de Québec for their work and advice regarding the fertility studies, especially M. V. St-Amant.
Disclaimer. The other authors declare that they do not have a commercial or other association that might pose a conflict of interest.
Financial support. This work was funded in part by a Foundation Grant from the Canadian Institutes of Health Research (grant no. 148361; to G. B.) and in part by the Canada Research Chair in Emerging and Re-Emerging Pathogens (Tier 1) (2016–23) program awarded to G. P. K.
Potential conflicts of interest. J. J. K. and N. Y. S. are employees of Inovio Pharmaceuticals, Inc., whereas Y. K. P., C. C. R., and J. N. M. are employees of GeneOne Life Science, Inc. As such, they receive salary and benefits, including ownership of stock and stock options from the respective companies. D. B. W. discloses grant funding, industry collaborations, speaking honoraria, and fees for consulting. His service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments, stock, or stock options. He notes potential conflicts associated with his work with Pfizer, Bristol Myers Squibb, Inovio Pharmaceuticals, Merck, GeneOne Life Science, and potentially others. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Quebec Medical Microbiology and Infectious Disease Annual Meeting, June 2017, Brômont, Québec.
References
- 1. Dick GW. Zika virus (II). Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–34. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Epidemiological alert - Zika virus infection Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=270&gid=32405. Accessed May 28, 2018.
- 3. Lazear HM, Diamond MS. Zika Virus: new clinical syndromes and its emergence in the Western Hemisphere. J Virol 2016; 90:4864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Fifth meeting of the Emergency Committee under the International Health Regulations (2005) regarding microcephaly, other neurological disorders and Zika virus. 2016. Available at: http://www.who.int/mediacentre/news/statements/2016/zika-fifth-ec/en/. Accessed May 28, 2018. [Google Scholar]
- 5. Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis 2016; 22:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen?Lancet Infect Dis 2016; 16:405. [DOI] [PubMed] [Google Scholar]
- 8. Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med 2016; 374:1552–63. [DOI] [PubMed] [Google Scholar]
- 10. Rowland A, Washington CI, Sheffield JS, Pardo-Villamizar CA, Segars JH. Zika virus infection in semen: a call to action and research. J Assist Reprod Genet 2016; 33:435–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venturi G, Zammarchi L, Fortuna C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill 2016; 21:1–4. [DOI] [PubMed] [Google Scholar]
- 12. Oduyebo T, Igbinosa I, Petersen EE, et al. Update: Interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure - United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:315–22. [DOI] [PubMed] [Google Scholar]
- 13. Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission - continental United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:215–6. [DOI] [PubMed] [Google Scholar]
- 14. Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect 2017; 23:296–305. [DOI] [PubMed] [Google Scholar]
- 15. Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:716–7. [DOI] [PubMed] [Google Scholar]
- 16. Deckard DT, Chung WM, Brooks JT, et al. Male-to-male sexual transmission of Zika virus–Texas, January 2016. MMWR Morb Mortal Wkly Rep 2016; 65:372–4. [DOI] [PubMed] [Google Scholar]
- 17. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A Mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep 2016; 17:3091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan JF, Zhang AJ, Chan CC, et al. Zika virus infection in dexamethasone-immunosuppressed mice demonstrating disseminated infection with multi-organ involvement including orchitis effectively treated by recombinant type I interferons. EBioMedicine 2016; 14:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffin BD, Muthumani K, Warner BM, et al. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat Commun 2017; 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uraki R, Hwang J, Jurado KA, et al. Zika virus causes testicular atrophy. Sci Adv 2017; 3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheng Z Y, Gao N, Wang Z Y, et al. Sertoli cells are susceptible to ZIKV infection in mouse testis. Front Cell Infect Microbiol 2017; 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yockey LJ, Varela L, Rakib T, et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 2016; 166:1247–56.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Govero J, Esakky P, Scheaffer SM, et al. Zika virus infection damages the testes in mice. Nature 2016; 540:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma W, Li S, Ma S, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell 2016; 167:1511–24.e10. [DOI] [PubMed] [Google Scholar]
- 25. Duggal NK, Ritter JM, Pestorius SE, et al. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep 2017; 18:1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pierson TC, Graham BS. Zika virus: immunity and vaccine development. Cell 2016; 167:625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. WHO vaccine pipeline tracker - Zika 2018. Available at: http://www.who.int/immunization/research/vaccine_pipeline_tracker_spreadsheet/en/. Accessed May 29, 2018.
- 28. Muthumani K, Griffin BD, Agarwal S, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. NPJ Vaccines 2016; 1:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization. Examination and processing of human semen 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241547789_eng.pdf. Accessed May 28, 2018.
- 31. Lazear HM, Govero J, Smith AM, et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016; 19:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uraki R, Jurado KA, Hwang J, et al. Fetal growth restriction caused by sexual transmission of Zika virus in mice. J Infect Dis 2017; 215:1720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cooper TG. Cytoplasmic droplets: the good, the bad or just confusing?Hum Reprod 2005; 20:9–11. [DOI] [PubMed] [Google Scholar]
- 34. Khakshooy A, Chiappelli F. Hypothalamus-pituitary-adrenal cell-mediated immunity regulation in the immune restoration inflammatory syndrome. Bioinformation 2016; 12:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miner JJ, Diamond MS. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 2017; 21:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jin JM, Yang WX. Molecular regulation of hypothalamus-pituitary-gonads axis in males. Gene 2014; 551: 15–25. [DOI] [PubMed] [Google Scholar]
- 37. Paz-Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika virus in body fluids — preliminary report. N Engl J Med 2017; doi: 10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.