Abstract
Background
Detection of Plasmodium antigens provides evidence of malaria infection status and is the basis for most malaria diagnosis.
Methods
We developed a sensitive bead-based multiplex assay for laboratory use, which simultaneously detects pan-Plasmodium aldolase (pAldo), pan-Plasmodium lactate dehydrogenase (pLDH), and P. falciparum histidine-rich protein 2 (PfHRP2) antigens. The assay was validated against purified recombinant antigens, monospecies malaria infections, and noninfected blood samples. To test against samples collected in an endemic setting, Angolan outpatient samples (n = 1267) were assayed.
Results
Of 466 Angolan samples positive for at least 1 antigen, the most common antigen profiles were PfHRP2+/pAldo+/pLDH+ (167, 36%), PfHRP2+/pAldo−/pLDH− (163, 35%), and PfHRP2+/pAldo+/pLDH− (129, 28%). Antigen profile was predictive of polymerase chain reaction (PCR) positivity and parasite density. Eight Angolan samples (1.7%) had no or very low PfHRP2 but were positive for 1 or both of the other antigens. PCR analysis confirmed 3 (0.6%) were P. ovale infections and 2 (0.4%) represented P. falciparum parasites lacking Pfhrp2 and/or Pfhrp3.
Conclusions
These are the first reports of Pfhrp2/3 deletion mutants in Angola. High-throughput multiplex antigen detection can inexpensively screen for low-density P. falciparum, non-falciparum, and Pfhrp2/3-deleted parasites to provide population-level antigen estimates and identify specimens requiring further molecular characterization.
Keywords: malaria, antigen detection, multiplex immunoassay, Pfhrp2 deletion, non-falciparum malaria
A multiplex malaria antigen detection assay was developed to measure pan-Plasmodium antigens aldolase and LDH, and P. falciparum HRP2. Multiplex antigen detection allowed for prediction of malaria infection status, species of infection, and detection of Pfhrp2/3-deleted parasites.
Detection of Plasmodium antigens in blood samples is an invaluable tool for malaria control and research. It has been employed for in vitro antimalarial drug sensitivity assays [1, 2], as a marker of clinical severity [3], a test to verify posttreatment parasite clearance [4], and, most importantly, as a field-deployable diagnostic tool for suspected malaria patients [5, 6]. The use of antigen detection for field diagnosis has greatly expanded in the past decade, with over 300 million rapid diagnostic tests (RDTs) used annually, at a cost of hundreds of millions of dollars [7]. Laboratory tests for Plasmodium antigens have also become a useful tool in malaria research, providing a sensitive measure for malaria protein detection and supplementing simple detection with the ability to quantify antigen concentrations [8]. Recently, a novel immunoassay for the Plasmodium falciparum histidine rich protein 2 (PfHRP2) has been developed. The assay utilizes a microbead capture and detection system on the Luminex platform, and has shown improvement in the limit of detection over other quantitative immunoassays [9]. As most RDTs currently detect the PfHRP2 antigen, this novel laboratory assay can compare RDT test results with actual blood PfHRP2 concentrations for the same individual—providing estimates for false positivity and false negativity RDT rates and estimating the in situ RDT limit of detection for the survey in which they were deployed [9–11].
The PfHRP2 antigen produced by P. falciparum is a unique protein in malaria biology, provides a species-specific marker for this parasite [12], and lingers in the blood circulation for weeks after successful clearance of parasites [13, 14]. The Plasmodium antigens aldolase (pAldo) and lactate dehydrogenase (pLDH) are also used for diagnostic purposes, but are thought to be cleared from the human host soon after resolution of infection [13, 15], thus potentially providing a more specific marker for active infection than PfHRP2. In addition, as antibodies recognizing pan-Plasmodium epitopes on pAldo and pLDH have been identified, detection of these 2 antigens in conjunction with PfHRP2 could allow estimation of non-falciparum malaria prevalence in coendemic areas of the world. Studies describing concentrations of all these 3 antigens within a single infection, or at a population level, have been lacking, in part due to the requirement to perform an immunoassay 3 times to obtain 3 unique assay signals.
Recent reports have emerged of P. falciparum parasites that do not produce the PfHRP2 and/or PfHRP3 antigens due to complete or partial genomic deletions of the Pfhrp2/3 gene(s) [16]. The potential for selection and spread of these parasites is a major emerging threat for future malaria diagnosis by HRP2-based RDT, as most RDTs deployed in malaria-endemic countries with predominant P. falciparum transmission detect only the PfHRP2 antigen. P. falciparum strains with partial or complete Pfhrp2/3 deletions in natural malaria infections were first reported in South America [17], but have now been reported in South Asia [18] and multiple countries in sub-Saharan Africa [19–22]. Systematic monitoring for Pfhrp2/3-deleted parasites in patient specimens is currently not applied globally, and discovery of mutant parasites has typically been triggered by a discordance between positive microscopy results and negative HRP2-based RDT results in patients undergoing both tests [17]. Genomic confirmation of complete or partial deletions of the Pfhrp2/3 gene requires time-intensive multireaction polymerase chain reaction (PCR) assays [23], and does not assay for the parasite-expressed antigen, which is the target of RDTs. Among other factors, the onerous nature of the current PCR assays hinders large-scale and timely screening of sample sets for Pfhrp2/3 deletions. The suitability of multiantigen detection for screening large sample sets in a high-throughput manner suggests a potential application for population screening for Pfhrp2/3 deletions in the laboratory.
Here, we describe an extension of our single-antigen bead-based immunoassay [9] to allow for multiantigen capture and detection, simultaneously assaying for the pan-Plasmodium antigens pAldo and pLDH, as well as P. falciparum-specific PfHRP2. The assay was validated using a panel of well-characterized single-species Plasmodium infections representing all 4 human malarias. To test its utility in an endemic setting, it was employed to assay dried blood samples from surveys of health facility patients in 2 provinces in Angola. The samples’ different multiantigen profiles were used to infer the presence of non-falciparum malaria parasites, low-density P. falciparum infections, and P. falciparum infections not expressing the PfHRP2 antigen.
METHODS
Study Design
A multiantigen assay for the simultaneous detection of PfHRP2, pAldo, and pLDH was developed and validated with purified recombinant antigens and on a panel of 239 known monoinfections in anonymized samples from returning US resident travelers with each of the 4 species of human malaria, as previously confirmed by PCR. The signal intensity cutoff for antigen positivity by the bead assay was determined using a panel of 73 malaria-negative US blood donors without travel to malaria endemic countries. The multiantigen assay was then used to screen 1267 dried blood spot samples previously collected during a 2016 health facility survey in Huambo and Uíge Provinces in Angola [24]. A subset of samples was chosen based on multiantigen profile and further assayed by quantitative reverse transcription PCR (qRT-PCR) for ultrasensitive detection of parasite nucleic acid to characterize malaria infection status. Separately, samples with an antigen profile showing no detected PfHRP2 but positive for pAldo and/or pLDH had another blood spot eluted and assay repeated to confirm antigen detection results. DNA was then extracted from these target samples and assayed by molecular methods for the presence of Plasmodium DNA and to determine species. Of these, samples that were confirmed to harbor P. falciparum infections were then analyzed using the standard confirmatory assay for Pfhrp2/3 gene deletions. See Supplementary Methods 1–4 for detailed sample collection and analysis methods.
Ethics Statement
All persons involved in the study provided informed consent before blood sample collection. For the samples from both the United States and Angola, testing was approved as research not involving identifiable human subjects by the Office of the Associate Director for Science in the Center for Global Health at the Centers for Disease Control and Prevention. For the Angola samples, collection and antigen detection was approved as part of the health facility survey by the Angolan Ministry of Health.
Statistical Analysis
In order to determine a signal intensity of the bead assay that would indicate a true positive signal for detecting a given antigen, 73 blood samples of US residents with no reported history of travel to malaria endemic countries (living in a malaria nonendemic setting) were tested at a 1:10 dilution. For each of the 3 antigens assayed in this sample set, the median fluorescence intensity minus background (MFI-bg) lognormal mean plus 3 standard deviations of this population was used as the positivity cutoff value to indicate which MFI-bg signal represented a true positive value when testing human blood samples [9], and these signals and estimated antigen detection limits of the assay are shown in Supplementary Table 1.
For the Angola survey, characteristics for each patient whose sample was profile positive for at least 1 antigen were compared to characteristics of patients testing negative for all 3 antigens. The proportion of patients with recorded fever or reported history of fever in the last 24 hours was compared using a Χ2 test. Differences in mean patient age were assessed using a 2-sample Student t test. The proportion of Pfhrp2/3-deleted parasites was calculated as the number of samples with genomically confirmed Pfhrp2/3 deletions divided by the total number of samples positive for any Plasmodium antigen.
The relationship between patient age and antigen concentration was modeled through locally weighted scatterplot smoothing (LOESS) regression in SAS v9.4 (Cary, NC).
RESULTS
Assay Validation
The MFI-bg assay signal titrated below 1000 pg/mL for all 3 antigens regardless of differences in the Plasmodium species isoforms of the recombinant proteins (Figure 1). Greater absolute detection capacity was shown when assaying for the pAldo and PfHRP2 antigens, with appreciable MFI-bg signals below 100 pg/mL and 10 pg/mL, respectively. For each recombinant antigen, signal cutoff values and limits of antigen detection appropriate for reporting (as described in Methods) are displayed in Supplementary Table 1.
Figure 1.
Titration of multiplex assay signal for recombinant aldolase, lactose dehydrogenase (LDH), and histidine-rich protein 2 (HRP2) Plasmodium proteins. Recombinant forms of Plasmodium vivax aldolase, P. falciparum, and P. vivax-specific isoforms of LDH, and P. falciparum type A, B, and C HRP2s were diluted to determine the minimal concentrations that gave appreciable median fluorescence intensity minus background (MFI-bg) assay signal. Error bars show ± standard error of the mean from 3 or 4 independent runs.
The assay was also found to reliably detect antigen from samples from known monospecies malaria infection. For all 4 major species of human malaria, the bead assay was able to detect 1 or more expressed antigens in 98.7% (236/239) of patient samples (Figure 2A and Supplementary Figure 1). Detection of antigens in P. vivax infection appeared to be most reliable, with all 54 P. vivax-infected patient samples testing positive for either pAldo or pLDH, and 81.5% (44/54) testing positive for both. All patient samples with P. falciparum infection were positive for the PfHRP2 antigen, with most P. falciparum-infected patient samples giving a high PfHRP2 assay signal (Figure 2A). For all 3 antigens, positive correlation was observed between antigen concentration and parasite density at the time of patient sampling with R2 correlation values ranging from 0.10 to 0.40 (Figure 2B and Supplementary Table 2). Using these regression estimates, and the detection capacity of the bead assay (Supplementary Table 1), it would be predicted that assaying for Plasmodium antigens would allow identification of active infections of less than 1 parasite/μL (p/μL) blood by using detection of any of the 3 antigens. The only exception to this finding was pLDH expression in P. falciparum infections, which modeled a substantially lower intercept. For all species, the absolute quantity of pLDH detected in an individual sample was on average greater than pAldo by a factor of 2- to 10-fold, with P. vivax infections showing the highest ratio and P. falciparum the lowest (Figure 2C and Supplementary Table 2).
Figure 2.
Quantification of Plasmodium aldolase (pAldo), Plasmodium lactate dehydrogenase (pLDH), and histidine-rich protein 2 (HRP2) from plasma of individuals with single-species Plasmodium infection. A, Median fluorescence intensity minus background (MFI-bg) signal for multiplex antigen assay from plasma of noninfected individuals (n = 73), or persons with monoinfection of P. falciparum (n = 153), P. vivax (n = 54), P. ovale (n = 23), or P. malariae (n = 9). B, Correlation of parasite density and antigen concentration by infecting species. Regression lines are shown by dashed lines in plots and regression estimates are shown in Supplementary Table 2. C, Correlation of pAldo and pLDH antigen concentrations within individual plasma samples for all 4 different types of infecting species. Dashed lines show regression lines.
Screening of Angola Samples for Different Antigen Profiles
Of 1267 samples from the Angola health facility survey, 302 (24%) were found to be positive for pAldo, 171 (13%) positive for pLDH, and 459 (36%) positive for PfHRP2, with a wide range of antigen levels (Supplementary Figure 2A). For antigen-positive samples, an inverse relationship was observed between antigen concentration and increasing patient age (Supplementary Figure 3). In the same manner as the confirmed monospecies infections in Figure 2, a direct correlation was seen between antigen concentration and parasite density in the Angola samples (Supplementary Figure 4), with the performance for detection of each antigen compared with qRT-PCR as the gold standard (Supplementary Table 3). A total of 801 (63%) samples were negative for all 3 antigens (Table 1). Of the 466 samples positive for at least 1 antigen, 167 (36%) of these were positive for all 3 antigens. A similar number (163, 35%) were positive only for PfHRP2. The next most frequent antigen profile was PfHRP2+/pAldo+/pLDH−, observed in 129 (28%) antigen-positive patients. No samples with the PfHRP2+/pAldo−/pLDH+ profile were observed. Different combinations of the multiantigen profile were predictive of P. falciparum nucleic acid carriage and mean parasite density as estimated by qRT-PCR (Figure 3). For samples with no antigen detected, 16% were positive for P. falciparum by qRT-PCR with a median parasite density amongst nucleic acid-positive samples of 0.41 p/μL (Table 1). Of samples positive only for the PfHRP2 antigen, 43% were qRT-PCR positive with a median parasite density of 2.0 p/μL. Amongst samples with the PfHRP2+/pAldo+/pLDH− profile, 96% were qRT-PCR positive, and the median parasite density in positive samples was 208 p/μL. All samples positive for all 3 antigens were qRT-PCR positive, with a median parasite density of 3104 p/μL. The prevalence of fever increased with cumulative antigen positivity, with 55%, 62%, 80%, and 88% of persons reporting fever for the 4 categories above, respectively (Table 1). Both PfHRP2+/pAldo+/pLDH+ and PfHRP2+/pAldo+/pLDH− patients were younger than patients negative for all 3 antigens, with mean ages of 9.4 and 17.3 years, respectively, compared to 23.8 years for the all-antigen–negative patients (P value < .01). The mean age of PfHRP2+/pAldo−/pLDH− patients (23.8 years) was not statistically different from the mean age of patients negative for all 3 antigens. The mean PfHRP2 concentration of individuals became reduced as persons were positive for fewer antigens (Supplementary Figure 2B).
Table 1.
Association Between Plasmodium Antigen Status and Fever Rates and Ages of 1267 Outpatients Attending Clinics in 2 Provinces in Angola, 2016
| Plasmodium Antigen Positivity | Fever | Age, y | Pf Parasite Density, median (IQR), p/µL |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PfHRP2 | pAldo | pLDH | n (%) | % | P valuea | Mean | P valuea | qRT-PCR, % Positive |
Interpretation | |
| − | − | − | 801 (63) | 55 | Ref | 23.8 | Ref | 16 | 0.41 (0.08–3.7) | Low association with malaria infection |
| + | − | + | 0 | … | … | … | … | … | … | … |
| + | − | − | 163 (13) | 62 | .10 | 23.8 | .99 | 43 | 2.0 (0.81–9.1) | Recently cleared or low-density P. falciparum infectionb |
| + | + | − | 129 (10) | 80 | <.01 | 17.3 | <.01 | 96 | 208 (75–639) | Low-density P. falciparum infectionb |
| + | + | + | 167 (13) | 88 | <.01 | 9.4 | <.01 | 100 | 3104 (1699–6430) | Acute P. falciparum infectionb |
| − | − | + | 1 (<1) | 100 | .99 | 16.0 | .99 | HRP2-mutant P. falciparum or non-falciparum malaria | ||
| − | + | − | 3 (<1) | 100 | .30 | 38.3 | .30 | |||
| − | + | + | 3 (<1) | 100 | .30 | 21.8 | .90 | |||
Abbreviations: pAldo, Plasmodium aldolase; Pf, Plasmodium falciparum; PfHRP2, Plasmodium histidine-rich protein 2; pLDH, Plasmodium lactate dehydrogenase; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
aCompared to reference, noninfected category (PfHRP2 −, pAldo −, pLDH −); P values in bold indicate statistical significance at alpha of 0.01.
bOr recent resolved P. falciparum infection followed by a current non-falciparum infection.
Figure 3.
Relationship of survey participants’ individual antigen profile with Plasmodium falciparum infection status and parasite density. Persons presenting to health facilities in Uige and Huambo, Angola had antigen levels for Plasmodium aldolase (pAldo), Plasmodium lactate dehydrogenase (pLDH), and P. falciparum histidine-rich protein 2 (PfHRP2) estimated by the multiplex bead assay, and had P. falciparum parasite density estimated by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Ratios on x axis for each antigen profile category indicate persons positive for P. falciparum nucleic acids divided by total number tested by qRT-PCR. Horizontal lines show mean parasite density for parasite-positive persons in each antigen category.
Use of Antigen Profile to Identify Non-falciparum and Pfhrp2/3 Deletions in Angola Samples
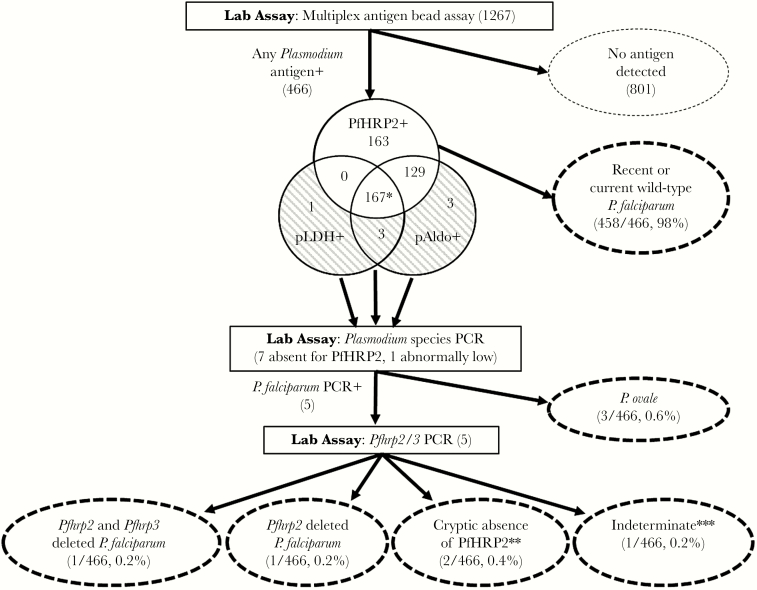
Of 466 Angolan samples positive for any antigens, 7 (1.5%) were negative for PfHRP2, but positive for at least 1 of the other antigens: 3 PfHRP2−/pAldo+/pLDH−, 3 PfHRP2−/pAldo+/pLDH+, and 1 PfHRP2−/pAldo−/pLDH+ (Figure 4 and Figure 5). There was 1 additional sample with an exceptionally low concentration of PfHRP2 (541 pg/mL) despite considerable concentrations of pAldo (140609 pg/mL) and pLDH (195205 pg/mL), and was clearly identified on scatterplots of antigen concentrations (Figure 4B). All 8 samples were pronounced outliers that did not show the characteristic relationship between PfHRP2 concentration and pAldo and pLDH concentration (Figure 4B). Photoinduced electron transfer polymerase chain reaction (PET-PCR) assays confirmed 3 of these samples to be monoinfections with Plasmodium ovale, all 3 from Uíge Province (Table 2). The remaining 5 (1.1%) antigen-positive samples were confirmed to be P. falciparum monoinfections and were assayed for Pfhrp2/3 deletions. One sample from Huambo was confirmed to be an infection with a P. falciparum showing a double Pfhrp2 and Pfhrp3 deletion (Table 2). One sample from Uíge was found to be an infection with a P. falciparum parasite with a deletion of the Pfhrp2 gene, but positive for the Pfhrp3 gene. In 2 samples from Uíge, the Pfhrp2 and Pfhrp3 gene targets were successfully amplified, despite the absence of detectable PfHRP2 antigen. Finally, the Pfmsp1 and Pfmsp2 single-copy genes could not be amplified in 1 sample from Uíge, and thus cannot be reported on for verification of Pfhrp2 and Pfhrp3 genotype [23]. The workflow algorithm and summary of results for the non-falciparum and Pfhrp2/3 screening are summarized in Figure 5 and Table 2.
Figure 4.
Identification of Plasmodium isolates from Angola not producing P. falciparum histidine-rich protein 2 (PfHRP2). A, Two by 2 tables of antigen positivity. Dashed circles show blood samples positive for Plasmodium aldolase (pAldo) or Plasmodium lactate dehydrogenase (pLDH) but not PfHRP2. B, Scatterplots of PfHRP2 antigen levels with pAldo and pLDH. Dashed ovals show samples positive for pan-Plasmodium antigens but not PfHRP2. Solid circle shows single sample from study with high level of pAldo and pLDH but very low PfHRP2.
Figure 5.
Screening algorithm for dried blood spots collected during health facility surveys in Angola, 2016. Percentages calculated using the total number of samples positive for any Plasmodium antigen as the denominator. *Includes 1 sample with abnormally low PfHRP2 concentration (<1000 pg/mL). **Absence of PfHRP2 protein despite successful amplification of Pfhrp2 and Pfhrp3 PCR targets. ***Does not meet World Health Organization reporting guidelines due to nonamplification of single-copy Pfmsp1 and Pfmsp2 genes. Abbreviations: pAldo, Plasmodium aldolase; PCR, polymerase chain reaction; PfHRP2, Plasmodium histidine-rich protein 2; pLDH, Plasmodium lactate dehydrogenase.
Table 2.
Results of Molecular and Genomic Analysis of Individual Samples With Antigen Profiles Suggestive of Non-falciparum or Pfhrp2/3-Deleted Plasmodium falciparum Infection
| Antigen Profile | PET-PCR Ct | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Age, y | Fever | HRP2 RDT | pAldo, pg/mL | pLDH, pg/mL | PfHRP2, pg/mL | Nested PCR, species | PET-PCR, Genus Ct | Estimated Parasite Density, p/μL | Pf | Pv | Po | Pm | Pfhrp2 Gene | Pfhrp3 Gene | Pfmsp1 Gene | Pfmsp2 Gene | Conclusion |
| Uíge | 4 | Yes | Neg | 36446 | 441214 | <LOD | Po | 28.2 | 1808.0 | … | … | 30.7 | … | … | … | … | … | P. ovale infection |
| Uíge | 1 | Yes | Neg | 62166 | 261949 | <LOD | Po | 27.5 | 2901.5 | … | … | 30.9 | … | … | … | … | … | P. ovale infection |
| Uíge | 60 | Yes | Neg | 20935 | 253044 | <LOD | Po | 29.9 | 573.3 | … | … | 35.0 | … | … | … | … | … | P. ovale infection |
| Huambo | 16 | Yes | Neg | <LOD | 279432 | <LOD | Pf | 38.4 | 1.8 | 39.2 | … | … | … | Neg | Neg | Pos | Pos | Pfhrp2 and Pfhrp3 deleted Pf infection |
| Uíge | 24 | No | Neg | 140609 | 195205 | 581 | Pf | 29.6 | 702.1 | 34.2 | … | … | … | Neg | Pos | Pos | Pos | Pfhrp2 deleted Pf infection |
| Uíge | 48 | Yes | Neg | 2284 | <LOD | <LOD | Pf | 39.3 | 1.0 | 41.4 | … | … | … | Neg | Neg | Neg | Neg | Indeterminatea |
| Uíge | 47 | Yes | Neg | 10384 | <LOD | <LOD | Pf | 32.4 | 105.9 | 34.5 | … | … | … | Pos | Pos | Pos | Pos | Cryptic absence of PfHRP2b |
| Uíge | 20 | Yes | Neg | 2131 | <LOD | <LOD | Pf | 31.1 | 254.8 | 33.2 | … | … | … | Pos | Pos | Pos | Pos | Cryptic absence of PfHRP2b |
Abbreviations: Ct, cycle threshold; LOD, limit of detection of bead-based assay; Neg, negative; pAldo, Plasmodium aldolase; PET-PCR, photoinduced electron transfer polymerase chain reaction; Pf, Plasmodium falciparum; PfHRP2, Plasmodium histidine-rich protein 2; pLDH, Plasmodium lactate dehydrogenase; Pm, P. malariae; Po, P. ovale; Pos, positive; Pv, P. vivax; RDT, rapid diagnostic test.
aDoes not meet World Health Organization reporting criteria due to nonamplification of single-copy Pfmsp1 and Pfmsp2 genes.
bAbsence of PfHRP2 protein despite successful amplification of Pfhrp2 and Pfhrp3 PCR targets.
DISCUSSION
Multiplexing detection of malaria antigens by the laboratory assay reported here allows for simultaneous detection of the pan-Plasmodium proteins aldolase and LDH, as well as the P. falciparum-specific protein PfHRP2. Once the appropriate platform has been procured (www.luminexcorp.com), at less than $1 USD per sample for reagents and supplies to assay for malaria antigens, this test can be used to quickly and inexpensively screen large sample sets for Plasmodium antigens to characterize the study population by antigen positivity. The assay was found to perform well with blood dried on filter paper, allowing for use of a practical sample type for collection of large numbers of field samples. Sample preparation simply includes dilution of blood sample in blocking buffer, so a single laboratory worker could process and collect data on upwards of 250 samples per day. Automated systems could increase this throughput even further.
The ability to characterize the multiantigen profiles (combinations of antigen positivity and concentrations) in individuals allows for a more nuanced characterization of malaria burden in an area. The finding that antigen profiles were also predictive of carriage and quantity of P. falciparum nucleic acids implies that low density (and more likely asymptomatic) parasite carriage in a population could be estimated simply by categorizing the study population by multiantigen profile. As currently formatted to be used in a laboratory setting, the utility of this assay does not likely extend to clinical use, but as a tool to provide epidemiological inference about circulating malaria in a population.
The assay was developed using the bead-based Luminex system, which allows multiplexing based on the gating principles of flow cytometry and causes analyte detection to be highly specific [25]. Many potential advantages are provided by the ability to simultaneously detect several Plasmodium antigens, including time saved by not performing multiple single-analyte assays, and ability to use less sample to obtain the same data. Additionally, formatting antigen detection to the bead-based immunoassay system has been found to be more sensitive than enzyme-linked immunosorbent assay (ELISA) for the P. falciparum antigen PfHRP2 [9].
A multiplex diagnostic test strategy is similar to the multiple antigen detection approach used by many malaria rapid diagnostic tests that are deployed in areas of known multispecies endemicity [6, 15, 26]. Data on the presence and absence of multiple antigens and their concentrations provides additional resolution over single-antigen detection and provides the opportunity to classify individuals based on their antigen profiles. By the bead assay, positivity to all 3 antigens was shown to be 100% reliable in estimating current infection with P. falciparum, and isolates positive for all 3 antigens displayed the highest median levels of estimated parasite density by an order of magnitude. This study found samples positive for PfHRP2 and pAldo, but negative for pLDH, generally represented low-density acute P. falciparum infections with 96% of these persons with detectable P. falciparum infection at time of sampling. For the PfHRP2+/pAldo+/pLDH− antigen profile, higher mean age, lower parasite density, and lower antigen levels when compared to persons in the all-antigen–positive profile may represent adults who have sufficient acquired immunity to suppress parasite densities. Of samples only positive for PfHRP2 but negative for the other 2 antigens, 43% were P. falciparum nucleic acid-positive, meaning 57% of PfHRP2+ persons were not found to have any detectable P. falciparum nucleic acids and had potentially cleared a P. falciparum infection in the recent past [14].
In the Angolan setting, the rarest antigen profile was the presence of pAldo or pLDH with the complete absence or very low levels of PfHRP2. This particular profile could be explained by 2 likely scenarios: infection with a non-falciparum malaria or infection with a P. falciparum strain not producing the PfHRP2/3 antigens. Eight of these antigen profiles were found from the Angolan survey, and in looking at the clinical history of the persons providing these samples (Table 2), 7 of these 8 individuals presented to a health facility with fever and all tested negative for an HRP2-based RDT, indicating they would not have been treated for malaria. Of these 8, we were able to confirm P. ovale infection in 3. This was not unexpected, as P. ovale infection has been confirmed in Angola [27] and in the contiguous nations of Zambia and Democratic Republic of the Congo (DRC), with population prevalence rates between 2.1% and 8.3% [28, 29]. Surveillance in nonendemic countries have traced P. ovale curtsi and wallikeri cases to migrant worker or tourist travel from Angola [30–33], and serological studies have provided evidence of substantial P. ovale transmission in other areas in Southern Africa [34]. However, the reliance on HRP2 RDTs for the majority of malaria diagnosis and the low sensitivity of microscopy in detecting non-falciparum infections and mixed infections precludes precise estimates of the rate of P. ovale infection in areas highly endemic for P. falciparum. Though this current study was not designed to estimate a point prevalence of malaria (falciparum or non-falciparum) in this region, the multiplex bead assay could be readily employed in large, population-based surveys to efficiently estimate antigen prevalence in a high-throughput manner, and specify samples requiring further molecular characterization and species identification. Of the 5 remaining samples with the PfHRP2−/pAldo+/pLDH+ antigen profile, 2 were confirmed to be mutant P. falciparum isolates lacking functional Pfhrp2 and/or Pfhrp3 genes. One sample did not have a sufficiently high parasite density to PCR amplify single-copy gene controls to infer presence or absence of the Pfhrp2/3 genes [23]. Interestingly, 2 additional samples did not have detectable PfHRP2 protein levels, but we were able to amplify regions from both the Pfhrp2 and Pfhrp3 genes, suggesting an unknown disruption in the pathway between the presence of the gene and the expression of the functional protein in these 2 cryptic cases.
The 2 confirmed Pfhrp2/3-deleted isolates represent the first report of Pfhrp2 and Pfhrp3 deletion in Angola. With recent reports of deletions in the south-west region of neighboring DRC [22], it may have been anticipated Pfhrp2/3 deleted parasites would be seen in Angola as well. Moreover, the identification of a parasite with a Pfhrp2 deletion but an intact Pfhrp3 gene in Uíge matches the major Pfhrp2/3 deletion profile reported in DRC [22]. The 2 samples represent less than 1% of the active or recent P. falciparum infections detected during the survey. However, the true prevalence of Pfhrp2/3-deleted parasites could be different, because mixed infections with wild-type and Pfhrp2/3-deleted P. falciparum would still produce PfHRP2. This study was not designed to provide estimates for Pfhrp2/3 deletion prevalence in these study areas but suggests a low prevalence of these parasites and provides empirical evidence that HRP2-based RDTs remain a strong and appropriate diagnostic tool in this setting.
As with any immunoassay, a primary limitation to the existing capacity of this assay are the currently available antibodies that are specific for these 3 malaria antigens. Specifically, the limit of detection for the pLDH antigen in blood was seen to only be reliable at approximately 4.5 ng/mL, which would be comparable or higher than currently published reports of ELISA limits of detection [35, 36]. Additionally, use of this multiplex assay by itself to identify species of infection (or prediction of Pfhrp2/3 genotype) assumes a monospecies and monogenomic infection. Lingering PfHRP2 from a previous P. falciparum infection could lead to misidentifying a current non-falciparum infection simply because of the presence of PfHRP2, and a mixed (wild-type) P. falciparum infection with another Plasmodium species would mask the presence of the non-falciparum malaria, as is the case for PfHRP2-based RDTs in the field [37]. Additionally, dichotomizing the antigen signal using a threshold determined in a nonimmune population might be biased by the influence of anti-HRP2 antibodies in endemic populations [38]. In order to reduce these errors in identification, molecular characterization of samples showing any unexpected patterns of antigen expression is suggested. This multiplex assay allows high-throughput and relatively inexpensive screening for the presence and amount of Plasmodium antigens at an individual and population level, and allows testing at both greatly reduced cost and time compared to exclusive testing by Pfhrp2/3 PCR assays. The multiplex antigen assay can efficiently screen large sample sets by narrowing the focus of molecular testing to a small proportion of samples that deserve further characterization.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. The authors thank all study participants providing blood samples, and Microcoat Biotechnologie GmbH for kindly providing the recombinant pLDH and PfHRP2 antigens.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the US President’s Malaria Initiative. Use of particular brand of instruments in this study does not mean CDC endorses such products.
Financial support. This work was supported by the US President’s Malaria Initiative; the Bill and Melinda Gates Foundation (grant number OPP1133622); and the National Institutes of Health (grant numbers AI-38858 and P30 AI027757). Atlanta Research and Education Foundation partially supported this work.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tritten L, Matile H, Brun R, Wittlin S. A new double-antibody sandwich ELISA targeting Plasmodium falciparum aldolase to evaluate anti-malarial drug sensitivity. Malar J 2009; 8:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noedl H, Wernsdorfer WH, Miller RS, Wongsrichanalai C. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob Agents Chemother 2002; 46:1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hendriksen IC, White LJ, Veenemans J, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis 2013; 207:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aydin-Schmidt B, Mubi M, Morris U, et al. Usefulness of Plasmodium falciparum-specific rapid diagnostic tests for assessment of parasite clearance and detection of recurrent infections after artemisinin-based combination therapy. Malar J 2013; 12:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 2002; 15:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mouatcho JC, Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol 2013; 62:1491–505. [DOI] [PubMed] [Google Scholar]
- 7. World Health Oganization. World malaria report 2015. www.who.int/malaria. Accessed 10 September 2018. [Google Scholar]
- 8. Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg 2006; 75:1205–8. [PubMed] [Google Scholar]
- 9. Rogier E, Plucinski M, Lucchi N, et al. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS One 2017; 12:e0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plucinski M, Dimbu R, Candrinho B, et al. Malaria surveys using rapid diagnostic tests and validation of results using post hoc quantification of Plasmodium falciparum histidine-rich protein 2. Malar J 2017; 16:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plucinski MM, Rogier E, Dimbu PR, Fortes F, Halsey ES, Aidoo M. Estimating the added utility of highly sensitive histidine-rich protein 2 detection in outpatient clinics in Sub-Saharan Africa. Am J Trop Med Hyg 2017; 97:1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parra ME, Evans CB, Taylor DW. Identification of Plasmodium falciparum histidine-rich protein 2 in the plasma of humans with malaria. J Clin Microbiol 1991; 29:1629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iqbal J, Siddique A, Jameel M, Hira PR. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J Clin Microbiol 2004; 42:4237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plucinski MM, Dimbu PR, Fortes F, et al. Posttreatment HRP2 clearance in patients with uncomplicated Plasmodium falciparum malaria. J Infect Dis 2018; 217:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nyunt MH, Kyaw MP, Win KK, Myint KM, Nyunt KM. Field evaluation of HRP2 and pan pLDH-based immunochromatographic assay in therapeutic monitoring of uncomplicated falciparum malaria in Myanmar. Malar J 2013; 12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. False-negative RDT results and implications of new reports of P. falciparum histidine-rich protein, 2016. www.who.int/malaria. Accessed 10 September 2018. [Google Scholar]
- 17. Gamboa D, Ho MF, Bendezu J, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 2010; 5:e8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bharti PK, Chandel HS, Ahmad A, Krishna S, Udhayakumar V, Singh N. Prevalence of pfhrp2 and/or pfhrp3 gene deletion in Plasmodium falciparum population in eight highly endemic states in India. PLoS One 2016; 11:e0157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amoah LE, Abankwa J, Oppong A. Plasmodium falciparum histidine rich protein-2 diversity and the implications for PfHRP 2: based malaria rapid diagnostic tests in Ghana. Malar J 2016; 15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koita OA, Doumbo OK, Ouattara A, et al. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg 2012; 86:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wurtz N, Fall B, Bui K, et al. Pfhrp2 and pfhrp3 polymorphisms in Plasmodium falciparum isolates from Dakar, Senegal: impact on rapid malaria diagnostic tests. Malar J 2013; 12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parr JB, Verity R, Doctor SM, et al. Pfhrp2-deleted Plasmodium falciparum parasites in the Democratic Republic of the Congo: a national cross-sectional survey. J Infect Dis 2017; 216:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng Q, Gatton ML, Barnwell J, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J 2014; 13:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plucinski MM, Ferreira M, Ferreira CMF, et al. Evaluating malaria case management at public health facilities in two provinces in Angola. Malar J 2017; 16:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jani IV, Janossy G, Brown DW, Mandy F. Multiplexed immunoassays by flow cytometry for diagnosis and surveillance of infectious diseases in resource-poor settings. Lancet Infect Dis 2002; 2:243–50. [DOI] [PubMed] [Google Scholar]
- 26. Hawkes M, Conroy AL, Opoka RO, et al. Use of a three-band HRP2/pLDH combination rapid diagnostic test increases diagnostic specificity for falciparum malaria in Ugandan children. Malar J 2014; 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. President’s Malaria Initiative. Angola National Malaria Strategic Plan 2016–2020. Luanda, Angola:Ministry of Health, 2015. [Google Scholar]
- 28. Doctor SM, Liu Y, Anderson OG, et al. Low prevalence of Plasmodium malariae and Plasmodium ovale mono-infections among children in the Democratic Republic of the Congo: a population-based, cross-sectional study. Malar J 2016; 15:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gabrielli S, Bellina L, Milardi GL, et al. Malaria in children of Tshimbulu (Western Kasai, Democratic Republic of the Congo): epidemiological data and accuracy of diagnostic assays applied in a limited resource setting. Malar J 2016; 15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao Y, Wang W, Liu Y, et al. The increasing importance of Plasmodium ovale and Plasmodium malariae in a malaria elimination setting: an observational study of imported cases in Jiangsu Province, China, 2011–2014. Malar J 2016; 15:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruas R, Pinto A, Nuak J, Sarmento A, Abreu C. Non-falciparum malaria imported mainly from Africa: a review from a Portuguese hospital. Malar J 2017; 16:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lupi O, Ridolfi F, da Silva S, et al. Dengue infection as a potential trigger of an imported Plasmodium ovale malaria relapse or a long incubation period in a non-endemic malaria region. Int J Infect Dis 2016; 44:20–4. [DOI] [PubMed] [Google Scholar]
- 33. Rojo-Marcos G, Rubio-Munoz JM, Ramirez-Olivencia G, et al. Comparison of imported Plasmodium ovale curtisi and P. ovale wallikeri infections among patients in Spain, 2005–2011. Emerg Infect Dis 2014; 20:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plucinski MM, Candrinho B, Chambe G, et al. Multiplex serology for impact evaluation of bed net distribution on burden of lymphatic filariasis and four species of human malaria in northern Mozambique. PLoS Negl Trop Dis 2018; 12:e0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bashir IM, Otsyula N, Awinda G, Spring M, Schneider P, Waitumbi JN. Comparison of PfHRP-2/pLDH ELISA, qPCR and microscopy for the detection of plasmodium events and prediction of sick visits during a malaria vaccine study. PLoS One 2013; 8:e56828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atchade PS, Doderer-Lang C, Chabi N, et al. Is a Plasmodium lactate dehydrogenase (pLDH) enzyme-linked immunosorbent (ELISA)-based assay a valid tool for detecting risky malaria blood donations in Africa?Malar J 2013; 12:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bharti PK, Chandel HS, Ahmad A, Krishna S, Udhayakumar V, Singh N. Prevalence of pfhrp2 and/or pfhrp3 gene deletion in Plasmodium falciparum population in eight highly endemic states in India. PLoS One 2016; 11:e0157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biswas S, Tomar D, Rao DN. Investigation of the kinetics of histidine-rich protein 2 and of the antibody responses to this antigen, in a group of malaria patients from India. Ann Trop Med Parasitol 2005; 99:553–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.