Abstract
Background
Initiation of antiretroviral therapy (ART) in human immunodeficiency virus (HIV)-infected individuals with cryptococcal meningitis places them at risk for Cryptococcus-associated immune reconstitution inflammatory syndrome (C-IRIS). The relationship between antibody immunity and C-IRIS risk has not been investigated.
Methods
We compared plasma levels of immunoglobulins, C. neoformans glucuronoxylomannan (GXM) capsule-specific and laminarin (Lam)-binding IgM and IgG, and percentages of peripheral blood total and memory B cells between 27 HIV-infected patients with CM who developed C-IRIS and 63 who did not, and evaluated associations of these parameters with risk of C-IRIS.
Results
Prior to initiation of ART, plasma IgM, Lam-binding IgM (Lam-IgM), Lam-IgG, and GXM-IgM levels were significantly lower in patients who developed C-IRIS than those who did not. Multivariate analysis revealed significant inverse associations between C-IRIS and IgM (P = .0003), Lam-IgM (P = .0005), Lam-IgG (P = .002), and GXM-IgM (P = .002) independent of age, sex, HIV viral load, CD4+ T-cell count, and cerebrospinal fluid fungal burden. There were no associations between C-IRIS and total or memory B cells.
Discussion
Antibody profiles that include plasma IgM, Lam-IgM, Lam-IgG, and/or GXM-IgM may have value in furthering our understanding of C-IRIS pathogenesis and hold promise as candidate biomarkers of C-IRIS risk.
Keywords: HIV, cryptococcal meningitis, Cryptococcus-associated immune reconstitution inflammatory syndrome, IgM, IgG, memory B cells, laminarin, GXM
Plasma IgM, glucuronoxylomannan-IgM, and β-glucan-binding IgM at the time of ART initiation were lower in HIV-infected individuals with cryptococcal meningitis who go on to develop C-IRIS than those who did not and inversely associated with development of C-IRIS.
(See the Editorial Commentary by Jarvis and Harrison on pages 344’6.)
Cryptococcal meningitis (CM) is a leading cause of human immunodeficiency virus (HIV)-associated mortality globally with an estimated 223100 cases annually and 181100 deaths in 2014 [1]. Individuals with CM are at risk for paradoxical Cryptococcus-associated immune reconstitution inflammatory syndrome (C-IRIS), a clinical worsening or recurrence of signs and symptoms of neurological deterioration due to the host immune response during antifungal treatment of cryptococcosis [2, 3]. Risk factors for C-IRIS include persistent cryptococcal burden in the cerebrospinal fluid (CSF), poor CD4+ T-cell count recovery after antiretroviral therapy (ART) initiation [3], elevated plasma interleukin-5 (IL-5) and IL-7 levels [4], and paucity of CSF inflammation prior to ART initiation [5]. At present, there are no validated predictive biomarkers for HIV-associated C-IRIS.
There are no published associations between antibody immunity and C-IRIS; however, ample data link specific antibody to resistance to human cryptococcosis. Glucuronoxylomannan (GXM) is the main constituent of the polysaccharide capsule of Cryptococcus neoformans. Levels of GXM-binding IgM were lower among HIV-infected than HIV-uninfected individuals [6, 7], HIV-infected individuals who developed cryptococcosis compared to those who did not [7], and HIV-uninfected solid organ transplant recipients who developed cryptococcosis posttransplant than those who did not [8]. Notably, GXM-IgG directly associates with risk for CM, being higher in HIV-infected than HIV-uninfected, and HIV-infected and HIV-uninfected individuals with CM than controls without CM [6, 9–11].
Prior work also shows that HIV-infected as well as HIV-uninfected individuals with a history of cryptococcosis had lower percentages of peripheral blood B, memory B, and IgM memory B cells than HIV-infected and HIV-uninfected persons who did not [7, 10]. IgM memory B cells are the main source of human serum IgM [12]. Antibodies produced by these cells, also called naturally occurring (natural) antibodies, have the ability to bind conserved carbohydrate moieties, such as β-glucans, found on microbes, including C. neoformans [13–15]. Data from experimental cryptococcosis models support a role for natural antibody in resistance to C. neoformans. Natural serum IgM and/or B-1 cells, mouse homologs of IgM memory B cells [12], reduced C. neoformans dissemination from lungs to brain in mice [7, 16, 17]. Natural IgM also enhanced alveolar macrophage phagocytosis of C. neoformans in IgM-deficient [7] and B and T-cell–deficient Rag1−/− mice [17], and naive B-1 cells enhanced lung antifungal immunity in B-1-cell–depleted wild-type mice and reduced C. neoformans dissemination to the brain [16]. Data showing a mouse β-(1,3)-glucan mAb reduced C. neoformans growth in vitro and protected mice against lethal C. neoformans infection [18] provides further evidence that natural antibody may enhance resistance to C. neoformans as there are β-(1,3)-glucans on the C. neoformans cell wall [13, 18, 19].
To investigate relationships between C-IRIS and antibody immunity, we determined levels of plasma immunoglobulins, naturally occurring, IgM, laminarin (Lam)-IgM and Lam-IgG, GXM-IgM and GXM-IgG, pustulan-IgM and pustulan-IgG, along with peripheral blood B-cell subset phenotypes in a previously described cohort of patients with HIV-associated cryptococcosis that did and did not develop C-IRIS after ART initiation [3].
MATERIALS AND METHODS
Study Population
The cohort was previously described [3]. It included 90 ART-naive, HIV-infected patients with a first episode of CM, of whom 27 developed possible or probable C-IRIS after ART initiation (C-IRIS group) and 63 who did not (no–C-IRIS group). Patient recruitment, inclusion criteria, the study protocol, and clinical outcomes were reported previously [3]. In brief, all patients received induction therapy with amphotericin B 1 mg/kg for 14 days followed by consolidation therapy with fluconazole 400 mg for 8–12 weeks, and ART was initiated on or about day 18 of antifungal therapy. The data reported herein were obtained with plasma and peripheral blood mononuclear cells (PBMCs) collected at ART initiation (W0), and 4 (W4) and 12 (W12) weeks after ART initiation. Written informed consent was given by the patients or their families and ethics approval to the parent study was granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BF053/09), Monash University (20161197839), and University of Western Australia (RA/4/1/2541) [3].
Measurement of Plasma Immunoglobulin Concentrations
Plasma IgM, IgG1, and IgG2 concentrations were determined on samples obtained at W0, W4, and W12 by Luminex according to the manufacturer’s instructions. In brief, a prediluted standard, assay buffer, and patient samples were added to the wells of a 96-well plate in duplicate. Then the plate was incubated with mixed beads at room temperature for 1 hour, the plate was washed and incubated with anti-human κ and λ light chain detection antibodies, incubated for 30 minutes at room temperature in the dark, and then with streptavidin-phycoerythrin. The content of the wells were read using Luminex 200 (EMD Millipore, Billerica, MA) with xPONENT software.
Measurement of Antibodies to GXM, Lam, and Pustulan
Titers of GXM-binding IgM and IgG, Lam-binding IgM and IgG, and pustulan (a β-(1,6)-linked linear glucan [20])-binding IgM and IgG, were determined using plasma obtained at W0, W4, and W12. GXM enzyme-linked immunosorbent assays (ELISAs) were performed as described [10, 21] using GXM from C. neoformans 24067 (serotype D) prepared as described [22]. This GXM exhibits cross reactivity with serotype A GXM [23] and is used extensively for serological analyses in mice and humans [6, 10, 17].
As Lam is an algal polysaccharide consisting mainly of β-(1–3)-glucan with rare β-(1–6)-glucan linkages [24], Lam antibodies were measured as a surrogate for β-(1–3)-glucan antibodies as described [14]. Lam (Sigma Chem, St. Louis, MO) and pustulan (InvivoGen, San Diego, CA) ELISAs were performed as described [14] with modification of the amount of coating antigen. First, we used 10 µg/mL rather than 50 µg/mL [14] of each glucan as the coating antigen. Then, to confirm Lam specificity and extend results to another β-glucan, pustulan, we repeated the assays with 5 µg/mL of coating antigen. Briefly, 96-well polystyrene plates (Costar; Corning, Kennebunk, ME) coated with 10 μg/mL (or 5 μg/mL) of Lam or pustulan were incubated with serially diluted plasma, washed, incubated with goat anti-human alkaline phosphatase-labeled IgM or IgG (Southern Biotech, Birmingham, AL), and then with p-nitrophenyl phosphatase (Sigma-Aldrich, St. Louis, MO). Absorbance was measured at 405 nm with a VERSAmax ELISA plate reader (Molecular Devices, Sunnyvale, CA). The highest dilution to give a signal at 2 times the optical density background (wells with no sera) was defined as the antibody titer.
Peripheral B-Cell Immunophenotyping
The phenotypes of peripheral blood B cells were determined by flow cytometry as previously described [7, 10]. Briefly, PBMCs obtained at W0, W4, and W12 stained with LIVE/DEAD Cell Stain (Invitrogen, Eugene, OR) were incubated with the following phenotyping reagents (BD Biosciences): mouse anti-human PerCP-Cy5.5-conjugated CD27, Alexa Fluor 700-conjugated CD19, phycoerythrin-conjugated IgD and allophycocyanin-conjugated IgM, after which cells were washed and fixed with 2% paraformaldehyde. Single-color controls and fluorescence-minus-one controls were included to ensure proper compensation and gating. Counting Beads (Invitrogen, Eugene, OR) were included in all samples. Data were collected on a LSR-II (Becton Dickinson) interfaced to a Windows FACSDiva software. Among living cells, percentages of the following cell types were identified with FlowJo version 10 software (Tree Star): total B cells (CD19+), total memory B cells (CD19+CD27+), IgM+-only memory B cells (CD19+CD27+IgM+IgD−), IgM+IgD+ memory B cells (CD19+CD27+IgM+IgD+), and class-switched memory cells (CD19+CD27+IgM−IgD−) (Supplementary Figure 1).
Statistical Analysis
We compared serological and B-cell measurements at W0, W4, and W12 by non-parametric Mann-Whitney U test without and with adjustment for previously reported age, sex, CD4+ T-cell counts, HIV viral load (VL), and CSF fungal burden [3]. Then, we estimated associations between ART initiation values and risk of C-IRIS using multivariable logistic regression. Included in the parametric model was previously reported age, sex, CD4+ T-cell counts, HIV VL, and CSF fungal burden [3]. We also looked at the correlation between baseline serological markers (Supplementary Table 1). A P value of <.05 was considered statistically significant.
RESULTS
IgM, Lam-IgM/IgG, GXM-IgM, and Pustulan-IgM/IgG Were Lower in the C-IRIS Group
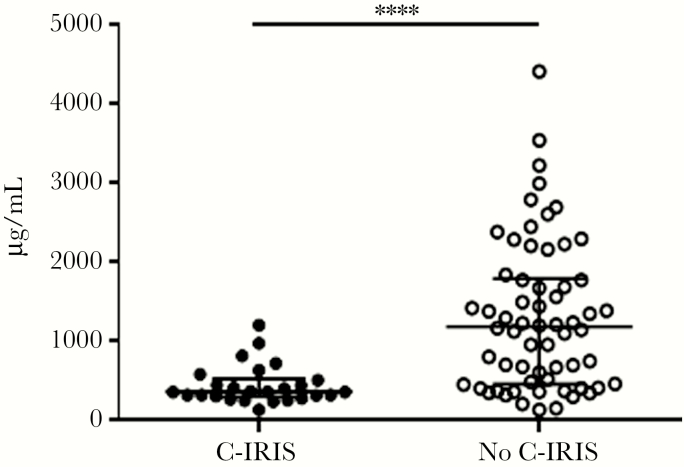
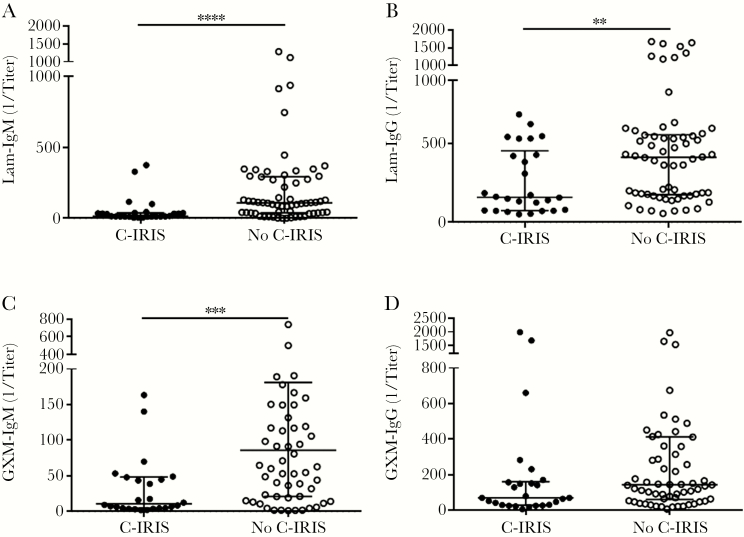
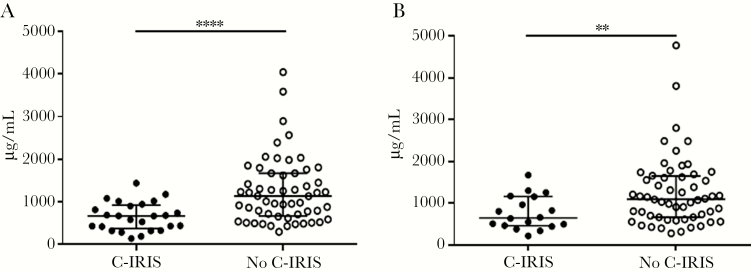
At ART initiation (W0), plasma IgM (P < .0001; Figure 1), Lam-IgM (P = .0001; Figure 2A), Lam-IgG (P = .004; Figure 2B), and GXM-IgM (P = .0003; Figure 2C) were each significantly lower in patients who developed C-IRIS than those who did not. Levels of Lam-IgM, but not Lam-IgG, were also significantly lower in the C-IRIS than no–C-IRIS group when 5 μg/mL was used as the coating antigen (P = .003; Supplementary Figure 2), as were titers of pustulan-IgM (P < .0001; Supplementary Figure 3A), and pustulan-IgG (P < .0001; Supplementary Figure 3B). There was no significant difference in titers when comparing Lam-IgM using 5 μg/mL versus 10 μg/mL of Lam as the coating agent. We also analyzed post-ART samples to ascertain the effect of ART on antibody profiles. The C-IRIS group had significantly lower Lam-IgM at W4 (P = .007; Supplementary Table 2), Lam-IgG at W4 (P = .01; Supplementary Table 2) and W12 (P = .007; Supplementary Table 2), and plasma IgM at W4 (P < .0001; Figure 3A) and W12 (P = .006; Figure 3B). Significant findings held after adjustment for age, sex, HIV VL, CD4+ T-cell counts, and CSF fungal burden (Supplementary Table 2).
Figure 1.
Plasma IgM concentration in Cryptococcus-associated immune reconstitution inflammatory syndrome (C-IRIS) and no–C-IRIS groups at week 0. Data were obtained by Luminex. Median values (horizontal lines in the center), and interquartile ranges (upper and lower horizontal lines) are shown. Asterisks indicate significant comparison between groups: ****P < .0001 by the Mann-Whitney U test. Significant findings remained after adjustment for age, sex, HIV viral load, CD4+ T-cell counts, and cerebrospinal fluid fungal burden.
Figure 2.
Titers of (A) laminarin (Lam)-IgM, (B) Lam-IgG, (C) glucuronoxylomannan (GXM)-IgM, and (D) GXM-IgG in Cryptococcus-associated immune reconstitution inflammatory syndrome (C-IRIS) and no–C-IRIS groups at week 0. Data were obtained by enzyme-linked immunosorbent assays (ELISA) using 10 µg/mL Lam or GXM as the coating antigen. Data are median values (horizontal lines in the center), and interquartile ranges (upper and lower horizontal lines). Asterisks indicate significant comparisons between groups: **P < .01, ***P < .001, ****P < .0001 by the Mann-Whitney U test. Significant findings remained after adjustment for age, sex, HIV viral load, CD4+ T-cell counts, and cerebrospinal fluid fungal burden.
Figure 3.
Plasma IgM concentration in Cryptococcus-associated immune reconstitution inflammatory syndrome (C-IRIS) and no–C-IRIS groups at (A) week 4, and (B) week 12. Data were obtained by Luminex. Median values (horizontal lines in the center), and interquartile ranges (upper and lower horizontal lines) are shown. Asterisks indicate significant comparisons between groups: **P < .01, ****P < .0001 by the Mann-Whitney U test. Significant findings remained after adjustment for age, sex, HIV viral load, CD4+ T-cell counts, and cerebrospinal fluid fungal burden.
Higher IgM, Lam-IgM, GXM-IgM, and Pustulan-IgM/IgG Levels at Baseline Were Associated With Lower Risk of C-IRIS
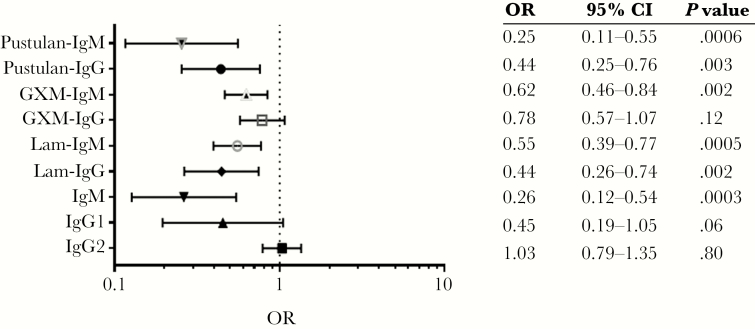
At ART initiation, log2 IgM was inversely associated with C-IRIS independent of age, sex, CD4+ T-cell count, HIV VL, and CSF fungal burden (odds ratio [OR] 0.26; 95% confidence interval [CI], 0.12–0.54; P = .0003). Inverse associations were also observed for Lam-IgM (OR 0.55; P = .0005), GXM-IgM (OR 0.62; P = .002), pustulan-IgM (OR 0.25; P = .0006), and pustulan-IgG (OR 0.44; P = .003) independent of adjustment factors (Figure 4). For Lam-IgG, an inverse association was found when 10 µg/mL (OR 0.44; P = .002), but not 5 µg/mL Lam was the ELISA coating agent. Total IgM levels at baseline were significantly correlated with GXM-IgM (r = 0.63; P < .05), GXM-IgG (r = 0.33; P < .05), and Lam-IgM (r = 0.45; P < .05) (Supplementary Table 1).
Figure 4.
Associations between log2 transformed plasma antibody titers and Cryptococcus-associated immune reconstitution inflammatory syndrome (C-IRIS) at week 0 (n = 90, 27 C-IRIS events). Associations of each serological marker with C-IRIS status were adjusted for age, sex, contemporaneous CD4+ T-cell count, log10 HIV viral load, and log10 cerebrospinal fluid fungal burden using logistic regression model. Laminarin (Lam)- and glucuronoxylomannan (GXM)-Ig data were obtained using 10 µg/mL of the coating agent and pustulan-Ig using 5 µg/mL of the coating agent. Significant association with Lam-IgG was lost when 5 µg/mL of the coating agent was used. Abbreviations: CI, confidence interval; OR, odds ratio.
Total and Memory B-Cell Subset Did Not Differ Between the C-IRIS and No–C-IRIS Group
There were no significant differences in the number or percentage of B-cell subsets (total or total memory, IgM+IgD+, IgM+-only, or switched memory B cells) between the C-IRIS and no–C-IRIS groups (Supplementary Figure 4). There were also no significant associations between any B-cell subset and C-IRIS or plasma IgM, IgG1, or IgG2, or GXM- or Lam-IgM or IgG at baseline (data not shown).
Baseline CD4+ T-Cell Counts Were Directly Correlated With Plasma IgM Level
There was a direct correlation between CD4+ T-cell counts and plasma IgM levels at ART initiation in an unadjusted analysis of the entire cohort (r = 0.39; P = .02). A previous report showed the C-IRIS group had lower CD4+ T-cell counts at baseline in an unadjusted analysis (median 16 vs 36 cells/μL; P = .015) [3]. No significant associations were identified between CSF fungal burden or HIV VL [3] at ART initiation with Ig levels or B-cell subsets of the entire cohort (data not shown). There were no correlations between changes in CD4+ T-cell counts and changes in serology or B-cell levels over time (data not shown).
DISCUSSION
The major finding of this study is that, at ART initiation, levels of plasma IgM, GXM-IgM, and β-glucan-binding (Lam and pustulan)-IgM were lower in the C-IRIS than no–C-IRIS group and inversely associated with development of C-IRIS after adjustment for age, sex, CD4+ T-cell count, HIV VL, and CSF fungal burden. Lam-IgG was significantly lower in the C-IRIS than no–C-IRIS group when 10 µg/mL, but not 5 µg/mL, Lam was the ELISA coating antigen. While association does not establish causation, these findings support the idea that GXM-IgM and naturally occurring β-glucan-reactive antibodies may play a role in preventing CM and/or C-IRIS. Though more work is needed to confirm this hypothesis, it is consistent with preclinical data in mice. Mouse and human monoclonal (mAb) GXM-IgMs promote monocyte/macrophage phagocytosis of C. neoformans, enhance antifungal immunity, and promote clearance of GXM, an immunosuppressant, in mice [25]. C. neoformans-selected mouse B-1 cells, which are IgM+ memory B-cell homologs, produced IgM that reacted with Lam and GXM [16]. In addition, naturally occurring serum IgM and/or B-1 cells enhanced antifungal immunity in the lungs, reduced C. neoformans dissemination to the brain [17, 21], and promoted a “wild type” inflammatory pulmonary response [17] in mice. The foregoing data, along with a study showing a mouse β-(1–3)-glucan mAb (2G8) inhibited C. neoformans budding and growth in vitro and protected mice against lethal C. neoformans [18], support the idea that natural antibody mediates host benefit against C. neoformans. The correlation between plasma IgM and Lam- and GXM-IgM suggests that these antibodies may be part of the same natural antibody repertoire, though separate studies are needed to determine the B-cell subset/s that produce these antibodies.
Human studies also support the idea Lam antibodies may contribute to antifungal immunity to C. neoformans. Human serum antibodies bind Lam and other β-glucans on the C. neoformans cell wall [13, 14, 16, 26]. Lam antibodies were associated with a better prognosis in patients with candidiasis [27], and C. neoformans β-glucosylceramide-binding antibodies from patients with cryptococcosis inhibited C. neoformans binding in vitro [26]. The ability of β-glucan antibodies to bind fungal elements was part of the rationale for an experimental Lam-based conjugate vaccine for fungi [28] that protected mice against Candida and Aspergillus [28, 29]. The aforementioned β-(1–3)-glucan mAb 2G8, which protected mice against C. neoformans [18] and Candida [28], was produced from mice immunized with this vaccine [28].
At present, we do not know how GXM-IgM or Lam-IgM may mediate a beneficial effect in patients being treated for CM. Mouse and human GXM-IgM mAbs promote effector cell phagocytosis of C. neoformans in mice [30, 31]. Mouse GXM mAbs also reduce C. neoformans budding in vitro and enhance clearance of GXM [32]; this effect was also observed in a clinical trial of a GXM mAb in patients with CM [33]. Thus, it is reasonable to posit that higher levels of GXM-IgM in the no–C-IRIS cohort may have contributed to their lower fungal burdens, while lower levels may contribute to C. neoformans persistence in the CNS, as reported for patients with C-IRIS [3, 5, 34]. Our data do not suggest a role for GXM-IgG in resistance to C-IRIS. In fact, higher levels of GXM-IgG were associated with risk for and/or active cryptococcosis in HIV-infected and HIV-uninfected patients [6, 10, 11]. Thus, our finding that GXM-IgG levels were not different between the cohorts may be a reflection of the similar fungal burdens of these groups at the time of ART initiation.
Available data suggest β-glucan antibodies may exert a direct antifungal effect. The aforementioned β-(1–3)-glucan mAb that protected mice against C. neoformans in vivo did not promote phagocytosis of C. neoformans [18]. Instead, it inhibited C. neoformans budding and growth, as did human antibodies to a β-glucan-linked C. neoformans cell wall moiety [18, 26]. We did not perform growth inhibition studies with samples from the cohort in this study because the patients were on antifungal therapy. However, fungal killing may not directly associate with protection; neither natural serum IgM nor B-1 B cells appreciably reduce C. neoformans lung fungal burdens in experimental cryptococcosis, even though they mediate protection against dissemination [7, 16, 17]. The inverse associations of total IgM, GXM-IgM, and Lam-IgM with C-IRIS in this study support the idea that some of these antibodies may reduce the inflammatory response to C. neoformans that can occur with ART initiation. Data from a B-cell–deficient mouse model show that absence of B cells and natural antibody led to a disorganized pulmonary inflammatory response to C. neoformans [17]. Along the same lines, natural serum IgM enhanced phagocytosis and restored wild-type lung histology in IgM and B and T-cell (Rag1−/−) deficient mice [17, 21]. Thus, natural antibodies may have dual activities, enhancing phagocytosis and dampening of inflammation. Regarding the latter, natural antibody can inhibit inflammation in mice [35] and has been proposed as adjuvant immunotherapy for inflammatory conditions [35]. Therefore, it is reasonable to posit that β-glucan IgM may contribute to beneficial immunomodulation in patients with cryptococcosis, resulting in reduced inflammation.
Plasma from patients in our study bound β-(1–3)-glucan in ELISAs using 10 and 5 µg/mL Lam as the capture antigen. These amounts of Lam are 5 and 10 times less than the amount used by others to establish β-glucan binding in normal human sera [14, 27]. The no–C-IRIS group had higher levels of Lam-IgM irrespective of the amount of coating antigen. On the other hand, there was no difference in Lam-IgG levels between the groups when 5 µg/mL Lam was used as the coating antigen. This may reflect the fact that IgM has greater avidity leading to continued reactivity at a similar titer. It may also reflect that Lam-IgM and Lam-IgG have different β-glucan specificities. Although studies to address this question were beyond the scope of this study, we note β-(1–3)- and β-(1–6)-glucan mouse mAbs differed by isotype and exhibited different biological activity against Candida [36]. Plasma from both cohorts also reacted with pustulan, a linear β-(1-6)-glucan. Pustulan-IgM and IgG were each significantly higher in the no–C-IRIS group, as reported for normal sera [14]. Although antibodies may also bind Lam via branched interchain hydrogen bonds [37], our data demonstrate reproducible differences in Lam-IgM between the cohorts at a substantially lower concentration than used in other studies [14]. In addition, a sample from 1 C-IRIS and 1 no–C-IRIS patient was inhibited by the aforementioned β-(1–3)-glucan mAb (data not shown). Unfortunately, we did not have enough of this mAb to test more sera.
HIV infection results in a deficiency of IgM+ memory B cells [38] and a myriad of B-cell defects, including apoptosis [39], reduced antibody levels [40], and dysfunctional B-cell differentiation [41]. Previous studies comparing HIV-infected and HIV-uninfected patients with cryptococcosis to normal (or HIV-infected) controls showed IgM+ memory B-cell levels were lower in patients with cryptococcosis. In our study, in which all patients had CM, B-cell levels did not differ between the C-IRIS and no–C-IRIS group. Notably, patients in both cohorts were acutely ill with extremely low CD4+ T-cell counts prior to ART initiation and had substantially lower IgM+ memory B-cell levels than those reported for other HIV-infected patients [7, 41]. Given that HIV infection is associated with impaired B-cell activation and deranged production of chemokines and cytokines [34, 42], lower antibody levels in C-IRIS patients in this study may reflect B-cell dysfunction. Consistent with this idea, pre-ART levels of plasma IL-5 and IL-7, each of which affect B-cell function [4], and CSF CCL2 and CCL3/CXCL10 ratios were higher in a subset of the C-IRIS cohort [43]. Human B cells also produce CXCL10 [44]. We did not seek associations between cytokine levels and our findings because ART-initiation cytokine levels were not available for more than half of the C-IRIS cohort we studied. Without a control group of HIV-infected patients that did not have C. neoformans, we could not examine effects of C. neoformans, HIV, ART, or coinfection on memory B-cell subsets or function.
We recognize that our data are descriptive and our discussion of how differences in natural antibodies between the C-IRIS and no–C-IRIS cohort may affect C-IRIS pathogenesis is speculative. Nonetheless, our findings are clear and suggest reduced levels of natural antibodies that react with β-glucans and/or GXM deserve further consideration as potential biomarkers of risk for C-IRIS at ART initiation. Lam-IgM levels increased post-ART in both cohorts, but the significant difference between the C-IRIS and no–C-IRIS group was unchanged. This leads us to wonder if the differences we observed may have been present at the time of presentation with CM. We can only speculate about this possibility, as preantifungal therapy samples were not available. Nonetheless, the pathogenesis of C-IRIS includes other factors in addition to ART initiation [2], including the inflammatory response to fungal antigens, which occurs in the setting of the proinflammatory effects of amphotericin [45]. As a shorter amphotericin induction period was noninferior to the standard 2-weeks regimen in resource-limited settings [46], it will be interesting to learn if this is also associated with a lower risk for C-IRIS. Notably, paradoxical clinical deterioration with antifungal therapy occurs in mice [47] and in HIV-uninfected patients [48, 49]. Therefore, control of the inflammatory response that may occur upon initiation of antifungal therapy may also be a critical determinant of clinical outcome in HIV-associated CM.
Our study has several limitations. As we did not have a control population with HIV infection alone, we cannot determine if HIV or C. neoformans infection or both affected antibody levels, and, as we did not have samples from the time of clinical presentation with CM, we could not assess the effect of antifungal therapy on risk for C-IRIS. Regarding our statistical analysis, we may have falsely claimed significance due to multiple comparisons done and the close correlation between some of the variables (Supplementary Table 1). However, associations between plasma IgM, Lam-IgM, and GXM-IgM further support the observed inverse association between natural antibodies and C-IRIS, as Lam and GXM antibodies may be produced by the same B-cell subsets [16]. Finally, given that C. neoformans sheds its capsule during infection, particularly in the presence of antifungals [50], shed GXM could form complexes and affect GXM antibody measurements. While this would underestimate antibody levels, acid dissociation of antibody complexes did not significantly alter GXM-Ig levels in another study [10].
In summary, our data revealed significant differences in plasma IgM, Lam-IgM, and GXM-IgM levels between patients who developed C-IRIS and those who did not before and after ART initiation. These findings are novel and important to the C. neoformans field as they contribute more data to support a role for antibody immunity in human cryptococcosis. We submit that one or a signature of these antibodies may predict risk for C-IRIS in patients on antifungal therapy. Given the devastating prognosis that C-IRIS carries, the ability to identify patients who may develop C-IRIS would improve the care and management of patients with CM. While more work is needed to determine mechanisms by which the antibody populations affect the pathogenesis of C-IRIS, we believe the associations identified in this study deserve further clinical and basic science investigation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. We thank Yiqun Wu (Albert Einstein College of Medicine) for assisting with the statistical analysis.
Financial support. This work was supported by the National Institutes of Health (grant number R01 AI 097096 to L. P.); Australian National Health and Medical Research Council Early Career Fellowship (grant number APP1092160 to C. C. C) and Practitioner Fellowship to S. R. L.; Howard Hughes Medical Institute International Early Career Scientist Award to T. N.; Wellcome Trust through the Sub-Saharan African Network for TB/HIV Research Excellence (grant number DEL-15-006 to T. N.); and the South African National Research Foundation Research Chairs Initiative to T. N.
Potential conflicts of interest. S. R. L. has received grants from National Health and Medical Research Council of Australia, American Foundation for AIDS Research, Danish Medical Council, University of Malaya, Gilead Sciences, Merck, ViiV, and Tetralogic/Shape Pharmaceuticals. T. N. has received grants from National Research Foundation of South Africa, Victor Daitz Foundation, and Wellcome Trust during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: ID Week 2016, New Orleans, LA, 28 October 2016, oral abstract 899, session 120.
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS 2013; 27:2089–99. [DOI] [PubMed] [Google Scholar]
- 4. Akilimali NA, Chang CC, Muema DM, et al. Plasma but not cerebrospinal fluid interleukin 7 and interleukin 5 levels pre-antiretroviral therapy commencement predict cryptococcosis-associated immune reconstitution inflammatory syndrome. Clin Infect Dis 2017; 65:1551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boulware DR, Bonham SC, Meya DB, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis 2010; 202:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subramaniam K, French N, Pirofski LA. Cryptococcus neoformans-reactive and total immunoglobulin profiles of human immunodeficiency virus-infected and uninfected Ugandans. Clin Diagn Lab Immunol 2005; 12:1168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Subramaniam K, Metzger B, Hanau LH, et al. IgM(+) memory B cell expression predicts HIV-associated cryptococcosis status. J Infect Dis 2009; 200:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jalali Z, Ng L, Singh N, Pirofski LA. Antibody response to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan in patients after solid-organ transplantation. Clin Vaccine Immunol 2006; 13:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rohatgi S, Gohil S, Kuniholm MH, et al. Fc gamma receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. MBio 2013; 4:e00573–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rohatgi S, Nakouzi A, Carreño LJ, et al. Antibody and B cell subset perturbations in human immunodeficiency virus-uninfected patients with cryptococcosis. Open Forum Infect Dis 2018; 5:ofx255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleuridor R, Lyles RH, Pirofski L. Quantitative and qualitative differences in the serum antibody profiles of human immunodeficiency virus-infected persons with and without Cryptococcus neoformans meningitis. J Infect Dis 1999; 180:1526–35. [DOI] [PubMed] [Google Scholar]
- 12. Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev 2004; 197:179–91. [DOI] [PubMed] [Google Scholar]
- 13. Feldmesser M, Kress Y, Mednick A, Casadevall A. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J Infect Dis 2000; 182:1791–5. [DOI] [PubMed] [Google Scholar]
- 14. Chiani P, Bromuro C, Cassone A, Torosantucci A. Anti-beta-glucan antibodies in healthy human subjects. Vaccine 2009; 27:513–9. [DOI] [PubMed] [Google Scholar]
- 15. Kondori N, Edebo L, Mattsby-Baltzer I. A novel monoclonal antibody recognizing beta(1-3) glucans in intact cells of Candida and Cryptococcus. APMIS 2008; 116:867–76. [DOI] [PubMed] [Google Scholar]
- 16. Rohatgi S, Pirofski LA. Molecular characterization of the early B cell response to pulmonary Cryptococcus neoformans infection. J Immunol 2012; 189:5820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dufaud C, Rivera J, Rohatgi S, Pirofski LA. Naive B cells reduce fungal dissemination in Cryptococcus neoformans infected Rag1-/- mice. Virulence 2018: 9:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rachini A, Pietrella D, Lupo P, et al. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun 2007; 75:5085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li SS, Ogbomo H, Mansour MK, et al. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat Commun 2018; 9:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de la Cruz J, Pintor-Toro JA, Benitez T, Llobell A. Purification and characterization of an endo-beta-1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J Bacteriol 1995; 177:1864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol 2010; 184:5755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cherniak R, Morris LC, Anderson BC, Meyer SA. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect Immun 1991; 59:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kozel TR. Characterization of cross-reactivity between cryptococcal polysaccharides by use of enzyme-linked immunosorbent assays and monoclonal antibodies. In: Drouhet E, ed. Fungal antigens. Boston, MA: Springer, 1988. [Google Scholar]
- 24. Read SM, Currie G, Bacic A. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr Res 1996; 281:187–201. [DOI] [PubMed] [Google Scholar]
- 25. Rohatgi S, Pirofski LA. Host immunity to Cryptococcus neoformans. Future Microbiol 2015; 10:565–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodrigues ML, Travassos LR, Miranda KR, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun 2000; 68:7049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torosantucci A, Tumbarello M, Bromuro C, et al. Antibodies against a beta-glucan-protein complex of Candida albicans and its potential as indicator of protective immunity in candidemic patients. Sci Rep 2017; 7:2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torosantucci A, Bromuro C, Chiani P, et al. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 2005; 202:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pietrella D, Rachini A, Torosantucci A, et al. A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine 2010; 28:1717–25. [DOI] [PubMed] [Google Scholar]
- 30. Zhong Z, Pirofski LA. Opsonization of Cryptococcus neoformans by human anticryptococcal glucuronoxylomannan antibodies. Infect Immun 1996; 64:3446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleuridor R, Lees A, Pirofski L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J Immunol 2001; 166:1087–96. [DOI] [PubMed] [Google Scholar]
- 32. Casadevall A, Cleare W, Feldmesser M, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother 1998; 42:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larsen RA, Pappas PG, Perfect J, et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother 2005; 49:952–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiesner DL, Boulware DR. Cryptococcus-related immune reconstitution inflammatory syndrome(IRIS): pathogenesis and its clinical implications. Curr Fungal Infect Rep 2011; 5:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. New JS, King RG, Kearney JF. Manipulation of the glycan-specific natural antibody repertoire for immunotherapy. Immunol Rev 2016; 270:32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torosantucci A, Chiani P, Bromuro C, et al. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS One 2009; 4:e5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lipke PN, Ovalle R. Cell wall architecture in yeast: new structure and new challenges. J Bacteriol 1998; 180:3735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol 2009; 9:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Milito A, Mörch C, Sönnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS 2001; 15:957–64. [DOI] [PubMed] [Google Scholar]
- 40. De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 2004; 103:2180–6. [DOI] [PubMed] [Google Scholar]
- 41. D’Orsogna LJ, Krueger RG, McKinnon EJ, French MA. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS 2007; 21:1747–52. [DOI] [PubMed] [Google Scholar]
- 42. Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med 2010; 7:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang CC, Omarjee S, Lim A, et al. Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome. J Infect Dis 2013; 208:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoff ST, Salman AM, Ruhwald M, et al. Human B cells produce chemokine CXCL10 in the presence of Mycobacterium tuberculosis specific T cells. Tuberculosis 2015; 95:40–7. [DOI] [PubMed] [Google Scholar]
- 45. Ben-Ami R, Lewis RE, Kontoyiannis DP. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis 2008; 47:226–35. [DOI] [PubMed] [Google Scholar]
- 46. Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018; 378:1004–17. [DOI] [PubMed] [Google Scholar]
- 47. Neal LM, Xing E, Xu J, et al. CD4+ T cells orchestrate lethal immune pathology despite fungal clearance during Cryptococcus neoformans meningoencephalitis. mBio 2017; 8:pii: e01415-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh N, Lortholary O, Alexander BD, et al. ; Cryptococcal Collaborative Transplant Study Group An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis 2005; 40:1756–61. [DOI] [PubMed] [Google Scholar]
- 49. Panackal AA, Wuest SC, Lin YC, et al. Paradoxical immune responses in non-HIV cryptococcal meningitis. PLoS Pathog 2015; 11:e1004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nosanchuk JD, Cleare W, Franzot SP, Casadevall A. Amphotericin B and fluconazole affect cellular charge, macrophage phagocytosis, and cellular morphology of Cryptococcus neoformans at subinhibitory concentrations. Antimicrob Agents Chemother 1999; 43:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.