Abstract
Purpose
Luminal breast cancer has a long natural history, with recurrences continuing beyond 10 years after diagnosis. We analyzed long-term follow-up (LTFU) of efficacy outcomes and adverse events in the Breast International Group (BIG) 1-98 study reported after a median follow-up of 12.6 years.
Patients and Methods
BIG 1-98 is a four-arm, phase III, double-blind, randomized trial comparing adjuvant letrozole versus tamoxifen (either treatment received for 5 years) and their sequences (2 years of one treatment plus 3 years of the other) for postmenopausal women with endocrine-responsive early breast cancer. When pharmaceutical company sponsorship ended at 8.4 years of median follow-up, academic partners initiated an observational, LTFU extension collecting annual data on survival, disease status, and adverse events. Information from Denmark was from the Danish Breast Cancer Cooperative Group Registry. Intention-to-treat analyses are reported.
Results
Of 8,010 enrolled patients, 4,433 were alive and not withdrawn at an LTFU participating center, and 3,833 (86%) had at least one LTFU report. For the monotherapy comparison of letrozole versus tamoxifen, we found a 9% relative reduction in the hazard of a disease-free survival event with letrozole (hazard ratio [HR], 0.91; 95% CI, 0.81 to 1.01). HRs for other efficacy end points were similar to those for disease-free survival. Efficacy of letrozole versus tamoxifen for contralateral breast cancer varied significantly over time (0- to 5-, 5- to 10-, and > 10-year HRs, 0.62, 0.47, and 1.35, respectively; treatment-by-time interaction P = .005), perhaps reflecting a longer carryover effect of tamoxifen. Reporting of specific long-term adverse events seemed more effective with national registry than with case-record reporting of clinical follow-up.
Conclusion
Efficacy end points continued to show trends favoring letrozole. Letrozole reduced contralateral breast cancer frequency in the first 10 years, but this reversed beyond 10 years. This study illustrates the value of extended follow-up in trials of luminal breast cancer.
INTRODUCTION
Hormone receptor–positive early breast cancer has a long natural history, with disease and potentially adverse events (AEs) occurring beyond 10 years since initiation of treatment.1,2 Because regulatory bodies often require, at most, 10 years of follow-up in clinical trials, pharmaceutical partners rarely fund data collection beyond this point. The current study describes extended follow-up of the Breast International Group (BIG) 1-98 trial, one of the pivotal studies establishing the role of aromatase inhibitors (AIs) in the adjuvant treatment of postmenopausal women with steroid hormone-receptor–positive early breast cancer.
The BIG 1-98 trial has been described elsewhere.3-8 Briefly, it compared 5 years of tamoxifen versus letrozole as monotherapy, and sequential treatment with 2 years of one of these drugs followed by 3 years of the other in postmenopausal women with hormone receptor–positive early breast cancer. In our most recent report,8 we found letrozole monotherapy provided a significant improvement in disease-free survival (DFS), overall survival, distant recurrence-free interval, and breast cancer–free interval (BCFI) compared with tamoxifen monotherapy at median follow-up of 8.1 years since randomization.
When the BIG 1-98 trial started as a Novartis-sponsored trial, it was intended to conduct long-term follow-up.3 In 2010, however, Novartis discontinued sponsorship and financing of follow-up. The International Breast Cancer Study Group (IBCSG), in collaboration with Breast International Group (BIG) partners, decided to continue follow-up and reporting from centers and groups that had conducted BIG 1-98 under contracts with academic organizations. This report presents results from the BIG 1-98 trial at a median follow-up of 12.6 years.
PATIENTS AND METHODS
Patients
BIG 1-98 was a randomized, phase III, double-blind trial that recruited postmenopausal women with early breast cancer positive for estrogen receptor, progesterone receptor, or both. The details of trial design, eligibility criteria, and study procedures for BIG 1-98 have been presented previously.3-8 Initially, from 1998 to 2000, in centers under contract with Novartis, women were randomly assigned to receive monotherapy with letrozole (Femara; Novartis, Basel, Switzerland) 2.5 mg orally daily or tamoxifen 20 mg orally daily for 5 years. In 1998, Novartis and IBCSG agreed to activate the four-arm BIG 1-98 trial in the BIG network, incorporate the prior two-arm randomizations and follow-up into BIG 1-98, and end random assignment to the two-arm option in 2000. Thus, from 1999 to 2003, patients were randomly assigned to one of four groups: monotherapy with tamoxifen or letrozole for 5 years or sequential therapy consisting of letrozole for 2 years followed by tamoxifen for 3 years, or tamoxifen for 2 years followed by letrozole for 3 years (four-arm option). The intention-to-treat (ITT) population included 8,010 patients from 247 participating centers in 27 countries. The schema for BIG 1-98 is shown in the Data Supplement.
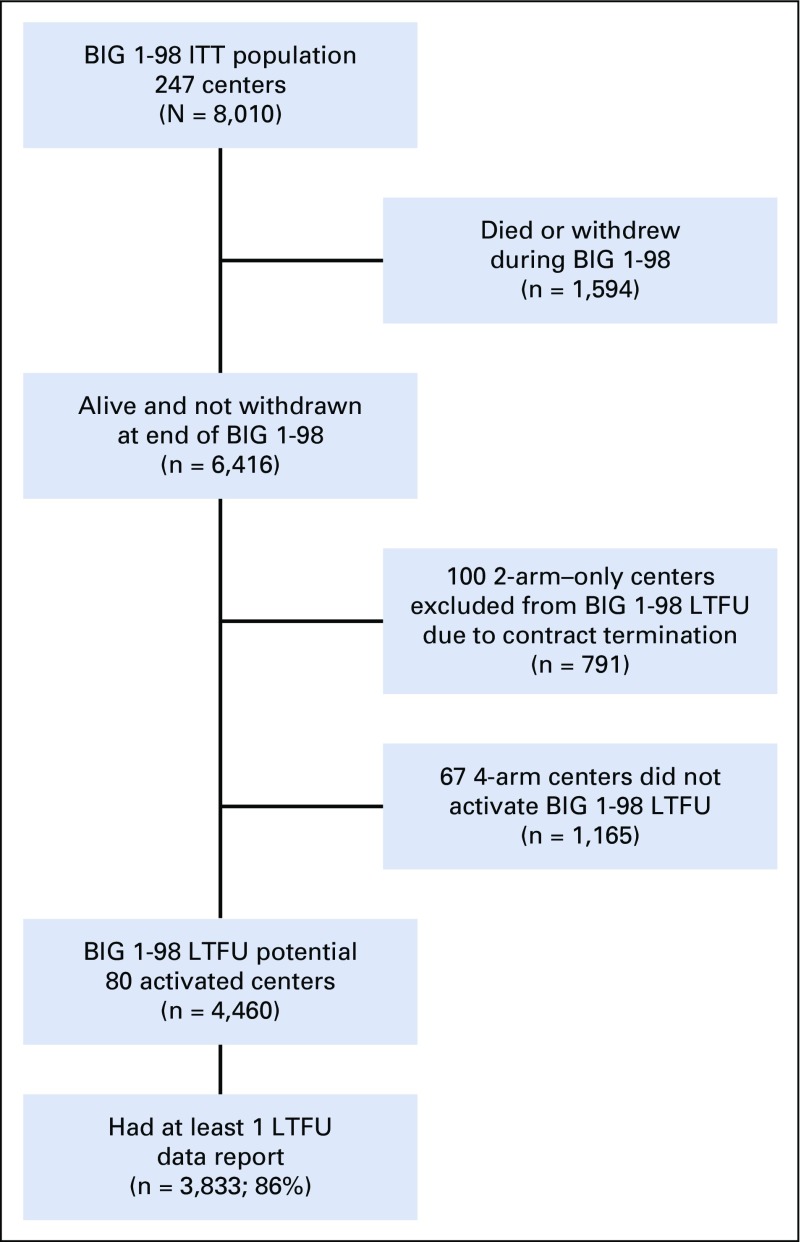
The BIG 1-98 long-term follow-up study (BIG 1-98 LTFU) was an observational extension of the BIG 1-98 trial, activated in 2011 to continue collection of a yearly update of survival, disease status, and long-term AEs beyond Novartis sponsorship. Patients who were alive and not withdrawn at the end of the original study could participate if they were enrolled from centers that had conducted the four-arm portion and had activated BIG 1-98 LTFU. A single-page data collection form was required annually for all patients, with additional pages as needed to document death, recurrence, second malignancy, specified AEs, or use of certain medications. Information was obtained by clinic visit, telephone contact with patient, general physician, or family member, or from the Danish Breast Cancer Cooperative Group (DBCG) Registry for Danish participants (Data Supplement). A summary of the number of patients participating in the LTFU study is shown in Figure 1. A detailed figure of patient disposition according to treatment group is available in the Data Supplement.
FIG 1.
Flow diagram of patients and centers providing data for efficacy analyses. Per contract, centers participating in the four-arm option (with or without prior participation in the two-arm option) could activate BIG 1-98 LTFU, whereas those participating only in the two-arm option could not activate BIG 1-98 LTFU. ITT, intention to treat; LTFU, long-term follow-up.
Statistical Analysis
The primary efficacy end point was DFS, defined as the time since random assignment to the first of the following events: invasive recurrence in local, regional, or distant sites; a new invasive cancer in the contralateral breast; any second (nonbreast) primary cancer; or death without a previous cancer event. Other end points previously reported were defined using standardized definitions for efficacy end points in adjuvant breast cancer trials criteria9 and include overall survival, distant recurrence-free interval, and invasive BCFI. We also analyzed breast cancer mortality, censoring deaths reported without a breast cancer event (ie, BCFI event), because median age at BIG 1-98 study entry was 61 years and deaths unrelated to breast cancer were expected during prolonged follow-up. Time to contralateral breast cancer was also analyzed, ignoring other events and censoring at last follow-up or death.
For each time-to-event end point, separate analyses were performed for the monotherapy comparison (letrozole v tamoxifen using patients randomly assigned during either two-arm or four-arm options) and for sequential therapy comparisons versus tamoxifen monotherapy. Analyses were by ITT principle according to random assignment, ignoring the fact that 619 (25.2%) of 2,459 patients assigned to tamoxifen for the monotherapy comparison and 612 (39.5%) of 1,548 assigned to tamoxifen for the four-arm comparisons (Data Supplement) selectively crossed over to receive letrozole after release of first results in 2005.3,5,8
Time-to-event end point analyses used data from all 8,010 patients in the BIG 1-98 ITT population. Because not all patients initially enrolled in BIG 1-98 were eligible for the BIG 1-98 LTFU study (Fig 1), weighted analyses as routinely applied in observational studies10-12 were used for Kaplan-Meier and Cox model estimates. The details of the weighting methods and other statistical analyses are presented in the Data Supplement. Briefly, intervals of risk and events recorded during the original trial follow-up received a weight of 1, whereas those recorded during LTFU received a weight > 1 such that the information provided by each patient observed in the LTFU study counted not only for herself but also for other women with similar disease, treatment, demographics, and original trial experiences who did not have LTFU data collection.
Cumulative incidence percentages for events defining efficacy end points were estimated as 100 minus the weighted Kaplan-Meier estimates. Weighted Cox models, stratified according to chemotherapy randomization stratum, estimated hazard ratios (HRs) and 95% CIs were determined on the basis of robust variance estimation. P values for tests of whether the HR differed from 1.0 were reported, not to make inference of whether the treatments were more efficacious than tamoxifen, but as a complement to the CI for judging the play of chance in these analyses. All Cox models were assessed for nonproportional hazards by including an interaction term between treatment and time (continuous) as a time-dependent covariate. In instances where the P value for interaction was < .15, a piecewise Cox model was used to estimate HRs over three predefined time intervals (0 to 5, 5 to 10, and > 10 years) to characterize the relationship between treatment effect and time (Data Supplement).
Prespecified AEs recorded during LTFU were summarized using number and incidence per 1,000 patient-years of follow-up. Because Danish centers reported a subset of the AE categories on the basis of the National Patient Registry, the incidences are presented overall and separately for Danish and non-Danish centers.
RESULTS
Population
Median follow-up for this extended analysis was 12.6 (interquartile range: 9.1 to 13.9) years, with a maximum follow-up of 17.7 years. This compares with a median follow-up of 8.1 (interquartile range: 7.3 to 9.5) years, with a maximum of 12.4 years at the most recent previous analysis of the original BIG 1-98 study.8 This LTFU report is based on a total of 83,271 patient-years of follow-up, a 32% increase in years of follow-up compared with the previous analysis.
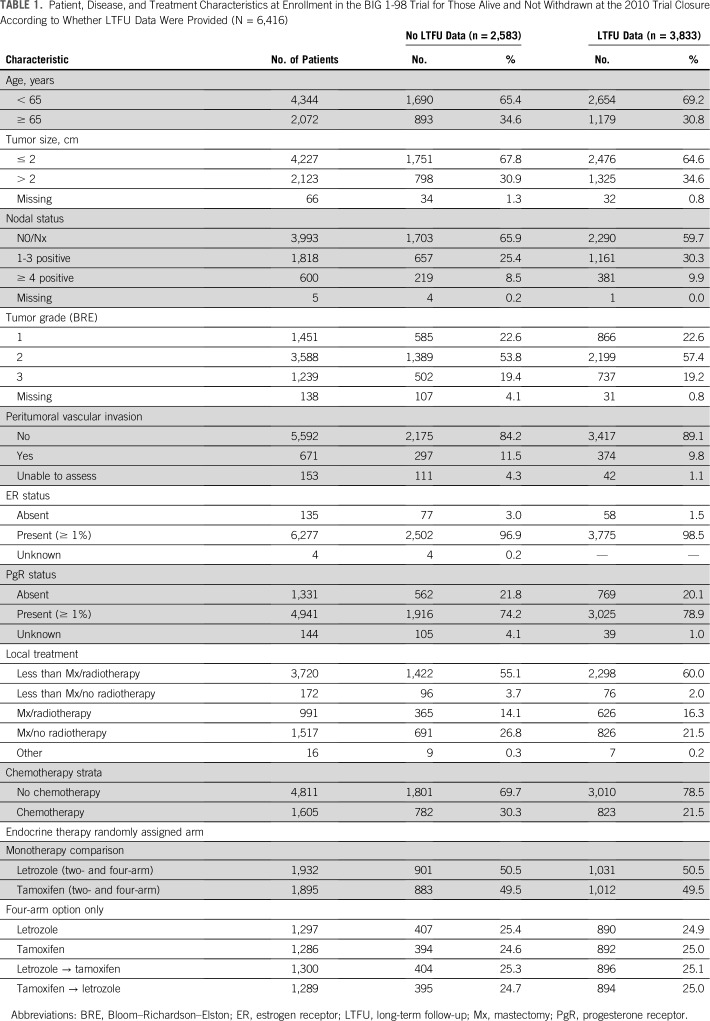
The characteristics of the patients with at least one LTFU contact were compared with those of the patients who were alive and not withdrawn at the end of the original BIG 1-98 but who did not have data provided for LTFU (Table 1). The patients with LTFU data were slightly younger at the time of enrolment in BIG 1-98 and had more advanced disease with slightly less node-negative disease and T1 stage compared with the cohort without any LTFU information. Fewer patients with BIG 1-98 LTFU data received adjuvant chemotherapy, and more received breast-conserving surgery than those not submitting LTFU data. There were no imbalances according to randomized treatment assignment.
TABLE 1.
Patient, Disease, and Treatment Characteristics at Enrollment in the BIG 1-98 Trial for Those Alive and Not Withdrawn at the 2010 Trial Closure According to Whether LTFU Data Were Provided (N = 6,416)
Efficacy Results
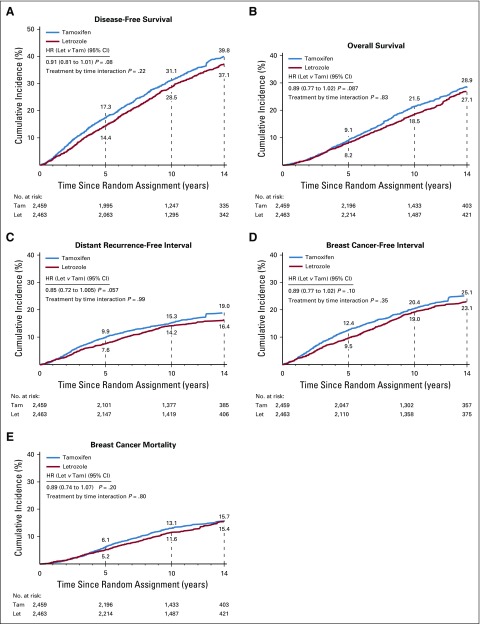
The cumulative incidence of efficacy end point events for the monotherapy comparison of letrozole for 5 years versus tamoxifen for 5 years are shown in Figure 2. There was a nonsignificant, 9% reduction in the hazard of a DFS event with letrozole compared with tamoxifen over the entire time of observation (HR, 0.91; 95% CI, 0.81 to 1.01; P = .08; Fig 2A). The early effect of letrozole was maintained over time; however, the magnitude in favor of letrozole seemed to be slightly diminished compared with the results at 8.1 years of median follow-up. For the monotherapy population, there was no evidence of variation of the treatment effect over time (treatment-by-time interaction P = .22).
FIG 2.
Cumulative incidence of events defining five efficacy end points for the monotherapy comparison of letrozole (Let; n = 2,463) versus tamoxifen (Tam; n = 2,459) based on weighted analyses incorporating data from the BIG 1-98 original study and the data collected during the BIG 1-98 LTFU observational component. HR, hazard ratio.
Other end points including overall survival, time to distant recurrence-free interval, and BCFI maintained similar HRs as in earlier reports, though the magnitude of the advantage for letrozole was numerically reduced (Fig 2B-2D; see Data Supplement for results of ITT analyses at 8.1 years and 12.6 years of follow-up).
Breast cancer mortality showed no evidence of variation of the treatment effect over time (treatment-by-time interaction P = .80) in the monotherapy comparison. The results showed a nonsignificant 11% reduction in breast cancer mortality with letrozole (HR, 0.89; 95% CI, 0.74 to 1.07; P = .20; Fig 2E).
Efficacy end point results according to nodal status are shown in the Data Supplement. The HRs comparing letrozole versus tamoxifen were similar across nodal groups, but, as expected, the absolute differences favoring letrozole were greater for the node-positive cohort.
Contralateral and Nonbreast Primaries
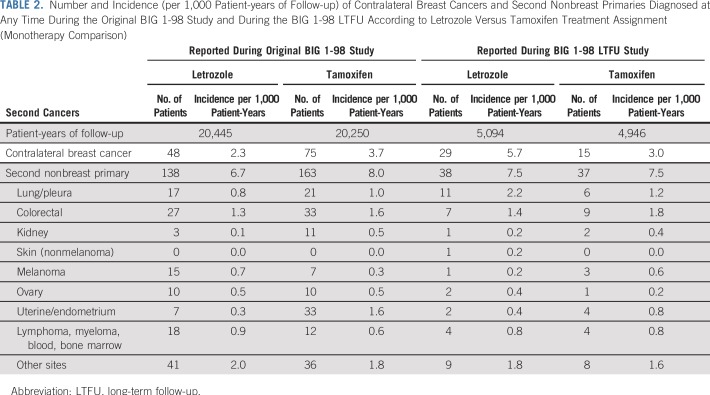
We detected a clear benefit of letrozole over tamoxifen in preventing contralateral breast cancer over the first 10 years, which was numerically reversed over the period beyond 10 years. During the original study, 48 contralateral breast cancer events were reported in the letrozole arm versus 75 in the tamoxifen arm, whereas during the LTFU study, there were 29 in the letrozole arm and 15 in the tamoxifen arm (Table 2). HRs for time to contralateral breast cancer for letrozole versus tamoxifen were 0.62 (95% CI, 0.36 to 1.09) for years 0 to 5, 0.47 (95% CI, 0.23 to 0.97) for years 5 to 10, and 1.35 (0.53 to 3.41) for years 10 or longer (treatment-by-time interaction P = .005).
TABLE 2.
Number and Incidence (per 1,000 Patient-years of Follow-up) of Contralateral Breast Cancers and Second Nonbreast Primaries Diagnosed at Any Time During the Original BIG 1-98 Study and During the BIG 1-98 LTFU According to Letrozole Versus Tamoxifen Treatment Assignment (Monotherapy Comparison)
Throughout follow-up, there was an 18% increase in the occurrence of second nonbreast primaries in patients receiving tamoxifen monotherapy, largely owing to an excess of endometrial cancer during the original study period (Table 2).
AEs Reported Clinically and by a Registry
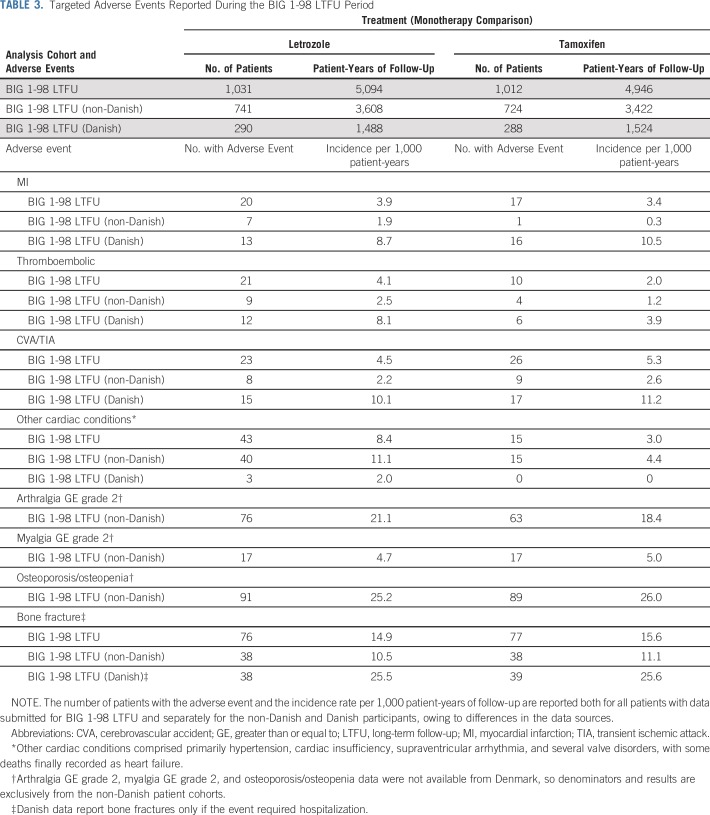
Most of the predefined AEs were reported at similar rates in the two arms during LTFU (Table 3). Of particular note is the lack of difference in osteoporosis/osteopenia and fracture rate between tamoxifen and letrozole. Myocardial infarction and cerebrovascular events showed no significant differences between the arms, but a higher rate of other cardiac events (hypertension, cardiac insufficiency, supraventricular arrhythmia and valve disorders) was reported in the letrozole arm. Thromboembolic events were more commonly reported in the letrozole arm during LTFU.
TABLE 3.
Targeted Adverse Events Reported During the BIG 1-98 LTFU Period
In this trial two different kinds of reporting of long-term AEs were used. The data of Danish patients were uploaded annually from DBCG (capturing data from nationwide Danish health registries). As seen in Table 3, the Danish DBCG registry reported more specific events like myocardial infarction, transient ischemic attack or cerebrovascular accident, and thromboembolism, whereas additional “other cardiac events” were recorded from centers using nonregistry reporting.
Comparisons of Sequential Arms Versus Monotherapy
The cumulative incidence of efficacy end points for the four-arm comparisons of letrozole, tamoxifen, and their sequences are shown in the Data Supplement. Outcomes achieved with the sequence of letrozole taken for 2 years followed by tamoxifen for 3 years were close to those obtained by assignment to 5 years of letrozole monotherapy. For example, cumulative incidence for a DFS event, comparing letrozole monotherapy versus the letrozole to tamoxifen sequence, were within one percentage point of each other during follow-up: 12.3% versus 12.6% at 5 years, 26.0% versus 26.8% at 10 years, and 35.5% versus 36.2% at 14 years, respectively.
DISCUSSION
Over the past 20 years, there has been a trend to cease follow-up of adjuvant therapy clinical trials after approximately 10 years, which has limited the available data about AIs for postmenopausal women. Other adjuvant endocrine therapy trials like the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial, and the Intergroup Exemestane Study (IES) reported their long-term outcome at 10 years,13-15 whereas the Austrian Breast and Colorectal Cancer Study Group (ABCSG) reported a cohort of their Trial 8 at 11 years correlating the risk of late recurrence with PAM50 risk of recurrence.16,17 The present report of LTFU at a median of 12.6 years in the BIG 1-98 study thus represents the longest currently available follow-up of trials investigating adjuvant AI therapy. Methodologically, the additional long-term follow-up constitutes a long-term observational study within a clearly defined study subpopulation from a prospective randomized trial. We used a weighted analysis methodology to include efficacy data for patients unavailable for LTFU. Another special feature of this investigation was the availability of efficacy end point data and AEs captured annually from a central registry for all Danish participants.
The protocol-defined primary DFS end point still showed a relative risk reduction of 9% in favor of the letrozole arm after a median follow-up of 12.6 years, the same as the 9% risk reduction seen with anastrozole in the 10-year analysis of the ATAC trial.13 In BIG 1-98, the letrozole advantage in terms of DFS in this ITT analysis was marginally less than in previous reports. The other efficacy end points also showed slightly attenuated HRs in favor of the letrozole arm compared with the previous ITT analysis after 8.1 years of follow-up (see Data Supplement for discussion).8
A reason for some attenuation of letrozole benefit in DFS over time may be the accumulation of nonbreast events as a result of the age of the observed population. The median age at inclusion was 61 years; after 12.6 years of follow-up, on average, many of these women are approaching their eighties and are at risk for death without a previous cancer event. The end point breast cancer mortality, perhaps the least likely to be diluted by intercurrent deaths, showed a relative hazard reduction of 11% in favor of letrozole therapy. Another reason for reduced DFS difference could be a more effective carryover of prevention of contralateral breast cancer seen with tamoxifen during LTFU. The long carryover effect of tamoxifen was also seen in the 16-year follow-up of the IBIS-l study, in which 5 years of preventive tamoxifen was compared with placebo and the preventive effect of tamoxifen remained similar throughout 20 years.18 In the long-term follow-up of ATAC and the IES, more contralateral breast cancer events were reported in the tamoxifen arm, but without analysis of the incidence during different time periods.13,14 New primary cancers at nonbreast sites were numerically similar in the various treatment arms during BIG 1-98 LTFU. Finally, the selective crossover to letrozole of 25.2% of patients assigned to tamoxifen for the monotherapy comparison and 39.5% of the patients assigned to tamoxifen for the four-arm comparison could also have contributed to improved outcome for those assigned to tamoxifen and the attenuation of letrozole benefit observed in these ITT analyses.
We found no relevant differences between the arms regarding occurrence of myocardial infarction, cerebrovascular events, osteoporosis, or fracture rates during LTFU. These reassuring observations are consistent with the result of the long-term follow-up of the ATAC trial.13 Previously expressed concerns about the potential long-term increased risk for ischemic cardiovascular events associated with AI therapy19 seem to be unfounded. The thromboembolic event rate was lower in the tamoxifen arm during LTFU. This may reflect more effective prophylaxis administered for patients deemed to be at higher risk as a result of earlier tamoxifen exposure. The higher incidence of other cardiac events in the letrozole arm included many different disorders and is difficult to interpret in an ageing population.
This study affords the opportunity to compare AE reports from clinical and registry sources. For the monotherapy comparison (Table 3), 29 (78%) of the 37 myocardial infarctions were reported from Danish centers, which had 28% of LTFU patients. The results illustrate the value of national health registries in those countries such as Denmark, which include detailed clinical information on registered patients with cancer.
An important feature of the BIG 1-98 study is the ability to compare letrozole monotherapy for 5 years versus the sequence of letrozole for 2 years followed by tamoxifen for 3 years. AIs and tamoxifen have toxicity profiles leading to nonadherence in a meaningful proportion of patients. The similar outcomes in the four-arm comparison for letrozole monotherapy or the sequence of letrozole followed by tamoxifen suggest switching to tamoxifen instead of stopping all therapy is a viable option for patients who are intolerant to initial AI therapy, especially because the toxicity profiles of an AI and tamoxifen are often individually different.
Although there are challenges to the successful conduct of LTFU,20 it is important to prospectively plan for the long-term follow-up of clinical trials that support regulatory approval of a new widely used treatment regimen, especially for a disease such as early, endocrine-responsive breast cancer. A limitation of our study is the closure of 167 of the 247 participating centers upon withdrawal of pharmaceutical company support, resulting in attenuated follow-up for approximately 30% of eligible patients (Fig 1). This limitation was mitigated by use of weighted analyses adjusting for differences in characteristics between patients with and without LTFU data, and the fact that closure of entire centers rather than exclusion of individual patients accounted for 75% of patients without LTFU data.
The BIG 1-98 LTFU study shows continued, albeit modest and slightly attenuated, benefit of initial letrozole rather than tamoxifen for postmenopausal women with endocrine-responsive early breast cancer. Contralateral breast cancer incidence was lower in the first 10 years with letrozole, whereas after year 10 it was less frequent following tamoxifen with a statistically significant interaction between treatment and time. Second nonbreast primary cancer was not different during LTFU. The reporting of some long-term AEs was apparently more complete from a nationwide clinical registry than with routine clinical follow-up.
ACKNOWLEDGMENT
We thank the women, pathologists, physicians, nurses, and data managers who participated in the BIG 1-98 clinical trial and the BIG 1-98 long-term follow-up observational study.
Footnotes
Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 6-10, 2016.
The Breast International Group (BIG) 1-98 trial was initially financed by Novartis and coordinated by the International Breast Cancer Study Group (IBCSG). IBCSG supported the BIG 1-98 LTFU observational study. Other support for the IBCSG was provided by The Swedish Cancer Society, The Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, US National Cancer Institute Grant No. CA-75362, Cancer Research Switzerland/Oncosuisse, and the Foundation for Clinical Cancer Research of Eastern Switzerland.
Written on behalf of the BIG 1-98 Collaborative Group and the International Breast Cancer Study Group (IBCSG). The investigators in the BIG 1-98 Collaborative Group and the IBCSG are listed in the Data Supplement pages 2-7.
Clinical trial information: NCT00004205
AUTHOR CONTRIBUTIONS
Conception and design: Anita Giobbie-Hurder, Maj-Britt Jensen, Bent Ejlertsen, Edda Simoncini, Richard D. Gelber, Alan S. Coates, Aron Goldhirsch, Beat Thürlimann, Meredith M. Regan
Provision of study material or patients: Thomas Ruhstaller, Marco Colleoni, Bent Ejlertsen, Evandro de Azambuja, Vernon J. Harvey, Carlo Tondini, Lucia Del Mastro, Corinne Veyret, Lorenzo Gianni, Christoph Rochlitz, Khalil Zaman, Jacek Jassem, Aron Goldhirsch, Beat Thürlimann
Collection and assembly of data: Thomas Ruhstaller, Anita Giobbie-Hurder, Marco Colleoni, Maj-Britt Jensen, Bent Ejlertsen, Evandro de Azambuja, Patrick Neven, István Láng, Erik Hugger Jakobsen, Laurence Gladieff, Hervé Bonnefoi, Vernon J. Harvey, Simon Spazzapan, Lucia Del Mastro, Corinne Veyret, Edda Simoncini, Lorenzo Gianni, Christoph Rochlitz, Elena Kralidis, Khalil Zaman, Richard D. Gelber, Aron Goldhirsch, Beat Thürlimann, and Meredith M. Regan
Data analysis and interpretation: Thomas Ruhstaller, Anita Giobbie-Hurder, Maj-Britt Jensen, Evandro de Azambuja, Patrick Neven, István Láng, Carlo Tondini, Corinne Veyret, Khalil Zaman, Jacek Jassem, Martine Piccart-Gebhart, Angelo Di Leo, Richard D. Gelber, Alan S. Coates, Aron Goldhirsch, Beat Thürlimann, Meredith M. Regan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Letrozole and Tamoxifen Alone or Sequentially for Postmenopausal Women With Hormone Receptor–Positive Breast Cancer: Long-Term Follow-Up of the BIG 1-98 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Thomas Ruhstaller
Honoraria: Pfizer
Consulting or Advisory Role: Novartis, Roche, AstraZeneca, Eli Lilly
Travel, Accommodations, Expenses: Roche, Amgen, Pierre Fabre
Marco Colleoni
Honoraria: Novartis
Consulting or Advisory Role: Pierre Fabre, Pfizer, OBI Pharma, Puma Biotechnology, Celldex, AstraZeneca
Maj-Britt Jensen
Honoraria: AstraZeneca
Travel, Accommodations, Expenses: Celgene, Novartis, AstraZeneca
Bent Ejlertsen
Research Funding: NanoString Technologies (Inst), Roche (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche; AstraZeneca
Evandro De Azambuja
Honoraria: Roche
Consulting or Advisory Role: Roche
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: Roche, GlaxoSmithKline, Novartis
Patrick Neven
Consulting or Advisory Role: Novartis (Inst), Eli Lilly (Inst), Ipsen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche
Erik Hugger Jakobsen
Consulting or Advisory Role: Novartis, Pfizer, Roche Eli Lilly
Travel, Accommodations, Expenses: Roche, Pfizer
Laurence Gladieff
Honoraria: AstraZeneca, Tesaro, Clovis Oncology, Roche
Travel, Accommodations, Expenses: Roche, PharmaMar, Tesaro
Hervé Bonnefoi
Consulting or Advisory Role: AbbVie, Astellas Pharma, Bayer, Innocrin Pharma, Puma Biotechnology, Pfizer
Research Funding: Bayer (Inst), Roche
Travel, Accommodations, Expenses: Pfizer
Simon Spazzapan
Honoraria: Takeda, Novartis, Roche, Eisai, Celgene, Pierre Fabre
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Roche Italia, Celgene, Teva
Carlo Tondini
Consulting or Advisory Role: Myriad Genetics
Speakers' Bureau: Amgen
Travel, Accommodations, Expenses: Roche, Novartis, Celgene
Lucia Del Mastro
Honoraria: Roche, Pfizer, Ipsen, Eli Lilly, Eisai, Novartis, Takeda
Consulting or Advisory Role: Eli Lilly, Roche, MSD
Travel, Accommodations, Expenses: Roche, Pfizer, Celgene
Edda Simoncini
Consulting or Advisory Role: Genomic Health, Amgen, Pfizer, Novartis, AstraZeneca
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Eli Lilly, Roche, Ipsen, Celgene
Lorenzo Gianni
Consulting or Advisory Role: Celgene, AstraZeneca, Amgen, Pfizer, Roche
Travel, Accommodations, Expenses: Roche, Novartis, Pfizer
Christoph Rochlitz
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Roche, Novartis, Amgen
Elena Kralidis
Honoraria: Novartis
Travel, Accommodations, Expenses: Eli Lilly, Astellas Pharma, Vifor Pharma, Amgen
Khalil Zaman
Consulting or Advisory Role: Amgen (Inst), AstraZeneca (Inst), Celgene (Inst), Genomic Health, Eli Lilly (Inst), Novartis (Inst) Pfizer (Inst), Eisai (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, Celgene, AstraZeneca
Jacek Jassem
Consulting or Advisory Role: AstraZeneca, AbbVie, G1 Therapeutics, Merck Boehringer, Bristol-Myers Squibb, Celgene, Eisai, Pfizer, Pierre Fabre, Roche
Travel, Accommodations, Expenses: Roche
Martine Piccart-Gebhart
Consulting or Advisory Role: AstraZeneca, Eli Lilly, MSD, Novartis, Pfizer, Roche, Crescendo Biologics, Periphagen, HUYA Bioscience International, Debiopharm Group, PharmaMar, Odonate Therapeutics, G1 Therapeutics, Menarini, Seattle Genetics
Research Funding: AstraZeneca (Inst), Eli Lilly (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Radius Health (Inst), Synthon (Inst), Servier (Inst)
Other Relationship: Radius Health
Angelo Di Leo
Honoraria: Roche, Novartis, Pfizer, AstraZeneca, Genomic Health, Eisai, Eli Lilly, Pierre Fabre, Bayer, Celgene, Amgen, Daiichi Sankyo, Ipsen, Roche
Consulting or Advisory Role: Roche, Novartis, Pfizer, AstraZeneca, Pierre Fabre, Eli Lilly, Bayer, Celgene, Puma Biotechnology, Daiichi Sankyo, Ipsen, Genomic Health, Genentech, Amgen, Eisai
Research Funding: Novartis, Pfizer, AstraZeneca
Travel, Accommodations, Expenses: Roche, Novartis, Pfizer, AstraZeneca, Eisai, Bayer, Celgene, Eli Lilly, Pierre Fabre, Puma Biotechnology, Daiichi Sankyo
Richard D. Gelber
Research Funding: AstraZeneca (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Merck (Inst), Pfizer (Inst), Ipsen (Inst), Ferring (Inst)
Beat Thürlimann
Stock and Other Ownership Interests: Roche (I), Novartis (I)
Consulting or Advisory Role: Roche, AstraZeneca, Pfizer, Amgen, Eli Lilly
Travel, Accommodations, Expenses: Roche
Meredith M. Regan
Consulting or Advisory Role: Merck, Ipsen (Inst)
Research Funding: Veridex (Inst), OncoGenex (Inst), Pfizer (Inst), Ipsen (Inst), Novartis (Inst), Merck (Inst), Ferring (Inst), Celgene (Inst), AstraZeneca (Inst), Pierre Fabre (Inst), Ipsen (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.1016/S0140-6736(11)60993-8. Early Breast Cancer Trialists′ Collaborative Group (EBCTCG), Davies C, Godwin J, et al: Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. 378: 771-784, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1056/NEJMoa1701830. Early Breast Cancer Trialists′ Collaborative Group (EBCTCG), Pan H, Gray R, et al: 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836-1846, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2005 Breast International Group (BIG) 1-98 Collaborative, Thürlimann B, Keshaviah A, et al: A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747-2757, [Google Scholar]
- 4.2007 Breast International Group (BIG) 1-98 Collaborative, Coates AS, Keshaviah A, et al: Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol 25:486-492, [Google Scholar]
- 5.2009 Breast International Group (BIG) 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, et al: Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 361:766-776, [Google Scholar]
- 6.Giobbie-Hurder A, Price KN, Gelber RD, et al. : Design, conduct, and analyses of Breast International Group (BIG) 1-98: A randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clin Trials 6:272-287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan MM, Price KN, Giobbie-Hurder A, et al. : Interpreting Breast International Group (BIG) 1-98: A randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Breast Cancer Res 13:209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan MM, Neven P, Giobbie-Hurder A, et al. : Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: The BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol 12:1101-1108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudis CA, Barlow WE, Costantino JP, et al. : Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol 25:2127-2132, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Curtis LH, Hammill BG, Eisenstein EL, et al. : Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care 45:S103-S107, 2007. (10, Suppl 2) [DOI] [PubMed] [Google Scholar]
- 11.Hirano K, Imbens GW: Estimation of causal effects using propensity score weighting: An application to data on right heart catheterization. Health Serv Outcomes Res Methodol 2:259-278, 2001 [Google Scholar]
- 12.Therneau TM, Grambsch PM: Modeling Survival Data: Extending the Cox Model. New York, NY, Springer-Verlag, 2000 [Google Scholar]
- 13.Cuzick J, Sestak I, Baum M, et al. : Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11:1135-1141, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Morden JP, Alvarez I, Bertelli G, et al. : Long-term follow-up of the Intergroup Exemestane Study. J Clin Oncol 35:2507-2514, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derks MGM, Blok EJ, Seynaeve C, et al. : Adjuvant tamoxifen and exemestane in women with postmenopausal early breast cancer (TEAM): 10-year follow-up of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 18:1211-1220, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Gnant M, Sestak I, Filipits M, et al. : Identifying clinically relevant prognostic subgroups of postmenopausal women with node-positive hormone receptor-positive early-stage breast cancer treated with endocrine therapy: A combined analysis of ABCSG-8 and ATAC using the PAM50 risk of recurrence score and intrinsic subtype. Ann Oncol 26:1685-1691, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Sestak I, Cuzick J, Dowsett M, et al. : Prediction of late distant recurrence after 5 years of endocrine treatment: A combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 33:916-922, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Cuzick J, Sestak I, Cawthorn S, et al. : Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I Breast Cancer Prevention Trial. Lancet Oncol 16:67-75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannock IF: 10-year analysis of the ATAC trial: Wrong conclusion? Lancet Oncol 12:216-217, author reply 217, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Cuzick J: Statistical controversies in clinical research: Long-term follow-up of clinical trials in cancer. Ann Oncol 26:2363-2366, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]