Abstract
Purpose
Infants with stage 4S neuroblastoma usually have favorable outcomes with observation or minimal chemotherapy. However, young infants with symptoms secondary to massive hepatomegaly or with unfavorable tumor biology are at high risk of death. Our aim was to improve outcomes for patients with symptomatic and/or unfavorable biology 4S neuroblastoma with a uniform treatment approach using a biology- and response-based algorithm.
Patients and Methods
The subset of patients with 4S disease with MYCN-not amplified tumors with impaired or impending organ dysfunction, or with unfavorable histology and/or diploid DNA index, were eligible. Patients were assigned to receive two, four, or eight cycles of chemotherapy on the basis of histology, diploid DNA index, chromosome arm 1p or 11q loss of heterozygosity (LOH) status, and symptoms.
Results
Forty-nine eligible patients were enrolled: 41 were symptomatic and 28 had unfavorable biology. Seventeen patients (symptomatic, favorable biology) were assigned two cycles, 21 patients (any unfavorable biologic feature without 1p or 11q LOH) were assigned four cycles, and 11 patients (unfavorable biology including 1p and/or 11q LOH [n = 7] or symptomatic with unknown biology [n = 4]), were assigned eight cycles. The 3-year overall survival was 81.4% ± 5.8%. Eight of nine deaths were in patients younger than 2 months of age at diagnosis (median, 9 days [range, 1 to 68 days]): five acute deaths were a result of hepatomegaly and associated toxicities; two were a result of late relapse in patients with unfavorable biology; and two were a result of treatment complications. No deaths occurred after protocol-mandated pre-emptive treatment of infants younger than 2 months with hepatomegaly, regardless of symptoms. A new scoring algorithm for emergent chemotherapy in patients with 4S disease was developed on the basis of this experience.
Conclusion
The outcome for 4S neuroblastoma can be improved with pre-emptive chemotherapy for evolving hepatomegaly or other baseline comorbidities in infants younger than 2 months of age.
INTRODUCTION
Since 1971,1 stage 4S neuroblastoma has been characterized by a unique pattern of disseminated disease in infants, with a surprisingly good prognosis. The International Neuroblastoma Staging System defines 4S neuroblastoma as age younger than 12 months, with International Neuroblastoma Staging System stage I or II primary tumor and metastases limited to liver, skin, and bone marrow (< 10%) involvement.2 Approximately 50% of infants with 4S neuroblastoma undergo spontaneous regression without any therapeutic intervention.3,4 However, there exists a subset of patients with 4S disease with a worse prognosis.5-8 Features associated with increased mortality include very young age at diagnosis, massive hepatomegaly with associated organ dysfunction such as coagulopathy, respiratory compromise, and abdominal compartment syndrome,4,6,9 or tumors with unfavorable biologic features.8 Reported 4S survival rates from clinical trials are generally greater than 90%, but many, including recent Children’s Oncology Group (COG) and European trials, had eligibility criteria including a requirement for tumor biopsy and/or strict organ performance guidelines, which may have biased the populations studied.10-14 To address these issues, the COG designed ANBL0531 to allow patients with clinically symptomatic 4S disease, or those at risk of impending organ dysfunction, to enroll without requirement for tissue biopsy for risk assignment. The objective of this study was to maintain 90% survival in this higher-risk subset of 4S with protocol-defined signs of organ dysfunction related to tumor progression, and to adjust chemotherapy cycles from the prior COG study according to tumor biology (including loss of heterozygosity [LOH] of 1p or 11q), excepting MYCN gene amplification (assigned to high-risk therapy). We aimed to evaluate specific baseline abnormalities present at diagnosis to determine whether these predicted for morbidity and mortality, and to reduce surgical morbidity by eliminating the requirement for complete resection of the primary tumor.
PATIENTS AND METHODS
Between October 8, 2007, and June 30, 2011, 49 eligible, evaluable patients with 4S neuroblastoma were enrolled. Patients were younger than 365 days of age, with newly diagnosed 4S and either clinically symptomatic or developing organ dysfunction from progressing disease. In addition, patients with 4S disease with tumors that were MYCN-not amplified (NA) but showed unfavorable histology by International Neuroblastoma Pathology Classification (high MKI or undifferentiated) and/or diploid DNA index (DI = 1) were defined as having unfavorable biology and were also eligible.15 After amendment in 2009, asymptomatic patients with 4S disease judged to have impending organ impairment caused by rapidly evolving hepatomegaly were to receive emergent chemotherapy, regardless of tumor biology or ability to undergo biopsy. Treatment group assignment was determined by tumor biology (Table 1). The duration of therapy was determined by resolution of symptoms and tumor response.
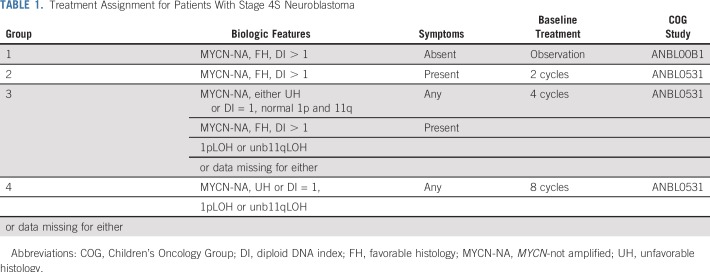
TABLE 1.
Treatment Assignment for Patients With Stage 4S Neuroblastoma
The International Neuroblastoma Pathology Classification, MYCN copy number, DI, and LOH at 1p36 and 11q23 (by multiplexed fluorescence polymerase chain reaction16) were determined in the COG Neuroblastoma Reference Laboratory. Asymptomatic patients with favorable biology tumors, defined as favorable histology (FH), MYCN-NA, hyperdiploidy (DI > 1), regardless of 1p/11q status, were designated as having low-risk disease and were assigned to group 1 for observation only (COG biology study ANBL00B1); therefore, they were not eligible for ANBL0531. Group 2 included symptomatic patients with favorable biology tumors (FH, DI > 1, normal 1p/11q), assigned to receive two cycles of chemotherapy. Group 3 patients, regardless of symptoms, had tumors with one unfavorable biologic feature (UH and any ploidy, or FH and DI = 1, with normal 1p/11q; or symptomatic patients with FH, DI greater than 1, with 1p and/or unbalanced 11q LOH) and were assigned four cycles of chemotherapy. Group 4 included patients whose tumors had 1p and/or 11q LOH with, in addition, either UH or DI = 1 and were assigned to eight cycles. Patients too ill to biopsy were assigned to group 4. If any biomarker was missing, it was considered to be unfavorable for the purpose of treatment assignment.
Chemotherapy consisted of alternating cycles of drug combinations with carboplatin, etoposide, cyclophosphamide, and doxorubicin, as reported previously for intermediate-risk neuroblastoma.10 Filgrastim was mandated for patients younger than 60 days of age. Emergent initiation of therapy was allowed before enrollment in patients who were symptomatic, or who were at impending risk to become symptomatic. Immediate initiation of chemotherapy for infants younger than 2 months of age with evolving hepatomegaly was strongly advised. This was made more explicit with a protocol amendment and specific education of treating physicians in COG after five deaths in young patients because of rapidly progressive hepatomegaly.
Resection of primary tumor was not required. Radiation therapy was allowed for symptomatic hepatomegaly unresponsive to chemotherapy. Successful completion of therapy was defined as completion of assigned chemotherapy, resolution of symptoms, achievement of greater than or equal to 50% reduction in primary tumor volume, and elimination of bone marrow and skin metastases. Liver metastases were not used in evaluation of response because of the known difficulty in interpreting imaging.17 Patients could receive up to eight cycles of chemotherapy as needed to meet the criteria for completion of therapy.
Baseline assessment of organ dysfunction was obtained for all patients at study entry to identify clinical predictors of early morbidity and mortality. Targeted baseline abnormalities using Common Terminology Criteria for Adverse Events (version 4.0) included respiratory compromise, coagulopathy (partial thromboplastin time international normalized ratio, fibrinogen), liver dysfunction (bilirubin, albumin, aspartate aminotransferase, alanine aminotransferase), and failure to thrive (anorexia). The protocol was approved by institutional review boards, and informed consent was obtained for all patients.
ANBL0531 was a prospective, nonrandomized, phase III clinical trial to evaluate the reduction of therapy for intermediate-risk neuroblastoma. Herein, the subgroup of patients with 4S disease has been analyzed separately. The relationships among toxicity, symptoms and vital status were explored using Fisher's exact test. For event-free survival (EFS), time to event was calculated from diagnosis to first occurrence of relapse, progression, death, or secondary malignancy, or time to last contact if no event occurred. For overall survival (OS), the event was death from any cause. Survival estimates were calculated according to Kaplan-Meier18 and are reported at 3 years ± SE,19 and curves were compared using a two-sided log-rank test.
In the subgroup of symptomatic patients, an optimal cut point for age at diagnosis was determined, to test when these patients were most at risk of death, by maximizing the Youden index (maximum of [sensitivity + specificity − 1] over possible values of age at diagnosis) with respect to how well age at diagnosis determined patient mortality within 3 years.20,21 In addition, logistic regression models for occurrence of death were fit to determine the age cut point at which the odds ratio was maximized and statistically significant. For all analyses, P values < .05 were considered statistically significant.
RESULTS
Patient Characteristics and Therapy
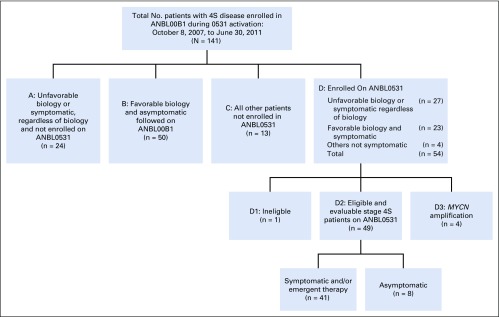
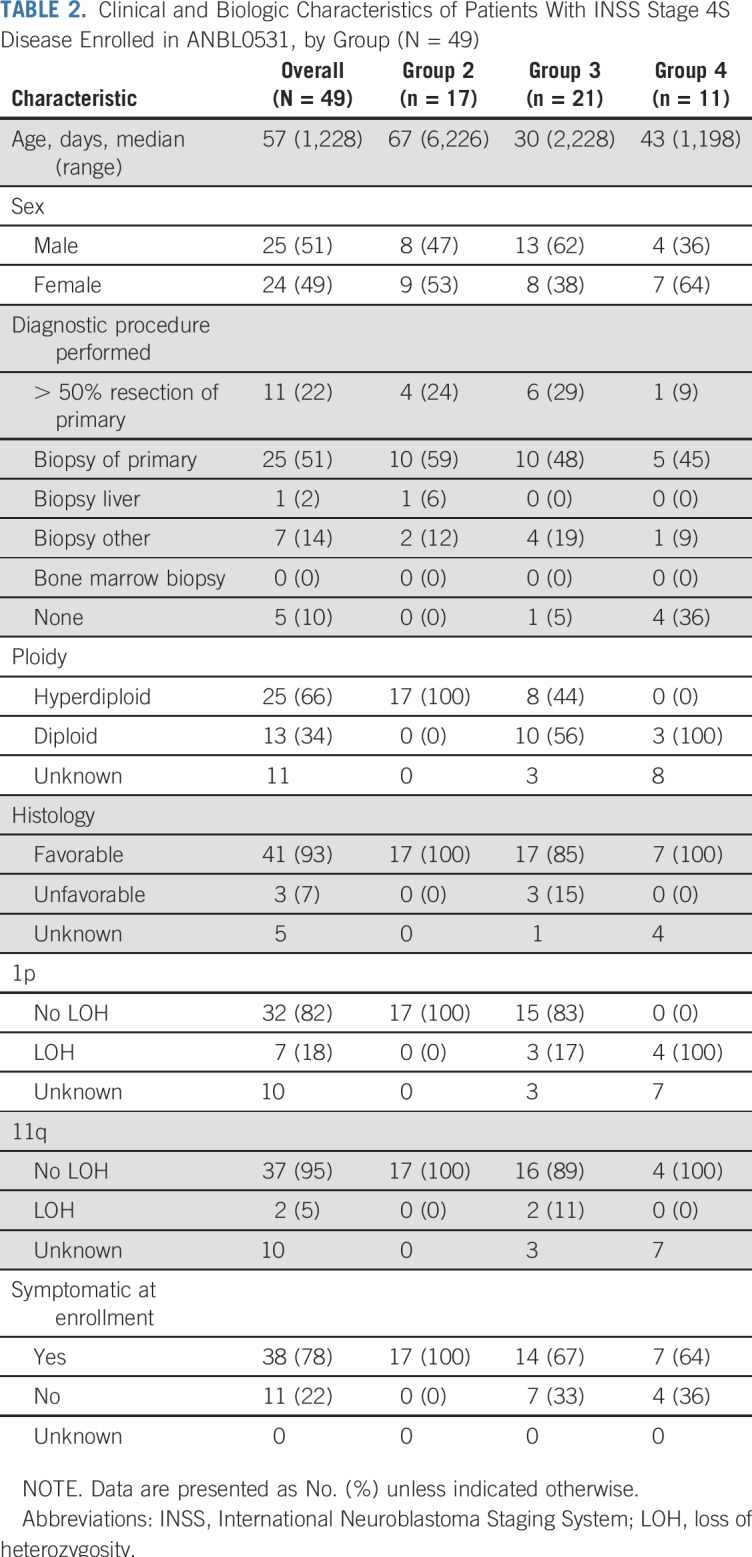
Forty-nine eligible patients with 4S disease enrolled in ANBL0531, all of whom were also evaluable and enrolled in the COG biology study ANBL00B1 (Fig 1). An additional five patients with 4S disease enrolled but were not eligible or evaluable because of asymptomatic low risk (n = 1) or MYCN amplification or high risk (n = 4) disease. Characteristics of eligible patients at enrollment are listed in Table 2. Twenty-eight patients had at least one unfavorable biologic characteristic. Seventeen patients were assigned to treatment group 2, 21 patients were assigned to group 3, and 11 patients to group 4 (unfavorable biology including 1p/11q LOH regardless of clinical symptoms [n = 7] or symptomatic with unknown biology [n = 4]). The median cycles of chemotherapy delivered to patients initially assigned to groups 2, 3, and 4, respectively, were two (range, two to six), four (range, two to four), and eight (range, two to eight).
FIG 1.
Diagram showing all 141 patients with International Neuroblastoma Staging System Stage 4S disease entered in Children’s Oncology Group biology study ANBL00B1 between October 8, 2007, and June 30, 2011. Forty-nine patients with 4S disease were eligible and evaluable and enrolled in ANBL0531.
TABLE 2.
Clinical and Biologic Characteristics of Patients With INSS Stage 4S Disease Enrolled in ANBL0531, by Group (N = 49)
Response to therapy was complete response (n = 6), very good partial response (n = 10), partial response (n = 23), stable disease (n = 1), and progressive disease (n = 1). Eight patients were missing response evaluation and were removed from therapy because of death (n = 5) or physician determination (n = 3). Nine patients in group 3 and five patients in group 4 received fewer than the assigned number of chemotherapy cycles for the following reasons: physician determination (n = 7), patient death (n = 5), and refusal of additional therapy by parent (n = 2). Thirty-eight patients were considered by their physician to be symptomatic at diagnosis, and an additional three patients received emergent chemotherapy because of impending risk of becoming symptomatic. Only five infants received hepatic radiotherapy. Among the 41 patients who were overtly symptomatic or at impending risk, the median age at diagnosis was 48 days (range, 1 to 228 days); 17 were in group 2, 17 were in group 3, and seven were in group 4.
Sixteen patients had greater than 50% surgical resection of the primary tumor (including 11 at diagnosis). There were two serious surgical complications reported in the 44 patients who underwent a diagnostic surgical procedure: one required partial liver resection together with partial tumor resection, and one required ventilator support postoperatively.
Baseline Abnormalities
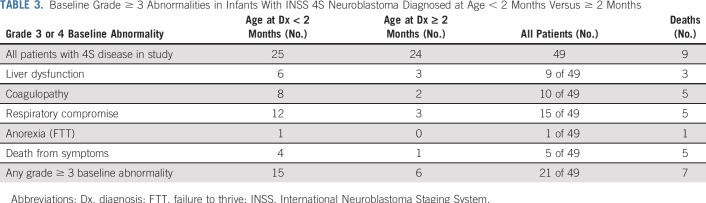
Assessment of at least one targeted baseline abnormality was obtained in 45 of the 49 patients; 25 had at least one targeted grade 2 abnormality (median, 1; range, 1 to 3). Seventeen patients had at least one grade 3 abnormality, and nine patients had at least one grade 4 abnormality, including hyperbilirubinemia (n = 1), hypofibrinogenemia (n = 2), and respiratory compromise (n = 7; Table 3). All nine patients who died reported at least one baseline abnormality of grade greater than or equal to 2, and seven had an abnormality of grade greater than or equal to 3 (Table 4). The severity of each of the targeted abnormalities between the patients who died and those who survived was only significant for coagulopathy. Five of nine patients (55.6%) who died experienced grade 3 or 4 coagulopathy versus five of 40 (12.5%) who survived (P = .01). Five of nine patients (55.6%) who died had baseline grade of greater than 3 respiratory compromise, and one of nine (11.1%) had grade 3 anorexia (failure to thrive [FTT]), compared with ten of forty infants (25.0%; P = .11) and zero of forty infants (0.0%; P = .18) who survived, respectively. Grade 3 and 4 liver dysfunction occurred more often in patients who died, although there was no significant difference in occurrence between those who died (three of nine [33.3%]) and those who survived (six of forty [15%]; P = .34).
TABLE 3.
Baseline Grade ≥ 3 Abnormalities in Infants With INSS 4S Neuroblastoma Diagnosed at Age < 2 Months Versus ≥ 2 Months
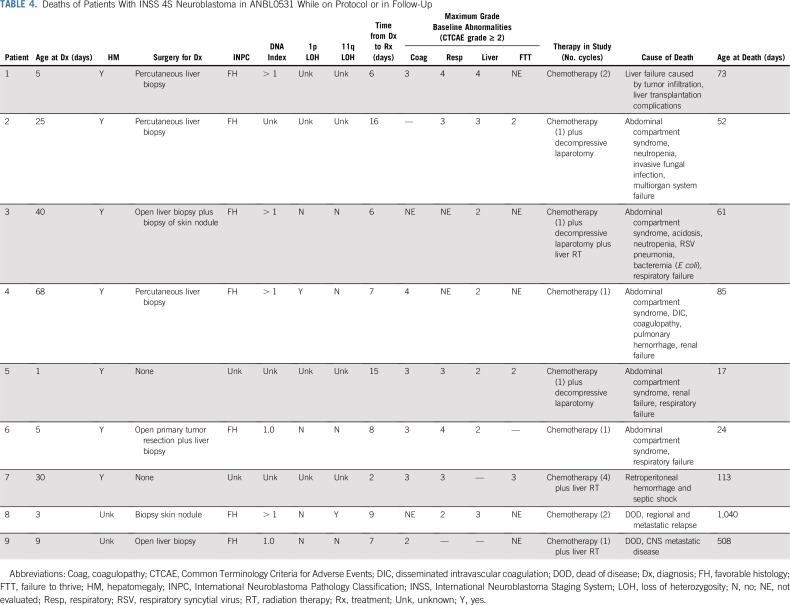
TABLE 4.
Deaths of Patients With INSS 4S Neuroblastoma in ANBL0531 While on Protocol or in Follow-Up
There was no difference in baseline liver dysfunction between symptomatic and asymptomatic patients. Two of the 11 asymptomatic patients (18.2%) had grade greater than or equal to 3 liver dysfunction compared with seven of the 38 symptomatic patients (18.4%; P = 1.00). None of the 11 asymptomatic patients had grade greater than or equal to 3 coagulopathy versus 10 of the 38 symptomatic patients (26.3%; P = .09).
EFS, OS, and Deaths
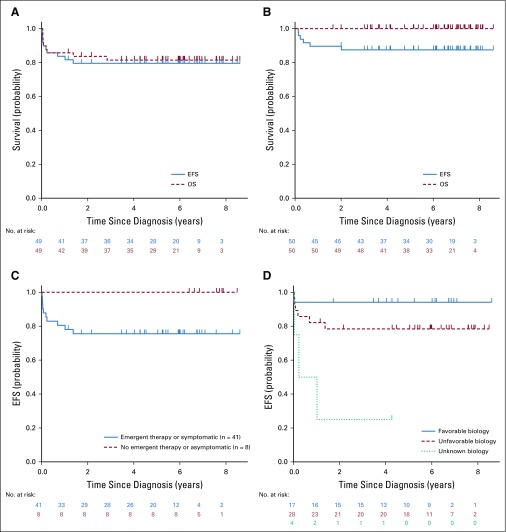
The 3-year EFS and OS for all 49 patients was 79.5% ± 6.0% and 81.4% ± 5.8%, respectively, (median follow-up, approximately 6 years; Fig 2A). This is apparently lower than the 3-year EFS (88.0% ± 4.7%) and OS (100%) for the 50 group 1 asymptomatic favorable biology (MYCN-NA, FH, hyperdiploid) patients with 4S disease registered in the COG biology study ANBL00B1 over this time period (and not enrolled in ANBL0531), who were followed with observation (Fig 2B). The median age of the asymptomatic patients in the non-ANBL0531 cohort was 72.5 days, slightly older than the ANBL0531 cohort (median age, 57 days). Figure 2C shows lower EFS for patients with organ impairment or emergent chemotherapy, compared with those without clinical signs of organ impairment (P = .14), and Fig 2D shows lower EFS for patients with unfavorable and unknown biology (P = .005).
FIG 2.
(A) Event-free survival (EFS) and overall survival (OS) for ANBL0531 4S eligible and evaluable patients (N = 49). The median follow-up for patients without an event was 2,196 days; the median follow-up for all surviving patients was 2,219 days. (B) EFS and OS for ANBL00B1 low-risk asymptomatic patients with 4S disease (n = 50) not enrolled in ANBL0531. Low-risk patients with 4S disease enrolled in ANBL00B1 but not enrolled in ANBL0531 had tumors that were MYCN nonamplified, hyperdiploid (regardless of 1p or 11q status), and had favorable histology by International Neuroblastoma Pathology Classification; the median age was 72 days, with 21 (42%) younger than 2 months at diagnosis. (C) EFS for ANBL0531 patients with 4S disease, symptomatic, and emergent therapy (n = 41) versus asymptomatic (n = 8; P = .14). (D) EFS for ANBL0531 patients with 4S disease, favorable (n = 17) versus unfavorable (n = 28) versus unknown biology (n = 4; P = .005).
Table 4 lists baseline characteristics and organ abnormalities, diagnostic procedure, time of treatment initiation, and outcome for all nine patients who died. Of these, five deaths were related to acute complications of rapidly progressing hepatomegaly, and all occurred over the initial 21 months of patient accrual. Two patients with unfavorable biology tumors assigned to treatment group 3 died after metastatic relapse, one with 11q LOH and one with DI = 1; both patients were taken off protocol therapy after receiving fewer than prescribed courses because of physician determination. Two additional deaths occurred: one as a result of septic shock and retroperitoneal hemorrhage after cycle 4 and one as a result of liver transplantation complications. Adverse events recorded in infants who died acutely because of complications of rapidly evolving hepatomegaly included abdominal compartment syndrome, renal failure, respiratory failure, coagulopathy, and infection. These five patients received treatment a median of 8 days from diagnosis (range, 6 to 16 days). They received one cycle of chemotherapy, three underwent decompressive laparotomy for compartment syndrome, and one received hepatic radiation.
Only four of 49 patients were considered to be too ill to undergo diagnostic biopsy, including one of the five infants who died as a result of complications of hepatomegaly. Baseline grade 4 toxicities were observed disproportionately in eight of nine patients younger than 3 months of age at diagnosis, and all deaths related to early complications of hepatomegaly also occurred in these young infants (Tables 3 and 4).
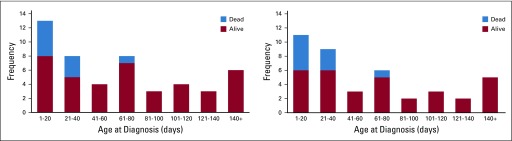
Because of the five deaths in the first 21 months of the study, an urgent safety memo was communicated to COG investigators, and the protocol was amended to mandate immediate chemotherapy for patients with 4S disease younger than 2 months of age at diagnosis with evolving hepatomegaly. No deaths related to complications of hepatomegaly occurred in the subsequent 31 4S infants enrolled, including 18 who were younger than 2 months of age (Appendix Fig A1, online only).
Among the 41 patients who were symptomatic and/or required emergent therapy, the age at diagnosis ranged from 1 to 228 days. All patients who died were diagnosed before 3 months of age, and eight of nine were diagnosed before 2 months of age. The optimal cut point for age at diagnosis and risk of death was in the range of 40 to 47 days, yielding a Youden index of 0.51 and an odds ratio of 13.33 (95% CI, 1.48 to 120.15; P = .02). Patients younger than 40 days had greater than 13 times the risk of dying compared with patients older than 47 days.
DISCUSSION
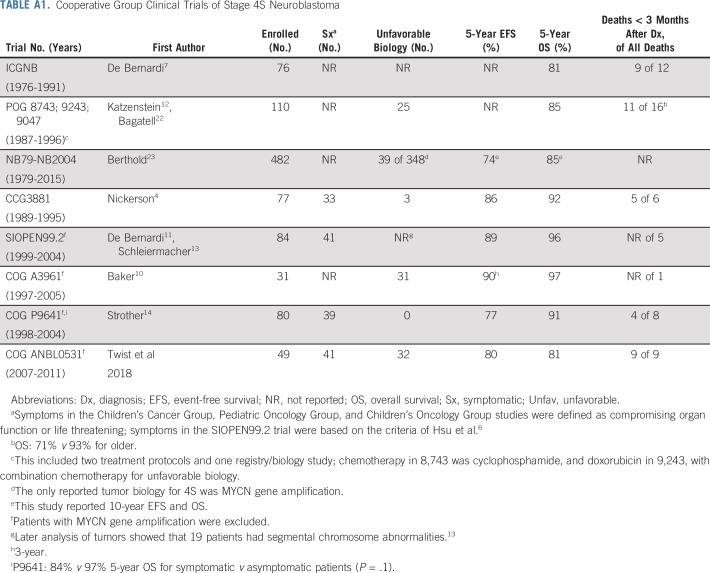
This clinical trial in patients with 4S neuroblastoma or at high risk of vital organ compromise from rapidly proliferating tumor and/or unfavorable tumor biology showed an 81% 3-year OS rate using biology- and response-based chemotherapy. We extended and refined prior reports suggesting that despite the well-documented complete and spontaneous multifocal tumor regressions observed in the majority of patients with 4S disease, identifiable subsets are at a higher risk of death from the extraordinarily rapid tumor proliferation, typically within the liver. Although the 81% 3-year OS is apparently inferior to the 85% to 97% 5-year OS in other cooperative trials,4,10-14,22,23 these studies mainly included asymptomatic patients, and the majority had favorable biology (Appendix Table A1, online only). In our study, 84% of patients had (or were at high risk of) vital organ impairment, and 57% showed at least one unfavorable tumor biomarker. Our study differed from prior trials because of its prospective, systematic allocation of treatment length that was based on symptoms, age, and tumor biology, including 1p and 11q aberrations. These data emphasize that despite the likelihood of spontaneous regression in many patients with 4S disease, those with evidence of rapid tumor growth in the first several weeks of life require immediate intervention with chemotherapy to avoid potentially irreversible abdominal compartment syndrome and hepatic and/or renal failure.
Our study confirmed the higher risk of death in patients diagnosed before 2 months of age, with eight of nine deaths in this trial occurring in patients in this group, and the Youden index showing a 13-fold increased risk of death in 4S neuroblastoma presenting before 40 to 47 days. Multiple reports in the literature have also suggested that the highest risk of death is in young infants.4,6,7,9,12,24 Once the investigators were alerted to the markedly increased risk of death in 4S neuroblastoma in infants younger than 2 months of age and the requirement for emergent treatment of evolving hepatomegaly, there were no additional deaths in the infants enrolled subsequently. This result provided sufficient evidence to support the clinical recommendation of a conservative cutoff of 2 months of age at diagnosis.
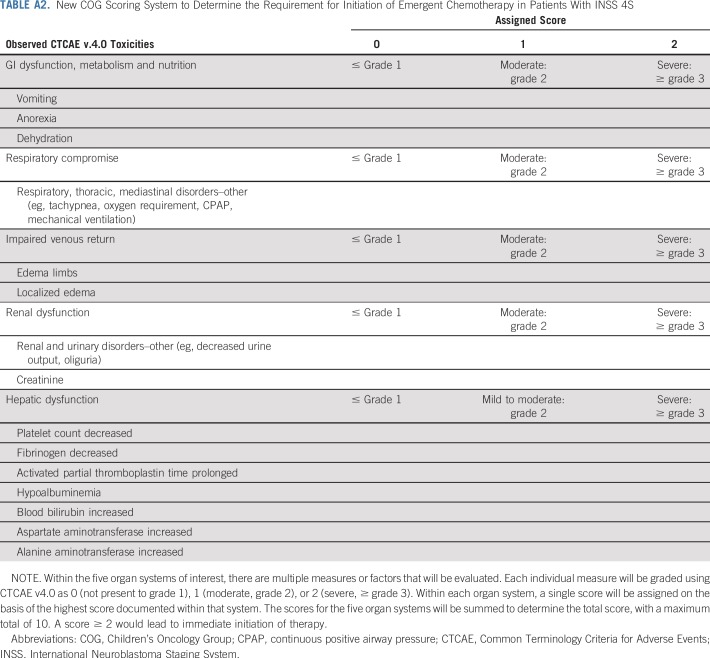
Hsu et al6 published guidelines in 1996 on the basis of a retrospective review of 35 patients with stage 4S neuroblastoma and suggested quantifiable clinical metrics to guide intervention with cytotoxic therapy. These guidelines have never been formally incorporated into COG clinical trials, likely because the most vulnerable patients with 4S disease were excluded on the basis of commonly used eligibility criteria. Moving forward, the COG has adapted a modified version of the Hsu system (Appendix Table A2, online only) that is based on our ANBL0531 results to guide chemotherapeutic intervention for 4S neuroblastoma, including International Neuroblastoma Risk Group stage MS.25 Prospective objective quantitation of organ function combined with age and tumor biology will validate appropriate therapy assignment in future trials.
Although late outcome was not an objective of our trial, one of the patients presenting with early hepatomegaly survived, only to die shortly after as a result of complications from a liver transplantation for hepatic failure. We found two reports of successful liver transplantations for 4S neuroblastoma.26,27 Other reported late effects include imaging abnormalities after hepatomegaly, with and without liver radiation, in patients with 4S disease who had hepatomegaly at diagnosis. The persistent lesions included focal nodular hyperplasia and hepatic fibrosis.17 Only five of our patients received hepatic radiation, which was less than the routine use prescribed in a prior trial.4
This study did not mandate resection of the primary tumor, because prior studies suggested no improvement in outcome for 4S neuroblastoma.12 Only 16 patients had greater than 50% resection of the primary tumor in this study, compared with the majority of patients in prior protocols. Furthermore, this trial allowed symptomatic patients without biopsy to enroll to encourage rapid treatment and avoid risky procedures, thus avoiding morbidity. Despite this recommendation, several patients underwent open surgical procedures at the time of diagnosis, which may have contributed to the development of comorbidities and a delay in the initiation of therapy (Table 4).
The second adverse outcome, in addition to early compartment syndrome or liver damage, is that even without MYCN amplification, other unfavorable biology (DI = 1, segmental chromosome aberrations, UH) are prognostic of a worse OS in 4S neuroblastoma.8,12,13,22,28-31 Our study confirmed the inferior outcome for patients with unfavorable biology compared with symptomatic patients with all favorable biology, despite the assigned increased length of therapy. Notably, both of the patients in our trial with late death as a result of metastatic disease had unfavorable biology. However, both patients received fewer than the prescribed four cycles of chemotherapy; therefore, it is unknown if more cycles of chemotherapy would have prevented relapse. It is also unknown if any of the four patients with unknown biology may have had MYCN-amplified tumors. Other tumor and host biology studies in 4S neuroblastoma may provide insight, such as the role of the immune system with tumor infiltrating invariant natural killer T cells, increased in 4S,32 epigenetic regulation,33 or telomerase activation.34-36
In conclusion, the outcome for 4S neuroblastoma can be further improved with pre-emptive chemotherapy for evolving hepatomegaly or other baseline comorbidities in infants younger than 2 months of age, and for any unfavorable biology characteristics, without surgical resection of the primary tumor. Recognition of the significant risk of morbidity and mortality in young infants with 4S neuroblastoma, even in those who initially seem asymptomatic, with prospective evaluation using the modified Hsu criteria, will allow prompt intervention with chemotherapy and vigilant supportive care.
ACKNOWLEDGMENT
We acknowledge all the participating institutions and investigators who entered patients in this trial, as well as the nurses and research coordinators.
Appendix
FIG A1.
Age at diagnosis and death in 4S neuroblastoma (n = 49). (A) Histogram showing the frequency of patients enrolled in each age group and the number of patients who died within that group. (B) Histogram of ANBL0531 enrollment showing the frequency of symptomatic patients in each age group and the number of patients who died of acute hepatomegaly complications (n = 5). No deaths occurred from hepatomegaly for 31 patients enrolled after the June 30, 2009, protocol amendment.
TABLE A1.
Cooperative Group Clinical Trials of Stage 4S Neuroblastoma
TABLE A2.
New COG Scoring System to Determine the Requirement for Initiation of Emergent Chemotherapy in Patients With INSS 4S
Footnotes
Supported in part by National Institutes of Health–National Cancer Institute Grant No.U10 CA180899 (Children’s Oncology Group Statistics and Data Center), National Clinical Trials Network Operations Center Grant No. U10 CA180886, and the St Baldrick’s Foundation.
Presented in part at the Advances in Neuroblastoma Research Meeting, Toronto, ON, Canada, June 18-21, 2012.
Clinical trial information: NCT00499616.
AUTHOR CONTRIBUTIONS
Conception and design: Clare J. Twist, Mary Lou Schmidt, Susan L. Cohn, E. Stanton Adkins, Wendy B. London, Julie R. Park, John M. Maris
Provision of study material or patients: Susan L. Cohn, John M. Maris
Collection and assembly of data: Clare J. Twist, Sheena C. Tenney, Susan L. Cohn, E. Stanton Adkins, Wendy B. London, John M. Maris
Data analysis and interpretation: Clare J. Twist, Arlene Naranjo, Sheena C. Tenney, Susan L. Cohn, Holly J. Meany, Peter Mattei, E. Stanton Adkins, Hiroyuki Shimada, Wendy B. London, Julie R. Park, John M. Maris
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Defining Risk Factors for Chemotherapeutic Intervention in Infants With Stage 4S Neuroblastoma: A Report From Children’s Oncology Group Study ANBL0531
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Susan L. Cohn
Stock and Other Ownership Interests: United Therapeutics (I), Varian Medical Systems (I), United Therapeutics, Vermillion, Resmed (I), Merck (I), Stryker (I), Amgen (I), Pfizer (I), AbbVie, Amgen, Jazz Pharmaceuticals, Eli Lilly, Sanofi, Varex Imaging, Pfizer
Research Funding: United Therapeutics (Inst), Merck (Inst)
Holly J. Meany
Consulting or Advisory Role: Bayer AG
Julie R. Park
Honoraria: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Roche
John M. Maris
Consulting or Advisory Role: Eli Lilly, Chrysalis, Illumina
No other potential conflicts of interest were reported.
REFERENCES
- 1.D’Angio GJ, Evans AE, Koop CE: Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet 1:1046-1049, 1971 [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM, Pritchard J, Berthold F, et al. : Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11:1466-1477, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Haas D, Ablin AR, Miller C, et al. : Complete pathologic maturation and regression of stage IVS neuroblastoma without treatment. Cancer 62:818-825, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Nickerson HJ, Matthay KK, Seeger RC, et al. : Favorable biology and outcome of stage IV-S neuroblastoma with supportive care or minimal therapy: A Children’s Cancer Group study. J Clin Oncol 18:477-486, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Hachitanda Y, Hata J: Stage IVS neuroblastoma: A clinical, histological, and biological analysis of 45 cases. Hum Pathol 27:1135-1138, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Hsu LL, Evans AE, D’Angio GJ: Hepatomegaly in neuroblastoma stage 4s: Criteria for treatment of the vulnerable neonate. Med Pediatr Oncol 27:521-528, 1996 [DOI] [PubMed] [Google Scholar]
- 7.De Bernardi B, Pianca C, Boni L, et al. : Disseminated neuroblastoma (stage IV and IV-S) in the first year of life. Outcome related to age and stage. Cancer 70:1625-1633, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Taggart DR, London WB, Schmidt ML, et al. : Prognostic value of the stage 4S metastatic pattern and tumor biology in patients with metastatic neuroblastoma diagnosed between birth and 18 months of age. J Clin Oncol 29:4358-4364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Noesel MM, Hählen K, Hakvoort-Cammel FG, et al. : Neuroblastoma 4S: A heterogeneous disease with variable risk factors and treatment strategies. Cancer 80:834-843, 1997 [PubMed] [Google Scholar]
- 10.Baker DL, Schmidt ML, Cohn SL, et al. : Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 363:1313-1323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bernardi B, Gerrard M, Boni L, et al. : Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. J Clin Oncol 27:1034-1040, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Katzenstein HM, Bowman LC, Brodeur GM, et al. : Prognostic significance of age, MYCN oncogene amplification, tumor cell ploidy, and histology in 110 infants with stage D(S) neuroblastoma: The pediatric oncology group experience—A pediatric oncology group study. J Clin Oncol 16:2007-2017, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Schleiermacher G, Michon J, Ribeiro A, et al. : Segmental chromosomal alterations lead to a higher risk of relapse in infants with MYCN-non-amplified localised unresectable/disseminated neuroblastoma (a SIOPEN collaborative study). Br J Cancer 105:1940-1948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strother DR, London WB, Schmidt ML, et al. : Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: Results of Children’s Oncology Group study P9641. J Clin Oncol 30:1842-1848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada H, Ambros IM, Dehner LP, et al. : The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 86:364-372, 1999 [PubMed] [Google Scholar]
- 16.Attiyeh EF, London WB, Mossé YP, et al. : Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med 353:2243-2253, 2005 [DOI] [PubMed] [Google Scholar]
- 17.French AE, Irwin MS, Navarro OM, et al. : Long-term hepatic outcomes in survivors of stage 4S and 4 neuroblastoma in infancy. Pediatr Blood Cancer 58:283-288, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 19.Peto R, Pike MC, Armitage P, et al. : Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer 35:1-39, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepe MS: The Statistical Evaluation of Medical Tests for Classification and Prediction. New York, NY, Oxford University Press, 2003 [Google Scholar]
- 21.Youden WJ: Index for rating diagnostic tests. Cancer 3:32-35, 1950 [DOI] [PubMed] [Google Scholar]
- 22.Bagatell R, Rumcheva P, London WB, et al. : Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J Clin Oncol 23:8819-8827, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Berthold F, Spix C, Kaatsch P, et al. : Incidence, survival, and treatment of localized and metastatic neuroblastoma in Germany 1979-2015. Paediatr Drugs 19:577-593, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickerson HJ, Nesbit ME, Grosfeld JL, et al. : Comparison of stage IV and IV-S neuroblastoma in the first year of life. Med Pediatr Oncol 13:261-268, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Monclair T, Brodeur GM, Ambros PF, et al. : The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol 27:298-303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holsten T, Schuster T, Grabhorn E, et al. : Liver transplantation as a potentially lifesaving measure in neuroblastoma stage 4S. Pediatr Hematol Oncol 34:17-23, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Steele M, Jones NL, Ng V, et al. : Successful liver transplantation in an infant with stage 4S(M) neuroblastoma. Pediatr Blood Cancer 60:515-517, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Bénard J, Raguénez G, Kauffmann A, et al. : MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: A molecular portrait of stage 4S. Mol Oncol 2:261-271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourhis J, Dominici C, McDowell H, et al. : N-myc genomic content and DNA ploidy in stage IVS neuroblastoma. J Clin Oncol 9:1371-1375, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Spitz R, Hero B, Ernestus K, et al. : Deletions in chromosome arms 3p and 11q are new prognostic markers in localized and 4s neuroblastoma. Clin Cancer Res 9:52-58, 2003 [PubMed] [Google Scholar]
- 31.Spitz R, Hero B, Simon T, et al. : Loss in chromosome 11q identifies tumors with increased risk for metastatic relapses in localized and 4S neuroblastoma. Clin Cancer Res 12:3368-3373, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hishiki T, Mise N, Harada K, et al. : Invariant natural killer T infiltration in neuroblastoma with favorable outcome. Pediatr Surg Int 34:195-201, 2018 [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1080/15592294.2016.1226739. Decock A, Ongenaert M, De Wilde B, et al: Stage 4S neuroblastoma tumors show a characteristic DNA methylation portrait. Epigenetics 11:761-771, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coco S, Theissen J, Scaruffi P, et al. : Age-dependent accumulation of genomic aberrations and deregulation of cell cycle and telomerase genes in metastatic neuroblastoma. Int J Cancer 131:1591-1600, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Peifer M, Hertwig F, Roels F, et al. : Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 526:700-704, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawashima M, Kojima M, Ueda Y, et al. : Telomere biology including TERT rearrangements in neuroblastoma: A useful indicator for surgical treatments. J Pediatr Surg 51:2080-2085, 2016 [DOI] [PubMed] [Google Scholar]