Abstract
The E6 and E7 oncoproteins of human papillomavirus (HPV) are ideal targets for developing immunotherapeutic approaches to treat HPV-associated tumors. Our previous studies showed that a recombinant lipidated HPV16 E7 mutant (rlipo-E7m) with inactivation of the E7 oncogenic functions can activate antigen presenting cells through Toll-like receptor 2 (TLR2) and induce antitumor immunity. Given that some HPV-associated tumors overexpress E6 but not E7, it is necessary to include therapeutic agents containing HPV E6 in therapeutic vaccine development to broaden the utility of the vaccine. In this study, we further incorporated a mutant HPV16 E6 (E6m) into rlipo-E7m to generate rlipo-E6mE7m, which could elicit both E6- and E7-specific immune responses after immunization. The rlipo-E6mE7m immunization induced higher levels of T cell proliferation and cytotoxic T lymphocyte response than the nonlipidated recombinant E6mE7m (rE6mE7m) immunization. Accordingly, a single-dose administration of rlipo-E6mE7m at day 7 after tumor inoculation in mice showed complete inhibition of tumor growth, whereas administration of rE6mE7m did not. These results demonstrated that rlipo-E6mE7m could be used in tumors with E6 and/or E7 expression via the induction of E6- and E7-specific immunity.
Keywords: Lipoprotein, TLR2, human papillomavirus, E6, E7
Introduction
Human papillomavirus (HPV) infection is the main risk factor for the development of several cancers, such as head and neck cancer and cervical cancer [1]. The number of newly diagnosed cervical cancer cases per year worldwide is over 600,000 [2]. Although prophylactic HPV vaccination to prevent HPV infection has been applied in health care and is expected to reduce the incidence of HPV-mediated cancers [3], cervical cancer is still the leading cause of cancer-related death in women because of the lack of therapeutic vaccines to treat patients with developed tumors. Therefore, therapeutic medicine to efficiently reduce cervical cancer development is an unmet medical need that could decrease cancer mortality.
Currently, the two major viral oncoproteins, E6 and E7, are considered targets for developing therapeutic vaccines because they drive cellular immortalization and maintain the transformed phenotype during tumor progression. They exert functions by binding with many cellular proteins to activate cancer hallmarks [4,5]. For example, E6 and E7 can interact with the pro-apoptotic proteins p53 and retinoblastoma tumor suppressor protein (pRB), respectively, to promote the targeted degradation of proteins with different crucial roles in tumorigenesis [6]. Moreover, the joint functioning of the E6 and E7 oncoproteins is required from early to later stages of HPV-induced malignancy [7]. In addition, the sequences of both proteins are well-conserved across a broad range of HPV subtypes [8,9]. Thus, the two oncoproteins are considered to be potential targets for therapeutic interventions using vaccines against HPV-associated tumors.
Different forms of HPV therapeutic vaccines have been developed and tested in preclinical and clinical trials, such as peptide- and protein-based vaccines [10]. Although these kinds of vaccines are safe, stable, and easy to manufacture, adjuvants and immunostimulatory molecules are needed to effectively elicit dendritic cell (DC) and T cell functions. In other words, a vaccine candidate with intrinsic adjuvant activity would be the best design.
Bacterial lipoproteins, a set of membrane proteins with lipid modifications, have been shown to play key roles in adhesion to host cells, modulation of inflammatory processes, and virulence-associated functions [11]. Several of the lipoproteins, such as lipidated outer surface protein A (OspA) of Borrelia burgdorferi [13], outer membrane lipoprotein I (OprI) from Pseudomonas aeruginosa [14], and the 19-kDa antigen of M. tuberculosis [15], have been identified as potential vaccine components that could induce proinflammatory effects and immune activation [12]. It has been demonstrated that the use of lipoproteins is a superior approach for the development of prophylactic vaccines against infectious diseases. Recently, we developed a platform for producing a high yield of recombinant lipoproteins [16] and proved that the lipid moiety of the lipo-immunogen, derived from bacterial lipoproteins, can be recognized as a danger signal to induce both innate and adaptive immunity in animals receiving lipoprotein vaccinations [17,18]. Moreover, we demonstrated that recombinant lipo-immunogen can induce higher virus-neutralizing antibody responses than the nonlipid form [16].
Continued expression of the two HPV oncoproteins is required to maintain the malignant phenotype; however, both E6 and E7 can individually transform primary cells through interacting with different cellular targets, and their major roles in tumorigenesis are different. In cervical tissue, E7 appears to serve as the dominant oncogene to produce the early stages of reproductive tract carcinomas, whereas E6 promotes the later stage of tumor progression [19]. A similar effect of the dominant oncogene E7 was detected in transgenic models of head and neck cancer [20]. In our previous study, we demonstrated that a recombinant lipidated HPV E7 mutant (rlipo-E7m) can provide protective immunity against cervical cancer in a mouse model [21]. Since the E6 and E7 oncoproteins are dominant at different stages during tumor development, targeting both the E6 and E7 oncoproteins would be a promising approach for the development of therapeutic vaccines that could provide comprehensive therapeutic effects in HPV-mediated cancers.
Materials and methods
Cloning and expression of recombinant protein rE6mE7m and rlipo-E6mE7m
The synthetic nucleotide sequence of the HPV16 E6mE7m gene in the pBR322 vector with added Nde I and Xho I restriction enzyme sites flanking the genes at the 5’- and 3’-ends, respectively, were purchased from Invitrogen (Carlsbad, CA, USA). The gene fragments were cloned into the Nde I-Xho I sites of the expression vector pET-22b(+) (Novagen, Madison, WI) to generate the plasmids pET-22b E6mE7m and pET-22b A23E6mE7m. An additional hexahistidine tag (HisTag) sequence was inserted at the C-terminal end of each of the recombinant proteins.
For the production of recombinant E6mE7m (rE6mE7m), the expression plasmid pET-22b E6mE7m was transformed into the E. coli strain BL21 Star (DE3) (Invitrogen, CA, USA). The transformed cells were cultured in LB medium at 37°C and then induced with 1 mM IPTG for 4 hr at O.D.=0.6. The cells were harvested and resuspended in 20 mM Tris-Cl, 500 mM NaCl, 50 mM sucrose, pH 8.0 buffer for further purification.
For the production of rlipo-E6mE7m, plasmid pET-22b A23E6mE7m was transformed into the CD43 (DE3) E. coli strain [16]. The transformed cells were cultured in M9 medium at 37°C overnight and then induced with 1 mM IPTG at 12°C for 3 days. The cells were harvested and resuspended in 20 mM Tris-Cl, pH 8.9 buffer for further purification.
Purification of recombinant proteins
Recombinant E6E7m (rE6mE7m) and lipidated E6E7m (rlipo-E6mE7m) were purified by disrupting the harvested cells in a French press (Constant Systems, Daventry, UK) at 27 Kpsi in homogenization buffer [500 mM NaCl; 50 mM sucrose; 20 mM Tris-HCl (pH 8.0)]. The cell lysate was clarified by centrifugation (80,000 × g for 40 min). The pellet was solubilized with urea buffer [8 M urea; 50 mM sucrose; 20 mM Tris-HCl (pH 8.0)] and homogenized with a Dounce homogenizer. The mixture was again clarified by centrifugation (80,000 × g for 40 min). The recombinant proteins were extracted in guanidine hydrochloride (GdnHCl) buffer and purified by using a Ni-NTA resin column. The purified proteins were dialyzed against phosphate buffer. The endotoxin levels in the final product were below 0.03 EU/mg.
Characterization of the recombinant proteins
Purified rE6mE7m and rlipo-E6mE7m were detected by an anti-His antibody (Santa Cruz, CA, USA) and a mouse anti-human papillomavirus 16 E7 antibody (VALDOSPAN GmbH, cat VS13004) by Western blotting. To identify the N-terminal fragment of rlipo-E6mE7m, rlipo-E6mE7m was first dialyzed against 25 mM ammonium bicarbonate at pH 8.5 and then treated with trypsin (Promega Co., Madison, WI) at a rlipo-E6mE7m:trypsin ratio of 50:1 (Wt/Wt) in 25 mM ammonium bicarbonate (pH 8.5) for 16 hrs at 37°C. The reaction mixture was further purified using Ziptip (Millipore, Massachusetts). One microliter of the trypsin-digested protein was mixed with 1 μL saturated α-ciano-4-hydrozycinnamic acid solution (Sigma-Aldrich, St. Louis, MO) in 75% acetonitrile/0.1% trifluoroacetic acid. One microliter of the mixture was placed on the target plate of a matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometer (Bruker Daltonics, Germany) for analysis. The results obtained from the MALDI-TOF analysis described above indicated that the partial trypsin digestion products corresponded to the N-terminal fragments of rlipo-E6mE7m, and these peptides were lipidated.
Activation of bone marrow-derived dendritic cells (BM-DCs)
BM-DCs derived from C57BL/6 mice were assessed as previously described [16]. Briefly, mouse bone marrow cells were cultured at a density of 2 × 106 cells/ml in petri dishes containing 10 ml complete medium (CM: RPMI 1640 containing 10% FCS, 1% P/S, 50 μM 2-ME, 2 mM glutamine, 25 mM HEPES, and 1 mM sodium pyruvate) with 20 ng/ml recombinant mouse GM-CSF. On day 3, an additional 10 ml CM medium containing 20 ng/ml GM-CSF was added. On day 6, the nonadherent cells were collected and transferred into 24-well plates. BM-DCs (1 × 106 cells/ml) were stimulated with LPS (0.1 μg/ml) or the indicated concentrations of rE6mE7m or rlipo-E6mE7m for 24 hrs. At this time point, the supernatants from the different cultures were collected and stored at -20°C for cytokine assays. The cell surface markers (CD40 and CD80) of BM-DCs were analyzed using flow cytometry (FACSCalibur, BD bioscience, San Jose, CA). The production of cytokines (TNFα) by BM-DCs was determined using ELISA kits (eBioscience, CA, USA).
Lymphocyte proliferation assay
Lymphocytes from the spleen and lymph nodes of C57BL/6 mice were plated at a density of 2 × 105/well in flat bottom 96-well plates and stimulated with 10 μg/mL rE6mE7m or rlipo-E6mE7m for 48 hrs at 37°C in the presence of 5% CO2 in a humidified incubator. In the final 24 hrs of culture, 1 μCi [3H]-thymidine (5 μCi/mmol; Amersham, Arlington Heights, IL) was added to each well, and the plates were again incubated overnight in a humidified CO2 atmosphere at 37°C. Following the incubation, the supernatant was aspirated, and the cells were harvested using a FilterMate (Packard, Meriden, CT) automatic cell harvester. The incorporated radioactivity was determined using a Top Count microplate scintillation counter (Packard, Meriden, CT). All results are presented as the mean cpm ± standard deviation (SD).
T cell response assay
C57BL/6 mice were injected subcutaneously with 10 μg rE6mE7m or rlipo-E6mE7m twice at two-week intervals. Seven days after the second immunization, the splenocytes and lymphocytes from lymph nodes were isolated and restimulated with rE6mE7m or rE6mE7m (10 μg/ml) for 4-5 days. The supernatants were collected, and the levels of IFN-γ, IL-5, IL-10 or IL-17 were measured by ELISA. The cells were simultaneously assessed by an IFN-γ ELISPOT assay as previously described [21]. Briefly, splenocytes (5 × 105/well) were added to anti-IFN-γ antibody-coated plates and cultured in the presence of 10 mg/ml HPV E7-derived peptide RAH (RAHYNIVTF) in a final volume of 200 μL CM. Two peptides containing HPV E6 epitopes, KQQ peptides (KQQLLRREVYDFAFRDLCIVYRDGN) or EVY peptides (EVYDFAFRDL), were also included. After 48 hrs of incubation, the cells were removed, and the plates were washed with 0.05% (w/v) Tween 20 in PBS. A biotinylated anti-IFN-γ antibody was added to each well, and the samples were incubated for 2 hrs. After the detection antibody solution was discarded, a diluted streptavidin-HRP solution was added to the wells for another 1 hr. The spots were developed using 3-amine-9-ethyl carbazole (Sigma-Aldrich, St. Louis, MO) and counted by an ELISPOT reader (Cellular Technology Ltd., Shaker Heights, OH). The data are expressed as the mean ± SD of each sample (n=3).
In vivo tumor protection experiments
Six- to twelve-week-old female C57BL/6 mice were subcutaneously inoculated with TC-1 cells (2 × 105/mouse). After 7 days, the tumor-bearing mice (5-10 mice per group) were injected once with RAH/IFA (10 mg/mouse), rE6mE7m (30 mg/mouse), rlipo-E6mE7m (30 mg/mouse) or PBS. The tumor size was measured at 2-day intervals with calipers in two dimensions, and the tumor volumes were calculated as width2 × length/2. The mice were sacrificed when the tumor size reached 2,000 mm3 or the mice lost >20% of their initial body weight, in accordance with the Guide for the Institutional Animal Care and Use Committee of NHRI. The protocol was approved by the Institutional Animal Care and Use Committee of NHRI (Permit Number: NHRI-IACUC-103020-A). After the vaccination, the tumor growth was recorded for 60 days.
Results
Construction of inactive oncoprotein-HPV E6mE7m immunogens
To construct the inactive HPV E6mE7m immunogens for vaccination, mutations in the amino acid sequences of E6 and E7 were designed to inactivate their oncogenic functions, and the E6 mutant (E6m) and E7 mutant (E7m) were combined to obtain the fused protein E6mE7m (Figure 1). For the HPV E6 mutant, a deletion of the PRKLP element and a leucine to valine mutation were included to influence the interactions of E6 with E6AP and p53 [22]. For the HPV E7 mutant, we demonstrated that the inactive-E7 immunogen (E7m) still contains the major CTL epitopes [21]. Previously, we identified a fusion sequence (D1, MKKLLIAAMMAAALAACSQEAKQEVKEAVQAVESDVKDTA) that can be fused with the nonlipidated immunogen to achieve high expression levels of the recombinant lipo-immunogen [16]. To generate rlipo-E6mE7m, a fusion sequence (D1) was inserted at the N-terminus of the E6mE7m gene, and a HisTag was included at the C-terminus of the E6mE7m gene for purification.
Figure 1.
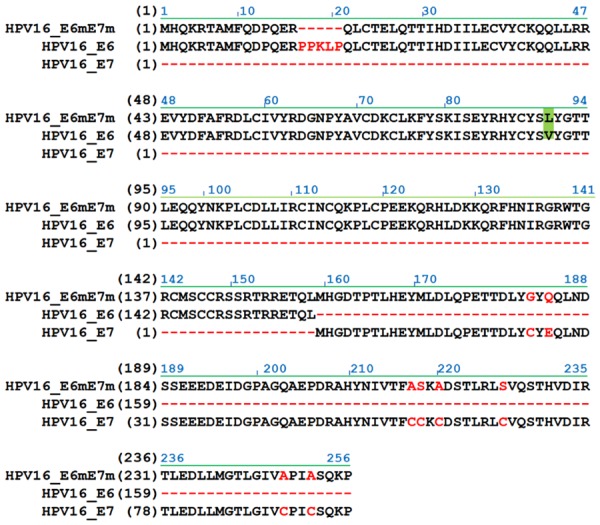
The amino acid sequence of the constructed HPV16 E6mE7m immunogen. E6mE7m proteins were combined with mutated E6 and E7. The mutated sites were highlighted or marked for inactivating the oncogenic activity of E6 and E7. The alignment of E6mE7m and wild-type E6 and E7 was representative. Additionally, a HisTag sequence was included at the C-terminus of the proteins, and the DNA sequence of E6mE7m was-modified using the codon usage of E. coli. For rlipo-E6mE7m protein expression, the region containing the lipid signal peptide was inserted into the expression plasmid.
Identification and characterization of purified immunogens
First, the recombinant immunogen rE6mE7m was produced with IPTG induction in the E. coli system and then purified using an immobilized metal affinity chromatography (IMAC) column (Figure 2A). The rE6mE7m proteins were detected by anti-HisTag antibodies (Figure 2B). The rlipo-E6mE7m proteins were produced and purified with a strategy similar to that used for the rE6mE7m proteins (Figure 2C) and were detected by anti-HisTag antibodies (Figure 2D). In addition, the purified rE6mE7m and rlipo-E6mE7m proteins were detected with an anti-HPV E7 antibody (Figure 2E). To identify whether the lipid moiety was added at the N-terminus of the rlipo-E6mE7m proteins, peptide mass fingerprinting (PMF) was performed to measure the major peaks in the mass spectral data derived from rlipo-E6mE7m. After processing the data, three major peaks with masses of 1453, 1467, and 1480.2 Da were identified (Figure 2F). Based on the observation that the distance of the mass between the major peaks was approximately 14 Da, which was the predicted mass of the CH2 structure from our previous study [21], the spectrum of these major peaks could be considered the signature of the lipid modifications in rlipo-E6mE7m [16].
Figure 2.
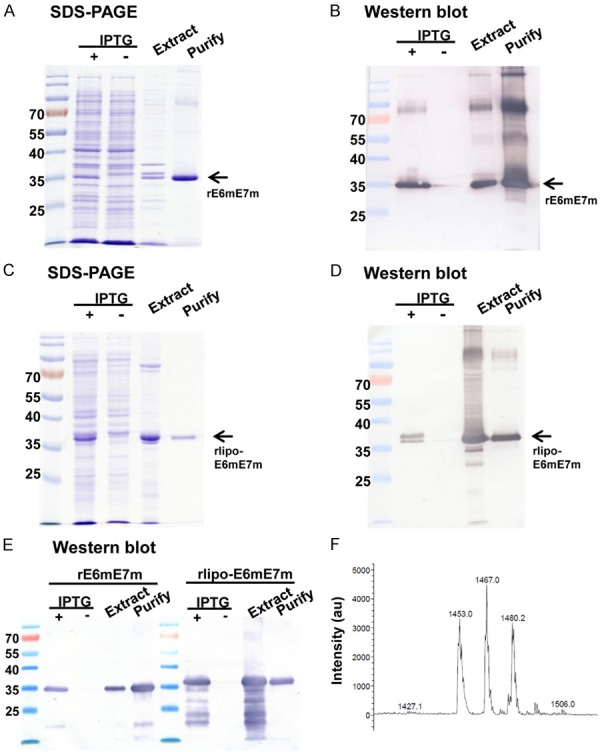
Production and purification of rE6mE7m and rlipo-E6mE7m. (A) The rE6mE7m proteins were monitored in the fraction subjected to IPTG induction and the extracted fraction. The final purified recombinant rE6mE7m proteins were detected using 15% reducing SDS-PAGE followed by Coomassie Blue staining. (B) The rE6mE7m proteins were validated using anti-HisTag antibodies in Western blotting. The nonlipidated form of rE6mE7m was expressed in the E. coli BL21 Star (DE3) strain. The arrows indicate the electrophoretic positions of rE6mE7m. (C) The rlipo-E6mE7m protein expression was also monitored by SDS-PAGE and validated using anti-HisTag antibodies in Western blotting (D). The lipidated form of rE6mE7m, rlipo-E6mE7m, was expressed in the E. coli BL21 (C43) strain. The arrows indicate the electrophoretic positions of rlipo-E6mE7m. (E) The Western blot analysis of rE6mE7m and rlipo-E6mE7m using an anti-HPV16 E7 antibody is shown. (F) The mass spectrometry analysis of rlipo-E6mE7m is shown. The N-terminal rlipo-E6mE7m fragments were obtained and identified after the digestion of rlipo-E6mE7m with trypsin. The digested sample was analyzed on a Waters® MALDI micro MX™ mass spectrometer. The MALDI-TOF MS spectra revealed the existence of three peaks with m/z values of 1453, 1467, and 1480.
rlipo-E6mE7m activates bone marrow-derived dendritic cells through TLR2
To evaluate the function of rlipo-E6mE7m and compare it with that of nonlipidated rE6mE7m, mouse bone marrow-derived DCs (BM-DCs) were used as a model to study the immunostimulatory properties of the immunogens. The lipopolysaccharide (LPS) levels of the purified rE6mE7m and rlipo-E6mE7m proteins were lower than 0.003 EU/μg, and then the immunogens were comparatively analyzed for their immunogenicity and efficacy in animal models. Polymyxin B (Pmb), which has been used to clear endotoxin contamination, was added to detect any interference caused by residual LPS. We found that rlipo-E6mE7m was capable of stimulating and upregulating the expression of the costimulatory molecules CD40 and CD80 on BM-DCs, while rE6mE7m was ineffective (Figure 3A and 3B). Similar levels of CD40 and CD80 expression on BM-DCs were seen with rlipo-E6mE7m stimulation regardless of Pmb addition, indicating that the effects were not caused by residual LPS. Furthermore, with or without Pmb addition, the secretion of TNF-α from BM-DCs was induced by rlipo-E6mE7m in a dose-dependent manner, but no TNF-α secretion was detected in the rE6mE7m groups (Figure 3C). Moreover, the secretion of TNF-α from BM-DCs was induced by rlipo-E6mE7m in wild-type mice but not in Toll-like receptor 2 knockout (TLR2 KO) mice (Figure 3D), indicating that TLR2 is required for the immunostimulatory activity of rlipo-E6mE7m. These results indicate that the activation of BM-DCs by rlipo-E6mE7m was not due to any residual LPS, and rlipo-E6mE7m provided stronger immunostimulatory activity than rE6mE7m. Since previous results have demonstrated that the amino acid sequence of the D1 domain (the fusion partner for recombinant lipoprotein production) is unable to stimulate the production of cytokines [16], these data clearly demonstrated that the lipidated form of E6mE7m is able to activate dendritic cells and trigger innate immunity.
Figure 3.
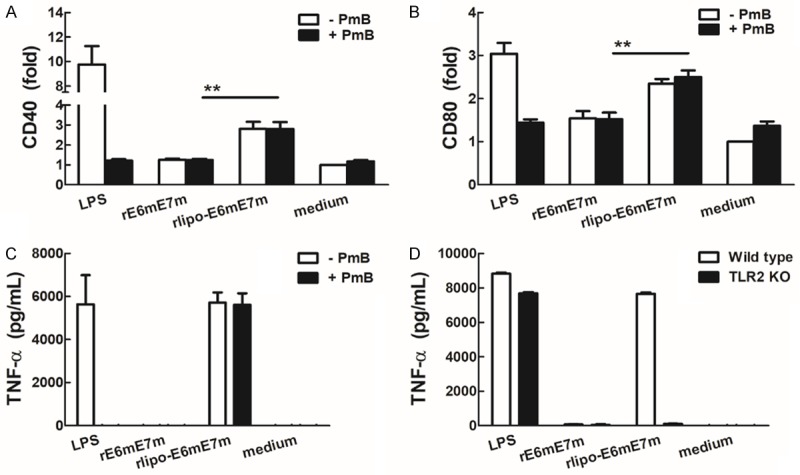
rlipo-E6mE7m proteins activate bone marrow-derived dendritic cells (BM-DCs). BM-DCs from wild-type mice were cultured in medium supplemented with LPS (0.1 μg/ml), rE6mE7m (10 μg/ml) or rlipo-E6mE7m (10 μg/ml) in the presence or absence of polymyxin B (20 μg/ml). After a 24-hr incubation, the expression of the dendritic cell surface markers CD40 (A) and CD80 (B) was analyzed using flow cytometry. The experiments were performed in triplicate, and the mean fluorescence intensity (MFI) of the cells cultured in medium alone was defined as 1. (C) TNF-α secretion by dendritic cells was stimulated by rlipo-E6mE7m. BM-DCs were cultured in medium supplemented with LPS (0.1 μg/ml), rE6mE7m (10 μg/ml) or rlipo-E6mE7m (10 μg/ml) in the presence or absence of polymyxin B (20 μg/ml). After a 24-hr incubation, the supernatants were harvested and analyzed for TNF-α production by ELISA. (D) BM-DCs isolated from wild-type and TLR2 knockout (TLR2 KO) mice were cultured in medium supplemented with LPS (0.1 μg/ml), rE6mE7m (10 μg/ml) or rlipo-E6mE7m (10 μg/ml), and then TNF-α production was detected by ELISA after a 24-hr incubation.
Enhancement of antigen-specific antibody levels and Th1 responses by immunization with rlipo-E6mE7m
Next, we examined lymphocyte proliferation in response to rE6mE7m and rlipo-E6mE7m in cells from mice treated with immunogen immunization using [3H]-thymidine to measure DNA synthesis. Both immunogens stimulated the proliferation of splenocytes, but rlipo-E6mE7m induced higher proliferation than rE6mE7m (Figure 4A). In addition, the secretion of IFN-γ, IL-5, IL-10, and IL-17A by T helper cells isolated from immunogen-immunized mice was increased by stimulation with either of the two immunogens, and rlipo-E6mE7m induced higher levels of cytokine secretion than the rE6mE7m (Figure 4B). Thus, the results demonstrated that the lipidated immunogen has better adjuvant properties than the nonlipid immunogen, which allows the lipidated immunogen to activate T helper cell proliferation and cytokine secretion.
Figure 4.
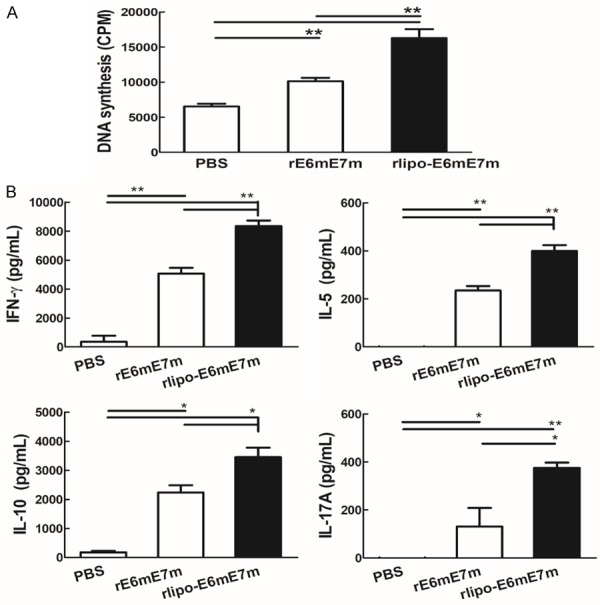
Immunization with rlipo-E6mE7m induced higher levels of T cell proliferation than immunization with rE6mE7m. (A) Lymphocytes from the spleen were isolated from protein-immunized C57BL/6 mice and plated at a density of 2 × 105 cells/well in 96-well plates. The cells were incubated with 10 μg/ml rE6mE7m and rlipo-E6mE7m for 48 hrs. In the final 24 hrs, 1 μCi [3H]-thymidine was added to each well to measure DNA synthesis. The data are represented as the mean ± SD of triplicate samples. (B) The immunization with rlipo-E6mE7m induced higher levels of cytokine release than the immunization with rE6mE7m. Mice were injected subcutaneously with 10 μg rE6mE7m or rlipo-E6mE7m twice at two-week intervals. Seven days after the second immunization, the splenocytes were isolated and stimulated with rE6mE7m or rE6mE7m (10 μg/ml) for 4-5 days. The supernatants were collected, and the levels of IFN-γ, IL-5, IL-10 or IL-17 were measured by ELISAs. The data are expressed as the mean ± SD of the samples (n=3).
Immunization with rlipo-E6mE7m induces E6- and E7-specific T cell responses
We then investigated whether rlipo-E6mE7m immunization can induce E7-specific CTL responses. After C57BL/6 mice were subcutaneously immunized twice (day 0 and 7) with the immunogens, the splenocytes from immunized mice were harvested 7 days after the last boost. Since the RAHYNIVTF peptide (RAH) of HPV16 E7 is a known H-2Db-restricted CTL epitope, the RAH peptide was used to stimulate the cultured splenocytes to assess the E7-specific CTL responses using an IFN-γ ELISpot assay (see Materials and Methods). The mice immunized with rlipo-E6mE7m exhibited a higher number of IFN-γ secreting cells than those immunized with rE6mE7m (Figure 5A). Furthermore, to investigate the E6-specific T cell response, the EVY peptides of HPV E6 that have been identified as H-2Kb-restricted CTL epitopes were used for specificity evaluation [23]. With the stimulation by the HPV E6 peptides KQQ (longer peptides containing EVY sequence) and EVY, more IFN-γ secreting cells were activated in the rlipo-E6mE7m immunized groups than in the rE6mE7m groups (Figure 5B). These results clearly demonstrated that the HPV E6- and E7-specific CTL responses can be induced using rlipo-E6mE7m vaccination.
Figure 5.
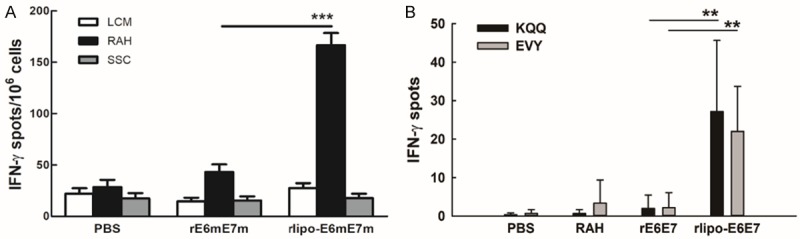
Immunization with rlipo-E6mE7m induced higher levels of E7-specific IFN-γ-secreting cells than the immunization with rE6mE7m. (A) Splenocytes or lymph node cells (2 × 105 cells/well) from immunized mice were incubated with lymphocyte culture medium control (LCM), 10 mg/ml RAHYNIVTF (RAH) peptide, or irrelevant peptide (SSC) for 48 hrs in an anti-IFN-γ-coated 96-well ELISPOT plate. The IFN-γ-secretion spots were measured using an ELISPOT reader. The data are expressed as the mean ± SD of three animals per group. (B) The immunization with rlipo-E6mE7m induces E6-specific IFN-γ secretion. Splenocytes (2 × 105 cells/well) from immunized mice were incubated with or without 10 mg/ml HPV16 E6 peptides KQQLLRREVYDFAFRDLCIVYRDGN (KQQ) or EVYDFAFRDL (EVY) for 48 hrs in an anti-IFN-γ antibody-coated 96-well ELISPOT plate. The IFN-γ-secretion spots were measured using an ELISPOT reader. The data are expressed as the mean ± SD of three animals per group.
Evaluation of the therapeutic effect of the rlipo-E6mE7m immunogens
To evaluate the antitumor efficacy of the immunogens in vivo, mouse lung cancer TC-1 cells, which were transformed with the E6 and E7 genes, were subcutaneously injected into C57BL/6 mice. Seven days later, these mice were subcutaneously injected with 30 μg rE6mE7m or rlipo-E6mE7m in the abdomen. In the PBS or rE6mE7m treatment, the tumors grew quickly within one month (Figure 6). However, the tumors were scarcely detected after day 55 in the mice receiving rlipo-E6mE7m. The results demonstrated that the therapeutic efficacy of the recombinant immunogens was dramatically increased in the lipidated form.
Figure 6.
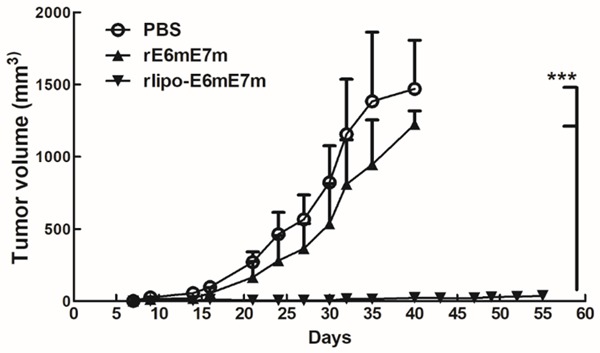
Treatment with rlipo-E6mE7m at day 7 after tumor inoculation inhibited tumor growth, but treatment with rE6mE7m did not. Mice were subcutaneously inoculated with TC-1 cells (2 × 105/mouse). After 7 days, the tumor-bearing mice (5-10 mice per group) were injected once with RAH/IFA (10 μg/mouse), rE6mE7m (30 μg/mouse), rlipo-E6mE7m (30 μg/mouse) or PBS. The tumor volumes were calculated as length × width × width/2 (mm3). The data are expressed as the mean ± SEM.
Discussion
In our previous studies, rlipo-E7m was shown to induce Th1 immune responses, E7-specific CTL responses, and tumor-protective immunity in a mouse tumor model [21]. We further demonstrated that rlipo-E7m combined with the TLR9 agonist CpG ODN not only inhibited tumor growth but also reduced the number of immunosuppressive cells in the tumor microenvironment [24]. Interestingly, the therapeutic effects of rlipo-E7m/CpG can be enhanced when rlipo-E7/CpG is combined with the anticancer drug gemcitabine. Moreover, the combination therapy can reduce the number of PD-1-positive E7-specific T lymphocytes [25]. Despite this result, E6 still needs to be incorporated into the formulation of a therapeutic vaccine. In particular, E6 can bind to p53 to induce the degradation of p53, which plays an important role in cell transformation.
In this study, we designed a lipidated fusion protein that contained mutant E6 and E7 (rlipo-E6mE7m), in which p53 and pRb binding activity are lost, respectively. The nonlipidated recombinant E6mE7m (rE6mE7m) was produced for comparison. HPV16 E6E7 fusion proteins have been designed for therapeutic vaccines using different approaches [26-30]. Because the protein-based vaccine has a high safety profile, it would be easier to fit into the regulatory requirement. However, the challenges of developing a protein-based therapeutic vaccine against tumors are low immunogenicity and difficulty in eliciting CTL responses. To overcome these limitations, the delivery of the antigen to antigen presenting cells in a way that activates the antigen presenting cells is key. The lipidated antigen rlipo-E6mE7m can effectively target antigen presenting cells through TLR2 and induce robust CTL responses.
In fact, the recombinant E6E7 fusion protein was a highly immunogenic protein that induced T cell responses in the absence of an adjuvant (Figure 4). However, the number of the CTL responses induced by recombinant E6mE7m was still lower than that of those induced by rlipo-E6mE7m (Figure 5). The induction of CTL responses in the rlipo-E6mE7m group may have occurred due to the increasing cross-presentation of antigenic peptides to CD8+ T cells mediated by TLR2 signaling. Our previous study showed that the TLR2-conjugated peptide can be taken up by dendritic cells and activate the MyD88-dependent signaling pathway to increase the cross-presentation of the peptide [31]. A nonadjuvanted HPV E6E7 multiepitope vaccine that can induce antitumor effects after three doses of immunization has also been developed [27]. Similarly, we observed that rE6mE7m alone can induce antigen-specific T cell responses, but it induces fewer CTL responses than rlipo-E6mE7m. These results demonstrated that the induction of efficient CTL responses requires efficient antigen targeting to antigen presenting cells and the activation of antigen presenting cells. The targeting and activation of antigen presenting cells could occur through TLR signaling or through other innate receptors. Here, we used lipidating technology to generate rlipo-E6mE7m, which has TLR2 agonist activity to efficiently induce antitumor immunity. The technology can be applied to different tumor-associated antigens to generate therapeutic cancer vaccines for different cancers.
Acknowledgements
This work was supported by a Research Investigator Award from the National Health Research Institutes (IV-106-PP-17 to S.-J. L.) in Taiwan and Chinese Medicine Research Center, China Medical University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan (CMRC-CHM-8).
Disclosure of conflict of interest
None.
References
- 1.Schwarz TF. AS04-adjuvanted human papillomavirus-16/18 vaccination: recent advances in cervical cancer prevention. Expert Rev Vaccines. 2008;7:1465–1473. doi: 10.1586/14760584.7.10.1465. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009;8:1663–1679. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 4.Tan S, de Vries EG, van der Zee AG, de Jong S. Anticancer drugs aimed at E6 and E7 activity in HPV-positive cervical cancer. Curr Cancer Drug Targets. 2012;12:170–184. doi: 10.2174/156800912799095135. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26:158–168. doi: 10.1016/j.tim.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Vande Pol SB, Klingelhutz AJ. Papillomavirus E6 oncoproteins. Virology. 2013;445:115–137. doi: 10.1016/j.virol.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 8.Trimble CL, Frazer IH. Development of therapeutic HPV vaccines. Lancet Oncol. 2009;10:975–980. doi: 10.1016/S1470-2045(09)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23:75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs-Simon A, Titball RW, Michell SL. Lipoproteins of bacterial pathogens. Infect Immun. 2011;79:548–561. doi: 10.1128/IAI.00682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christodoulides A, Boyadjian A, Kelesidis T. Spirochetal lipoproteins and immune evasion. Front Immunol. 2017;8:364. doi: 10.3389/fimmu.2017.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanassi WT, Schoen RT. The Lyme disease vaccine: conception, development, and implementation. Ann Intern Med. 2000;132:661–668. doi: 10.7326/0003-4819-132-8-200004180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Rau H, Revets H, Cornelis P, Titzmann A, Ruggli N, McCullough KC, Summerfield A. Efficacy and functionality of lipoprotein OprI from Pseudomonas aeruginosa as adjuvant for a subunit vaccine against classical swine fever. Vaccine. 2006;24:4757–4768. doi: 10.1016/j.vaccine.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson KA, Newton SM, Stewart GR, Martineau AR, Patel J, Sullivan SM, Herrmann JL, Neyrolles O, Young DB, Wilkinson RJ. Genetic determination of the effect of post-translational modification on the innate immune response to the 19 kDa lipoprotein of Mycobacterium tuberculosis. BMC Microbiol. 2009;9:93. doi: 10.1186/1471-2180-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HW, Liu SJ, Liu HH, Kwok Y, Lin CL, Lin LH, Chen MY, Tsai JP, Chang LS, Chiu FF, Lai LW, Lian WC, Yang CY, Hsieh SY, Chong P, Leng CH. A novel technology for the production of a heterologous lipoprotein immunogen in high yield has implications for the field of vaccine design. Vaccine. 2009;27:1400–1409. doi: 10.1016/j.vaccine.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Infante-Duarte C, Kamradt T. Lipopeptides of Borrelia burgdorferi outer surface proteins induce Th1 phenotype development in alphabeta T-cell receptor transgenic mice. Infect Immun. 1997;65:4094–4099. doi: 10.1128/iai.65.10.4094-4099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oftung F, Wiker HG, Deggerdal A, Mustafa AS. A novel mycobacterial antigen relevant to cellular immunity belongs to a family of secreted lipoproteins. Scand J Immunol. 1997;46:445–451. doi: 10.1046/j.1365-3083.1997.d01-150.x. [DOI] [PubMed] [Google Scholar]
- 19.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strati K, Lambert PF. Role of Rb-dependent and Rb-independent functions of papillomavirus E7 oncogene in head and neck cancer. Cancer Res. 2007;67:11585–11593. doi: 10.1158/0008-5472.CAN-07-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CY, Chen JJ, Shen KY, Chang LS, Yeh YC, Chen IH, Chong P, Liu SJ, Leng CH. Recombinant lipidated HPV E7 induces a Th-1-biased immune response and protective immunity against cervical cancer in a mouse model. PLoS One. 2012;7:e40970. doi: 10.1371/journal.pone.0040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper B, Schneider S, Bohl J, Jiang Y, Beaudet A, Vande Pol S. Requirement of E6AP and the features of human papillomavirus E6 necessary to support degradation of p53. Virology. 2003;306:87–99. doi: 10.1016/s0042-6822(02)00012-0. [DOI] [PubMed] [Google Scholar]
- 23.Stauss HJ, Davies H, Sadovnikova E, Chain B, Horowitz N, Sinclair C. Induction of cytotoxic T lymphocytes with peptides in vitro: identification of candidate T-cell epitopes in human papilloma virus. Proc Natl Acad Sci U S A. 1992;89:7871–7875. doi: 10.1073/pnas.89.17.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang LS, Leng CH, Yeh YC, Wu CC, Chen HW, Huang HM, Liu SJ. Toll-like receptor 9 agonist enhances anti-tumor immunity and inhibits tumor-associated immunosuppressive cells numbers in a mouse cervical cancer model following recombinant lipoprotein therapy. Mol Cancer. 2014;13:60. doi: 10.1186/1476-4598-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang LS, Yan WL, Chang YW, Yeh YC, Chen HW, Leng CH, Liu SJ. Gemcitabine enhances antitumor efficacy of recombinant lipoimmunogen-based immunotherapy. Oncoimmunology. 2016;5:e1095433. doi: 10.1080/2162402X.2015.1095433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almajhdi FN, Senger T, Amer HM, Gissmann L, Ohlschlager P. Design of a highly effective therapeutic HPV16 E6/E7-specific DNA vaccine: optimization by different ways of sequence rearrangements (shuffling) PLoS One. 2014;9:e113461. doi: 10.1371/journal.pone.0113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira LM, Morale MG, Chaves AA, Cavalher AM, Lopes AS, Diniz Mde O, Schanoski AS, de Melo RL, Ferreira LC, de Oliveira ML, Demasi M, Ho PL. Design, immune responses and anti-tumor potential of an HPV16 E6E7 multi-epitope vaccine. PLoS One. 2015;10:e0138686. doi: 10.1371/journal.pone.0138686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng S, Wang JW, Karanam B, Wang C, Huh WK, Alvarez RD, Pai SI, Hung CF, Wu TC, Roden RB. Sequential cisplatin therapy and vaccination with HPV16 E6E7L2 fusion protein in saponin adjuvant GPI-0100 for the treatment of a model HPV16+ cancer. PLoS One. 2015;10:e116389. doi: 10.1371/journal.pone.0116389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Bates TM, Kim E, Concha-Benavente F, Trivedi S, Mailliard RB, Gambotto A, Ferris RL. Enhanced cytotoxic CD8 T cell priming using dendritic cell-expressing human papillomavirus-16 E6/E7-p16INK4 fusion protein with sequenced anti-programmed death-1. J Immunol. 2016;196:2870–2878. doi: 10.4049/jimmunol.1502027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atherton MJ, Stephenson KB, Nikota JK, Hu QN, Nguyen A, Wan Y, Lichty BD. Preclinical development of peptide vaccination combined with oncolytic MG1-E6E7 for HPV-associated cancer. Vaccine. 2018;36:2181–2192. doi: 10.1016/j.vaccine.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 31.Shen KY, Song YC, Chen IH, Leng CH, Chen HW, Li HJ, Chong P, Liu SJ. Molecular mechanisms of TLR2-mediated antigen cross-presentation in dendritic cells. J Immunol. 2014;192:4233–4241. doi: 10.4049/jimmunol.1302850. [DOI] [PMC free article] [PubMed] [Google Scholar]


