Abstract
Recently SPAG9 has been reported to show aberrant expressions in numerous human malignancies and act as a crucial role in tumor’s proliferation and invasion. Human enhancer of filamentation 1 (HEF1, also known as CasL and NEDD9) is a non-catalytic scaffolding protein belonging to CAS (Crk-associated substrate) protein family that interacts with multiple signaling cascades. Due to the diversified function of HEF1, abnormal expression of HEF1 frequently combines with malignant phenotypes and poor prognosis. However, little is known between the relationship of SPAG9 and HEF1 in bladder tumorigenesis. In this study, expression of SPAG9 in vivo and in vitro has been detected by quantitative real-time PCR and Western blot analysis after transfected with SPAG9 overexpression/inhibitor vector. We also found that HEF1 expression shows consistency and is regulated by SPAG9. Overexpression of SPAG9 promotes bladder cancer cells migration through HEF1 upregulation and emerges protein level of activated Rac1. Silencing SPAG9 inhibits cell migration through HEF1 downregulation and reduces protein level of activated Rac1. Also, we found that expression of EMT marker such as E-cadherin, Vimentin is regulated by SPAG9. Considering EMT plays a crucial role in tumor cells spreading and invasion, SPAG9 and HEF1 may potentially set a new therapeutic approach to bladder cancer treatment.
Keywords: HEF1, EMT, bladder cancer, metastasis, rac1 signaling pathway, SPAG9
Introduction
Bladder transitional cell carcinoma is ranking as the fifth high-occurrence cancer in the United States and the ninth most common malignancy worldwide [1,2,26] and accounts for 80-90% of bladder tumor. The morbidity is increasing steadily and its death rates share the same trend [3]. The survival rate is only 50-70% after receiving cystectomy 5 years due to bladder cancer cells’ high recurrence and metastasis. Therefore, a whole new therapeutic method is urgently needed.
Sperm-associated antigen 9 (SPAG9), a novel member of cancer testis antigens, is involved in c-Jun N-terminal kinase (JNK)-signaling characterized as a scaffold protein for JNKs [4]. SPAG9 has been critically involved in cell proliferation, apoptosis, and tumorigenesis. SPAG9 is also reported to exhibit aberrant expression in various human malignancies such as gastrointestinal cancers [5], thyroid cancers [6], ovarian cancers [7] and renal carcinoma [8]. Overexpression of SPAG9 in tumors is also correlated with poor prognosis.
Human enhancer of filamentation 1, also known as Neural precursor cell-expressed developmentally downregulated protein 9 (NEDD9) or CasL, is a multidomain scaffolding protein of Crk-associated substrate (CAS) protein family. Previous research has demonstrated that HEF1 serves as a mediator to interact with focal adhesion kinase (FAK), SRC (Proto-oncogene tyrosine-protein kinase Src), Aurora-A [9,10,20]. Thanks to its pleiotropic connection, HEF1 is crucially involved in numerous biological processes like cell cycle, apoptosis, invasion, migration and mitosis [11]. Altered expression of HEF1 has been reported in many malignant properties such as colorectal cancer, lung cancer and melanoma [12-14].
Rac1, small GTPase belonging to Rho GTPase family, has been reported to intervene in various biological processes, especially in cell mobility and proliferation [15,16]. Mediated by NEDD9 and DOCK3 complex, Rac1 and its downstream cascades direct cell from amoeboid movement to Mesenchymal type movement transformation, featuring as cells cytoskeletal remodeling such as losing cell-matrix interaction and converting into stretched morphology. This may finally contribute to the EMT (epithelial mesenchymal transition) occurrence [17-19].
EMT (epithelial mesenchymal transition) is a crucial regulation mechanism in embryogenesis characterized as cell losing polarity, intercellular junction, adjusting cytoskeleton structure and adapting morphology to gain migratory and invasive properties, which contributes to cancer’s recurrence and metastasis. Thus cell-cell adhesion glycoproteins such as E-cadherin and SNAI1, structural protein in mesenchymal cytoskeleton as Vimentin, Fibronectin and matrix metalloproteinase family involved in degradation of extracellular matrix as MMP-2/MMP-9 could all conduct a distinctive change during EMT process [21-24].
In this study, we are aiming to elucidate that SPAG9 affects bladder cancer cells’ migration through regulating HEF1 and Rac1 signaling pathway, which is also correlated with EMT.
Material and methods
Plasmid construction
Plasmid vectors are obtained from GenePharma (Shanghai, China), vector shSPAG9 and shHEF1 are constructed under manufacturer’s recommendation. Three different specific sequences of SPAG9 and HEF1 are tested, the most effective sequences are selected. Relative non-specific shRNAs were used as negative control.
Overexpression vector pcDNATM3.1-SPAG9 and pcDNATM3.1-HEF1 are constructed (Invitrogen, USA) and relative non-specific empty vectors were used as negative control.
Tumor specimens and cell lines
Total 136 bladder transitional cancer specimens including different TNM stages are obtained from China-Japan Union Hospital of Jilin University with patients’ consent and in accordance to local ethic committee. T24 and 5637 cell lines were obtained from Chinese Academy of Sciences (Shanghai, China), cultured with DMEM with 10% FBS and 1% penicillin-streptomycin antibiotics in humidified atmosphere containing 5% CO2 in 37°C. Cell transfection was conducted using lipofectamineTM 3000 as mediator. All reagents are purchased from Invitrogen USA.
Immunohistochemistry
The formalin-fixed, paraffin-embedded bladder cancer sections and matched adjacent non-cancerous tissues (ANCT) were cut into 4 um specimens, analyzed with standard procedures as mentioned previously [25]. Two independent pathologists were invited to examine and score every specimen’s immunostaining intensity. The staining intensity was set at 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The proportion of SPAG9/HEF1 positive cells was evaluated as: 0 (<10%), 1 (11%-25%), 2 (26%-50%), 3 (>51%). Intensity value and proportion value are multiplied. Threshold index was set at 3. Index value less than 3 was considered to be a lower expression.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from shSPAG9 and pcDNA3.1TM-SPAG9 transfected cancer cells and control group using TRIzol (Invitrogen). Real-time PCR was performed to detect SPAG9 expression using 2-ΔΔCT methods. The sequence of primer was as follows: SPAG9: forward, 5’-GGCGGCTCGAGAAAATCCGTTCTACCATAAC-3’; reverse, 5’-AATGCGGCCGCAACTCAATCAACATCACCAT-3’. HEF1: forward, 5’-CCACCCTCCTACCAGAATCA-3’; reverse, 5’-ATACCCCTTGAGTGCTGTGG-3’. Quantitative real-time PCR was performed on an ABI Prism 7000 Sequence Detection System using QuantiTect SYBR Green. GAPDH was used as an internal control.
Migration and Invasion assays
Transwell migration assay was conducted using 24-well chamber with 8 μm pore size containing polycarbonate membrane (Corning USA). After 48 h transfection, total 9*104 cells were suspended in serum-free medium and seeded in the upper chamber. 10% FBS was filled into lower chamber as a chemotactic factor. Incubation at 37 for 48 h, the remaining cells on upper level was removed with cotton swab, lower chamber cells were fixed, stained with 0.1% crystal violet and counted under microscope with four different fields (Olympus Japan 40×). As for invasion assay, Matrigel (BD Biosciences USA) was covered on the membrane to form matrix barriers, other procedures were exhibited under the same protocol with Migration assay.
Rac1 activation assays
After transfected with previous vectors, 5637 and T24 cells were well prepared and detected for active Rac1 protein using the Active Rac1 Detection kit (#8815, cell signaling technology, USA). Briefly, GST-PAK1-PBD fusion protein was deployed to bind the active GTP-bound Rac1, Western blot analysis was used to examined the activation form of Rac1-GTP protein.
Western blot analysis
Cells were lysed in RIPA buffer mixed with 1% PMSF and Western blot process was performed under standard experiment protocol. Primary mouse antibodies were listed as below: anti-SPAG9 (1:1000 Cell Signaling Technology), anti-HEF1 (1:2000 Abcam), anti-E-cadherin (1:500 Cell Signaling Technology) and anti-Vimentin (1:500 Cell Signaling Technology), and anti-MMP2 (1:500 MMP-2 (D2O4T) Rabbit mAb #87809 Cell Signaling Technology), anti-GADPH (1:1000 Cell Signaling Technology). The protein bar was incubated with fluorescent anti-Mouse second antibody (IRDye® 800CW Goat anti-Mouse IgG (H + L), 0.1 mg, P/N 925-32210) and visualized on Odyssey® CLx Imaging System (LI-COR Biosciences USA).
Xenograft model
The T24 and 5637 cells were transfected with treated vector. Transfected cells (4*106) were harvested, washed in PBS, suspended by 200 uL serum-free DMEM culture medium and injected into nude mice (6 weeks old) in the armpit area. Total 50 mice (2 weeks old) were divided into 5 groups with each contains 10. All mice were sacrificed 3 weeks after implantation. The tumors were excised and fixed in 10% formalin for immunohistochemistry.
All animal experiments were conducted according to Institutional Animal Care and Use Committee guidelines, China-Japan Union Hospital of Jilin University. Mice were purchased from Shanghai SLAC Laboratory Animal (Shanghai China).
Statistical analysis
Final results are presented as mean ± SD with each experiment repeated at least three times. Statistical analysis between groups was evaluated using two-tailed Student’s t-test with GraphPad Prism 7. P<0.05 or less was considered statistically significant.
Results
HEF1 expression shows consistency with SPAG9 in different bladder cancer tumor stage
Aiming to understand the clinical relationship of SPAG9 and HEF1. IHC tests are carried out on samples from total 136 patients. Both SPAG9 and HEF1 protein expression were mainly located in cytoplasm (Figure 1A) and overexpression was pervasive. High expression of SPAG9 was observed in 61.8% (84/136) of all cases whereas HEF1 expression was 69.1% (94/136) (Table 1). Graphic of immunostaining index was shown in Figure 1B. Both SPAG9 and HEF1 were significantly overexpressed compared to adjacent non-cancerous tissue (ANCT) (P<0.005; Figure 1A, 1B).
Figure 1.
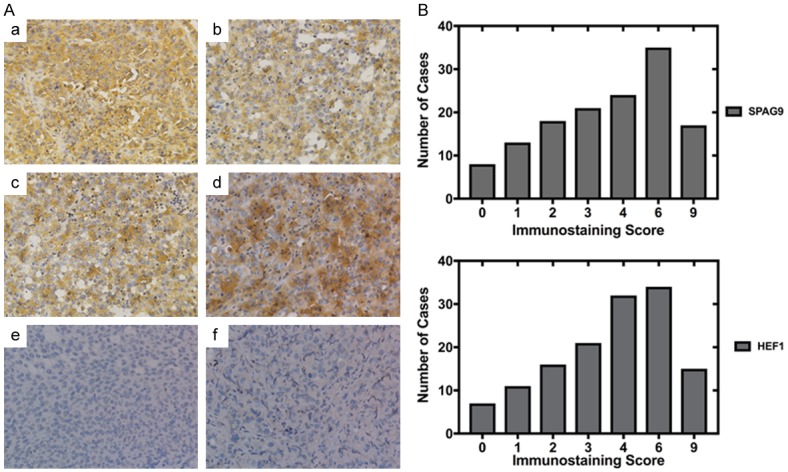
SPAG9 and HEF1 expression exhibits similar tendency in bladder transitional cells carcinoma of different tumor stage. A. a. Strong staining of SPAG9 in muscle invasion bladder transitional cells carcinoma. b. Strong staining of SPAG9 in non-muscle invasion bladder transitional cells carcinoma. c. Strong staining of HEF1 in muscle invasion bladder transitional cells carcinoma. d. Strong staining of HEF1 in non-muscle invasion bladder transitional cells carcinoma. e. Negative control of SPAG9 immunostaining in adjacent non-cancerous tissue (ANCT) with normal saline. f. Negative control of HEF1 immunostaining in adjacent non-cancerous tissue (ANCT) with normal saline. B. SPAG9 and HEF1 immunostaining index in graphical form. Magnification, ×40.
Table 1.
Relationship between SPAG9 and HEF1 expression and clinicopathological parameters in patients with bladder transitional carcinoma
| Variable | Cases | SPAG9 expression | P-Value | HEF1 expression | P-Value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Low (%) | High (%) | Low (%) | High (%) | ||||
| Total | 136 | 52 (38.2) | 84 (61.8) | 42 (30.9) | 94 (69.1) | ||
| Age (years) | 0.966 | 0.213 | |||||
| <60 | 52 | 19 (36.5) | 33 (63.5) | 21 (40.4) | 31 (59.6) | ||
| ≥60 | 84 | 31 (36.9) | 53 (63.1) | 26 (31.0) | 60 (69.0) | ||
| Gender | 0.023 | 0.174 | |||||
| Male | 91 | 39 (42.9) | 52 (57.1) | 35 (38.5) | 56 (61.5) | ||
| Female | 45 | 10 (22.3) | 35 (77.7) | 12 (26.7) | 33 (73.3) | ||
| Tumor Size | 0.001 | 0.005 | |||||
| <3.5 cm | 89 | 40 (44.9) | 49 (55.1) | 45 (50.6) | 44 (49.6) | ||
| ≥3 cm | 47 | 8 (17.0) | 39 (83.0) | 12 (25.5) | 35 (74.5) | ||
| Muscle Invasion | 0.000 | 0.000 | |||||
| Ta-I | 47 | 32 (68.1) | 15 (31.9) | 41 (87.2) | 6 (12.8) | ||
| T2-T3 | 89 | 18 (20.3) | 71 (79.7) | 24 (26.9) | 65 (73.1) | ||
| Distant Metastasis | 0.235 | 0.655 | |||||
| No | 117 | 54 (46.1) | 63 (54.9) | 43 (36.8) | 74 (63.2) | ||
| Yes | 19 | 6 (31.6) | 13 (68.4) | 8 (42.1) | 11 (57.9) | ||
| Pathologic Tumor Grade | 0.001 | 0.049 | |||||
| Low Grade | 54 | 6 (11.2) | 48 (88.8) | 9 (16.7) | 45 (83.3) | ||
| High Grade | 82 | 30 (36.6) | 52 (63.4) | 26 (31.7) | 56 (68.3) | ||
In Table 1, we also observed that the proportion of overexpression of SPAG9 and HEF1 in low grade bladder cancer was relatively higher than in high grade. High SPAG9 expression in low grade was 88.8% (48/54) in comparison of 63.4% (52/82) in high grade (P<0.001). High HEF1 expression in low grade was 83.3% (45/54) in comparison of 68.3% (56/82) in high grade (P<0.05). SPAG9 and HEF1 expression were both significantly associated with clinical parameters as tumor size, muscle invasion and tumor grade.
HEF1 expression significantly regulated by SPAG9 in bladder transitional cancer cells in vitro and in vivo
5637 and T24 cells were transfected with upregulated vector (pcDNA3.1TM-SPAG9) or downregulated vector (shSPAG9), SPAG9 expression was shown below (Figure 2A). Western Blot and qPCR analysis were performed to determine whether HEF1 was regulated by SPAG9. As shown in Figure 2B, expression of HEF1 did increase significantly after 48 h SPAG9 transfection. Silencing of SPAG9 attenuated HEF1 expression in 5637 and T24 cells.
Figure 2.
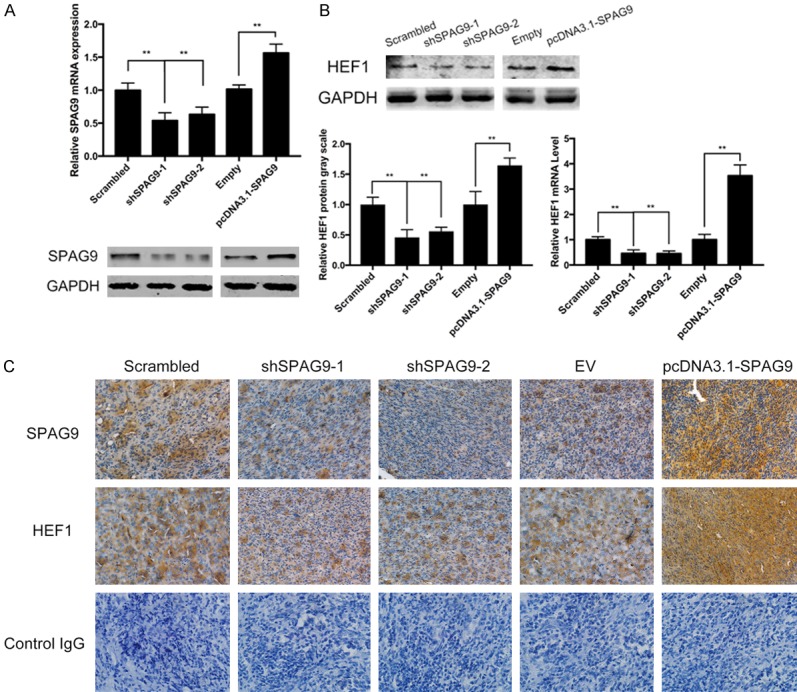
SPAG9 regulates HEF1 expression in bladder TCC. A. Western blot and quantitive real-time PCR analysis of SPAG9 expression after transfected with shSPAG9 or pcDNATM3.1-SPAG9 in 5637 cells. GAPDH was chosen as internal control. B. Western blot and quantitive real-time PCR analysis of HEF1 expression after transfected with shSPAG9 or pcDNATM3.1-SPAG9 in 5637 cells. GAPDH was chosen as internal control. C. In vivo experiment of immunohistochemical pictures of SPAG9 and HEF1 expression in nude mice. **for P<0.01. All experiments were repeated in triplicate.
To further validate the regulation of HEF1 by SPAG9, xenograft model was conducted using transfected cells, immunohistochemistry images shows SPAG9 regulates HEF1 in a significant manner (Figure 2C).
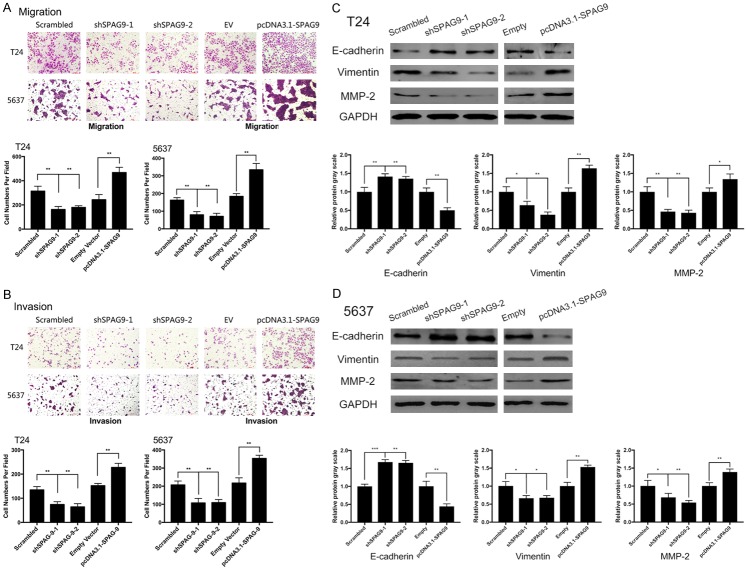
SPAG9 promotes migration and invasion in bladder transitional cancer cells and regulates EMT
Numerous protein markers such as E-cadherin, Vimentin have wildly been considered as characteristic protein in evaluating EMT and are closely tied to malignant phenotypes in bladder cancer [21,24]. In this study, we determined to explore the effect on EMT process in bladder cancer after SPAG9 transfection. Silencing SPAG9 attenuated the invasion and migration, upregulating SPAG9 promoted invasion and migration (Figure 3A, 3B). Stable knockdown of SPAG9 in 5637 and T24 cells elevated E-cadherin protein level along with inhibiting MMP-2/Vimentin expression, whereas overexpression of SPAG9 effectively reduced E-cadherin expression but emerged MMP-2/Vimentin level (Figure 3C, 3D).
Figure 3.
SPAG9 enhances migration and invasion and regulates EMT process in bladder transitional cancer cells. A and B. Transwell assays were set to evaluate the influence of SPAG9 expression on migration/invasion abilities in 5637 and T24 cells after transfection with shSPAG9 or pcDNATM3.1-SPAG9. C and D. Western blot analysis on EMT associated proteins E-cadherin/Vimentin/MMP-2 in 5637 and T24 cells after transfection with shSPAG9 or pcDNATM3.1-SPAG9. **for P<0.01. All experiments were repeated in triplicate.
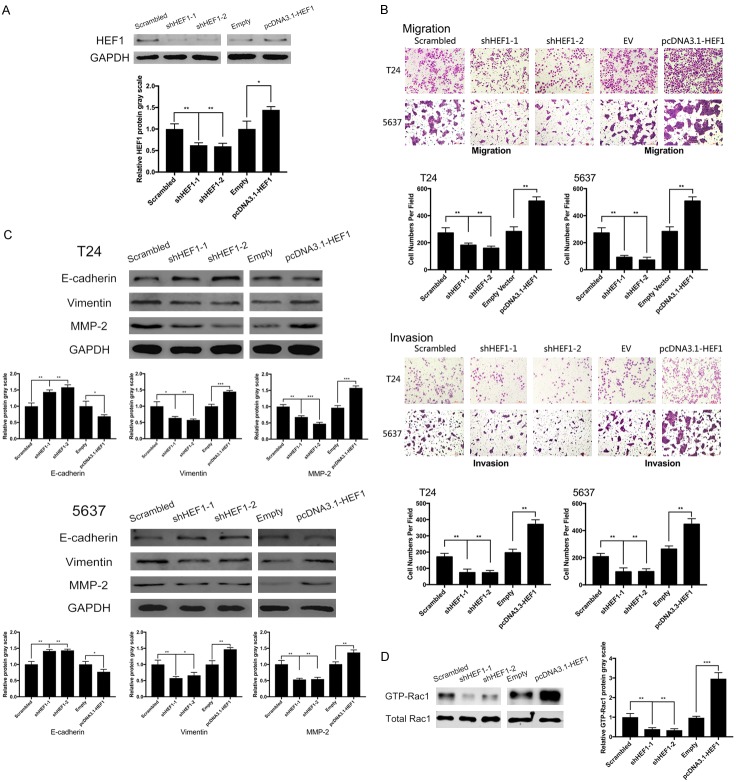
HEF1 expression regulates migration and invasion in bladder cancer along with EMT and promotes rac1 activation
To validate biological effect of HEF1 on bladder cancer, knockdown and upregulation was performed on HEF1 in 5637 and T24. Transfection efficiency was detected using Western blot analysis (Figure 4A). Similar to SPAG9, knocking down HEF1 reduced cell’s potency on migration and invasion. Overexpression of HEF1 promoted migration and invasion in 5637 and T24 cell lines (Figure 4B). Furthermore, we evaluated the role of HEF1 on EMT, knockdown of HEF1 promoted E-cadherin protein level and lowered MMP-2 and Vimentin expression. Increasing HEF1 expression declined E-cadherin and lifted both MMP-2 and Vimentin level (Figure 4C). Also, as shown in Figure 4D, inhibition of HEF1 resulted in inactivation of Rac1, whereas overexpression of HEF1 contributed to the emerged activated form of Rac1.
Figure 4.
HEF1 regulates migration and invasion and induces Rac1 activation with EMT. A. Western blot analysis on HEF1 expression in 5637 cells after transfected with shHEF1 or pcDNATM3.1-HEF1. B. Transwell assays on influences of HEF1 in 5637 and T24 cells after transfection above. C. HEF1 influences on EMT related protein analyzed by Western blot in 5637 and T24 cells. D. Western blot analysis on Rac1 protein after transfected with shHEF1 or pcDNATM3.1-HEF1 in 5637 cells. **for P<0.01. All experiments were repeated in triplicate.
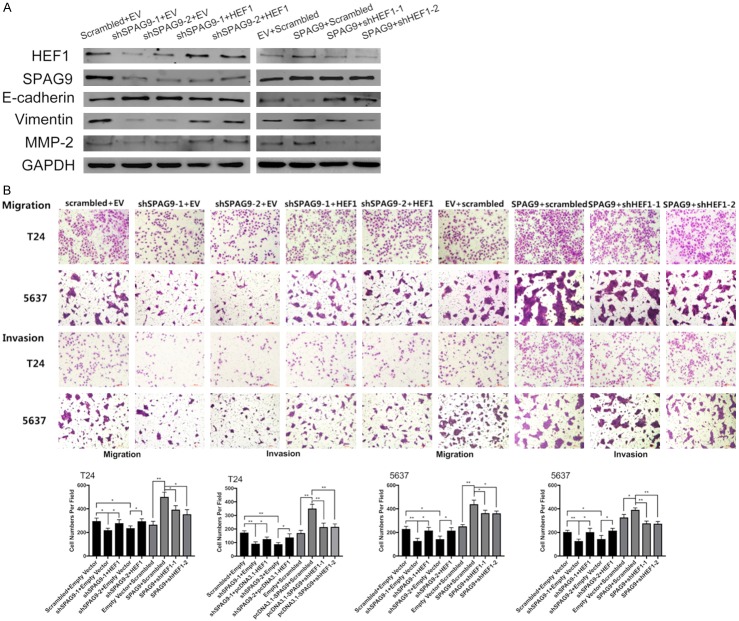
Downregulating HEF1 antagonized the malignant phenotype mediated by SPAG9
Previous results have demonstrated that SPAG9 and HEF1 exhibits similar biological effect on bladder cancer’s migration and invasion ability. Providing that SPAG9 regulates HEF1, an additional experiment was designed to determine whether the effects of SPAG9 on bladder cancer cells was induced by HEF1 variations. Transfection combinations of shSPAG9 + pcDNATM3.1-HEF1 and shHEF1 + pcDNATM3.1-SPAG9 was each carried out to verify our hypothesis. As shown in Figure 5A, 5B, downregulation of HEF1 attenuated the potency of invasion and migration lifted by pcDNATM3.1-SPAG9, whereas upregulation of HEF1 restored T24 and 5637 cells’ aggressive phenotype and lifts the decreasing HEF1 protein level induced by shSPAG9. Besides, we also evaluated the co-transfection’s influences on EMT index proteins. As shown in Figure 5A, upregulation of HEF1 could effectively antagonize the upregulation of E-cadherin and downregulation of Vimentin/MMP-2 induced by shSPAG9, meanwhile inhibiting HEF1 level could restore the suppression of E-cadherin and reduce the high expression of Vimentin/MMP-2 induced by pcDNATM3.1-SPAG9. Interestingly, whether its pcDNATM3.1-HEF1 or shHEF1 could not redress the SPAG9 inhibition or elevation induced by shSPAG9 and pcDNATM3.1-SPAG9.
Figure 5.
HEF1 knockdown restores the malignant properties induced by SPAG9. A. Transfection combinations of shSPAG9 + pcDNATM3.1-HEF1 or pcDNATM3.1-SPAG9 + shHEF1 and its control vectors were first implemented in 5637 and T24 cells, then protein was extracted and analyzed by Western blot. B. Transwell assays on evaluating the migration and invasion abilities after co-transfection above in 5637 and T24 cells. *for P<0.05, **for P<0.01. All experiments were repeated in triplicate.
SPAG9 directs rac1 signaling pathway through regulating HEF1
It has been confirmed in the previous experiment that HEF1 could affect Rac1 activation and SPAG9 regulates the expression of HEF1. Thus, we come up with a speculation that SPAG9 could affect Rac1 activation though mediation of HEF1. We verify our hypothesis both in vivo and in vitro. As shown blow, SPAG9 influenced Rac1 activation in vivo (Figure 6A). Knockdown/overexpression of SPAG9 resulted in Rac1 inactivation/activation in vitro (Figure 6B). These indicated that SPAG9 regulates Rac1 signaling pathway. To further prove that HEF1 is the mediator of spag9 affecting Rac1, we designed two transfection combinations: shSPAG9 + pcDNATM3.1-HEF1 and shHEF1 + pcDNATM3.1-SPAG9. As shown in Figure 6C, overexpression of HEF1 could rescue the inactivation of Rac1 caused by shSPAG9, whereas inhibition of HEF1 could attenuate the activation of Rac1 caused by pcDNATM3.1-SPAG9. These results confirmed our speculation that SPAG9 directs Rac1 signaling pathway through mediated by HEF1.
Figure 6.
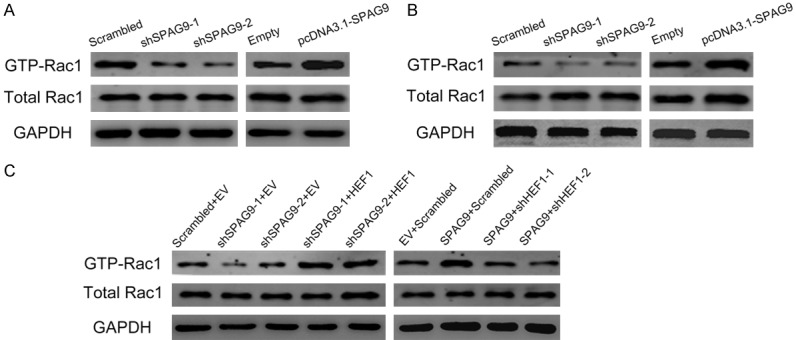
HEF1 acts as mediator in SPAG9 induced Rac1 signaling pathway. A. Western blot analysis on SPAG9 transfection affecting Rac1 activation in vivo. B. Western blot analysis of SPAG9 transfection influences on Rac1 in vitro in 5637 cells. C. Western blot analysis on HEF1 antagonism after transfection of pcDNATM3.1-HEF1 and pcDNATM3.1-HEF1 on Rac1 activation in 5637 cells. All experiments were repeated in triplicate.
Discussion
Bladder transitional cell carcinoma, which accounts for 90% in all type of bladder cancer [27], is ranked as the second most common cancer in human genitourinary malignancies and the most common cancer in bladder neoplasms in China [28]. Nearly 25% transitional cell carcinoma (TCC) patients was diagnosed with muscle-invasion and distant metastasis and especially bone metastasis has been wildly reported in 30%-40% TCC patients [29]. Distant metastasis in lung, breast and liver is often related with poor survival rate [30-32]. Usual treatments include chemotherapy, radical surgery and radiation, yet only chemotherapy which involves methotrexate/vinblastine/adriamycin/cisplatin or cisplatin/gemcitabine has been proven with significant survival improvement [33,34]. But drug tolerance particularly of platinum resistance has grew rapidly and severely decreased patient’s objective response rates (ORR), progression-free survival (PFS) and overall survival [35-37]. Novel clinical therapeutic agents targeting specific tumorigenesis pathway are wildly developed, although still in its infancy, this could exploit an innovative method in treating bladder transitional cell carcinoma.
SPAG9, a scaffolding protein belonging to CT antigen family, has been reported to show its abundant expression in cervical carcinoma, melanoma and lung cancer, acting as a potential novel biomarker in diagnosis [4-8]. It’s reported that SPAG9 influence hepatocellular carcinoma migration and invasion through regulating ELK 1. In prostate cancer, protein-kinase C-related kinase 1 (PRK1/PKN1) induces cell metastasis mainly by assembling with SPAG9, then co-localization leads to a p38 phosphorylation and finally activates p38 downstream effector ELK 1 to promote cell migration [38,39]. A recent research indicates that SPAG9 is also overexpressed in bladder transitional carcinoma and involved in multifarious tumorigenesis such as cell migration, proliferation and invasion [40]. However, the mechanism of SPAG9 regulating these biological processes is still unexplored yet in bladder cancer.
HEF1, also known as NEDD9, Cas-L, is also a pleiotropic protein involved in cytoskeletal reorganization. Previous study has reported that HEF1 may act as a major contributor in stabilizing focal adhesion and maintaining the homeostasis of extra-cellular matrix [9,20]. In cancer cells, GEF-NEDD9-DOCK3 complex could promote the amoeboid-to-mesenchymal transition by activating Rac1 signaling pathway thus to entitle cancer cells an aggressive phenotype, enhancing their invasion and migration ability [18,19,41]. Considering HEF1 exhibits an abnormal expression in bladder cancer [42] and no research has been performed between HEF1 and SPAG9 in bladder cancer, we are determined to explore the inner relationship of SPAG9 and HEF1.
In this study, first we examined the SPAG9 and HEF1 expression in clinical samples; our results showed consistency with previous research that both SPAG9 and HEF1 are aberrantly expressed in bladder transitional cell carcinoma. Meanwhile we analyzed the correlation between the expression of SPAG9/HEF1 and the sample’s clinical characteristics. We conclude that both SPAG9 and HEF1 expression are in close tie with patient’s muscle invasion, tumor size, tumor stages. By using gene-silencing technology, verified by qPCR and Western blot analysis, we conclude that SPAG9 regulates HEF1 both at mRNA and protein level. Xenograft model was also conducted to test our results in vivo. All experiments indicated that HEF1 was significantly regulated by SPAG9.
EMT (epithelial mesenchymal transition) is a critical physiological programme both in embryonic development and pathogenesis, mainly executes its function by EMT-activating transcription factors (EMT-TFs). Those protein variates their expression in all major tumorigenesis from tumor growth to metastasis even involved in double-strand DNA repair allowing cancer cells to evolve an anti-apoptotic and pro-survival properties [22,24]. In our research, we found that knocking down SPAG9 could inhibit migration and invasion. After choosing E-cadherin, Vimentin and MMP-2 as EMT evaluation proteins, we found that downregulation of SPAG9 could lead to emerged E-cadherin protein level along with reduction of Vimentin and MMP-2 expression. Overexpression of SPAG9 could result in inhibition of E-cadherin but promoting protein level of Vimentin and MMP-2. Thus, we conclude that SPAG9 regulates EMT process in bladder transitional carcinoma.
However, the biological role of HEF1 in bladder cancer still remains uncertain. In our study, we found silencing HEF1 could inhibit migration and invasion in 5637 and T24 cells, meanwhile we also have detected the inactivation of Rac1 caused by silencing HEF1. Overexpression of HEF1 will promote migration and invasion along with the activation of Rac1.
Yet to confirm our speculations that SPAG9 regulates bladder cancer cells’ malignant phenotype through mediation of HEF1, co-transfection of shSPAG9 and pcDNATM3.1-HEF1 was exhibited. We found that overexpression of HEF1 could rescue the inhibition in migration and invasion capacity caused by SPAG9 silencing. The other co-transfection combination pcDNATM3.1-SPAG9 and shHEF1 was also conducted, we found that knocking down HEF1 expression could antagonize the malignant phenotype caused by SPAG9 overexpression. Those data indicated that SPAG9 regulates at least partially the migration and invasion abilities through the mediation of HEF1.
Rho family small guanosine triphosphatases (GTPases) are another crucial proteins participating in various cell movements like lamellipodia, filopodia, invadopodia [43]. Typical proteins representing Rho family are ROCK, RAC1 and CDC42. It is reported that mesenchymal movement require deformation of extracellular matrix for the release of Rac-dependent actin. Anne J. Ridley previously reported that Rac1 could promoting lamellipodia formation and regulating invadopodial turnover and stability along with CDC42 [44-47]. These functions are key regulator in cell migration and metastasis.
Providing that SPAG9 and HEF1 exhibits similar biological effect on regulating migration and invasion in bladder cancer cells, we are determined to explore whether SPAG9 could affect Rac1 signaling pathway via HEF1 variation. We found that SPAG9 inhibition could deactivate Rac1, whereas overexpression of SPAG9 will result in activation of Rac1 both in vivo and in vitro. Then we co-transfected shSPAG9 and pcDNATM3.1-HEF1, inactivation of Rac1 caused by shPAG9 was effectively remitted. In addition, knocking down HEF1 could significantly reduce the GTP-Rac1 level lifted by pcDNATM3.1-SPAG9. All these results conclude that SPAG9-HEF1-Rac1 cascade could be a potential signaling pathway in regulating EMT process in bladder transitional cell carcinoma.
Conclusion
Our research revealed that SPAG9 could influence HEF1 expression and induced EMT process in bladder transitional cell carcinoma (TCC) through Rac1 signaling pathway.
Acknowledgements
Supported by China-Japan Union Hospital of Jilin University Innovation Project (Project ID: 3D517Q053430). Special appreciation to He Liang for cell culture advices.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J, Rouprêt M European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Jagadish N, Rana R, Selvi R, Mishra D, Garg M, Yadav S, Herr John C, Okumura K, Hasegawa A, Koyama K, Suri A. Characterization of a novel human sperm-associated antigen 9 (SPAG9) having structural homology with c-Jun N-terminal kinase-interacting protein. Biochem J. 2005;389:73–82. doi: 10.1042/BJ20041577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9 is a novel biomarker for colorectal cancer and is involved in tumor growth and tumorigenicity. Am J Pathol. 2011;178:1009–1020. doi: 10.1016/j.ajpath.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg M, Kanojia D, Suri S, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9: a novel diagnostic marker for thyroid cancer. J Clin Endocrinol Metab. 2009;94:4613–4618. doi: 10.1210/jc.2009-0703. [DOI] [PubMed] [Google Scholar]
- 7.Garg M, Chaurasiya D, Rana R, Jagadish N, Kanojia D, Dudha N, Kamran N, Salhan S, Bhatnagar A, Suri S, Gupta A, Suri A. Sperm-associated antigen 9, a novel cancer testis antigen, is a potential target for immunotherapy in epithelial ovarian cancer. Clin Cancer Res. 2007;13:1421–1428. doi: 10.1158/1078-0432.CCR-06-2340. [DOI] [PubMed] [Google Scholar]
- 8.Garg M, Kanojia D, Khosla A, Dudha N, Sati S, Chaurasiya D, Jagadish N, Seth A, Kumar R, Gupta S, Gupta A, Lohiya NK, Suri A. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res. 2008;68:8240–8248. doi: 10.1158/0008-5472.CAN-08-1708. [DOI] [PubMed] [Google Scholar]
- 9.O’Neill GM, Seo S, Serebriiskii IG, Lessin SR, Golemis EA. A new central scaffold for metastasis: parsing HEF1/Cas-L/NEDD9. Cancer Res. 2007;67:8975–8979. doi: 10.1158/0008-5472.CAN-07-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shagisultanova E, Gaponova AV, Gabbasov R, Nicolas E, Golemis EA. Preclinical and clinical studies of the NEDD9 scaffold protein in cancer and other diseases. Gene. 2015;567:1–11. doi: 10.1016/j.gene.2015.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh M, Cowell L, Seo S, O’Neill G, Golemis E. Molecular basis for HEF1/NEDD9/Cas-L action as a multifunctional coordinator of invasion, apoptosis and cell cycle. Cell Biochem Biophys. 2007;48:54–72. doi: 10.1007/s12013-007-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai J, Van Wie PG, Fai LY, Kim D, Wang L, Poyil P, Luo J, Zhang Z. Downregulation of NEDD9 by apigenin suppresses migration, invasion, and metastasis of colorectal cancer cells. Toxicol Appl Pharmacol. 2016;311:106–112. doi: 10.1016/j.taap.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JX, Gao F, Zhao GQ, Zhang GJ. Effects of lentivirus-mediated RNAi knockdown of NEDD9 on human lung adenocarcinoma cells in vitro and in vivo. Oncol Rep. 2014;32:1543–1549. doi: 10.3892/or.2014.3347. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, Flotte TJ, Duncan LM, Granter SR, Chin L. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Wang B, Li P, Zhang Q, Liu P. Reduced RAC1 activity inhibits cell proliferation and induces apoptosis in neurofibromatosis type 2(NF2)-associated schwannoma. Neurol Res. 2017;39:1086–1093. doi: 10.1080/01616412.2017.1376494. [DOI] [PubMed] [Google Scholar]
- 16.Zegers MM, Friedl P. Rho GTPases in collective cell migration. Small GTPases. 2014;5:e28997. doi: 10.4161/sgtp.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang WH, Lan HY, Huang CH, Tai SK, Tzeng CH, Kao SY, Wu KJ, Hung MC, Yang MH. RAC1 activation mediates Twist1-induced cancer cell migration. Nat Cell Biol. 2012;14:366–374. doi: 10.1038/ncb2455. [DOI] [PubMed] [Google Scholar]
- 18.Jansen S, Gosens R, Wieland T, Schmidt M. Paving the Rho in cancer metastasis: Rho GTPases and beyond. Pharmacol Ther. 2018;183:1–21. doi: 10.1016/j.pharmthera.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Yoon C, Cho SJ, Chang KK, Park DJ, Ryeom SW, Yoon SS. Role of Rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol Cancer Res. 2017;15:1106–1116. doi: 10.1158/1541-7786.MCR-17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Guerrero MS, Parsons JT, Bouton AH. Cas and NEDD9 contribute to tumor progression through dynamic regulation of the cytoskeleton. Genes Cancer. 2012;3:371–381. doi: 10.1177/1947601912458585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripathi K, Garg M. Mechanistic regulation of epithelial-to-mesenchymal transition through RAS signaling pathway and therapeutic implications in human cancer. J Cell Commun Signal. 2018;12:513–527. doi: 10.1007/s12079-017-0441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 23.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Xing T, Yang Z, Dudek R, Lu Q, Chen YH. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 2017;7 doi: 10.3390/jcm7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Jiang F, Song H, Li X, Xian J, Gu X. MicroRNA-200a-3p suppresses tumor proliferation and induces apoptosis by targeting SPAG9 in renal cell carcinoma. Biochem Biophys Res Commun. 2016;470:620–626. doi: 10.1016/j.bbrc.2016.01.095. [DOI] [PubMed] [Google Scholar]
- 26.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Beltran A. Bladder cancer: clinical and pathological profile. Scand J Urol Nephrol Suppl. 2008:95–109. doi: 10.1080/03008880802325226. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 29.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 30.Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, Herr H, Higgins G, Boyle MG. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J. Clin. Oncol. 1999;17:3173–81. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 31.Bellmunt J, Albanell J, Paz-Ares L, Climent MA, González-Larriba JL, Carles J, de la Cruz JJ, Guillem V, Díaz-Rubio E, Cortés-Funes H, Baselga J Spanish Oncology Genitourinary Group. Pretreatment prognostic factors for survival in patients with advanced urothelial tumors treated in a phase I/II trial with paclitaxel, cisplatin, and gemcitabine. Cancer. 2002;95:751–757. doi: 10.1002/cncr.10762. [DOI] [PubMed] [Google Scholar]
- 32.Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, Winquist E, Culine S, von der Maase H, Vaughn DJ, Rosenberg JE. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J. Clin. Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 33.von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000;18:3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 34.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 35.Galsky MD, Mironov S, Iasonos A, Scattergood J, Boyle MG, Bajorin DF. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs. 2007;25:265–70. doi: 10.1007/s10637-006-9020-9. [DOI] [PubMed] [Google Scholar]
- 36.Witte RS, Elson P, Bono B, Knop R, Richardson RR, Dreicer R, Loehrer PJ Sr. Eastern cooperative oncology group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J. Clin. Oncol. 1997;15:589–93. doi: 10.1200/JCO.1997.15.2.589. [DOI] [PubMed] [Google Scholar]
- 37.Witte RS, Manola J, Burch PA, Kuzel T, Weinshel EL, Loehrer PJ. Topotecan in previously treated advanced urothelial carcinoma: an ECOG phase II trial. Invest New Drugs. 1998;16:191–5. doi: 10.1023/a:1006159525793. [DOI] [PubMed] [Google Scholar]
- 38.Yan Q, Lou G, Qian Y, Qin B, Xu X, Wang Y, Liu Y, Dong X. SPAG9 is involved in hepatocarcinoma cell migration and invasion via modulation of ELK1 expression. Onco Targets Ther. 2016;9:1067–1075. doi: 10.2147/OTT.S98727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jilg CA, Ketscher A, Metzger E, Hummel B, Willmann D, Rüsseler V, Drendel V, Imhof A, Jung M, Franz H, Hölz S, Krönig M, Müller JM, Schüle R. PRK1/PKN1 controls migration and metastasis of androgen-independent prostate cancer cells. Oncotarget. 2014;5:12646–12664. doi: 10.18632/oncotarget.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang YJ, Kanojia D, Garg M, Saini S, Agarwal S, Parashar D, Jagadish N, Seth A, Bhatnagar A, Gupta A, Kumar R, Lohiya NK, Suri A. Sperm associated antigen 9 plays an important role in bladder transitional cell carcinoma. PLoS One. 2013;8:e81348. doi: 10.1371/journal.pone.0081348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Wang HJ, Zhang DH, Ru GQ, He XJ, Ma YY. High expression of HEF1 is associated with poor prognosis in urinary bladder carcinoma. Onco Targets Ther. 2014;7:1319–1326. doi: 10.2147/OTT.S64418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawson CD, Ridley AJ. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol. 2018;217:447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science. 1997;275:1308. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 45.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–22. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 46.Haga RB, Ridley AJ. Rho GTPases: regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vega FM, Thomas M, Reymond N, Ridley AJ. The Rho GTPase RhoB regulates cadherin expression and epithelial cell-cell interaction. Cell Commun Signal. 2015;13:6. doi: 10.1186/s12964-015-0085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]