Abstract
MicroRNA-485-5p (miR-485-5p) has been reported to be involved in the development and progression of human cancers; however, its role in glioma remains unclear. In the present study, we found that miR-485-5p was significantly down-regulated in both glioma tissues and cell lines. Functional experiments indicated that enhanced expression of miR-485-5p attenuated glioma cell proliferation in vitro and in vivo, and induced glioma cells cycle arrest in G1. MiR-485-5p was found to directly bind to the 3’-UTR of paired box 3 (PAX3) and decrease its expression of protein level, which further inhibits the proliferation of glioma. The decreasing of PAX3 was found to lead to the accumulation of p-JNK. Mechanistic studies revealed that restoring the expression of PAX3 alleviated miR-485-5p-induced inhibition of proliferation of glioma cells. Taken together, these findings suggest that PAX3 modulation by miR-485-5p has an important role in regulating glioma proliferation, and miR-485-5p might be a novel therapeutic target for glioma.
Keywords: Glioma, miR-485-5p, proliferation, cell cycle arrest, PAX3
Introduction
Glioma is the most common form of primary brain tumors in adults, which accounts for over 70% of human brain tumours [1,2]. Glioma shows a high percentage of recurrence [3,4]. The standardized treatment for glioma is surgical resection followed by chemotherapy and radiation. Despite advances in therapy, the median survival time is about 12-15 months [5,6]. Therefore, studying the molecules mechanisms of glioma development and progression is critical for prolonging the survival time of patients.
MicroRNAs (miRs) are small non-coding RNAs with an length of 22 or so nucleotides, which exist in many organisms [7,8]. MiRs play important roles in cancer development and progression as promoters or suppressors, which are usually downregulated in various cancers [9-11]. Recently, studies have shown that the aberrant expression of miR-485-5p in lots of human cancer. Several evidence demonstrated that miR-485-5p plays as an oncogene in gastric cancer [12], breast cancer [13] and hepatocellular carcinoma [14]. However, the mechanism of miR-485-5p in glioma is not clear.
PAX3 (paired box 3) is a developmental critical transcription factor for neuronal, and displays a regionalized expression pattern in the neural crest and neural tube [15,16]. Aberrant PAX3 expression is known to contribute to the proliferation, metastasis, and invasion of various types of cancer cells, such as rhabdomyosarcoma [17] and melanoma [18]. However, molecular mechanisms of PAX3 in glioma are not known.
In this study, we demonstrated that decreased expression of miR-485-5p in human gliomas is related to tumor progression, and overexpression of miR-485-5p can inhibit glioma cells proliferation in vitro and in vivo. Moreover, miR-485-5p acts as a novel tumor suppressor by directly targeting PAX3. Our results provide new insights into the molecular function of miR-485-5p as well as highlight new therapeutic opportunities for the development of glioma treatments.
Materials and methods
Database and human tissue samples
198 glioma cases for miRNA expression were obtained from the CGGA data portal (http://www.cgga.org.cn.portal.phpg). The 198 glioma samples included 107 low-grade glioma samples (LGG) and 91 high-grade glioma samples (HGG). Gene expression data for miRNA were obtained from the Cancer Genome Atlas (TCGA) database (http://tcga-data.nci.nih.gov) and CGGA database. 29 glioma tissue specimens were obtained from the Department of Neurosurgery, First Affiliated Hospitals of Nanjing Medical University, from 2015 to 2017. Of these 29 samples, 6 were normal brain tissues (NBTs), 8 were low-grade glioma samples (LGG) (grades I and II) and 15 were high-grade glioma samples (HGG) (grades III and IV). The histological features of all specimens were confirmed by pathologists according to the WHO criteria. All the patients involved in this study gave written informed consent.
Cell culture and transfection
Glioma cell lines (U251 and U87) were obtained from Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM with 10% FBS. All the cells were cultured at 37°C in a humidified 5% CO2 environment.
Cell transfection was performed using Lipo-fectamine 2000 (Thermo Fisher Scientific, Waltham, USA) according to manufacturer’s protocols. miR-485-5p mimic and its negative control were synthesized by Shanghai Gene-Pharma (Shanghai, China). Small interfering RNA (siRNA) against PAX3 and its negative control siRNA were also purchased from Gene-Pharma (Shanghai, China).
RNA isolation and qRT-PCR (quantitative real-time PCR)
RNA extraction and qRT-PCR were performed as our previously described. To detect the expression of miR-485-5p and U6 RNA, qRT-PCR was performed using TaqMan miRNA assays. To detect the expression of PAX3, qRT-PCR was performed using Fermentas reverse transcription reagents and SYBR Green PCR Master Mix (Applied Biosystems, USA). Each experiments was performed in triplicate.
Western blot analysis
The expression patterns of CDK2, CDK4, CDK6, CCND1, PAX3, JNK, p-JNK were all detected using specific antibodies and Actin was used as the loading control. Each experiment was carried out thrice biological replicates.
Cell viability assay
Cell Counting Kit-8 (CCK8) assay assessed Cell proliferation. The glioma cells (1 × 103) were trypsinized and seeded into 96-well culture plates 24 h after transfection. Ten microliters of CCK8 was added to each well. After 2 h incubation, the absorbance was measured at 450 nm using a spectrophotometer 20.
EdU assay
The U87 and U251 cells after transfection were seeded into 96-well plates at 1 × 104 cells. After 48 h incubation, Cell-Light TM EdU DNA cell proliferation kit (Ruibo Biotech, Guangzhou, China) was used according to the manufacturer’s instructions. Finally, the cells were examined with fluorescence microscopy and photographed (Leica, Wetzlar, Germany).
Colony formation assay
The transfected glioma cells about 400 seeded in 6-well culture plates. After a 14-day incubation, cells were fixed with methanol for 30 min and stained with 0.1% crystal violet for 30 min. Colonies (> 50 cells/colony) were counted. Colony formation assay was used to evaluate the possible differences in long-term effects.
Cell cycle assays
After 2 days transfection, U87 and U251 cells were trypsinized and fixed in 70% ethanol at -20°C. Afterwards, U87 and U251 cells were washed in PBS for twice and incubated with PI solution that contained 50 μg/mL PI and 25 μg/mL Rnase in the dark for 20 min. Subsequently, the cells were assayed on flow cytometry (BD Bioscience, Franklin Lakes, NJ, USA).
Dual luciferase reporter assay
The 3’-UTR of PAX3 containing a binding site for miR-485-5p was amplified from a human cDNA library and cloned into the downstream region of the luciferase gene. For reporter assay, cells were co-transfected with miR-485-5p mimics and wild-type or mutant 3’ UTR of PAX3 by Lipofectamine 2000. Data were normalized against the activity of the Renilla luciferase gene.
Tumor xenograft
Male BALB/c-A nude mice (4-5 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Tumors were generated by injection of 1 × 107 U87 cells for in vivo tumor growth assay. Each group (n = 5) was treated with miR-NC or miR-485-5p mimic in 15 μL Lipofectamine 2000 through local injection of the tumor at multiple sites. After 28 days of injection, mice were sacrificed and the tumors were photographed. Tumor volume was calculated using the formula: V = 0.5 × Length × Width2.
Immunohistochemistry
Immunohistochemical (IHC) analysis was conducted to study Ki-67 protein expression in xenografts. Firstly, glioma xenografts were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm-sections. Then, the sections were immunohistochemically stained using Ki-67 antibody (1:100, Cell Signaling Technologies). Lastly, Slides were imaged under a light microscope (Leica).
Statistical analysis
All statistical analyses are performed using Prism GraphPad version 6.0 (GraphPad Software Inc., San Diego, USA) and are presented as the means ± SEM of three to five independent experiments. Comparisons between miR-485-5p mimic or its negative control were calculated using Student’s t test. P values < 0.05 were considered statistically significant.
Results
miR-485-5p is significantly downregulated in glioma tissues and cell lines
To identify the roles of miR-485-5p in the development and progression of glioma, we first analyzed the expression level of miR-485-5p in database of CGGA. MiR-485-5p levels in high-grade glioma samples (HGG) (grades III and IV) were significantly decreased compared with those in low-grade glioma (LGG) (grade II) (Figure 1A). Then, we investigated miR-485-5p expression in 29 samples, including 6 normal brain tissues (NBTs), 8 low-grade glioma cases and 15 high-grade glioma cases by qRT-PCR. miR-485-5p expression was decreased observed LGG and HGG compared with NBTs. In addition, HGG had lower miR-485-5p expression than LGG (Figure 1B). Futhermore, we examined the expression levels of miR-485-5p in U251 and U87 cells as well as normal human astrocytes (NHA). Compared with NHA, miR-485-5p was downregulated in U251 and U87 cells (Figure 1C).
Figure 1.
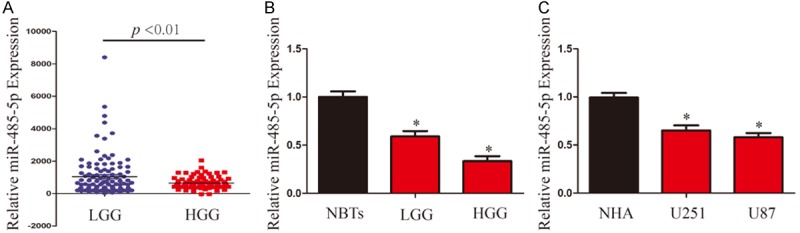
MiR-485-5p is downregulated in glioma tissues and cell lines. A. CGGA database showing miR-485-5p expression in LGG and HGG. B. qRT-PCR analysis of miR-485-5p expression in NBTs, LGG and HGG. C. qRT-PCR analysis of miR-485-5p expression in NHAs and glioma cell lines (U87 and U251). Transcript levels were normalized by U6 expression. *P < 0.05.
Overexpression of miR-485-5p inhibits glioma cell proliferation
To explore function of miR-485-5p in glioma, U251 and U87 cells were transfected with miR-485-5p mimics or its negative control. The transfection efficiency was assessed by qRT-PCR (Figure 2A). Ectopic miR-485-5p expression blocked proliferation of U251 and U87 cells proliferation in colony formation assays (Figure 2B and 2C). EdU assays demonstrated that the growth of miR-485-5p transfected cells were significantly inhibited by miR-485-5p relative to miR-NC (Figure 2D and 2E). CCK8 assays demonstrated that the growth of miR-485-5p transfected cells were significantly inhibited by miR-485-5p relative to miR-NC (Figure 2F).
Figure 2.
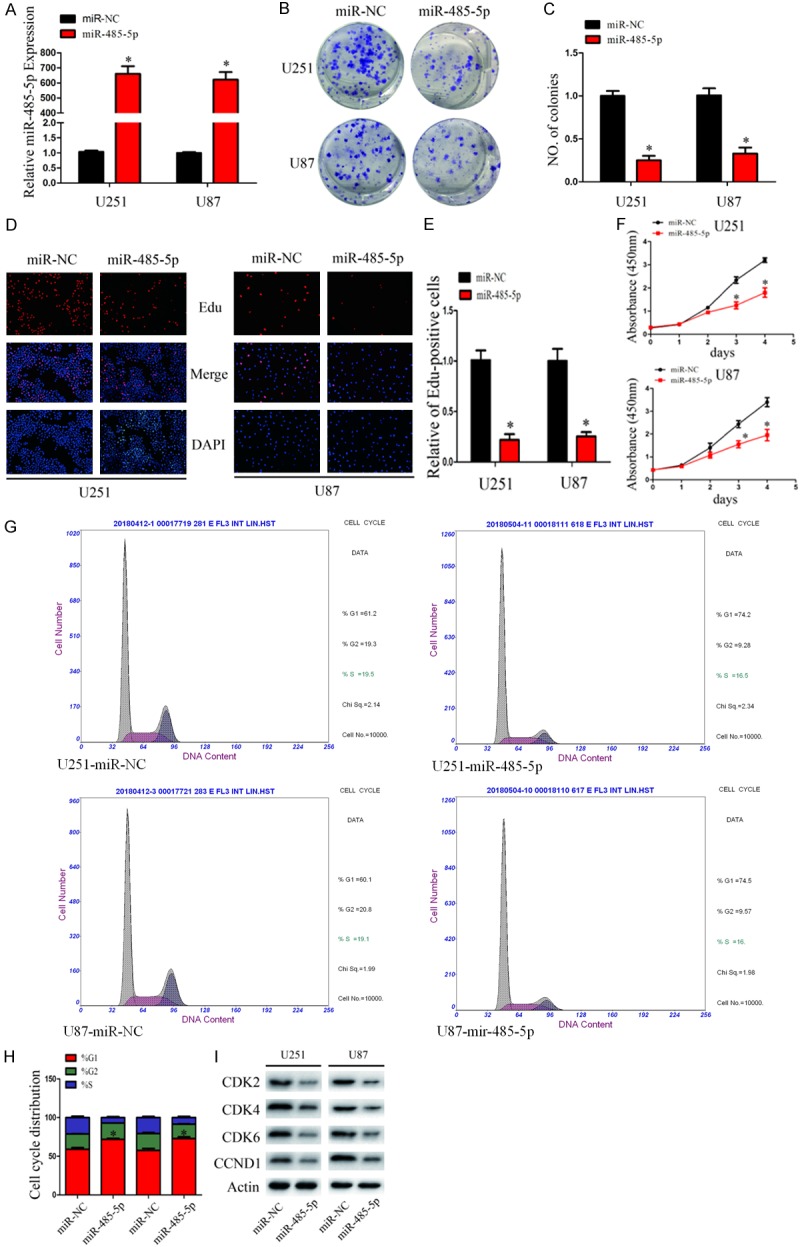
MiR-485-5p inhibits glioma cell proliferation and induces cell cycle arrest. A. qRT-PCR analysis of miR-485-5p expression in U251 and U87 cells transfected with miR-485-5p mimic and miR-NC. *P < 0.05. B and C. Effect of miR-485-5p and miR-NC on U251 and U87 proliferative ability as determined by colony formation assays. *P < 0.05. D and E. The effect of miR-485-5p and miR-NC on the growth of U251 and U87 cells was examined by EdU assays (original magnification 200 X). *P < 0.05. F. The effect of miR-485-5p and miR-NC on the growth of U251 and U87 cells was examined by CCK8 assays. *P < 0.05. G and H. Effect of miR-485-5p and miR-NC on cell cycle distribution of U251 and U87 cells. *P < 0.05. I. Western blot analysis of CDK2, CDK4, CDK6, and CCND1 protein levels in U251 and U87 cells after transfection with miR-485-5p and miR-NC. Actin was used as control. Data were expressed as the mean ± SEM from three independent experiments.
Overexpression of miR-485-5p induces cell cycle G1 arrest
To determine cell cycle effected by overexpression of miR-485-5p, cell cycle analysis was carried out in U87 and U251 were transfected with miR-485-5p mimics and its negative control. As shown in Figure 2G and 2H, the U251 and U87 cells transfected with miR-485-5p were arrested at G1 phase of the cell cycle. To investigate the molecules involved in this cell cycle block, we measured the expression of cyclin-dependent kinases (CDKs; CDK2, CDK4 and CDK6) and Cyclin D1 (CCND1) in miR-485-5p transfected cells and miR-NC transfected cells by western blot (Figure 2I). These Proteins all have been previously reported as important regulators of G1 phase. Collectively, these results demonstrated that overexpression of miR-485-5p induces G1 cell cycle arrest.
miR-485-5p directly targets PAX3 3’-UTR
After observing the inhibitive effect of miR-485-5p on glioma cell proliferation, we next investigated its potential specific targets. TargetScan and miRanda algorithms were applied to predict the potentital targets of miR-485-5p. PAX3, frequently reported to be involved in the tumorigenesis of malignancies of multiple types of human cancers, was selected as a candidate target of miR-485-5p (Figure 3A). To confirm whether miR-485-5p mediated the expression of PAX3, a dual-luciferase reporter system was employed. The results demonstrate that the pGL3 vector with 3’-UTR WT of PAX3 resulted in a significant decrease in luciferase activity after transfection with miR-485-5p mimic, whereas the vector with mutated 3’-UTR of PAX3 had no change in luciferase activity (Figure 3B). PAX3 plays an important role in p-JNK degradation, we investigated JNK, p-JNK protein levels in glioma cells transfected with miR-485-5p mimics and its negative control. Overexpression of miR-485-5p suppressed expression levels of PAX3, and promoted expression levels of p-JNK as assessed by Western blot (Figure 3C). We further examined PAX3 mRNA expression pattern in TCGA and CGGA database. As shown in Figure 3D, the mRNA levels of PAX3 in glioma were significantly upregulated compared with those in NBTs. The mRNA levels of PAX3 in HGG were also significantly upregulated compared with those in LGG (Figure 3E). To determine whether the expression of Pearson’s correlation analysis revealed a significant negative correlation between miR-485-5p and PAX3 in HGG (Figure 3F).
Figure 3.
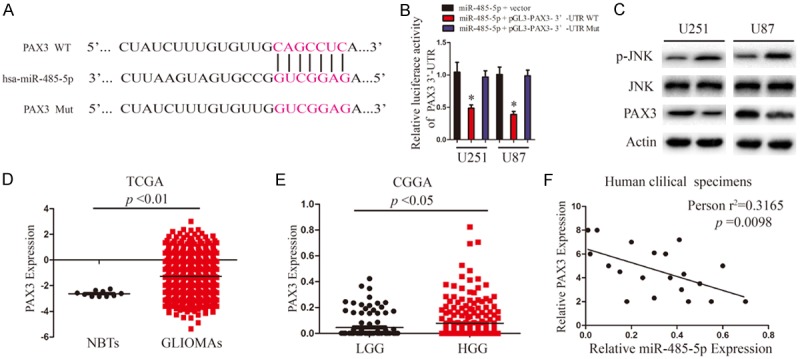
PAX3 is direct target of miR-485-5p in U251 and U87 cells. A. Predicted binding sites of wild-type (WT) and mutated sequences of miR-485-5p in the 3’-UTR of PAX3 mRNA. B. Luciferase assays of U251 and U87 cells transfected with pGL3-PAX3 3’-UTR WT or pGL3-PAX3 3’-UTR Mut reporter with miR-485-5p mimic. *P < 0.05. C. Western blot analysis of PAX3, JNK and p-JNK protein levels in U251 and U87 cells after transfection with miR-485-5p and miR-NC. D. TCGA database showing PAX3 expression in NBTs and GLIOMAs. E. CGGA database showing PAX3 expression in LGG and HGG. F. Pearson’s correlation analysis of the relative expression levels of miR-485-5p and the relative PAX3 mRNA levels in HGG.
PAX3 functions to promote glioma cell proliferation and cell cycle progression
To determine whether the biological functions of PAX3 and miR-485-5p were same or not, PAX3 was knocked down using siRNA. Then, the cell proliferation and cell cycle distribution were detected. The expression of PAX3 protein decreased more than 50% in cells transfected with siRNA-PAX3 after 48 h (Figure 4A). Colony formation was significantly reduced following PAX3 silencing (Figure 4B and 4C). Additionally, EdU incorporation assays revealed that PAX3 knockdown significantly reduced the EdU positive rates compared with control groups (Figure 4D and 4E). Then, PAX3 knockdown induced glioma cell cycle arrest at G1 phases (Figure 4F and 4G). Collectively, these results demonstrated that PAX3 knockdown inhibits glioma cell proliferation and induces G1 cell cycle arrest the same as miR-485-5p overexpression.
Figure 4.
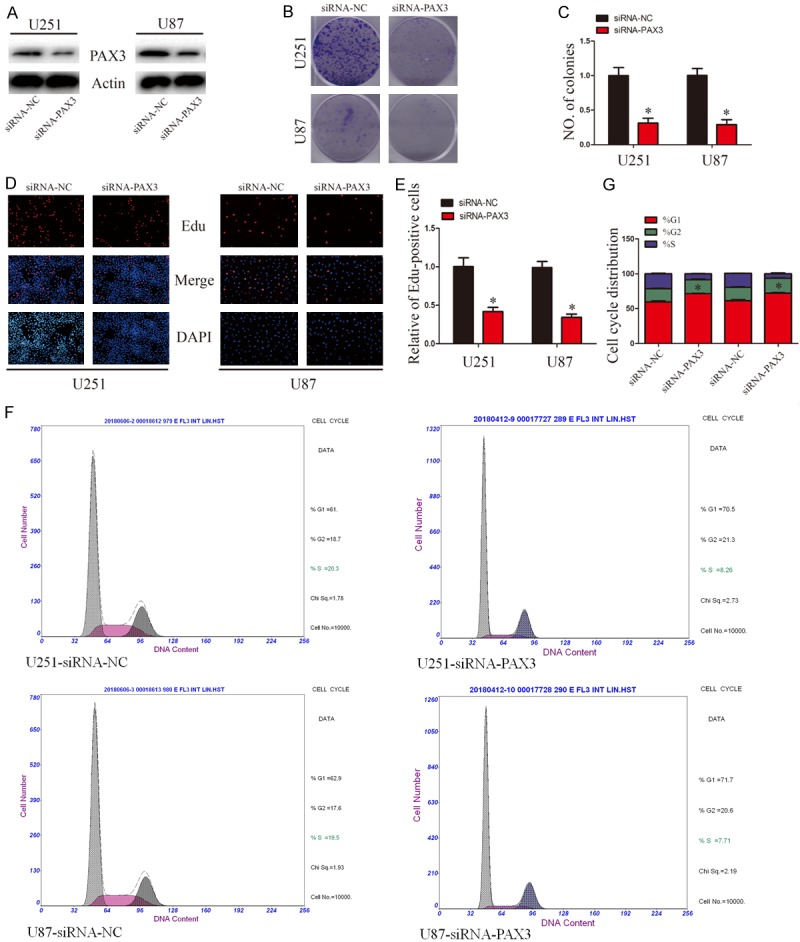
PAX3 knockdown suppresses glioma cells proliferation and cell cycle progression. A. Western blot analysis of PAX3 protein levels in U251 and U87 cells after knockdown PAX3. B and C. Effect of PAX3 knockdown on U87 and U251 proliferative ability as determined by colony formation assays. *P < 0.05. D and E. The effect of PAX3 knockdown on the growth of U251 and U87 cells was examined by EdU incorporation assays (original magnification 200 ×). *P < 0.05. F and G. Effect of PAX3 knockdown on cell cycle distribution of U251 and U87 cells. *P < 0.05.
Ectopic expression of PAX3 reverses miR-485-5p suppression of glioma cell proliferation
To determine whether miR-485-5p targeting PAX3 was responsible for inhibition of the proliferation of glioma cells. We constructed an expression vector that encode the entire PAX3 coding sequence but lacks the 3’-UTR. Then, we cotransfected this vector or its control with miR-485-5p mimic or miR-NC into U251 and U87 cells. Glioma Cell proliferation data showed that concomitant overexpression of miR-485-5p and PAX3 abrogated the inhibitory effects of miR-485-5p (Figure 5B-F). Meanwhile, the expression levels of p-JNK also recovered after exogenous introduction of PAX3 (Figure 5A). These results indicate that PAX3 is a functional target of miR-485-5p in glioma cells.
Figure 5.

Reintroduction of miR-485-5p abrogated the inhibitory effects of miR-485-5p on glioma cell proliferation. A. Western blot analysis of PAX3 and p-JNK protein levels in cells transfected with vector or PAX3 in the presence of miR-NC or miR-485-5p. B. Effect of PAX3 reintroduction on miR-NC or miR-485-5p transfected U251 and U87 growth rates as measured by CCK-8 assays. C and D. Effect of PAX3 reintroduction on miR-NC or miR-485-5p transfected U251 and U87 growth rates as measured by EdU assays. E and F. Effect of PAX3 reintroduction on miR-NC or miR-485-5p transfected on U251 and U87 proliferative ability as determined by colony formation assays. Data were expressed as the mean ± SEM from three independent experiments. *,#,$ P < 0.05.
MiR-485-5p suppresses tumor growth in vivo
To assess the therapeutic potential of miR-485-5p in vivo, nude mice were injection of 1 × 107 U87 cells for in vivo tumor growth assay. Each group (n = 5) was treated with miR-NC or miR-485-5p mimic in 15 μL Lipofectamine 2000 through local injection of the tumor at multiple sites. Xenograft tumors from the miR-485-5p-treated group exhibited a dramatic reduction in tumor volume compared with the miR-NC-treated group (Figure 6A and 6B). Tumor weights were also significantly reduced in the miR-485-5p group (Figure 6C). The expression of miR-485-5p in xenograft tumors was assessed by qRT-PCR (Figure 2D). The expression levels of PAX3 and p-JNK in xenograft tumors of two groups was assessed by WB (Figure 2E). As shown in Figure 6F, Ki-67 staining showed that tumors of miR-485-5p-treated group had fewer proliferative cells than miR-NC-treated group. These results suggest that miR-485-5p could suppress tumorigenesis of glioma cells.
Figure 6.
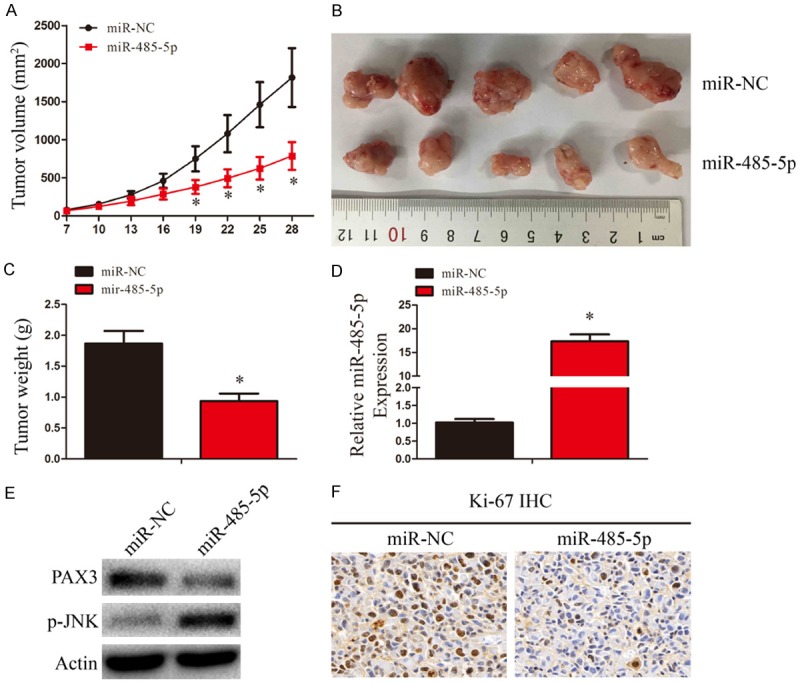
MiR-485-5p overexpression suppresses glioma growth in vivo. (A) Tumor growth curves after subcutaneous injection of nude mice with U87 cells. MiR-NC or miR-485-5p was injected intratumorally into each subcutaneous tumor every 3 d. Tumor volumes were measured every 3 d from days 7 to 28. (B-F) Analysis of miR-NC group and miR-485-5p group tumors on day 28 after injection: tumor images (B); tumor weights (C); miR-485-5p expression (D); PAX3 and p-JNK expression (E); immunohistochemical staining of Ki-67 (F). Data were expressed as the mean ± SEM from three independent experiments. *P < 0.05.
Discussion
MiRNAs serve as major regulators during development and progression of glioma [19,20]. Gliomas are known to display aberrant miRNA expression [21-23], and the biological functions of the miRNAs have been studied extensively. Understanding of the biological functions of miRNAs may be useful for seaching therapeutic strategies and identifying diagnosis markers for glioma patients. In this study, the biological roles of miR-485-5p and its target gene PAX3 in glioma proliferation was explored.
A lot of evidence has showed that dysregulated miRNAs are usually correlated with malignant biological behaviors including proliferation, cell cycle progression, chemoresistance, angiogenesis, apoptosis, migration and invasion [24-26]. In the present study, we initially found that miR-485-5p was decreased in miRNA expression of CGGA datasets, glioma tissue samples and glioma cell lines (especially U87 and U251). To explore the biological function of miR-485-5p in glioma, we overexpressed miR-485-5p in U251 and U87 cells transfecting with miR-485-5p mimics. The results showed that overexpression of miR-485-5p inhibited glioma cell proliferation in vitro, induce cell cycle arrest at G1 phases and inhibit CDK2, CDK4, CDK6 and Cyclin D1 expression. Then, in vivo studies revealed a marked decrease in xenograft tumor growth by overexpression of miR-485-5p, which indicating its therapeutic potential for glioma patients. Moreover, the tumor-suppressing effects of miR-485-5p in glioma were abolished by miR-485-5p inhibitor treatment. These data suggested that miR-485-5p may serve as a potential diagnostic biomarker and therapeutic target for glioma patients.
To explore the potential molecular mechanisms of miR-485-5p in glioma, we performed bioinformatics analysis by TargetScan and miRanda algo-rithms and predicted that PAX3 was a target of miR-485-5p. Subsequently, luciferase reporter assays identified PAX3 as a direct target of miR-485-5p in glioma. PAX3 has been shown to play a vital role in cancer development. PAX3 is a critical transcription factor for neuronal development, as its expression is required for the migration and differentiation of neural crest cells [27,28]. PAX3 has also been found to play an important role in oncogenesis [29].
In this study, PAX3 expression level was notably decreased in U87 and U251 cells transfected with miR-485-5p mimics compared with miR-NC treatment. To further determine the link between miR-485-5p expression and PAX3 expression, we measured the expression of PAX3 and miR-485-5p in glioma to further determine the link between miR-485-5p and PAX3. PAX3 was found to be upregulated in glioma tissues and inversely correlated with miR-485-5p expression. We also observed that slience of PAX3 significantly suppressed glioma cell proliferation, similar to the miR-485-5p overexpression. More importantly, reintroducing PAX3 into miR-485-5p overexpressing cells blocked the effect of miR-485-5p on glioma cell proliferation.
In summary, our results showed that the expression of miR-485-5p is significantly downregulated in glioma tissues and cell lines and is negatively associated with PAX3 expression levels. Overexpression of miR-485-5p can inhibit glioma cells proliferation and induce cell cycle arrest by directly targeting PAX3. Thus, miR-485-5p/PAX3 axis may serve as a promising therapeutic target for glioma treatment.
Disclosure of conflict of interest
None.
References
- 1.Jantas D, Grygier B, Golda S, Chwastek J, Zatorska J, Tertil M. An endogenous and ectopic expression of metabotropic glutamate receptor 8 (mGluR8) inhibits proliferation and increases chemosensitivity of human neuroblastoma and glioma cells. Cancer Lett. 2018;432:1–16. doi: 10.1016/j.canlet.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Xiong Z, Wang L, Wang Q, Yuan Y. LncRNA MALAT1/miR-129 axis promotes glioma tumorigenesis by targeting SOX4. J Cell Mol Med. 2018 doi: 10.1111/jcmm.13667. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang W, Wang X, Chen Y, Zhang J, Chen Y, Lin Z. CXCL12 and CXCR4 as predictive biomarkers of glioma recurrence pattern after total resection. Pathol Biol (Paris) 2015;63:190–198. doi: 10.1016/j.patbio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 4.van der Sanden B, Ratel D, Berger F, Wion D. Glioma recurrence following surgery: peritumoral or perilesional? Front Neurol. 2016;7:52. doi: 10.3389/fneur.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andronesi OC, Arrillaga-Romany IC, Ly KI, Bogner W, Ratai EM, Reitz K, Iafrate AJ, Dietrich J, Gerstner ER, Chi AS, Rosen BR, Wen PY, Cahill DP, Batchelor TT. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun. 2018;9:1474. doi: 10.1038/s41467-018-03905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu G, Wang W, Liu Y, Zhuang K, Cai T, Wang ZF, Yang L. NY-SAR-35 is involved in apoptosis, cell migration, invasion and epithelial to mesenchymal transition in glioma. Biomed Pharmacother. 2018;97:1632–1638. doi: 10.1016/j.biopha.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 7.Seo M, Kim SM, Woo EY, Han KC, Park EJ, Ko S, Choi EW, Jang M. Stemness-attenuating miR-503-3p as a paracrine factor to regulate growth of cancer stem cells. Stem Cells Int. 2018;2018:4851949. doi: 10.1155/2018/4851949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong P, Xiong Y, Yu J, Chen L, Tao T, Yi S, Hanley SJB, Yue J, Watari H, Sakuragi N. Control of PD-L1 expression by miR-140/142/340/383 and oncogenic activation of the OCT4-miR-18a pathway in cervical cancer. Oncogene. 2018;37:5257–5268. doi: 10.1038/s41388-018-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, Gao X, Chen Z, Xue H, Li G. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten pathways. Oncogene. 2018;37:4239–4259. doi: 10.1038/s41388-018-0261-9. [DOI] [PubMed] [Google Scholar]
- 10.Gu JJ, Gao GZ, Zhang SM. miR-218 inhibits the migration and invasion of glioma U87 cells through the Slit2-Robo1 pathway. Oncol Lett. 2015;9:1561–1566. doi: 10.3892/ol.2015.2904. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Guo M, Zhang X, Wang G, Sun J, Jiang Z, Khadarian K, Yu S, Zhao Y, Xie C, Zhang K, Zhu M, Shen H, Lin Z, Jiang C, Shen J, Zheng Y. miR-603 promotes glioma cell growth via Wnt/beta-catenin pathway by inhibiting WIF1 and CTNNBIP1. Cancer Lett. 2015;360:76–86. doi: 10.1016/j.canlet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Jing LL, Mo XM. Reduced miR-485-5p expression predicts poor prognosis in patients with gastric cancer. Eur Rev Med Pharmacol Sci. 2016;20:1516–1520. [PubMed] [Google Scholar]
- 13.Wang M, Cai WR, Meng R, Chi JR, Li YR, Chen AX, Yu Y, Cao XC. miR-485-5p suppresses breast cancer progression and chemosensitivity by targeting survivin. Biochem Biophys Res Commun. 2018;501:48–54. doi: 10.1016/j.bbrc.2018.04.129. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Liu Y, Li M, Wang M, Wang Y. Involvement of miR-485-5p in hepatocellular carcinoma progression targeting EMMPRIN. Biomed Pharmacother. 2015;72:58–65. doi: 10.1016/j.biopha.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Li Y, Du J, Lin H, Cao S, Mao Z, Wu R, Liu M, Liu Y, Yin Q. PAX3 promotes cell migration and CXCR4 gene expression in neural crest cells. J Mol Neurosci. 2018;64:1–8. doi: 10.1007/s12031-017-0995-9. [DOI] [PubMed] [Google Scholar]
- 16.Wachtel M, Schafer BW. PAX3-FOXO1: zooming in on an “undruggable” target. Semin Cancer Biol. 2018;50:115–123. doi: 10.1016/j.semcancer.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Kendall GC, Watson S, Xu L, LaVigne CA, Murchison W, Rakheja D, Skapek SX, Tirode F, Delattre O, Amatruda JF. PAX3-FOXO1 transgenic zebrafish models identify HES3 as a mediator of rhabdomyosarcoma tumorigenesis. Elife. 2018:7. doi: 10.7554/eLife.33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamaraju AK, Bertolotto C, Chebath J, Revel M. Pax3 down-regulation and shut-off of melanogenesis in melanoma B16/F10.9 by interleukin-6 receptor signaling. J Biol Chem. 2002;277:15132–15141. doi: 10.1074/jbc.M200004200. [DOI] [PubMed] [Google Scholar]
- 19.Li FF, Xing C, Wu LL, Xue F. MiR-205 enhances cisplatin sensitivity of glioma cells by targeting E2F1. Eur Rev Med Pharmacol Sci. 2018;22:299–306. doi: 10.26355/eurrev_201801_14172. [DOI] [PubMed] [Google Scholar]
- 20.Diao Y, Jin B, Huang L, Zhou W. MiR-129-5p inhibits glioma cell progression in vitro and in vivo by targeting TGIF2. J Cell Mol Med. 2018;22:2357–2367. doi: 10.1111/jcmm.13529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Yan W, Zhang W, Sun L, Liu Y, You G, Wang Y, Kang C, You Y, Jiang T. Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res. 2011;1411:108–115. doi: 10.1016/j.brainres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Lavon I, Zrihan D, Granit A, Einstein O, Fainstein N, Cohen MA, Cohen MA, Zelikovitch B, Shoshan Y, Spektor S, Reubinoff BE, Felig Y, Gerlitz O, Ben-Hur T, Smith Y, Siegal T. Gliomas display a microRNA expression profile reminiscent of neural precursor cells. Neuro Oncol. 2010;12:422–433. doi: 10.1093/neuonc/nop061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Mao P, Song L, Wu J, Huang J, Lin C, Yuan J, Qu L, Cheng SY, Li J. miR-182 as a prognostic marker for glioma progression and patient survival. Am J Pathol. 2010;177:29–38. doi: 10.2353/ajpath.2010.090812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Peng B, Han Y. MiR-23a regulates the proliferation and migration of human pulmonary artery smooth muscle cells (HPASMCs) through targeting BMPR2/Smad1 signaling. Biomed Pharmacother. 2018;103:1279–1286. doi: 10.1016/j.biopha.2018.04.172. [DOI] [PubMed] [Google Scholar]
- 25.Yan CQ, Lu YH, Tang SM, Fan WX. MiR-519d inhibits prostate cancer cell proliferation, cycle and invasion via targeting NRBP1. Eur Rev Med Pharmacol Sci. 2018;22:2985–2990. doi: 10.26355/eurrev_201805_15054. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Liu S, Su L, Su Q, Lin J, Huang X, Wang C. miR-570 inhibits proliferation, angiogenesis, and immune escape of hepatocellular carcinoma. Cancer Biother Radiopharm. 2018;33:252–257. doi: 10.1089/cbr.2017.2389. [DOI] [PubMed] [Google Scholar]
- 27.Xia L, Huang Q, Nie D, Shi J, Gong M, Wu B, Gong P, Zhao L, Zuo H, Ju S, Chen J, Shi W. PAX3 is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Brain Res. 2013;1521:68–78. doi: 10.1016/j.brainres.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Su X, Liu X, Ni L, Shi W, Zhu H, Shi J, Chen J, Gu Z, Gao Y, Lan Q, Huang Q. GFAP expression is regulated by Pax3 in brain glioma stem cells. Oncol Rep. 2016;36:1277–1284. doi: 10.3892/or.2016.4917. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Xia L, Wu X, Xu L, Nie D, Shi J, Xu X, Ni L, Ju S, Wu X, Zhu H, Shi W. Clinical significance and prognostic value of PAX3 expression in human glioma. J Mol Neurosci. 2012;47:52–58. doi: 10.1007/s12031-011-9677-1. [DOI] [PubMed] [Google Scholar]


