Abstract
The aim of this study was to facilitate the clinical treatment and prognosis of stroke-associated pneumonia (SAP) by examining changes in T-lymphocyte subsets. Stroke patients admitted in Suzhou Hospital between 2014 and 2016 participated in the study. Patients were divided into a pneumonia group (50 patients) and a non-pneumonia group (254 patients) based on a diagnosis of pneumonia. Information regarding risk factors for ischemic stroke was collected from all patients using a questionnaire. Compared with non-SAP patients, SAP patients were older, dysphagic, smokers, had higher NIH stroke scale (NIHSS) scores and neutrophil: lymphocyte ratio, had higher leukocyte, neutrophil, and CD8 levels, had lower CD3, CD4, and lymphocyte levels, and had a lower CD4:CD8 ratio. Patients with a higher NIHSS score had higher CD8 levels, lower CD3 and CD4 levels, and a lower CD4:CD8 ratio. No significant differences in T-lymphocyte subsets were found between the left and right cerebral hemispheres. After adjusting for other variables, smoking, dysphagia, NIHSS score, and CD4:CD8 ratio were positively associated with SAP. The areas under the receiver operating characteristic curve for dysphagia, NIHSS score, CD4:CD8 ratio, CD4:CD8 ratio + NIHSS score, and Dysphagia+ CD4:CD8 ratio + NIHSS score were 0.583 (95% CI: 0.490-0.675), 0.791 (95% CI: 0.724-0.859), 0.676 (95% CI: 0.593-0.759), 0.846 (95% CI: 0.790-0.902), and 0.867 (95% CI: 0.815-0.918), respectively. A few T-lymphocyte subsets may increase susceptibility to pneumonia after acute ischemic stroke. Thus, the detection of T-lymphocyte subsets may predict the risk of SAP in such patients.
Keywords: T-lymphocyte, acute ischemic stroke, pneumonia, predictive biomarker
Introduction
Stroke-associated pneumonia (SAP) is a common inpatient medical complication after stroke, with a reported incidence between 5.6% and 37.98% [1-4]. Studies have shown that SAP, which is influenced by many factors, is associated with death, high medical costs, and prolonged hospital stays [5-10]. Based on published evidence, stroke-induced immunosuppression is a major cause of SAP [11-13] and is characterized by the downregulation of systemic immune responses, such as a rapid decrease in the number of peripheral blood lymphocyte subpopulations and the functional deactivation of monocytes, resulting in an increased susceptibility to SAP [14-17]. Therefore, understanding the immune status of patients with SAP will help identify patients at high risk for SAP, which, if treated early, could reduce SAP incidence and mortality. The aim of this study is to investigate the value of peripheral blood T-lymphocyte subsets in predicting SAP.
Materials and methods
Study subjects
Three hundred and four patients with stroke admitted in Suzhou Hospital between 2014 and 2016 participated in the study. A diagnosis of stroke was confirmed by a consulting physician within 24 h of admission [18]. Patients with intracranial hemorrhage, cerebral tumor, transient ischemic attack, head trauma, severe multiple organ dysfunction, signs and/or symptoms of infection at the time of admission, reported use of antibiotics, immunosuppressants, or corticosteroids during the 3 months prior to the stroke, and significant disability (modified Rankin scale, mRS >2) before the stroke were excluded from the study. Our study design was approved by the Ethics Committee of Suzhou Hospital, which is affiliated with Nanjing Medical University.
Sociodemographic data including age, sex, smoking history, alcohol history, and medical history were collected from all patients. Clinical information including NIHSS score [classified as mild (≤4) or severe (>4)] and evidence of hypertension (blood pressure ≥140/90 mm Hg on repeated measurements or taking antihypertensive medication), diabetes (fasting plasma glucose level ≥7.0 mmol/L, plasma glucose level ≥11.1 mmol/L at any time on repeated measurements, or taking antidiabetic medication), coronary heart disease (a reported history of diagnosis), and atrial fibrillation (AF) was also collected. Fasting venous blood samples were collected within 24 h of admission. A complete blood count and blood biochemical analyses (levels of blood glucose, urea, creatinine, low-density lipoprotein-cholesterol, total homocysteine (tHCY), and C-reactive protein, numbers of leukocytes, neutrophils, and lymphocytes, and neutrophil: lymphocyte ratio levels) were reviewed. All patients underwent swallow screening using a local protocol based on the water swallow test, as recommended by international guidelines, and were classified as having dysphagia if they failed an initial swallowing screen or were too ill or drowsy to swallow.
Flow cytometry testing
Twenty microliters of fluorescein isothiocyanate (FITC) (Becton Dickinson company, USA)-labeled anti-CD3, anti-CD4, and anti-CD8 monoclonal antibodies were added to 100-uL blood samples and incubated in the dark for 20 min. Then, 100 µL Hemolytic agent was added, and the mixture was incubated in the dark for 20 min. The mixture was then buffered with three times of Phosphateand immediately placed in a flow cytometer (BD, USA). The Cell Quest software system (BD, USA) was used for data collection and analysis.
SAP diagnostic criteria
Clinical diagnostic criteria for SAP included at least one of the following clinical signs: (1) Fever greater than or equal to 38°C; (2) New or worsening cough, new onset of purulent sputum, change in the character of the sputum, or new wheezing or crackling upon breathing; (3) Leucopenia (<4000 × 109 cells/L) or leukocytosis (>11.4 × 109 cells/L); or (4) New and persistent infiltrates/consolidation on at least one chest X-ray (or at least two serial chest X-rays in the case of underlying lung disease). Patients were divided into SAP and non-SAP groups.
Statistical analysis
SPSS 17.0 software was used for statistical analysis. Continuous variables that were normally distributed are reported as the mean ± standard deviation and compared using the Student’s t test. Continuous variables that were not normally distributed were compared using the Mann-Whitney U test. Frequency data are expressed as percentages and compared using the χ2 test. Logistic regression analysis was performed using SAP as the dependent variable and risk factors (P<0.2) as independent variables. Logistic regression analysis was used to select variables (inclusion, P<0.05; exclusion, P>0.10) and performed to determine independent risk factors for SAP. Areas under the receiver operating characteristic (ROC) curve were used to determine dysphagia, NIH stroke scale (NIHSS) score, CD4:CD8 ratio, NIHSS score + CD4:CD8 ratio, and Dysphagia+ CD4:CD8 ratio + NIHSS score. P<0.05 was considered statistically significant (two-sided).
Results
Comparison of baseline characteristics between SAP and non-SAP groups
Compared with non-SAP patients, SAP patients were older, smokers, dysphagic, and had higher NIHSS scores, neutrophil: lymphocyte ratios, and levels of leukocytes, neutrophils, and lymphocytes (P<0.05). No statistically significant differences were found between groups in terms of the male: female ratio or histories of alcohol use, hypertension, diabetes mellitus, coronary heart disease, atrial fibrillation, previous stroke/transient ischemic attack, blood pressure, temperature, or levels of low-density lipoprotein-cholesterol, blood sugar, tHCY, creatinine, urea, and C-reactive protein (Table 1).
Table 1.
Comparison of baseline characteristicT between SAP and Non-SAP
| SAP (n=50) | Non-SAP (n=254) | T or X2-value | P-value | |
|---|---|---|---|---|
| Age (years) | 73.04±8.65 | 68.59±12.20 | 2.458 | 0.015 |
| Male:female | 26/24 | 158/96 | 1.821 | 0.177 |
| Hypertention (n, %) | 39 (78.00) | 218 (85.52) | 1.958 | 0.162 |
| Diabetes mellitus (n, %) | 21 (42.00) | 96 (37.79) | 0.312 | 0.576 |
| Coronary heart disedse (n, %) | 12 (24.00) | 45 (17.71) | 1.083 | 0.298 |
| Atrial fibrillation (n, %) | 7 (14.00) | 27 (10.60) | 0.478 | 0.489 |
| Previous Stroke/TIA (n, %) | 4 (8.00) | 15 (5.90) | 0.530 | |
| Smoking (n, %) | 15 (30.00) | 26 (10.20) | 11.537 | 0.001 |
| Alcohol drinking (n, %) | 12 (24.00) | 45 (17.70) | 1.083 | 0.298 |
| Dysphagia (n, %) | 13 (26.00) | 24 (9.40) | 10.706 | 0.001 |
| Systolic blood pressure (mmHg) | 157.92±19.95 | 155.42±22.24 | 0.738 | 0.461 |
| Diastolicblood pressure (mmHg) | 80.60±13.02 | 82.67±12.63 | -1.055 | 0.292 |
| Temperature (°C) | 36.55±0.55 | 36.47±0.37 | 1.136 | 0.257 |
| NIHSS score | 7.36±4.77 | 3.55±2.54 | 8.149 | <0.001 |
| Low-densitylipoprotein (mmol/L) | 3.13±0.96 | 2.95±0.83 | 1.405 | 0.161 |
| Blood sugar (mmol/L) | 6.97±2.67 | 6.65±4.91 | 0.443 | 0.658 |
| Homocysteine (umol/L) | 21.66±5.24 | 19.50±7.65 | 1.908 | 0.057 |
| Creatinine (umol/L) | 71.26±24.92 | 71.25±28.77 | 0.003 | 0.997 |
| Urea (mmol/L) | 5.64±1.83 | 5.22±1.51 | 1.728 | 0.085 |
| Leukocyte (× 109/L) | 8.38±2.39 | 7.29±2.43 | 2.912 | 0.004 |
| Neutrophil (× 109/L) | 8.54±2.31 | 7.07±2.24 | 4.194 | <0.001 |
| lymphocyte (× 109/L) | 1.46±0.45 | 1.66±0.50 | -2.610 | 0.009 |
| Neutrophil-to-lymphocyte | 6.30±2.37 | 4.60±1.96 | 5.387 | <0.001 |
| CRP (mmol/L) | 5.83±2.90 | 4.86±3.60 | 1.800 | 0.073 |
Comparison of T-lymphocyte subsets between SAP and non-SAP patients
Compared with non-SAP patients, SAP patients had significantly lower CD3 and CD4 levels, a lower CD4:CD8 ratio, and higher CD8 levels (Table 2 and Figure 1).
Table 2.
Comparison of T-lymphocyte subsets between SAP and non-SAP patients
| SAP | Non-SAP | Test-value | P value | |
|---|---|---|---|---|
| CD3 | 63.96±13.61 | 67.40±10.06 | -2.074 | 0.039 |
| CD4 | 35.54±9.63 | 41.14±8.68 | -4.094 | P<0.001 |
| CD8 | 28.52±10.32 | 23.29±8.16 | 3.947 | P<0.001 |
| CD4/CD8 | 1.39±0.68 | 2.06±1.07 | -4.224 | P<0.001 |
P<0.05.
Figure 1.

Comparison of CD4 CD8 between SAP (A, B) and Non-SAP (C, D). P<0.05.
Comparison of T-lymphocyte subsets between patients with mild or severe NIHSS scores
Compared with patients with an NIHSS score less than 4, patients with an NIHSS score greater than 4 had significantly lower CD3 and CD4 levels, a lower CD4:CD8 ratio, and higher CD8 levels (Table 3).
Table 3.
Comparison of T-lymphocyte subsets between patients with mild or severe NIHSS scores
| Mild (n=263) | Sever (n=43) | Test-value | P value | |
|---|---|---|---|---|
| CD3 | 66.93±11.11 | 60.73±10.35 | -3.353 | 0.001 |
| CD4 | 40.09±9.27 | 36.34±5.71 | -2.511 | 0.013 |
| CD8 | 25.91±9.13 | 22.73±7.38 | -2.118 | 0.035 |
| CD4/CD8 | 1.98±1.04 | 1.54±0.65 | -2.577 | 0.010 |
P<0.05.
Comparison of T-lymphocyte subsets between left and right cerebral hemispheres of all patients
CD3, CD4, and CD8 levels, and the CD4:CD8 ratio were measured in both left and right cerebral hemispheres. No significant differences were found between the two hemispheres with regard to these risk factors (Table 4).
Table 4.
Comparison of T-lymphocyte subsets between left and right cerebral hemispheres of all patients
| Left (n=158) | Right (n=146) | Test-value | P value | |
|---|---|---|---|---|
| CD3 | 67.69±10.23 | 65.90±11.29 | 1.453 | 0.147 |
| CD4 | 41.19±8.78 | 39.18±9.30 | 1.939 | 0.053 |
| CD8 | 23.51±7.87 | 25.24±9.56 | -1.730 | 0.085 |
| CD4/CD8 | 2.05±1.01 | 1.84±1.07 | 1.691 | 0.092 |
P>0.05.
Logistic regression of associations between risk factors for SAP
Results of the logistic regression analysis showed that factors such as smoking, dysphagia, NIHSS score, and the CD4:CD8 ratio, were positively associated with SAP (Table 5).
Table 5.
Logistic regression of associations between risk factors for SAP
| B | S.E | Wals | P-value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Smoking | 2.636 | 0.600 | 19.276 | 0.000 | 13.956 | 4.303-45.270 |
| Dysphagia | 1.236 | 0.620 | 3.980 | 0.046 | 3.443 | 1.022-11.602 |
| NIHSS score | 0.331 | 0.061 | 29.286 | <0.001 | 1.393 | 1.230-1.570 |
| CD4/CD8 | -1.336 | 0.493 | 7.336 | 0.007 | 0.250 | 0.100-0.691 |
Comparison of risk factors as predictive biomarkers for SAP
Results from the ROC curve showed that dysphagia, NIHSS score, CD4:CD8 ratio, CD4:CD8 ratio + NIHSS score, Dysphagia+ NIHSS score, and Dysphagia+ CD4:CD8 ratio + NIHSS score were all relatively good predictive biomarkers for SAP, but Dysphagia+ CD4:CD8 ratio + NIHSS score was the best predictor of SAP, with a larger predictive value than any of these risk factors alone (Table 6 and Figures 2, 3, 4 and 5).
Table 6.
Comparison of risk factors as predictive biomarkers for SAP
| Area under the curve | P-value | 95% CI | |
|---|---|---|---|
| Dysphagia | 0.583 | 0.064 | 0.490-0.675 |
| NIHSS score | 0.791 | <0.001 | 0.724-0.859 |
| CD4/CD8 | 0.676 | <0.001 | 0.593-0.759 |
| CD4/CD8+NIHSS score | 0.846 | <0.001 | 0.790-0.902 |
| Dysphagia+ NIHSS score | 0.702 | <0.001 | 0.616-0.788 |
| Dysphagia+ CD4/CD8+NIHSS score | 0.867 | <0.001 | 0.815-0.918 |
Figure 2.
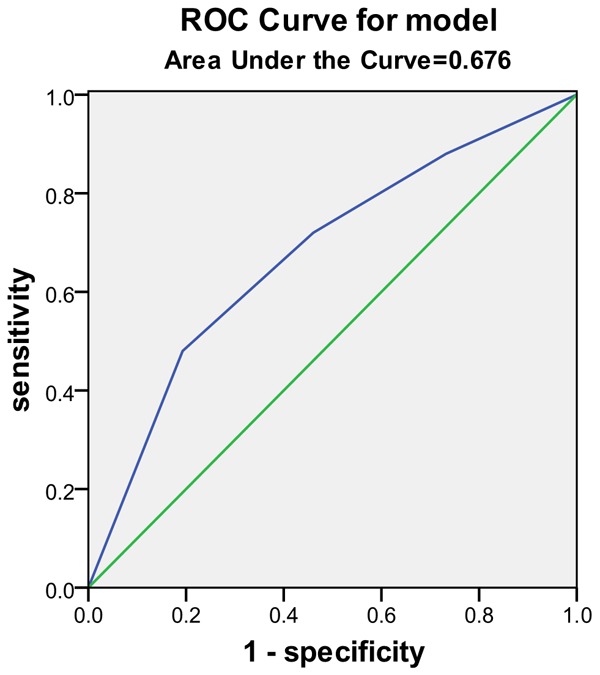
CD4/CD8 Area under the curve.
Figure 3.
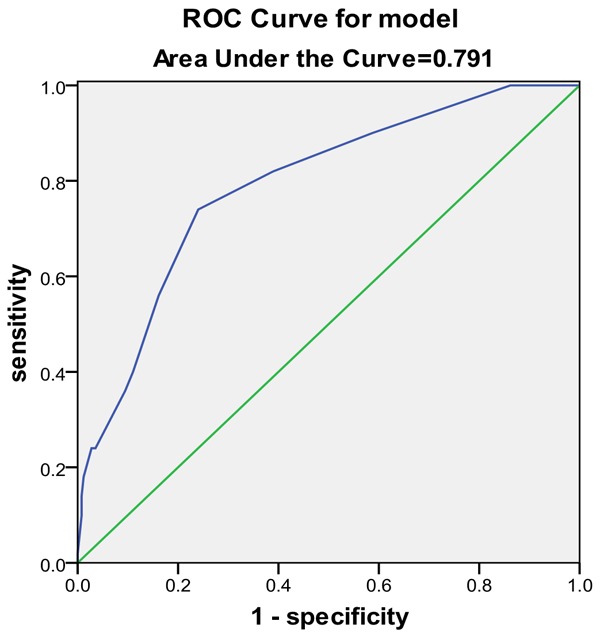
NIHSS score Area under the curve.
Figure 4.
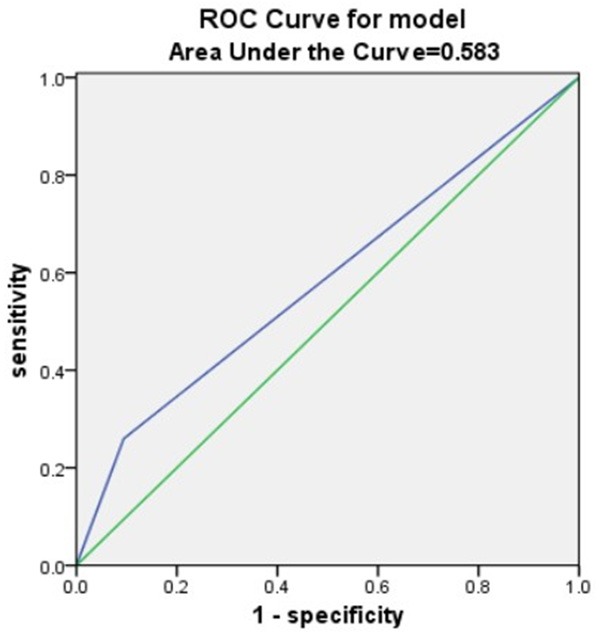
Dysphagia Area under the curve.
Figure 5.
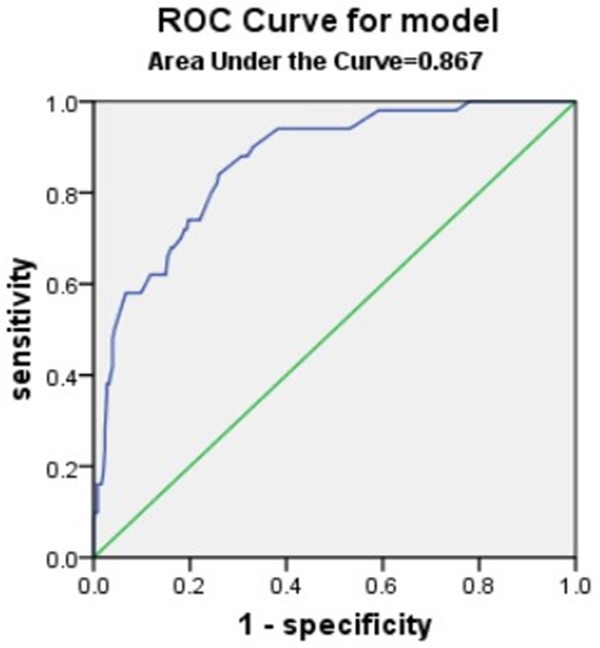
Dysphagia+ CD4/CD8+NIHSS score Area under the curve.
Discussion
Stroke has high incidence and recurrence rates, and is a common cause of both disability and mortality. Acute ischemic stroke is the most common type of stroke, accounting for 80% of all cases [19]. Pneumonia is the most common complication after stroke. However, due to different design methods, the reported incidence of SAP is inconsistent across studies. The current study showed that the incidence of SAP is 16.44%.
In this study, a single-factor analysis showed significant differences in age, smoking, dysphagia, and baseline NIHSS scores between SAP and non-SAP groups. A multivariate regression analysis showed that smoking, dysphagia, and baseline NIHSS scores were independent risk factors for SAP. Factors, such as smoking, increased lung secretions after long-term bed rest, and poor coughing ability, cause a decrease in lung resistance, which increases the risk of lung infection. Stroke can cause varying degrees of dysphagia, a decrease in protective respiratory reflexes, and aspiration pneumonia when eating and drinking. Previous studies have shown that the NIHSS score is a risk factor for SAP [20-22]. The higher the NIHSS score, the greater the likelihood of SAP. The reason for this observation is that patients with high NIHSS scores often have a reduced level of consciousness or are on bed rest, and are prone to gastroesophageal reflux, leading to aspiration pneumonia.
Increasing evidence indicates that immune function declines after stroke, which then leads to complications from infection [23-25]. Prass [26] confirmed that immune dysfunction can be caused by stroke and confirmed a lack of antibacterial immune responses in an experimental stroke model. This study found that blocking β-adrenergic receptors can prevent aspiration pneumonia after cerebral ischemia. Vogelgesang [27] confirmed that CD4 cell dysfunction can contribute to immunosuppression in stroke patients. Klehmet [11] showed that a common feature of stroke patients is a rapid decrease in the number of T-lymphocytes and the production of interferon-gamma. A reduction in the number of T-lymphocytes is more pronounced during infection. The results of this study showed that the peripheral blood CD4:CD8 ratio was significantly lower in SAP patients after acute cerebral infarction compared with stroke-only patients. Results of a multivariate regression analysis suggested that the CD:CD8 ratio was an independent risk factor for SAP.
Lymphocytes play an important role in the immune system and participate in both cellular and humoral immune responses. A reduction in the number of lymphocytes or their dysfunction can cause severe immunosuppression, leading to infection. Recent studies have found that changes in the relative number of T-lymphocyte subsets are closely related to patient immune function status and disease progression [28-30]. Therefore, the detection of T-lymphocyte subsets is critical for determining immune status, and the CD4:CD8 ratio is the simplest and most specific indicator of these subsets [31-33].
Compared with the mild NIHSS score group, levels of CD3, CD4, and the CD4:CD8 ratio were decreased in the severe NIHSS score group, suggesting that patients with more severe neurological deficits had a greater degree of cellular immunosuppression. There were no statistically significant differences in T-lymphocyte subsets between patients with ischemic stroke that occurred in different hemispheres, consistent with results from previous studies [23]. This finding suggested that lesions on different sides of the brain had no significant effects on cellular immune function after a stroke.
Although stroke-induced immunosuppression has received widespread attention, its mechanism of action has not been fully elucidated. To date, the proposed pathological mechanisms include local brain tissue necrosis, hypoperfusion or reperfusion injury, and activation of immune cells by inflammatory transmitters, followed by the release of cytokines that enter the hypothalamus via an impaired blood-brain barrier. Dysfunction of the hypothalamic-pituitary-adrenal axis leads to decreased cellular immune function and SAP. In addition, sympathetic nerves continue to fire during acute stroke, reducing local respiratory immune function, airway clearance, and the phagocytic capacity of white blood cells, which can easily lead to pneumonia [34-40].
Traditional indicators of primary infection include high white blood cell and neutrophil counts, but these indicators are not typically diagnostic. According to a previous study, there were no significant differences in leukocyte or neutrophil counts between patients with and without infection at admission, suggesting that the predictive value of leukocyte and neutrophil counts for infection is limited [41], which is consistent with the results of our study. Our single-factor analysis found that neutrophil and lymphocyte counts, and neutrophil: lymphocyte ratios differed between the SAP and non-SAP groups, but the results of the multivariate analysis did not support the predictive values of these risk factors for SAP. This apparent discrepancy may be related to the small number of cases examined. Therefore, we plan to increase the sample size in future studies of SAP to verify the role of the neutrophil: lymphocyte ratio.
While some studies have found that SAP can be predicted, small sample sizes and complicated scoring methods limit the determination of SAP risk [42-45]. We showed that the areas under the ROC curve were 0.676 for the CD4:CD8 ratio and 0.867 for the CD4:CD8 ratio + NIHSS score + dysphagia. These results suggested that the CD4:CD8 ratio had a good predictive value for SAP, but the combination of the CD4:CD8 ratio, NIHSS score, and dysphagia was an even better predictor than the CD4:CD8 ratio alone.
Our study suggested that acute stroke can lead to changes in the peripheral blood T-lymphocyte population, impaired immune function, and increased risk of concurrent infection. Detection of peripheral blood T-lymphocyte subsets early in stroke, in particular, the CD4:CD8 T-cell ratio, can help predict the risk of SAP.
Acknowledgements
This work was funded by the Science and Technology Development Foundation of Nanjing Medical University (No. 2014NJMU106), the Municipal Science and technology plan guidance project of Suzhou (No. SYSD2016114), the Municipal Technology Program-Applied Basic Research Project of Suzhou (No. SYS201768).
Disclosure of conflict of interest
None.
References
- 1.Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke. 2003;34:975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 2.Harms H, Grittner U, Droge H, Meisel A. Predicting post-stroke pneumonia: the PANTHERIS score. Acta Neurol Scand. 2013;128:178–184. doi: 10.1111/ane.12095. [DOI] [PubMed] [Google Scholar]
- 3.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G Canadian Stroke Network; Stroke Outcome Research Canada (SORCan) Working Group. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 4.Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, Di Napoli M, Kalra L, Langhorne P, Montaner J, Roffe C, Rudd AG, Tyrrell PJ, van de Beek D, Woodhead M, Meisel A, Smith CJ. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke. 2015;46:1202–1209. doi: 10.1161/STROKEAHA.114.007843. [DOI] [PubMed] [Google Scholar]
- 5.Yu YJ, Weng WC, Su FC, Peng TI, Chien YY, Wu CL, Lee KY, Wei YC, Lin SW, Zhu JX, Huang WY. Association between pneumonia in acute stroke stage and 3-year mortality in patients with acute first-ever ischemic stroke. J Clin Neurosci. 2016;33:124–128. doi: 10.1016/j.jocn.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Ali AN, Howe J, Majid A, Redgrave J, Pownall S, Abdelhafiz AH. The economic cost of stroke-associated pneumonia in a UK setting. Top Stroke Rehabil. 2018;25:214–223. doi: 10.1080/10749357.2017.1398482. [DOI] [PubMed] [Google Scholar]
- 7.Spratt N, Wang Y, Levi C, Ng K, Evans M, Fisher J. A prospective study of predictors of prolonged hospital stay and disability after stroke. J Clin Neurosci. 2003;10:665–669. doi: 10.1016/j.jocn.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Miller CM, Behrouz R. Impact of infection on stroke morbidity and outcomes. Curr Neurol Neurosci Rep. 2016;16:83. doi: 10.1007/s11910-016-0679-9. [DOI] [PubMed] [Google Scholar]
- 9.Shah SV, Corado C, Bergman D, Curran Y, Bernstein RA, Naidech AM, Prabhakaran S. Impact of poststroke medical complications on 30-day readmission rate. J Stroke Cerebrovasc Dis. 2015;24:1969–1977. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, Meisel A, Meisel C. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Meisel A, Meisel C, Harms H, Hartmann O, Ulm L. Predicting post-stroke infections and outcome with blood-based immune and stress markers. Cerebrovasc Dis. 2012;33:580–588. doi: 10.1159/000338080. [DOI] [PubMed] [Google Scholar]
- 13.Folyovich A, Biro E, Orban C, Bajnok A, Varga V, Beres-Molnar AK, Vasarhelyi B, Toldi G. Relevance of novel inflammatory markers in stroke-induced immunosuppression. BMC Neurol. 2014;14:41. doi: 10.1186/1471-2377-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 15.Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 16.Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, Liesz A, Veltkamp R. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- 17.Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16:7–18. doi: 10.1007/pl00021290. [DOI] [PubMed] [Google Scholar]
- 18.Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 19.Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 20.Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR GAIN International Steering Committee and Investigators. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 21.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 22.Maeshima S, Osawa A, Hayashi T, Tanahashi N. Elderly age, bilateral lesions, and severe neurological deficit are correlated with stroke-associated pneumonia. J Stroke Cerebrovasc Dis. 2014;23:484–489. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. 2009;158:1174–1183. doi: 10.1016/j.neuroscience.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann S, Harms H, Ulm L, Nabavi DG, Mackert BM, Schmehl I, Jungehulsing GJ, Montaner J, Bustamante A, Hermans M, Hamilton F, Gohler J, Malzahn U, Malsch C, Heuschmann PU, Meisel C, Meisel A PREDICT Investigators. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia - The PREDICT study. J Cereb Blood Flow Metab. 2017;37:3671–3682. doi: 10.1177/0271678X16671964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu DD, Chu SF, Chen C, Yang PF, Chen NH, He X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP) Neurochem Int. 2018;114:42–54. doi: 10.1016/j.neuint.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogelgesang A, Grunwald U, Langner S, Jack R, Broker BM, Kessler C, Dressel A. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39:237–241. doi: 10.1161/STROKEAHA.107.493635. [DOI] [PubMed] [Google Scholar]
- 28.Snauwaert S, Verstichel G, Bonte S, Goetgeluk G, Vanhee S, Van Caeneghem Y, De Mulder K, Heirman C, Stauss H, Heemskerk MH, Taghon T, Leclercq G, Plum J, Langerak AW, Thielemans K, Kerre T, Vandekerckhove B. In vitro generation of mature, naive antigen-specific CD8(+) T cells with a single T-cell receptor by agonist selection. Leukemia. 2014;28:830–841. doi: 10.1038/leu.2013.285. [DOI] [PubMed] [Google Scholar]
- 29.Theodorou GL, Marousi S, Ellul J, Mougiou A, Theodori E, Mouzaki A, Karakantza M. T helper 1 (Th1)/Th2 cytokine expression shift of peripheral blood CD4+ and CD8+ T cells in patients at the post-acute phase of stroke. Clin Exp Immunol. 2008;152:456–463. doi: 10.1111/j.1365-2249.2008.03650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 31.Read AJ, Erickson S, Harmsen AG. Role of CD4+ and CD8+ T cells in clearance of primary pulmonary infection with Coxiella burnetii. Infect Immun. 2010;78:3019–3026. doi: 10.1128/IAI.00101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JE, Bauer S, La KS, Lee KH, Choung JT, Roh KH, Lee CK, Yoo Y. CD4+/CD8+ T lymphocytes imbalance in children with severe 2009 pandemic influenza A (H1N1) pneumonia. Korean J Pediatr. 2011;54:207–211. doi: 10.3345/kjp.2011.54.5.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill D, Veltkamp R. Dynamics of T cell responses after stroke. Curr Opin Pharmacol. 2016;26:26–32. doi: 10.1016/j.coph.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Winklewski PJ, Radkowski M, Demkow U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflammation. 2014;11:213. doi: 10.1186/s12974-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorrance AM, Fink G. Effects of stroke on the autonomic nervous system. Compr Physiol. 2015;5:1241–1263. doi: 10.1002/cphy.c140016. [DOI] [PubMed] [Google Scholar]
- 36.Raditic DM, Bartges JW. Evidence-based integrative medicine in clinical veterinary oncology. Vet Clin North Am Small Anim Pract. 2014;44:831–853. doi: 10.1016/j.cvsm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Sundboll J, Horvath-Puho E, Schmidt M, Dekkers OM, Christiansen CF, Pedersen L, Botker HE, Sorensen HT. Preadmission use of glucocorticoids and 30-day mortality after stroke. Stroke. 2016;47:829–835. doi: 10.1161/STROKEAHA.115.012231. [DOI] [PubMed] [Google Scholar]
- 38.Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60:620–625. doi: 10.1212/01.wnl.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- 39.Mracsko E, Liesz A, Karcher S, Zorn M, Bari F, Veltkamp R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun. 2014;41:200–209. doi: 10.1016/j.bbi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Engel O, Akyuz L, da Costa Goncalves AC, Winek K, Dames C, Thielke M, Herold S, Bottcher C, Priller J, Volk HD, Dirnagl U, Meisel C, Meisel A. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke. 2015;46:3232–3240. doi: 10.1161/STROKEAHA.115.008989. [DOI] [PubMed] [Google Scholar]
- 41.Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis. 2012;21:61–67. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon HM, Jeong SW, Lee SH, Yoon BW. The pneumonia score: a simple grading scale for prediction of pneumonia after acute stroke. Am J Infect Control. 2006;34:64–68. doi: 10.1016/j.ajic.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Chumbler NR, Williams LS, Wells CK, Lo AC, Nadeau S, Peixoto AJ, Gorman M, Boice JL, Concato J, Bravata DM. Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology. 2010;34:193–199. doi: 10.1159/000289350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, Walter G, Meisel A, Heuschmann PU Berlin Stroke Register and the Stroke Register of Northwest Germany. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. 2012;43:2617–2623. doi: 10.1161/STROKEAHA.112.653055. [DOI] [PubMed] [Google Scholar]
- 45.Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, Li H, Wang Y China National Stroke Registry Investigators. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. 2013;44:1303–1309. doi: 10.1161/STROKEAHA.111.000598. [DOI] [PubMed] [Google Scholar]


