Abstract
Keloids are raised, red, hard and irregular tumors that are prone to extend beyond the wound borders. Surgical excision is not sufficient to eradicate a keloid. Adjuvant therapy with radiation is a recommended treatment that reportedly achieves improved efficacy. However, radiation does not only kill cells in the keloid tissue but also stimulates their resistance, and intractable cases can display continuous recurrence. Quercetin was initially extracted from natural products and is used as a dietary supplement. The role of quercetin as an oxidant scavenger has been highlighted in many studies and has drawn interest to the application of ionizing radiation (IR) sensitization. In this study, we first demonstrate that keloid fibroblasts acquire resistance after IR treatment, and this can be relieved by treatment with quercetin. Further, we showed that hypoxia-inducible factor 1 (HIF-1), a prognostic marker used in clinical practice after radiation therapy, was associated with stronger radioresistance in keloid fibroblasts, which was downregulated after quercetin treatment. The inhibition of HIF-1 expression by quercetin was found to be dependent on the phosphatidylinositol-3-kinase (PI3K)/Akt pathway. Quercetin has been reported to reduce the phosphorylation of Akt. Taken together, we revealed one mechanism underlying the suppression of radioresistance by quercetin, which involved the regulation of HIF-1α by the PI3K/Akt pathway. Our study provides a molecular basis for the application of quercetin in radiation sensitization in the treatment of keloids.
Keywords: Keloid, quercetin, radioresistance, hypoxia-inducible factor 1, phosphatidylinositol-3-kinase/Akt pathway
Introduction
A keloid is a benign tumor of the derma, which is characterized by infiltration into adjacent normal tissues. In people of Asian descent, keloids occur at a frequency of 4%-16% [1], primarily in young adults. Keloids appear red, hard, tickling, raised, and irregular and may extend outside the area of an injury [2]. Long-existing keloids can cause necrosis, recurrent hemorrhage, and suppuration. Histopathologic sections showed overabundance of fibroblasts undergoing active mitotic division within keloid tissues, accompanied by dense collagen fibers and an abnormal increase in myxoid stroma [3]. The etiology of keloids is reminiscent of tumorigenesis in that it involves characteristics such as dysfunction of the tissues, an abnormally excessive extracellular matrix, and dysregulation of apoptosis [4-7]. However, there is still a lack of knowledge regarding definitive mechanisms that explain keloid pathogenesis. Recurrence is an intricate problem in the treatment of keloids. Berman and Bieley et al. comprehensively compared the efficacy of different therapies and showed that combining surgical excision and radiation minimized recurrence; however, some patients suffered from radioresistance [8,9]. Therefore, it is imperative to develop novel means to increase the sensitivity of keloids to radiation, which would then reduce secondary insults.
Adjuvant therapy has emerged as an effective means to reduce resistance. Quercetin, a flavonoid that can be extracted from plants such as vegetables, bark roots, flowers and grains, has been found to be beneficial to health [10,11]. Although a natural product, quercetin has drawn extensive research interest regarding its pharmacological effects, and a substantial amount of evidence now exists for potential therapeutic uses in a variety of conditions, including allergies, asthma, anti-bacterial activity, and cancer. Among these, cancer is a condition that is similar to keloids. Indeed, effective inhibition of radiation-induced PKC activity, one of the ways that radioresistance arises in tumor cells, has been achieved by quercetin treatment [12]. However, the role of quercetin in the treatment of keloids and the feasibility of its clinical application remain elusive.
In this study, we first compared the radioresistance of keloid fibroblasts, the major constituent cells of keloids, with that of normal fibroblasts. Results showed that compared to normal fibroblasts, keloid fibroblasts exhibited less severe apoptosis, which was increased by treatment with quercetin. Subsequently, we found that the expression level of hypoxia-inducible factor-1 (HIF-1), an indicator of radioresistance, was correlated with quercetin concentration and associated with the degree of radioresistance. We further employed a phosphorylation inhibitor and agonist to examine whether quercetin inhibits HIF-1α through the PI3K/Akt pathway, which has been highly implicated in tumorigenesis, tumor development, and escape from apoptosis. Quercetin was found to reduce the protein level of p-Akt, and the Akt phosphorylation agonist IGF-1 attenuated, but did not completely block, inhibition by quercetin. This indicates involvement of PI3K/Akt in the suppression of radioresistance by quercetin and suggests the existence of another potential inhibitory pathway.
Materials and methods
Source of keloid fibroblasts
The keloid fibroblasts used in this study were sampled from the disposed skin keloid tissues of five patients who received surgery in the plastic surgery department of Peking Union Medical College Hospital, and the normal fibroblasts were derived from normal skin tissues of non-keloid patients. None of the patients had been administrated any therapy prior to sampling or complicating disease that affects wound healing. All keloid tissue specimens were examined and confirmed pathologically, and the full-thickness biopsy specimens were used in subsequent experiments. The keloid fibroblasts from different patients were used as biological replicates. Normal fibroblasts were sampled from surgically excised normal skin from nonkeloid people. Written, informed consent of all patients was obtained and this study was approved by the Ethics Committee of the hospital.
Cell culture and treatment
Tissues were obtained from central parts of each keloid lesion, dissected into small pieces (<1 mm in diameter), and cultured in DMEM supplemented with 10% FBS, in a 5% CO2 humidified atmosphere at 37°C. The edges of the explanted tissues at 8-10 days post-primary culture were passaged, and the cells harvested from the center of the keloid colonies in passages 3-6 were used for subsequent experiments. Cells were divided into the normal fibroblast group (Norm) and the keloid fibroblast group (KLD). For radiation treatment, cells were cultured in DMEM supplemented with FBS and were subjected to radiation at 15 Gray, 20 Gray and 25 Gray for 24 or 48 hrs. Each treatment had 5 replicates. For quercetin treatment, different quantities of quercetin were added to DMEM supplemented with FBS to obtain a final concentration of 20 μmol/l, 40 μmol/l and 80 μmol/l. The time period that cells were cultured in the modified mediums depended on the experiment.
Apoptosis assessment by flow cytometry
Flow cytometry using annexin V and propidium iodide (PI) was used to measure apoptosis in fibroblasts. Fibroblasts were plated in 6-well plates at a density of 5×104 cell/cm2. After 24 or 48 hours of exposure to ionizing radiation or treatment with quercetin for 24 hrs, 100 μL of cells were suspended in 1× Annexin-binding buffer and incubated with 5 μL fluorescein isothiocyanate (FITC), annexin V, and 5 μL PI (Invitrogen, US) for 15 min at room temperature in darkness. After adding 400 μL of annexin-binding buffer to each replicate, viability and apoptosis were analyzed by flow cytometry at 530 nm and >575 nm, respectively (BD Bioscience).
Western blotting
Total proteins were extracted from cells using RIPA buffer. Equivalent quantities of total protein were then subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes. Blocking was done using 5% skimmed milk and 0.1% Tween 20 resolved in PBS (pH 7.4). Membranes were then incubated with primary antibodies for 60 min at 37°C. The following antibodies were used: mouse-anti-human HIF-1α (1:250, Abcam), p-Akt (1:1000, Abcam), Akt (1:1000, Abcam), and β-actin (1:1000, Abcam). Horseradish peroxidase (HRP) conjugated secondary antibody (diluted in 0.01 M PBS) was added, followed by 4 washes using 0.01 M PBS. An enhanced chemiluminescence solution was used to visualize antigens, and Image J software was employed to assess the signal intensities.
Quantitative RT-PCR
Total RNA was extracted using Trizol reagent. Revert Aid M-MulV Reverse Transcriptase was used to synthesize cDNA which served as a template for PCR. The primers used were: β-actin forward: 5’-GAGACCTTCAACACCCCAGCC-3’; β-actin reverse: 5’-AATGTCACGCACGATTTCCC-3’; HIF-1α forward: 5’-ACAAGTCACCACAGGACAG-3’; HIF-1α: 5’-AGGGAGAAAATCAAGTCG-3’. SYBR mix (Roche Applied Science) was added to the PCR mixture as per the manufacturer’s instructions. Each sample contained 3 replicates.
Immunohistochemistry
Tissues were fixed in 10% formaldehyde solution, and then rehydrated by gradually decreasing the concentration of ethanol followed by immersion in EDTA (pH 8.0) at 270°C to recover the antigens. Repair of peroxidase was done by adding 50 μl of 3% H2O2. Goat serum was used to block uninteresting epitopes. After removal of serum, the specimens were incubated with the mouse anti human HIF-1α monoclonal antibody (1:100), followed by 3 washes with PBS. Horseradish peroxidase labelled goat anti-mouse antibody was added and the mixture was incubated for 30 min at room temperature. Newly prepared DAB was added to visualize antigens.
siRNA transfection
siRNA for HIF-1α was purchased from Santa Cruz (Santa Cruz, CA, USA), and negative control siRNAs were purchased from QIAGEN. Cells were transfected with HIF-1α siRNAs or negative control siRNAs (QIAGEN) using the Lipofectamine 2000 Transfection Reagent once the cells grew to 70-90% confluence. Two days after incubation at 37°C, cells were harvested for subsequent assays.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature and then permeated with 0.5% Triton at 4°C for 10 min. After blocking with 2% BSA at 37°C for 30 min, the primary mouse anti-human HIF-1α antibody was diluted in 3% BSA and then added to the plate. After incubation overnight, the secondary antibody (1:100) was added and incubation was performed in the darkness for 1 hr. Hochest33342 was diluted at 1:800 with PBS and added to the cells. Samples were visualized under a fluorescence microscope.
Statistical analysis
Each biological replicate had three technical replicates which were used to calculate the average value of each parameter. Data are reported as mean ± SD. Student’s t test was used to test the significance of differences, and P<0.05 was considered statistically significant.
Results
Keloid fibroblasts are more resistant to ionizing radiation than normal fibroblasts
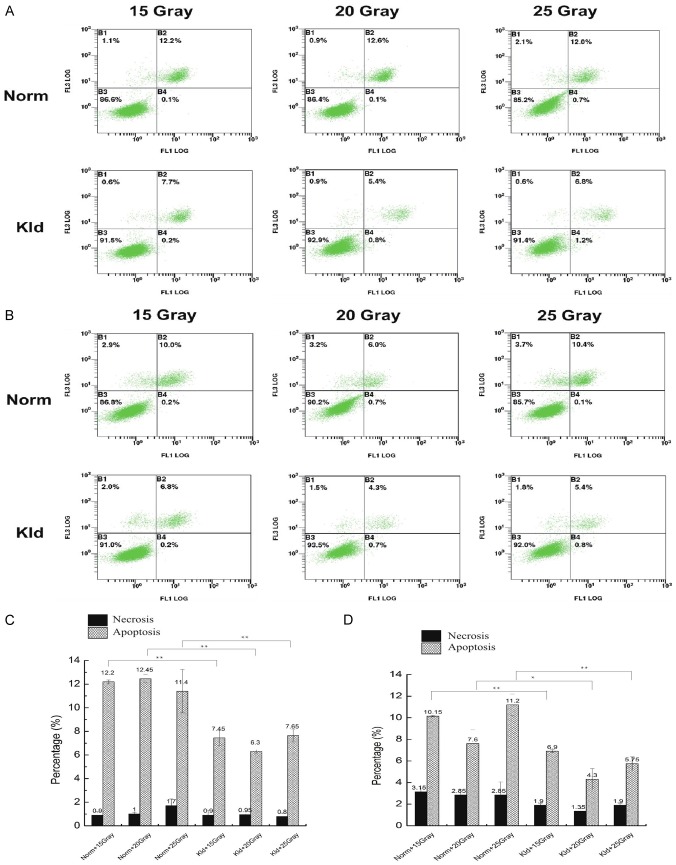
We treated keloid fibroblasts and normal fibroblasts with different doses of ionizing radiation (IR). After 24 hours, we observed no significant difference in the percentage of necrosis between normal fibroblasts and keloid fibroblasts (Figure 1A, 1C). The percentage of apoptotic cells in the normal group showed no significant difference among different doses (P>0.05). In the keloid group, 20 Gray induced the lowest amount apoptosis, and this was lower than the parallel contrast in the normal group (P<0.05), suggesting that keloid fibroblasts possess resistance to ionizing radiation. Because the normal and the keloid groups exhibited the largest difference under 20 Gray, we chose 20 Gray as an optimal radiation dose in subsequent experiments.
Figure 1.
Keloid fibroblasts have stronger resistance at 24 hrs and 48 hrs post-IR treatment. The y-axis indicates propidium iodide (PI) staining, and the x-axis shows Annexin V-FITC staining. The upper left quadrant represents necrosis, the upper right shows late apoptosis, and the lower right shows early apoptosis. A. Annexin V flow cytometry analysis at 24 hrs post-IR treatment. B. Annexin V flow cytometry analysis at 48 hrs post-IR treatment. C. The percentage of necrosis and apoptosis at 24 hrs post-IR treatment. No significant difference in the percentage of necrosis was observed between normal fibroblasts and keloid fibroblasts. D. The percentage of necrosis and apoptosis at 24 hrs post-IR treatment. At different doses, the keloid group exhibited a consistently lower degree of apoptosis suggesting keloid maintained resistance to radiation. (*, P<0.05; **, P<0.01).
After 48 hours, the normal and keloid groups showed no significant difference in the percentage of necrosis. However, at different doses, the keloid group exhibited a consistently lower degree of apoptosis suggesting keloid maintained resistance to radiation (Figure 1B, 1D). The percentage of apoptosis was not dose-dependent since under 20 Gray, both groups showed the lowest degree of apoptosis while under 15 Gray, apoptosis was higher. Compared with 24 hours post-radiation, we observed some degree of apoptosis recovery at 48 hours post-radiation. Therefore, we reasoned that 24 hours is a proper time point to examine apoptosis and detect radioresistance.
Quercetin sensitized keloid fibroblasts to ionizing radiation in a dose-dependent manner
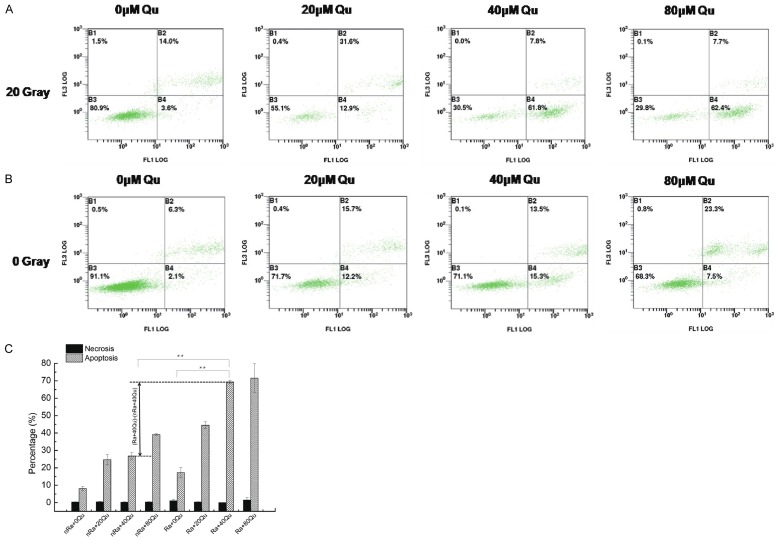
Keloid fibroblasts were treated with 0, 20, 40, and 80 μmol/L quercetin with and without ionizing radiation treatment. As expected, there was no significant difference in the degree of necrosis between the different doses. At 24 h post-radiation, the degree of apoptosis increased in a dose-dependent manner, and the radiation-treated group showed a higher degree of apoptosis than the parallel untreated contrast group. This indicates that a combination of quercetin and radiation may be conducive to the removal of keloid after excision. After treatment with 80 μmol/L, both groups demonstrated the largest percentage of apoptosis. It is obvious that the degree of apoptosis between cells with and without radiation treatment differed to the largest extent at 40 μmol/L quercetin. At this level, the quercetin treated cells showed a 3-fold increase in sensitization to radiation compared to the untreated cells (Ra+40 Qu vs. Ra+0 Qu; 69.7% vs. 17.6%) (Figure 2).
Figure 2.
Quercetin increased apoptosis in IR treated keloid fibroblasts. A. Flow cytometry analysis of keloid fibroblasts treated with different concentrations of quercetin and IR. B. Flow cytometry analysis of keloid fibroblasts treated with quercetin alone. C. Histogram representing the percentage of necrosis and apoptosis in both experiments. At 24 h post-radiation, the degree of apoptosis increased in a dose-dependent manner, and the radiation-treated group showed a higher degree of apoptosis than the parallel untreated contrast group. (*, P<0.05; **, P<0.01).
Quercetin treatment reduced HIF-1α expression in keloid fibroblasts
Ionizing radiation can lead to chemical and biological changes in living cells. The reactive chemical species generated from the radiolysis of water may cause damage to nucleic acids, proteins, and lipids. In contrast, the anti-oxidant mechanisms that cells have evolved can protect cells from oxidant hazards. Previous studies have shown that hypoxic conditions confer radioresistance to cells [13,14]. Moreover, patients with tumors exposed to a highly hypoxic environment have a significantly poorer outcome. As a critical regulator of the cellular response to hypoxia, hypoxia inducible factor 1 (HIF-1) has been shown to play a role in tumor radioresistance [15,16]. Therefore, we performed western blotting and RT-PCR to examine whether HIF-1 expression at the protein and mRNA level is correlated with radioresistance in keloid fibroblasts. HIF-1α protein expression in the western blot of normal fibroblasts was so minimal that it could not be detected (Figure 3A). To facilitate quantification of the protein level of HIF-1α, we employed Image J software to standardize the blots (Figure 3C). All keloid fibroblasts showed diverse amounts of HIF-1α protein expression, approximately 10 times more than normal fibroblasts. In particular, keloid fibroblasts treated with 20 Gray IR displayed a 20% increase in HIF-1α compared to non-IR treated cells, suggesting that IR may promote the expression of HIF-1α.
Figure 3.
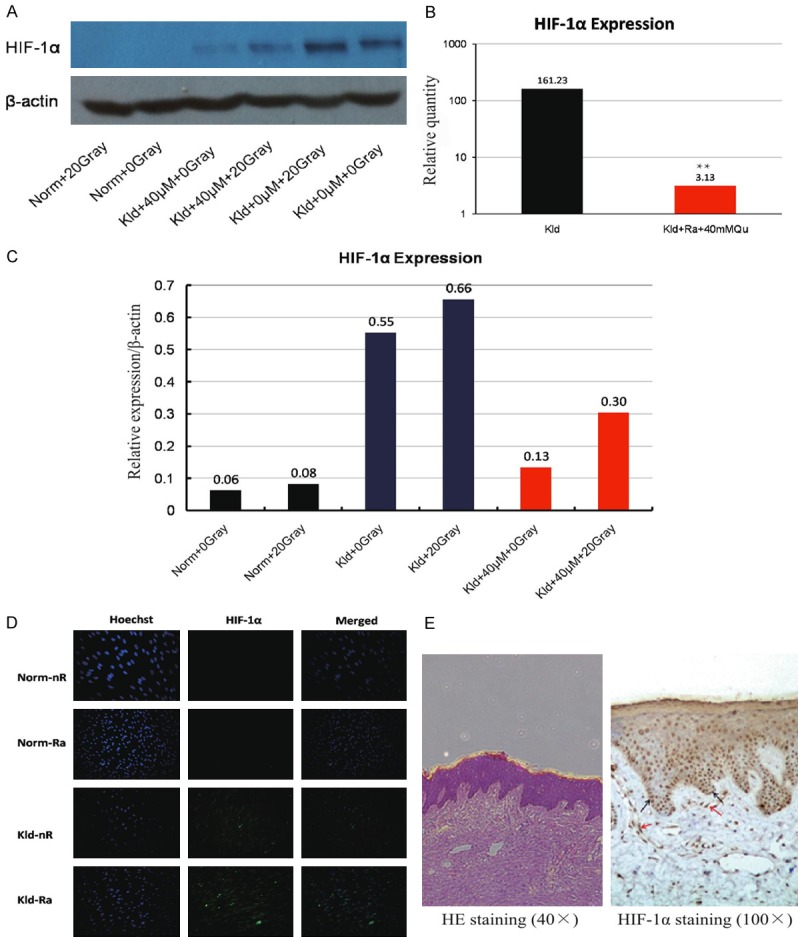
HIF-1α expression in keloid fibroblasts. (A) Western blot of HIF-1α after treatment with 20 Gray IR, 40 μM quercetin, or a combination of both in normal fibroblasts and keloid fibroblasts. (B) The relative quantity of HIF-1α mRNA as measured by RT-PCR. The combination treatment of 40 μmol/L quercetin and 20 Gray IR increased the transcription of HIF-1α by 50-fold. (C) The relative expression of HIF-1α corresponding to (A). keloid fibroblasts treated with 20 Gray IR displayed a 20% increase in HIF-1α compared to non-IR treated cells, suggesting that IR may promote the expression of HIF-1α. (D) Immunofluorescence staining of HIF-1α. HIF-1α was present in the area surrounding the nucleus, and abundant in basal cells, some stratum cells, and keloid fibroblasts of the corium layer. (E) Immunohistochemistry staining of HIF-1α.
Figure 3E demonstrates the distribution of HIF-1α in keloid tissues, which is indicated by brown dots, as visualized by immunohistochemistry. HIF-1α was present in the area surrounding the nucleus, and abundant in basal cells, some stratum cells, and keloid fibroblasts of the corium layer. The immunofluorescence staining also confirmed these findings (Figure 3D). At the mRNA level, the combination treatment of 40 μmol/L quercetin and 20 Gray IR increased the transcription of HIF-1α by 50-fold (Figure 3B). Together, these findings suggest the involvement of HIF-1α in the radioresistance of keloid fibroblasts.
Knockdown of HIF-1α promotes apoptosis of keloid fibroblasts exposed to IR
We detected elevated expression of HIF-1α in IR-treated keloid fibroblasts, and quercetin was found to reduce HIF-1α, suggesting that quercetin may function by targeting HIF-1α. To address this, we knocked down HIF-1α in keloid fibroblasts and treated them with IR. The knockdown efficiency was confirmed by western blotting (Figure 4A). Corresponding percentages of apoptosis and necrosis were measured by flow cytometry. The HIF-1α deficient cells showed a substantially higher degree of apoptosis than their non-deficient counterparts at 24 h and 48 h post IR (Figure 4B, 4C), which implies that HIF-1α may be implicated in inducing apoptosis under IR.
Figure 4.
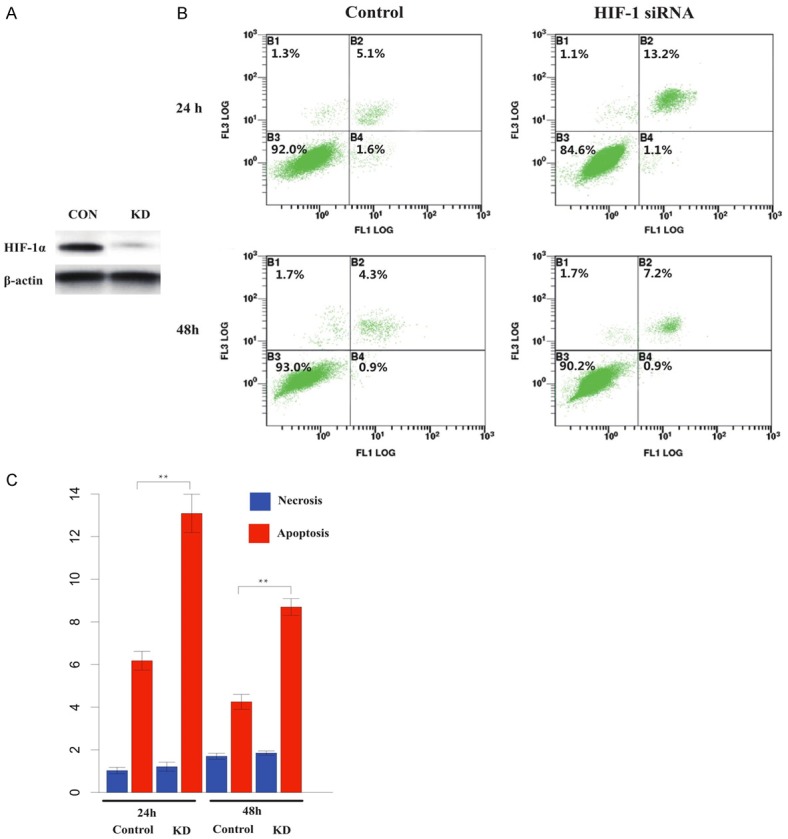
Apoptosis was increased after knock-down of HIF-1α. A. Western blot of HIF-1α in keloid fibroblasts transfected with HIF-1α siRNAs (right) and control (right). B. Annexin-V flow cytometry analysis of HIF-1α deficient keloid fibroblasts and controls at 24 h and 48 h post 20 Gray IR. C. Histogram indicating percentage of necrosis and apoptosis in HIF-1α deficient keloid fibroblast. The HIF-1α deficient cells showed a substantially higher degree of apoptosis than their non-deficient counterparts at 24 h and 48 h post IR (*, P<0.05; **, P<0.01).
The PI3K/Akt pathway is involved in the quercetin-induced decrease of HIF-1α in IR-treated keloid fibroblasts
Previous studies reported that the phosphatidylinositol 3’-kinase (PI3-kinase)/Akt signaling pathway is involved in the regulation of HIF-1α [17,18]. We sought to further examine the downregulation of HIF-1α by quercetin during IR treatment and the involvement of the PI3K signaling pathway. We treated the cells with quercetin, LY294002 (10 µmol/L), or IGF-1 (30 ng/mL), alone or in combination. LY294002 inhibited the phosphorylation of Akt, which can be seen in the blot as the weaker band compared to the blank control (Figure 5A). Of note, treatment with quercetin alone also resulted in a similar phenomenon, indicating that quercetin may also be an inhibitor of the PI3K/Akt pathway. Furthermore, HIF-1α expression decreased along with protein level of p-Akt in the LY294002 and quercetin treated groups, and IGF-1 was able to attenuate suppression of HIF-1α (Figure 5B, 5C). Taken together, we surmised that quercetin inhibits HIF-1α expression via the PI3K/Akt pathway.
Figure 5.
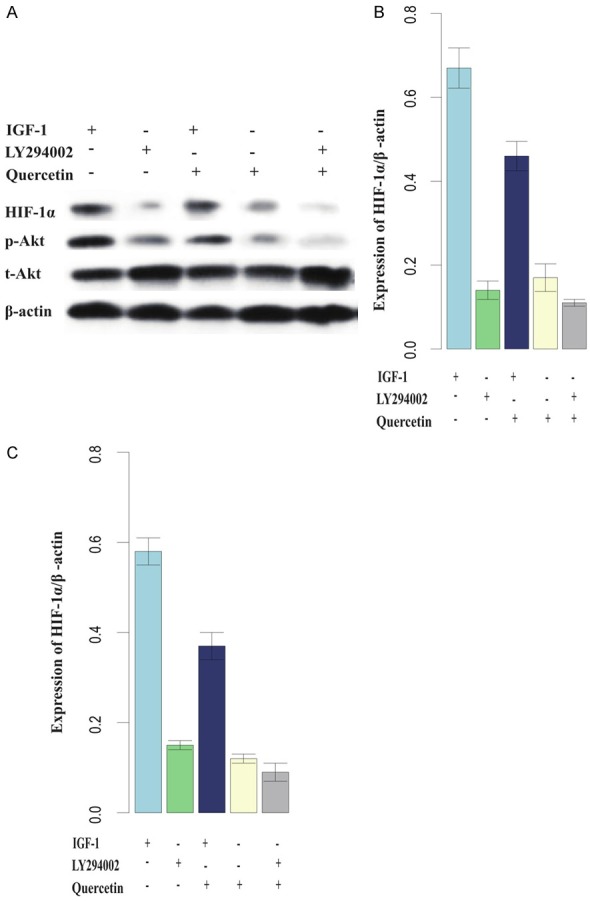
Downregulation of HIF-1α was dependent on the PI3K/Akt pathway. (A) Western blot of HIF-1α, total Akt and phosphorylated Akt (p-Akt) after treatment with IGF-1, LY294002, and quercetin; (B and C) are histograms indicating the relative quantities of HIF-1α and p-Akt protein. HIF-1α expression decreased along with protein level of p-Akt in the LY294002 and quercetin treated groups, and IGF-1 was able to attenuate suppression of HIF-1α. (*, P<0.05; **, P<0.01).
Discussion
Histologically, keloids are a class of benign tumor composed of irregular fibroblasts and accumulation of extracellular matrix proteins, such as collagen, annexin, proteoglycan, and elastin, which confer oncogenic properties to the inflexible scars [19]. Apoptosis is the major mechanism by which keloid tumors regress [20,21]. Currently, excision followed by radiation therapy has been reported as an effective treatment. Radiation is a useful therapy to treat topical tumors as a single modality and is highly cost-efficient [22]. Ionizing radiation (IR), which can penetrate through tissues, is the most commonly employed type of radiation. Ions resulting from the fissure produced by IR are the major components that kill or cause severe damage to living cells. IR causes diverse forms of cell death, including necrosis, apoptosis, autophagy, senescence, and mitotic catastrophe. However, IR exposure not only causes cell death but also induces resistance in the tumor cells, which constitutes another pitfall of this treatment [23]. In the present study, we observed that necrosis increased in normal cells with increasing IR doses, while this was not observed in the keloid tissues. In addition, apoptosis decreased as the treatment continued, demonstrating that keloid tissues acquired radioresistance after IR treatment.
Radioresistance remains an intractable clinical problem that leads to poor outcomes in cancer patients. Radioresistance induces survival signaling pathways to sustain cell viability and antagonize anti-oncogenic pathways [24-26]. Multiple research reports have unveiled a number of signaling pathways that contribute to IR resistance. Originally, cells activate these pathways to escape or remove hazards in an attempt to adapt to the extracellular environment, which becomes oncogenic when augmented. Among them, the hypoxia pathway is one of the most extensively studied pathways due to its intimate association with ionizing conditions [27-29]. For the past few decades, a plethora of studies has been dedicated to developing radiosensitizers for specific tumors. Chemotherapeutic agents have been utilized as an adjuvant for radiation therapy, which has achieved superior outcomes compared to using radiation alone. Yamawaki et al. reported that adjuvant therapy after excision and radiation could force keloids into remission [30]. Here, we demonstrated that quercetin sensitized keloid fibroblasts to IR treatment, and the effect was associated with the expression of HIF-1α, a critical component of the radioresistance-related hypoxia pathway.
Quercetin is abundant in broccoli, berries, apples, and onions and is frequently reported as an antioxidant. Previous studies showed that quercetin exerts protective effects in reperfusion ischemic tissue damage by scavenging free radicals [31,32]. Because the HIF-1α pathway is usually activated in response to low oxygen levels, the link between quercetin and HIF-1α with hypoxia-related radioresistance does not seem intuitive. However, it has been reported that low oxygen tension is not related to HIF-1α both in vivo and in vitro [33]. Rather, reoxygenation markedly increased the expression of HIF-1α [34,35]. The oxidative stress generated through release of oxygen from deceased cells leads to the activation of HIF-1α, and removal of oxidants by quercetin can abolish reoxygenation, thereby decreasing accumulation of HIF-1α. In the present study, we observed that keloid fibroblasts contain more HIF-1α than normal fibroblasts, which is in accordance with previous findings showing that mast cells may increase the expression of HIF-1α in keloid fibroblasts in vitro during hypoxia. The accumulation of HIF-1α decreased in a dose-dependent manner, where a higher dose of quercetin was related to lower protein levels of HIF-1α. HIF-1α ablation substantially equalized apoptosis. Taken together, we deem that quercetin sensitized keloid cells to IR via targeting HIF-1α. Quercetin as a sensitizer of anticancer therapies has been suggested elsewhere [36], while we initially reported this property of quercetin in IR-treated keloid fibroblasts in relation with HIF-1α downregulation.
Accumulation of HIF-1α promotes its binding with HIF-1β to form active dimers, which were identified as transcriptional factors of over 100 genes implicated in cellular proliferation, apoptosis, and angiogenesis [33,37]. The links among proliferation, apoptosis, angiogenesis, hypoxia, and radioresistance are well established. The PI3K/Akt pathway is highly involved in these biological processes and has been documented as an oncogenic pathway [38-40]. Nguyen et al. revealed that quercetin treatment stalled the human lung adenocarcinoma cell line A549 at G0/G1, which then entered apoptosis [41,42]. The apoptosis induced by quercetin acts through inhibition of PI3K/Akt and the apoptotic inhibitor Bcl-2, as well as via activation of proapoptotic Bax and caspase-2/-3/-7. In the present study, we employed LY294002 to abrogate the phosphorylation of Akt, which inhibited the PI3K/Akt pathway. Quercetin exerted an inhibitory effect on both Akt phosphorylation and HIF-1α accumulation, which suggests that the PI3K/Akt pathway might be implicated in the suppression of HIF-1α by quercetin. When treated with IGF-1, an agonist of Akt phosphorylation, the inhibition of quercetin on HIF-1α was attenuated but did not recover completely, suggesting it as an alternative regulator of HIF-1α in addition to p-Akt. Since PI3K/Akt was affected by quercetin, the sensitizing effect might arise from the promotion of apoptosis resulting from HIF-1α downregulation, as well as diminished proliferation via blockage of the PI3K/Akt pathway, which warrants further comprehensive investigation.
In conclusion, this study revealed that stronger radioresistance of keloid fibroblasts, characterized by aggravated apoptosis, is associated with HIF-1α downregulation, which can be achieved by quercetin treatment. The promotion of apoptosis by quercetin is PI3K/Akt-dependent, which implies potential inhibition of cell proliferation by quercetin in keloid fibroblasts. These findings provide potent experimental support for quercetin as an adjuvant therapy after excision and radiation treatment of keloid fibroblasts.
Disclosure of conflict of interest
None.
References
- 1.Satish L, Lyons-Weiler J, Hebda PA, Wells A. Gene expression patterns in isolated keloid fibroblasts. Wound Repair Regen. 2006;14:463–470. doi: 10.1111/j.1743-6109.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 2.Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 3.Viera MH, Vivas AC, Berman B. Treatment of keloids and scars. Ethn Dermatology Princ Pract. 2013:159–172. [Google Scholar]
- 4.Estrem SA, Domayer M, Bardach J, Cram AE. Implantation of human keloid into athymic mice. Laryngoscope. 1987;97:1214–1218. doi: 10.1288/00005537-198710000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Shang Y, Yu D, Hao L. Liposome-adenoviral hTERT-siRNA knockdown in fibroblasts from keloids reduce telomere length and fibroblast growth. Cell Biochem Biophys. 2015;72:405–410. doi: 10.1007/s12013-014-0476-5. [DOI] [PubMed] [Google Scholar]
- 6.Sidgwick GP, Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatology Venereol. 2012;26:141–152. doi: 10.1111/j.1468-3083.2011.04200.x. [DOI] [PubMed] [Google Scholar]
- 7.Aoki M, Miyake K, Ogawa R, Dohi T, Akaishi S, Hyakusoku H, shimada T. siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I. J Invest Dermatol. 2014;134:818–826. doi: 10.1038/jid.2013.396. [DOI] [PubMed] [Google Scholar]
- 8.Berman B, Bieley HC. Adjunct therapies to surgical management of keloids. Dermatologic Surg. 1996;22:126–130. doi: 10.1111/j.1524-4725.1996.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 9.Berman B, Bieley HC. Keloids. J Am Acad Dermatol. 1995;33:117–123. doi: 10.1016/0190-9622(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 10.Lakhanpal P, Rai DK. Quercetin: a versatile Flavonoid. Internet J Med Updat. 2007;2:22–37. [Google Scholar]
- 11.Kobylińska A, Janas KM. Health--promoting effect of quercetin in human diet. Postepy Hig Med Dosw. 2014;69:51–62. doi: 10.5604/17322693.1135423. [DOI] [PubMed] [Google Scholar]
- 12.Varadkar P, Dubey P, Krishna M, Verma NC. Modulation of radiation-induced protein kinase C activity by phenolics. J Radiol Prot. 2001;21:361. doi: 10.1088/0952-4746/21/4/304. [DOI] [PubMed] [Google Scholar]
- 13.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 14.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 15.Harada H, Kizaka-Kondoh S, Li G, Itasaka S, Shibuya K, Inoue M, Hiraoka M. Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene. 2007;26:7508–7516. doi: 10.1038/sj.onc.1210556. [DOI] [PubMed] [Google Scholar]
- 16.Kessler J, Hahnel A, Wichmann H, Rot S, Kappler M, Bache M, Vordermark D. HIF-1α inhibition by siRNA or chetomin in human malignant glioma cells: effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer. 2010;10:605. doi: 10.1186/1471-2407-10-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Oh CK, Messadi DV, Duong HS, Kelly AP, Soo C, Wang L, Le AD. Hypoxia-induced HIF-1 alpha accumulation is augmented in a co-culture of keloid fibroblasts and human mast cells: involvement of ERK1/2 and PI-3K/Akt. Exp Cell Res. 2006;312:145–155. doi: 10.1016/j.yexcr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Gort EH, Groot AJ, Derks van de Ven TL, van der Groep P, Verlaan I, van Laar T, van Diest PJ, van der Wall E, Shvarts A. Hypoxia-inducible factor-1α expression requires PI 3-kinase activity and correlates with Akt1 phosphorylation in invasive breast carcinomas. Oncogene. 2006;25:6123–6127. doi: 10.1038/sj.onc.1209643. [DOI] [PubMed] [Google Scholar]
- 19.Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosom Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo S, Benathan M, Raffoul W, Panizzon RG, Egloff DV. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Srug. 2001;107:87–96. doi: 10.1097/00006534-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids-a review of their pathophysiology, risk factors, and therapeutic management. Dermatologic Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim BM, Hong Y, Lee S, Liu P, Lim JH, Lee YH, Lee TH, Chang KT, Hong Y. Therapeutic implications for overcoming radiation resistance in cancer therapy. Int J Mol Sci. 2015;16:26880–26913. doi: 10.3390/ijms161125991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 24.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akimoto T, Nonaka T, Ishikawa H, Sakurai H, Saitoh J, Takahashi T, Mitsuhashi N. Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines: possible involvement of inhibition of survival signal transduction pathways. Int J Radiat Oncol Biol Phys. 2016;50:195–201. doi: 10.1016/s0360-3016(00)01560-1. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Xia L, Lee LM, Khaletskiy A, Wang J, Wong JYC, Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res. 2001;155:543–553. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Ma NY, Tinganelli W, Maier A, Durante M, Kraft-Weyrather W. Influence of chronic hypoxia and radiation quality on cell survival. J Radiat Res. 2013;54:i13–i22. doi: 10.1093/jrr/rrs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helbig L, Koi L, Brüchner K, Gurtner K, Hess-Stumpp H, Unterschemmann K, Pruschy M, Baumann M, Yaromina A, Zips D. Hypoxia-inducible factor pathway inhibition resolves tumor hypoxia and improves local tumor control after single-dose irradiation. Int J Radiat Oncol Biol Phys. 2014;88:159–166. doi: 10.1016/j.ijrobp.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 29.Scott SD, Greco O. Radiation and hypoxia inducible gene therapy systems. Cancer MEtastasis Rev. 2004;23:269–276. doi: 10.1023/B:CANC.0000031766.58614.f1. [DOI] [PubMed] [Google Scholar]
- 30.Yamawaki S, Naitoh M, Ishiko T, Muneuchi G, Suzuki S. Keloids can be forced into remission with surgical excision and radiation, followed by adjuvant therapy. Ann Plast Surg. 2011;67:402–406. doi: 10.1097/SAP.0b013e31820d684d. [DOI] [PubMed] [Google Scholar]
- 31.Santos AC, Uyemura SA, Lopes JL, Bazon JN, Mingatto FE, Curti C. Effect of naturally occurring flavonoids on lipid peroxidation and membrane permeability transition in mitochondria. Free Radic Biol Med. 1998;24:1455–1461. doi: 10.1016/s0891-5849(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF. Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv. 2016;34:532–549. doi: 10.1016/j.biotechadv.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Conde E, Alegre L, Blanco-Sánchez I, Sáenz-Morales D, Aguado-Fraile E, Ponte B, Ramos E, Sáiz A, Jiménez C, Ordoñez A, López-Cabrera M, del Peso L, de Landázuri MO, Liaño F, Selgas R, Sanchez-Tomero JA, García-Bermejo ML. Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PLoS One. 2012;7:e33258. doi: 10.1371/journal.pone.0033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DW, Forman C, Vesey DA. Novel renoprotective actions of erythropoietin: new uses for an old hormone. Nephrology. 2006;11:306–312. doi: 10.1111/j.1440-1797.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Tao L, Qi K, Zhang H, Feng D, Wei W, Kong K, Chen T, Lin Q. Quercetin reverses tamoxifen resistance in breast cancer cells. J BUON. 2015;20:707–713. [PubMed] [Google Scholar]
- 37.Mancini M, Gariboldi MB, Taiana E, Bonzi MC, Craparotta I, Pagin M, Monti E. Co-targeting the IGF system and HIF-1 inhibits migration and invasion by (triple-negative) btreast cancer cells. Br J Cancer. 2014;110:1–9. doi: 10.1038/bjc.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: insibe-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 39.Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol. 2009;4:43. doi: 10.1186/1748-717X-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toulany M, Minjgee M, Kehlbach R, Chen J, Baumann M, Rodemann HP. ErbB2 expression through heterodimerization with erbB1 is necessary for ionizing radiation- but not EGF-induced activation of Akt survival pathway. Radiother Oncol. 2010;97:338–345. doi: 10.1016/j.radonc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lng cancer cells. Carcinogenesis. 2004;25:647–659. doi: 10.1093/carcin/bgh052. [DOI] [PubMed] [Google Scholar]
- 42.Yuan Z, Long C, Junming T, Qihuan L, Youshun Z, Chan Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol Biol Rep. 2012;39:7785–7793. doi: 10.1007/s11033-012-1621-0. [DOI] [PubMed] [Google Scholar]