Abstract
Intrauterine adhesion (IUA) is a common disease among women after uterus operation. BMSCs are commonly used as a therapeutic agent for IUA treatment, but the underlying mechanism is not fully delineated. Here we showed that BMSCs co-cultured with EPCs promotes proliferative ability and decreases apoptosis ratio of BMSCs and EPCs. In addition, BMSCs promote the differentiation of EPCs into vascular endothelial cells, and BMSCs derived epithelial cells are also induced by EPCs. We also found that the levels of Collagen Type I, vascular endothelial growth factor (VEGF), granulocyte-macrophage colony stimulating factor (GM-CSF) and bone morphogenetic protein (BMP-2) are significantly increased in the co-culturing system comparing to those of the BMSCs or EPCs alone group. Of note, PI3K/Akt/Cox2 axis is activated in the co-culturing system and LY294002 abrogates the co-culturing system’s effects on cell proliferation, apoptosis and cytokines secretion, which are reversed by synergistically overexpressing Cox2. In conclusion, our in vitro experiments proved that the interaction of BMSCs and EPCs might promote angiogenesis and alleviate IUA pathogenesis by regulating PI3K/Akt/Cox2 axis mediated modulation of cell apoptosis, proliferation, differentiation and angiogenesis-associated cytokines secretion.
Keywords: IUA, BMSCs, EPCs, angiogenesis
Introduction
Intrauterine adhesion (IUA) is a major problem causing infertility, menstrual irregularities and recurrent pregnancy losses in women, which seriously endangers women’s health and life quality [1]. It has been reported that approximately 90% of IUA patients are caused by the damage to the uterine due to the intrauterine surgery. Besides, IUA recurrence is at a high level after intrauterine surgery especially for patients with severe IUA [2]. However, there are still no effective treatments for IUA. Angiogenesis has been reported to be pivotal for IUA recovery, and the treatments targeted angiogenesis therapy were proved to be efficacious for IUA [3].
Bone mesenchymal stem cells (BMSCs) have been used to treat various diseases such as chronic kidney disease [4], osteoarthritis [5] and liver fibrosis [6] as a result of its differentiation and cytokines secretion abilities. BMSCs therapy has also been used for IUA treatments and proved to be efficacious for IUA recovery by promoting endometrium angiogenesis [3,7,8], but the mechanisms of BMSCs-mediated angiogenesis are still not fully depicted. Previous studies have proved that BMSCs promote angiogenesis and osteogenesis by interacting with endothelial progenitor cells (EPCs) [9], which further differentiate into vascular endothelial cells [10] and facilitate angiogenesis by secreting different angiogenesis associated cytokines [11,12]. EPCs are also reported to regulate the proliferation and differentiation of BMSCs by secreting EPCs derived extracellular vesicles [13]. Based on the above studies, we hypothesize that BMSCs and EPCs interaction might be critical for BMSCs mediated angiogenesis and IUA alleviation.
Cyclooxygenase 2 (COX2) is an inducible form of cyclooxygenase which is crucial for cell growth, proliferation and apoptosis [14,15]. Cox2 plays an important role in angiogenesis and EPCs mobilization [16], and certain stimulis such as cytokines and growth factors increase Cox2 expression levels [17]. COX2 overexpression has also been shown to promote BMSCs proliferation and differentiation [18,19], it plays an important role in regulating EPCs’ functions [20,21]. Cox2 is regulated by many factors and signal pathways [22,23], but it is still unclear which regulators participate in the regulation of BMSCs and EPCs interaction mediated angiogenesis. Among all the upstream regulators, PI3K/Akt pathway activation was found to be important for Cox2 upregulation [24], which further participates in tumor angiogenesis [25].
In this study, we aim at investigating whether BMSCs and EPCs interaction alleviate IUA pathogenesis by modulating PI3K/Akt/Cox2 axis. This study might provide new targets and strategies for IUA treatment in clinical practices.
Materials and methods
Cells separation and culture
EPCs were isolated from patient peripheral blood by Ficoll-Paque PLUS (GE, USA) and cultured in endothelial cell medium according to the previous study [26], cells adhering to the flask were collected after 6 days’ culture and stained with anti-CD34 (Abcam, CA, UK), anti-CD133 (Santa Cruz, USA) and anti-CD29 (Santa Cruz, USA) antibodies, Flow cytometry was conducted to identify and separate CD34+ CD133+ CD29+ cells, which were identified as the typical markers of EPCs. BMSCs were purchased from ATCC (#PCS-500-012TM, USA) and cultivated in DMEM medium with 5% CO2 and a constant temperature of 37°C for incubation.
Establishment of the co-culturing systems
Transwell chambers were purchased from Corning (USA), EPCs and BMSCs were cultured in the upper and lower chambers respectively at the ratio of 1:1, 1:2 and 2:1 respectively, cells were cultured with 5% CO2 and a constant temperature of 37°C for 24 h, 48 h, 72 h and 96 h. The experiments were divided into the five groups including BMSCs alone group, EPCs alone group, B:E (1:1) group, B:E (1:2) group and B:E (2:1) group.
CCK-8 cell proliferation assay
BMSCs and EPCs in the co-culturing systems in different time points were separated and collected in different 96-well plates respectively, and all the cells were cultured in 37°C and 5% CO2 conditions for 12 h. Cell Counting Kit-8 (Beyotime, China) and SpectraMax Paradigm (Molecular Devices, USA) were used to detect cells’ proliferative abilities following the manufacture’s instruction.
Western blot
EPCs and BMSCs in the co-culturing system were collected separately. Cell samples were lysed by incubating with RIPA lysis buffer containing 150 mM NaCl, 1% NP-40, 0.5% deoxycholate and 1% SDS on ice for 1 hour. The supernatant was collected and protein was diluted to 50 ug/10 ul according to the total protein content quantified by Bradford’s method (Bio-Rad). SDS-PAGE was used to separate proteins and HMGB1 was transferred from the gel to PVDF membranes (Immun-blot, Bio-Rad) using a Bio-Rad Mini PROTEAN III apparatus. The PVDF membranes were incubated with the first antibodies (#sc-56698, Santa, USA) for the whole night at 4°C. Then the anti-mouse IgG-peroxidase-conjugate secondary antibodies (Sigma, USA) were incubated with the membranes for 1 hour. The bands were visualized by Enhanced Chemiluminescence kit (Bio-Rad) and ChemiDoc (Bio-Rad). Each experiment has at three repetitions.
Flow cytometry
EPCs and BMSCs in the co-culturing system were collected separately and cell suspensions were prepared by GE Ficoll-Paque PLUS (GE, USA), cells were then treated with 10% DMSO and stored in -80°C. Ex vivo cellular staining for Annexin-V and PI was implemented by incubating cells with specific dyes (ThermoFisher, USA). Attune NxT Flow Cytometer (ThermoFisher, USA) was employed detect cell apoptosis.
ELISA
The cell culture medium from different groups in different time points were collected, and ELISA kits (Abcam, USA) were employed to detect VEGF, GM-CSF, Collagen type II and BMP-2 levels according to the manufacture’s instruction. SpectraMax Paradigm (Molecular Devices, USA) microplate reader was used to measure OD values to quantify expression levels of the cytokines.
Statistical analysis
The data were shown as the mean ± standard deviation (SD). SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) was used to analyze all the data, one-way analysis variance (ANOVA) was used for multiple groups comparison and the Student’s t-test was employed for two groups comparison. P<0.05 was considered statistically significance.
Results
The effects of BMSCs and EPCs interaction on cell proliferation
To investigate the impacts of BMSCs and EPCs interaction on cell proliferation, CCK-8 assay was performed to detect BMSCs and EPCs proliferation by co-culturing the two cells for 24 h, 48 h, 72 h and 96 h respectively. Comparing to the BMSCs or EPCs alone groups, the proliferative ability of both BMSCs and EPCs are significantly improved by the co-culturing system (Figure 1A, 1B). Western Blot results also suggested that cell cycle associated proteins including Cyclin D1, Cyclin E2, CDK2, CDK4 and CDK6 in the two cells are significantly upregulated by the co-culturing system comparing to the BMSCs and EPCs alone groups, specifically, the ratio of BMSCs and EPCs at 2:1 reaches the best effects on cell proliferation in contrast with 1:1 or 1:2 (Figure 1C-H). The results indicated that co-culturing BMSCs with EPCs significantly improves the proliferative ability of both cells.
Figure 1.
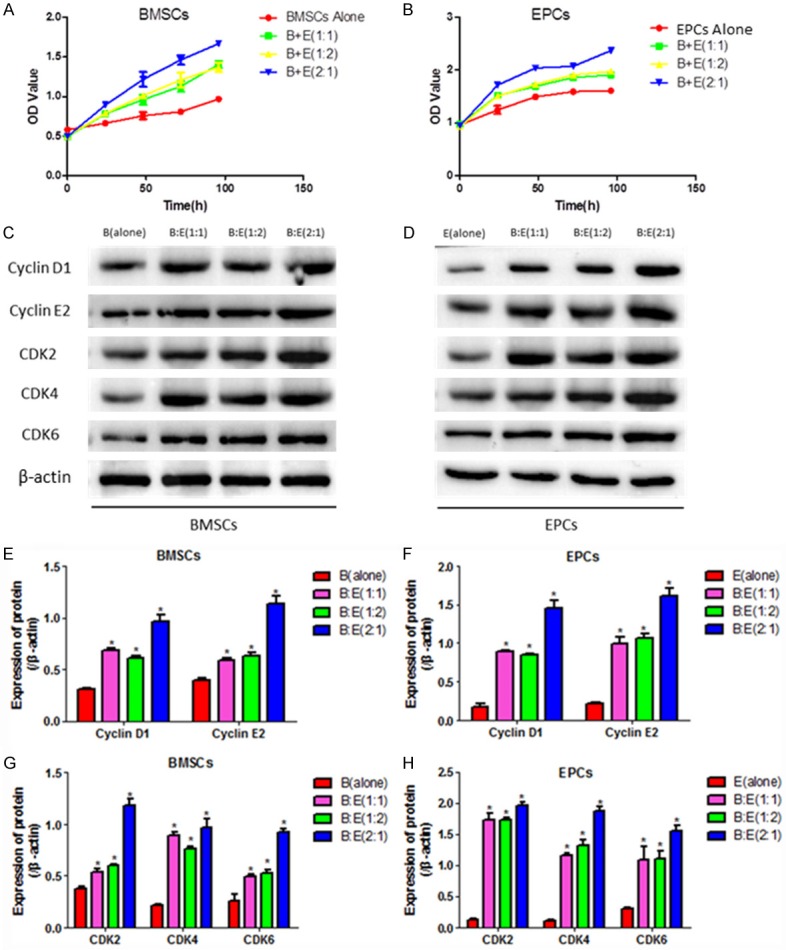
BMSCs and EPCs proliferations in the co-culturing system. A, B: The proliferative abilities of BMSCs and EPCs were detected by CCK-8 assay, the experiments were divided into five groups including BMSCs alone, EPCs alone, co-culturing BMSCs with EPCs at the ratio of 1:1, 1:2 and 2:1 respectively. The OD values were used to quantify cell proliferation. C, D: Western blot was performed to detect cell cycle associated proteins including cyclin D1, cyclin E2, CDK2, CDK4 and CDK6, the intensity of the protein bands were employed to evaluate protein levels. E-H: Image J software was used to quantify proteins according to the gray values. (*, P<0.05 VS B(alone) or E(alone) control groups).
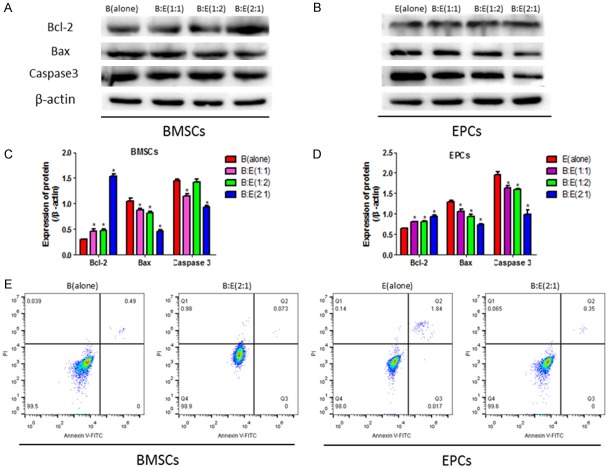
Co-culturing BMSCs with EPCs affects cell apoptosis
Based on the proliferation results, we hypothesized that the co-culturing system might have inhibiting effects on cell apoptosis. Western Blot results showed that the co-culturing system increases the anti-apoptotic protein Bcl-2 and downregulates the pro-apoptotic proteins Bax as well as Caspase3 levels comparing to the BMSCs or EPCs alone groups, specifically, co-culturing BMSCs with EPCs at the ratio of 2:1 has better inhibiting effects on cell apoptosis than the 1:1 and 1:2 groups (Figure 2A-D). Flow cytometry results also showed that the co-culturing system (BMSCs:EPCs = 2:1) decreases both BMSCs and EPCs apoptosis rates after the two cells were co-cultured for 4 hours (Figure 2E), which was in accordance with the Western Blot results.
Figure 2.
Cell apoptosis was affected by co-culturing BMSCs with EPCs at the ratio of 1:1, 1:2 and 2:1 respectively. A, B: Western Blot was employed to investigate apoptosis associated proteins including Bcl-2, Bax and Caspase3 in BMSCs and EPCs after culturing the two cells alone or co-culturing the BMSCs with EPCs at the ratios of 1:1, 1:2 or 2:1 respectively for 4 hours, the intensity of the protein bands were employed to evaluate protein levels. C, D: Image J software was used to analyze the grey values of the protein bands, the proteins were normalized by β-actin. E: Flow cytometry (FCM) was performed to detect cell apoptosis rates in the BMSCs or EPCs alone groups and co-culturing the two cells at the ratio of 2:1 for 4 hours, BMSCs and EPCs were separated and stained with Annexin V-FITC and PI. (*, P<0.05 VS B(alone) or E(alone) control groups).
The effects of BMSCs and EPCs interaction on cell differentiation
Since BMSCs are potential to differentiate into epithelial cells and EPCs are the progenitor of vascular endothelial cells, we next investigated cell differentiation in the co-culturing system. BMSCs and EPCs were separated and Western Blot was used to detect the markers of epithelial cell (CK18, VIM and ESR1) in BMSCs and markers of vascular endothelial cell (VEGFA, VEGFR2 and VEGFR3) in EPCs respectively. The results showed that CK18, VIM and ESR1 in BMSCs are significantly increased by the co-culturing system comparing to the control group (BMSCs alone), and co-culturing BMSCs with EPCs at the ratio of 2:1 most significantly increases CK18, VIM and ESR1 comparing to the 1:1 and 1:2 groups (Figure 3A, 3C). In addition, VEGFA, VEGF2 and VEGFR3 in EPCs are also significantly increased by the co-culturing system comparing to the control group (EPCs alone), of note, the ratio of BMSCs and EPCs at 2:1 merely increases VEGFA and VEGFR3 except for VEGFR2 comparing to the 1:1 and 1:2 groups (Figure 3B, 3D).
Figure 3.
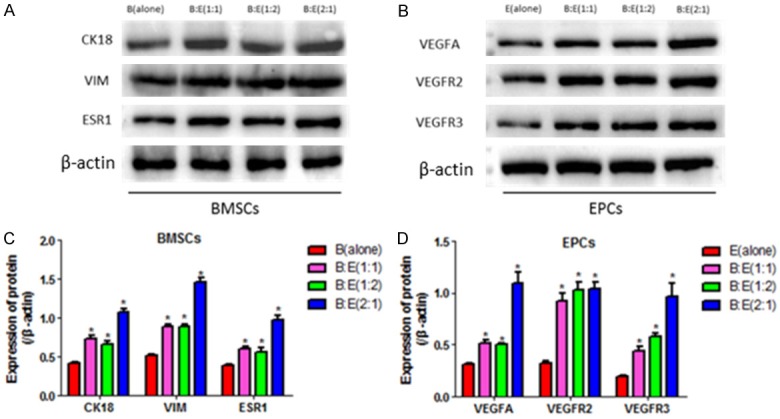
Detection of the markers of epithelial cells and vascular endothelial cells in BMSCs and EPCs. A: Western Blot was used to detect the markers of epithelial cells (CK18, VIM and ESR1) in BMSCs in control group (BMSCs alone) and the groups of co-culturing BMSCs with EPCs at the ratio of 1:1, 1:2 and 2:1 respectively for 4 hours. B: Western Blot was used to detect the markers of vascular endothelial cells (VEGFA, VEGFR2 and VEGFR3) in EPCs in control group (EPCs alone) and the groups of co-culturing BMSCs with EPCs at the ratio of 1:1, 1:2 and 2:1 respectively for 4 hours. C, D: Image J software was performed to analyze the grey values of the protein bands in A, and B respectively, the protein levels were normalized by β-actin. (*, P<0.05 VS B(alone) or E(alone) control groups).
Angiogenesis associated cytokines secretion in the culture medium of the co-culturing system
Angiogenesis associated cytokines including VEGF, GM-CSF, Collagen type I and BMP-2 in the culture medium were also detected by ELISA to investigate whether BMSCs and EPCs co-culturing system influences their levels. The results showed that VEGF, GM-CSF, Collagen type I and BMP-2 are significantly increased in the co-culturing system comparing to the BMSCs or EPCs alone groups, specifically, co-culturing BMSCs and EPCs at the ratio of 2:1 significantly increases VEGF, GM-CSF and BMP-2 instead of Collagen type I in contrast with the 1:1 or 1:2 groups (Figure 4A-D). Our results were in line with the previous study, which reported that BMSCs and EPCs interaction promotes angiogenesis associated cytokines secretion [9].
Figure 4.
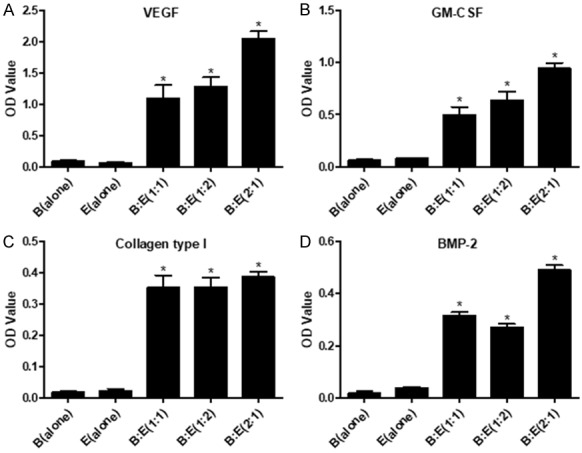
Detection of angiogenesis associated cytokines in the culture medium by ELISA. A: Detection of VEGF in BMSCs (alone), EPCs (alone), B:E (1:1), B:E (1:2) and B:E (2:1) groups respectively. B: Detection of GM-CSF in BMSCs (alone), EPCs (alone), B:E (1:1), B:E (1:2) and B:E (2:1) groups respectively. C: Detection of Collagen type I in BMSCs (alone), EPCs (alone), B:E (1:1), B:E (1:2) and B:E (2:1) groups respectively. D: Detection of BMP-2 in BMSCs (alone), EPCs (alone), B:E (1:1), B:E (1:2) and B:E (2:1) groups respectively. The OD value was employed to quantify cytokines in the culture medium. (*, P<0.05 VS B(alone) or E(alone) control groups).
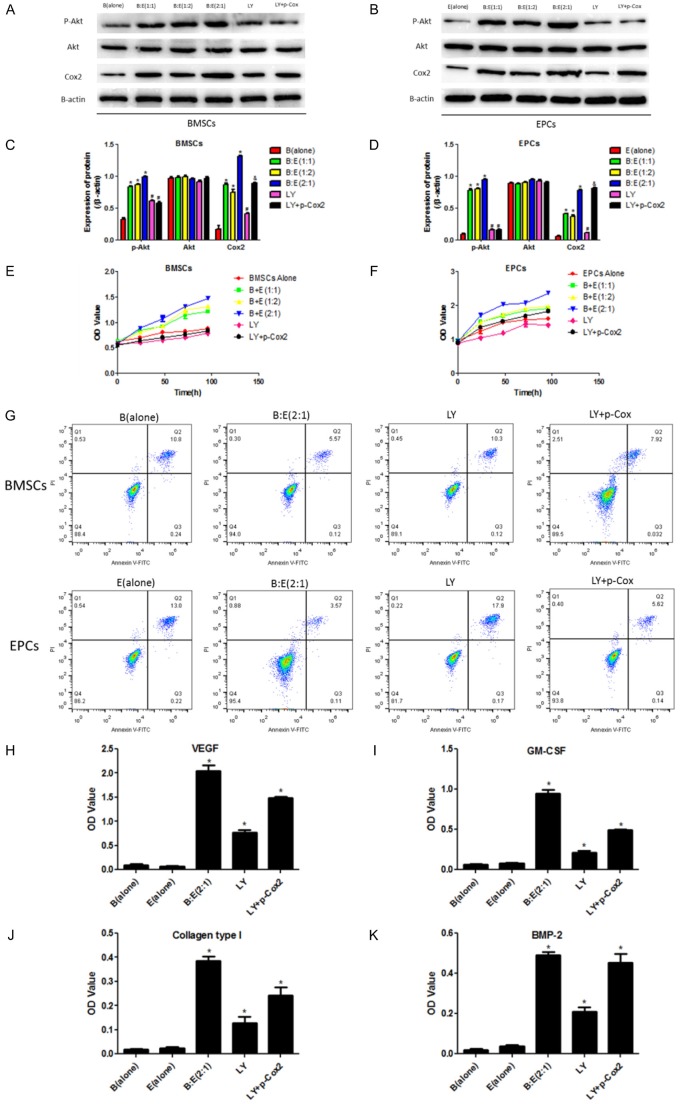
Involvement of PI3K/Akt/Cox2 signal pathway in BMSCs and EPCs interaction mediated modulation of cell proliferation, apoptosis and cytokines secretion
We next explored the mechanism of the effects of BMSCs and EPCs co-culturing system for cell proliferation, apoptosis and angiogenesis cytokines secretion. Our results showed that co-culturing BMSCs with EPCs significantly increases phosphorylated Akt and Cox2 in both cells. Of note, Cox 2 in BMSCs and EPCs from the co-culturing system is significantly decreased by treating cells with PI3K/Akt inhibitor LY29400-2, which are recovered by transfecting BMSCs and EPCs with Cox2 overexpression lentiviral vectors (Figure 5A-D). CCK-8 results showed that LY294002 abrogates BMSCs and EPCs interaction mediated promotion of cell proliferation in both the two cells, and the inhibiting effects of LY294002 on EPCs instead of BMSCs are reversed by Cox-2 overexpression (Figure 5E, 5F). LY294002 also abrogates the inhibiting effects of the co-culturing system on BMSCs and EPCs apoptosis rate, which are reversed by synergistically overexpressing Cox-2 (Figure 5G). In addition, LY294002 decreases VEGF, GM-CSF, Collagen type I and BMP-2 in the culture medium of the co-culturing system, which are also reversed by synergistically overexpressing Cox-2 (Figure 5H-K).
Figure 5.
Involvement of PI3K/Akt/Cox-2 axis in BMSCs and EPCs interaction mediated modulation of cell proliferation, apoptosis and angiogenesis associated cytokines secretion. A, B: Western Blot was used to detect p-Akt, Akt and Cox2 in different groups including BMSCs alone, EPCs alone, BMSCs and EPCs co-culturing system at the ratio of 1:1, 1:2 and 2:1, treating the co-culturing system (BMSCs:EPCs = 2:1) with LY294002 and synergistically treating the co-culturing system (BMSCs:EPCs = 2:1) with LY294002 and Cox-2 overexpression lentiviral vectors. The protein bands were used to quantify proteins. C, D: Image J software was performed to quantify proteins according to the grey values, the proteins were normalized by β-actin. E, F: Cell proliferation was evaluated by CCK-8 assay after treating the co-culturing system with LY294002 alone or synergistically treating the co-culturing system with LY294002 and Cox2 overexpression lentiviral vectors. The OD value was used to evaluate cell proliferative abilities. G: Flow cytometry (FCM) was employed to detect cell apoptosis rates of BMSCs and EPCs in the co-culturing system treated with LY294002 alone or synergistically treated with LY294002 and Cox2 overexpression lentiviral vectors. H-K: Detection of angiogenesis associated cytokines including VEGF, GM-CSF, Collagen type I and BMP-2 by ELISA in the culture medium of the co-culturing system treated with LY294002 alone or synergistically treated with LY294002 and Cox2 overexpression lentiviral vectors. The OD value was used to quantify the cytokines secretion. (*, P<0.05 VS B(alone) or E(alone) control groups).
Discussion
BMSCs therapy is an efficacious treatment for IUA recovery and prognosis by regulating angiogenesis [3,7,8], recent studies found that BMSCs and EPCs interaction plays an important role in IUA angiogenesis [9,13], and PI3K/Akt/Cox2 axis might be critical for BMSCs and EPCs interaction mediated angiogenesis [25]. Therefore, we speculated that BMSCs and EPCs interaction might alleviate IUA pathogenesis by modulating PI3K/Akt/Cox2 axis mediated angiogenesis, but the detailed mechanism is still unclear.
It has been reported that BMSCs promotes EPCs proliferation [9] and differentiation [10], EPCs also have the similar impacts on BMSCs in the BMSCs and EPCs co-culturing system [13]. Besides, increased angiogenesis associated cytokines secretion was also observed in the system [11,12]. In this study, we confirmed that co-culturing BMSCs with EPCs significantly increases proliferative ability of both cells and cell cycle associated proteins including Cyclin D1, Cyclin E2, CDK2, CDK4 and CDK6 are upregulated in the co-culturing system comparing to BMSCs or EPCs alone groups. Intrugingly, the ratio of BMSCs and EPCs is crucial for cell proliferation, which indicated that BMSCs and EPCs interaction promotes cell proliferation in a ratio dependent manner. In addition, we proved that the co-culturing system protects both BMSCs and EPCs from cell apoptosis.
Since both BMSCs and EPCs are potential to differentiate and previous study reported that BMSCs and EPCs differentiation participates in angiogenesis [9], we speculated that the co-culturing system might influence cell differentiation. To verify our hypothesis, BMSCs and EPCs were separated and purified in the transwell system, FCM results showed that the co-culturing system increases epithelial cell markers including CK18, VIM and ESR1 in BMSCs, in addition, the markers of vascular endothelial cell containing VEGFA, VEGFR2 and VEGFR3 are increased in EPCs in the co-culturing system. We also observed that the angiogenesis associated cytokines including VEGF, GM-CSF, Collagen type I and BMP-2 are upregulated in the medium of the co-culturing system. These results suggested that the co-culturing system induces BMSCs and EPCs into epithelial cell and vascular endothelial cell respectively, promotes angiogenesis associated cytokines secretion, which synergistically promotes angiogenesis and alleviates IUA pathogenesis.
We next investigated the possible mechanism of BMSCs and EPCs interaction mediated modulation of cell proliferation, apoptosis and angiogenesis associated cytokines secretion. The results showed that BMSCs and EPCs interaction activates PI3K/Akt signal pathway in both cells, and Cox2 is verified as the downstream target of this signal pathway, which indicated that the co-culturing system activates PI3K/Akt/Cox2 axis in both BMSCs and EPCs. Our further results showed that the proliferative ability of both cells in the co-culturing system is significantly inhibited by PI3K/Akt pathway inhibitor LY294002, which are reversed by synergistically overexpressing Cox2, which indicated that BMSCs and EPCs interaction promotes cell proliferation by activating PI3K/Akt/Cox2 axis. Besides, we also found that LY294002 promotes both BMSCs as well as EPCs apoptosis, and inhibits angiogenesis associated cytokines in the culture medium of the co-culturing system. These results indicated that BMSCs and EPCs interaction regulates cell proliferation, apoptosis and angiogenesis secretion by activating PI3K/Akt/Cox2 axis in both BMSCs and EPCs.
Although we have proved that PI3K/Akt/Cox axis is crucial for BMSCs and EPCs interaction, the communication methods of the two cells in the co-culturing system are still unclear. In our study, we cultured BMSCs and EPCs in the transwell system separately, which verified that BMSCs interacts with EPCs in a cell-cell contact independent manner. Previous study has reported that EPCs interacts with BMSCs by secreting EPCs derived extracellular vesicles in the co-culturing system [13], which is in line with our results. However, whether the two cells interacts with each other in a cell-cell contact dependent manner is still need to be elucidated.
Taken together, our results proved that BMSCs and EPCs interaction might promote angiogenesis and alleviate IUA pathogenesis by regulating PI3K/Akt/Cox2 axis mediated modulation of cell proliferation, apoptosis, differentiation and angiogenesis associated cytokines secretion.
Disclosure of conflict of interest
None.
References
- 1.Su L, You L, Huang HP. Risk factors of intrauterine adhesion after hysteroscopic resection of endometrial polyps. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39:812–816. doi: 10.3881/j.issn.1000-503X.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Dalton VK, Saunders NA, Harris LH, Williams JA, Lebovic DI. Intrauterine adhesions after manual vacuum aspiration for early pregnancy failure. Fertil Steril. 2006;85:1823.e1–3. doi: 10.1016/j.fertnstert.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Chang Y, Yao S. Role of angiogenesis in endometrial repair of patients with severe intrauterine adhesion. Int J Clin Exp Pathol. 2013;6:1343–50. [PMC free article] [PubMed] [Google Scholar]
- 4.Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, Hosseini SE, Falah N, Abbasi F, Baharvand H, Aghdami N. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: a safety study with 18 months of follow-up. Cytotherapy. 2018;20:660–669. doi: 10.1016/j.jcyt.2018.02.368. [DOI] [PubMed] [Google Scholar]
- 5.Jevotovsky DS, Alfonso AR, Einhorn TA, Chiu ES. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthritis Cartilage. 2018;26:711–729. doi: 10.1016/j.joca.2018.02.906. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda T, Takami T, Sasaki R, Nishimura T, Aibe Y, Paredes BD, Quintanilha LF, Matsumoto T, Ishikawa T, Yamamoto N, Tani K, Terai S, Taura Y, Sakaida I. A canine liver fibrosis model to develop a therapy for liver cirrhosis using cultured bone marrow-derived cells. Hepatol Commun. 2017;1:691–703. doi: 10.1002/hep4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Ju B, Pan C, Gu Y, Zhang Y, Sun L, Zhang B, Zhang Y. Application of bone marrow-derived mesenchymal stem cells in the treatment of intrauterine adhesions in rats. Cell Physiol Biochem. 2016;39:1553–60. doi: 10.1159/000447857. [DOI] [PubMed] [Google Scholar]
- 8.Xu HL, Xu J, Zhang SS, Zhu QY, Jin BH, ZhuGe DL, Shen BX, Wu XQ, Xiao J, Zhao YZ. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017;24:867–881. doi: 10.1080/10717544.2017.1333173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L, Zhao X, He B, Jiang J, Xie XJ, Liu L. The possible roles of biological bone constructed with peripheral blood derived EPCs and BMSCs in osteogenesis and angiogenesis. Biomed Res Int. 2016;2016:8168943. doi: 10.1155/2016/8168943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Zhang W, He G, Sha H, Quan Z. Method for in vitro differentiation of bone marrow mesenchymal stem cells into endothelial progenitor cells and vascular endothelial cells. Mol Med Rep. 2016;14:5551–5555. doi: 10.3892/mmr.2016.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider KH, Aziz S, Al-Reshidi MA. Endothelial progenitor cells for cellular angiogenesis and repair: lessons learned from experimental animal models. Regen Med. 2017;12:969–982. doi: 10.2217/rme-2017-0074. [DOI] [PubMed] [Google Scholar]
- 12.Ke X, Yang D, Liang J, Wang X, Wu S, Wang X, Hu C. Human endothelial progenitor cell-derived exosomes increase proliferation and angiogenesis in cardiac fibroblasts by promoting the mesenchymal-endothelial transition and reducing high mobility group box 1 protein B1 expression. DNA Cell Biol. 2017;36:1018–1028. doi: 10.1089/dna.2017.3836. [DOI] [PubMed] [Google Scholar]
- 13.Qin Y, Zhang C. Endothelial progenitor cellderived extracellular vesiclemeditated celltocell communication regulates the proliferation and osteoblastic differentiation of bone mesenchymal stromal cells. Mol Med Rep. 2017;16:7018–7024. doi: 10.3892/mmr.2017.7403. [DOI] [PubMed] [Google Scholar]
- 14.Siegfried JM, Gubish CT, Rothstein ME, Queiroz de Oliveira PE, Stabile LP. Signaling pathways involved in cyclooxygenase-2 induction by hepatocyte growth factor in non small-cell lung cancer. Mol Pharmacol. 2007;72:769–79. doi: 10.1124/mol.107.034215. [DOI] [PubMed] [Google Scholar]
- 15.Su B, Chen S. Lead optimization of COX-2 inhibitor nimesulide analogs to overcome aromatase inhibitor resistance in breast cancer cells. Bioorg Med Chem Lett. 2009;19:6733–5. doi: 10.1016/j.bmcl.2009.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Huang L, Jin J, Wu XJ, Fang YQ, Song MB, Zhao G, Zhao XH, Zhou YP. The role of cyclooxygenase on angiogenesis and endothelial progenitor cell mobilization in rat ischemic myocardium. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:833–6. [PubMed] [Google Scholar]
- 17.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 18.Chen YJ, Li HY, Chang YL, Yuan CC, Tai LK, Lu KH, Chang CM, Chiou SH. Suppression of migratory/invasive ability and induction of apoptosis in adenomyosis-derived mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil Steril. 2010;94:1972–9. 1979.e1–4. doi: 10.1016/j.fertnstert.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 19.Geesala R, Dhoke NR, Das A. Cox-2 inhibition potentiates mouse bone marrow stem cell engraftment and differentiation-mediated wound repair. Cytotherapy. 2017;19:756–770. doi: 10.1016/j.jcyt.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 20.Rudzitis-Auth J, Nickels RM, Menger MD, Laschke MW. Inhibition of cyclooxygenase-2 suppresses the recruitment of endothelial progenitor cells in the microvasculature of endometriotic lesions. Am J Pathol. 2018;188:450–460. doi: 10.1016/j.ajpath.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Santhanam AV, Smith LA, He T, Nath KA, Katusic ZS. Endothelial progenitor cells stimulate cerebrovascular production of prostacyclin by paracrine activation of cyclooxygenase-2. Circ Res. 2007;100:1379–88. doi: 10.1161/01.RES.0000265848.55035.5d. [DOI] [PubMed] [Google Scholar]
- 22.Jia H, Wang H, Yao Y, Wang C, Li P. MiR-136 inhibits malignant progression of hepatocellular carcinoma cells by targeting cyclooxygenase 2. Oncol Res. 2018;26:967–976. doi: 10.3727/096504018X15148192843443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YX, Li YZ, Zhang ZY, Wang JQ, Cui J, Qian XL. HPV16 E6 promotes breast cancer proliferation via upregulation of COX-2 expression. Biomed Res Int. 2017;2017:2948467. doi: 10.1155/2017/2948467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuang W, Deng Q, Deng C, Li W, Shu S, Zhou M. Hepatocyte growth factor induces breast cancer cell invasion via the PI3K/Akt and p38 MAPK signaling pathways to up-regulate the expression of COX2. Am J Transl Res. 2017;9:3816–3826. [PMC free article] [PubMed] [Google Scholar]
- 25.Sui W, Zhang Y, Wang Z, Wang Z, Jia Q, Wu L, Zhang W. Antitumor effect of a selective COX-2 inhibitor, celecoxib, may be attributed to angiogenesis inhibition through modulating the PTEN/PI3K/Akt/HIF-1 pathway in an H(2)(2) murine hepatocarcinoma model. Oncol Rep. 2014;31:2252–60. doi: 10.3892/or.2014.3093. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Fu S, Hou T, Wu X. Endothelial progenitor cells enhance the migration and osteoclastic differentiation of bone marrow-derived macrophages in vitro and in a mouse femur fracture model through Talin-1. Cell Physiol Biochem. 2018;49:555–564. doi: 10.1159/000492993. [DOI] [PubMed] [Google Scholar]