Abstract
Septic acute kidney injury (AKI) characterized as acute infection and renal inflammation, still lacks of effective therapies. Isoliquiritigenin (ISL) as a small molecular from licorice, is able to inhibit the expression of HMGB1. However, the role and mechanism of ISL in septic AKI has not been investigated. In this study, we used LPS injection to induce murine septic AKI. One hour before LPS injection, 50 mg/kg ISL was once orally given to the mice. For the in vitro study, HK2 human tubular cells were respectively treated with 50 μM and 100 μM ISL 5 hrs before 2 μg/ml LPS stimulation. Then we observed that ISL ameliorated renal dysfunction and attenuated renal tubular injury. ISL inhibited the phosphorylation of IκB-α and NF-κB p65 after LPS induction both in vivo and in vitro. ISL also inhibited NF-κB p65 translocation from cytoplasm to the nucleus upon LPS stimulation. Further, NF-κB p65 translocation could trigger macrophage polarization, neutrophil activation and pro-inflammatory cytokines secretion in LPS-induced inflammation. These results showed that ISL could alleviate LPS-induced AKI by suppressing NF-κB p65 translocation and inhibiting inflammatory responses, indicating protective effects of ISL in LPS-induced acute renal inflammation. This study might be useful for designing potential clinical trials to prevent and treat sepsis induced AKI in patients with serious illness.
Keywords: Septic acute kidney injury, isoliquiritigenin, NF-κB p65, inflammation response
Introduction
Sepsis, as a life-threatening organ dysfunction, can induce acute kidney injury (AKI) and cause the mortality of patients in the intensive care unit [1]. However, due to the undefined pathophysiological mechanism of AKI in sepsis [2,3], there is still a lack of preventive therapy in early stage.
Isoliquiritigenin (ISL), isolated from the roots of licorice, is one of the flavonoid components from licorice (Figure 1A) [4,5]. Licorice is a well-known Chinese traditional herb used for centuries. Among 300 kinds of flavonoids from licorice, ISL was considered to be a potent candidate with anti-inflammatory and immunomodulatory effect on the digestive system and cardiovascular system [4-7]. Recent studies reported that ISL could activate PPAR-γ [8] and inhibit Nuclear factor κ light chain enhancer of activated B cells (NF-κB) pathway [9] to protect the lung organ against acute pulmonary injury. It is also thought to be a potent Nrf2 inducer [10,11]. These studies indicate ISL has anti-oxidant and anti-inflammatory effects on major organs. Unfortunately, the role of ISL in sepsis induced AKI is still unknown.
Figure 1.
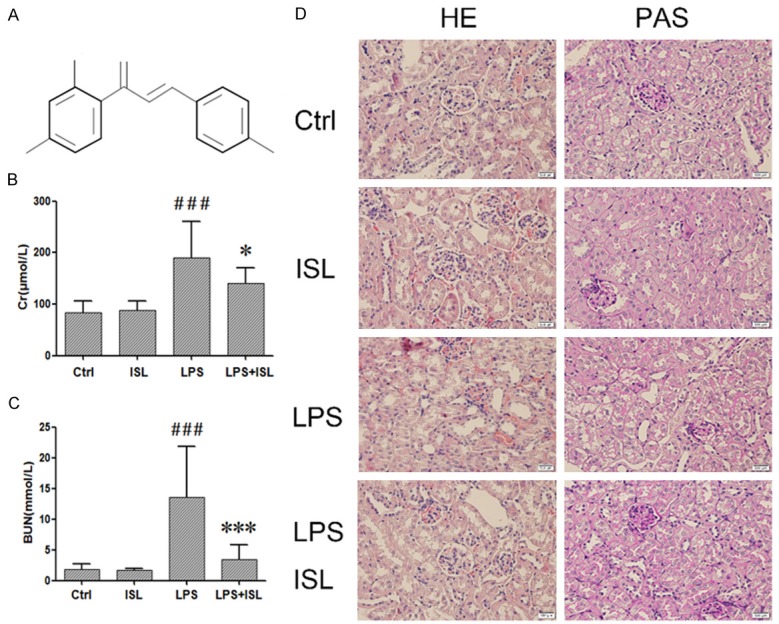
ISL ameliorated LPS-induced AKI renal dysfunction in mouse model. A. The formula of Isoliquiritigenin (ISL). B and C. LPS increased the CREA and BUN which represent serious kidney inflammation. ISL can decrease CREA and BUN to protect renal function. The level of CREA and BUN was measured by assay kit. D. Histological changes were assessed using HE and PAS staining. LPS treatment induced severe renal pathological lesions compared to normal control mice. In contrast, ISL treatment reduced the pathological features in murine kidney (magnification, ×400); *P < 0.05 vs. the LPS group, ***P < 0.001 vs. the LPS group, ###P < 0.001 vs. the Ctrl group.
High mobility group protein-1 (HMGB-1), belonging to high mobility protein group, contains HMG-box domain to regulate cellular inflammatory response [12,13]. It specifically binds to Toll like receptor-4 (TLR-4) and then stimulates NF-κB to induce the secretion of pro-inflammatory cytokine [14]. TNF-α dependent secretion of HMGB-1 could be induced by HDAC activation in systemic inflammation and multiple organ failure during AKI [15]. ISL was reported to reduce the secretion of HMGB-1 activated by HDAC against inflammatory bowel disease [16]. Though HMGB-1 could be considered as a novel biomarker for AKI [17], due to a lack of early effective therapy about HMGB-1 for AKI patients, the role and mechanism of ISL upon HMGB-1 in septic AKI still need to be further elucidated. In our study, we explored the protective role of ISL in LPS-induced AKI, associated with its interference to innate immune and inflammation. This finding might be useful for designing potential clinical trials to prevent and treat sepsis induced AKI in critically ill patients.
Material and methods
Chemicals and reagents
Isoliquiritigenin was purchased from Med-ChemExpress, USA (10208, MCE, USA) (Figure 1A). Escherichia coli O111:B4 LPS was purchased from Sigma-Aldrich (L4130, Sigma, USA).
Cell culture and treatment
The human kidney proximal tubular cell line HK2 was obtained from the American Type Culture Collection. According to the manufacturer’s instructions, cells was cultured in DME/F-12 (SH30023.01, HyClone, USA), supplemented with 10% FBS (Gibco, Life Technologies, Lofer, Austria), 100 units/ml penicillin and 100 units/ml streptomycin (1705694, HyClone) at 37°C in a humidified atmosphere condition of 95% air and 5% carbon dioxide. Cells were divided into 6 groups. They were control, ISL 50 (50 μM), ISL 100 (100 μM), LPS (2 μg/ml), LPS plus ISL 50 (50 μM) and LPS plus ISL 100 (100 μM). ISL were respectively treated with two concentrations (50 μM/100 μM) for 5 hrs. And then induced the cells with 2 μg/ml LPS.
Animals and LPS-induced AKI model
Male, six to eight-week-old C57BL/6 mice were provided by Experimental Animal Center of Sichuan Provincial People’s Hospital. Mice were treated with a standard laboratory diet. LPS was dissolved into normal saline. And ISL was dissolved into 0.5% Tween-20/saline. All the mice were randomly divided into four groups (n=10): control, ISL, LPS and ISL plus LPS group. LPS was i.p. injected at a dosage of 10 mg/kg. For ISL treatment, 50 mg/kg ISL was given to the mice via gavage before LPS injection. Mice were sacrificed at 8th hr after LPS injection. At the same time, the kidney and serum samples were collected.
Renal function assessment
Blood samples were collected when mice were sacrificed. Serum was separated by 3,000 rpm for 5 min at 4°C. Blood urea nitrogen (BUN) and serum creatinine (CREA) were detected by BUN Assay Kit (C013-2, Jiancheng, Nanjing, China) and Creatinine Assay Kit (C011-2, Jiancheng, Nanjing, China).
Renal morphological changes
The kidney samples harvested from mice were fixed in formalin at least for 24 hrs and then embedded in paraffin after dehydrated. Two-micrometer sections were respectively stained by PAS staining and Hematoxylin and eosin (HE) staining. The images were captured with microscope under 400×.
Quantitative real-time PCR
The total RNA of HK2 cells and kidney issue were isolated with TRIzol reagent (15596026, Thermo, USA). RNA was reverse-transcribed with the PrimeScript RT reagent kit (#RR037A, TaKaRa, Japan) following the manufacturer’s instruction. The analysis of gene expression was performed by the 7500 Fast Real Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Premix EX TaqTM II (RR820, TaKaRa). The reaction volume was 20 μl which contained 0.8 μmol/L primers, and 2 μL of template cDNA. Thermal cycling was 30 s at 95°C, followed by 40 cycles of 5 s at 95°C, 30 s at 55°C and 30 s at 72°C. The changes of mRNA levels were normalized by the levels of the control (GAPDH). The information of primers is listed in Table 1.
Table 1.
Primers of RT-PCR analysis (5’→3’)
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | AGGCACCAAGATACTTACAAAAAC | GGTGGTGAACTTGTTTTGCGA |
| IL-6 | CACTTCACAAGTCGGAGGCT | TCTGACAGTGCATCATCGCT |
Western blot
The total proteins of kidney tissue and HK2 cells was lysed by a RIPA lysis buffer (#P0013B, Beyotime, Shanghai, China). The nuclear protein and cytoplasmic protein were obtained by the Nuclear and Cytoplasmic Protein Extraction kit (CW0199s, CWBIO, China). Proteins were loaded onto SDS-PAGE and transferred to polyvinylidene difluoride membranes (R7CA6580A, Thermo Fisher Scientific, USA). The membranes were blotted with NF-κB p65 (1:1000, 66418-1-lg, Proteintech, China), phosphorylated NF-κB pp65 (1:1000, ab86299, abcam, USA), IκB-α (1:1000, 380682, Zen BioScience, China), p-IκB (1:1000, ab133462, abcam), HMGB-1 (1:1000, ab18256, abcam), PCNA (1:1000, 10205-2-AP, Proteintech). And then, the membranes were respectively incubated with HRP labeled goat anti-rabbit IgG (1:5000, 511203, Zen BioScience) and, HRP labeled goat anti-mouse IgG (1:5000, 511103, Zen BioScience). The bands were detected with enhanced chemiluminescence (1701102, MILLIPORE, USA). The total protein levels were normalized by β-actin (1:5000, HRP-60008, Proteintech). The nuclear protein was normalized by PCNA (1:1000, 10205-2-AP, Proteintech).
Immunohistochemistry
Renal tissues were from the formalin-fixed, paraffin-embedded, and cut into 2 μm. The tissues were subjected to immunohistochemical staining for CD68 (1:200, abcam, ab955), CD206 (1 µg/ml, ab64693, abcam), iNOS (1:100, abcam, ab15323), MPO (1:50, ab9535, abcam) and HMGB1 (1:400, ab18256, abcam). The process was conducted in strict accordance with the kit protocol.
Statistical analysis
The results were shown as normal controls ± SD. Statistical analyses were performed by one-way ANOVA and post hoc test using Graph Pad Prism 5 software for comparison between groups. Newman-Keuls multiple comparison test was used to compare differences. P < 0.05 indicated statistically significant.
Result
ISL ameliorated renal dysfunction in LPS-induced AKI murine model
To observe the therapeutic effect of ISL on renal function against LPS-induced AKI in vivo, mice were divided randomly into control, ISL, LPS and LPS plus ISL groups (n=10). Blood urea nitrogen (BUN) and serum creatinine (CREA) were detected after mice were sacrificed. LPS increased the levels of CREA and BUN (Figure 1B and 1C). The mice in LPS plus ISL group had reduced levels of CREA and BUN (Figure 1B and 1C). In addition, HE staining and PAS staining both showed that LPS led to severe histological injury particularly in terms of moderate/severe degree to tubular necrosis, compare to that of normal control group. In contrast, ISL treatment ameliorated the injury of renal tubular epithelial cells, and reduced the injury degree to mild. Moreover, ISL had no effect to normal mice (Figure 1D). It indicated that ISL significantly ameliorated LPS-induced renal function.
ISL inhibited the secretion of inflammatory factors, macrophage polarization and neutrophil activation
We determined these factors related to macrophage polarization and neutrophil activation upon inflammatory response of septic AKI by immunohistochemistry. CD68 and iNOS represented M1 macrophage polarization, CD206 represented M2 macrophage polarization and MPO stood for neutrophil activation. These markers were detected in murine kidney. LPS increased the expression of CD68, iNOS and MPO then decreased the expression of CD206 in murine kidney, while ISL reduced the expression of CD68, iNOS and MPO then increased the expression of CD206 in mice kidney after LPS injection (Figure 2).
Figure 2.
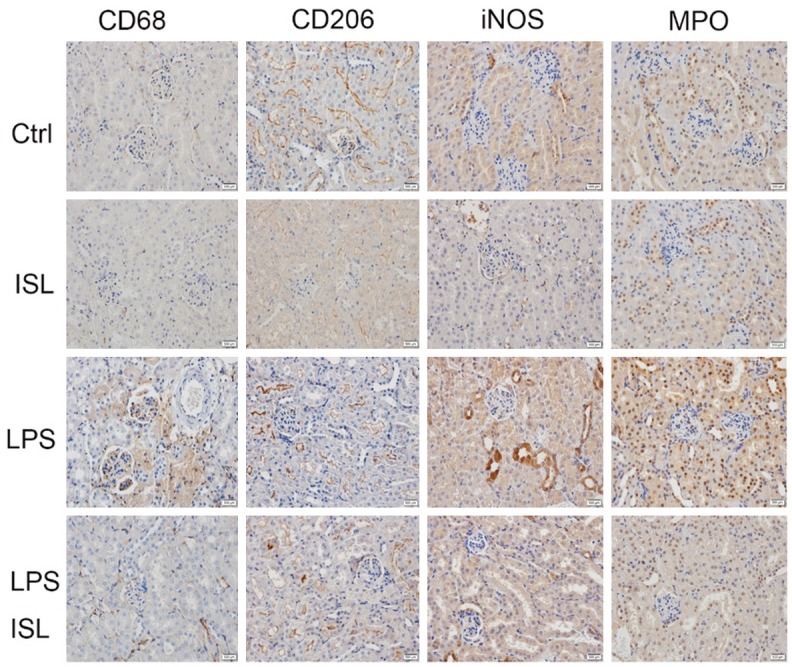
ISL inhibited M1 macrophage polarization and neutrophil activation while stimulated M2 macrophage polarization. The expression of CD68, CD206, iNOS, MPO was determined by immunohistochemistry in four animal groups. LPS increased the expression of CD68, iNOS and MPO but decreased the expression of CD206 in murine kidney, while ISL reduced the expression of CD68, iNOS and MPO then increased the expression of CD206 in mice kidney after LPS injection. (magnification, ×400).
ISL downregulated expressions of IL-6 and HMGB-1 in LPS-induced AKI murine model and renal tubular epithelium cells
To investigate the effect of ISL on IL-6 both in vivo and in vitro, the level of IL-6 in the murine kidney tissue and renal tubular epithelium cells were measured by RT-PCR. As shown in Figure 3A and 3B, the mRNA level of IL-6 was increased in LPS induction both in vitro and in vivo, whereas ISL reduced the expression of IL-6 after LPS stimulation, compared to that of normal control group (Figure 3A and 3B). The immunohistochemistry shows LPS upregulated HMGB-1 but ISL reduced the expression of HMGB-1 (Figure 3C). Western blot showed that LPS increased the expression of HMGB1. However, ISL inhibited the expression of HMGB-1 following LPS induction both in vitro and in vivo (Figure 3D and 3G).
Figure 3.
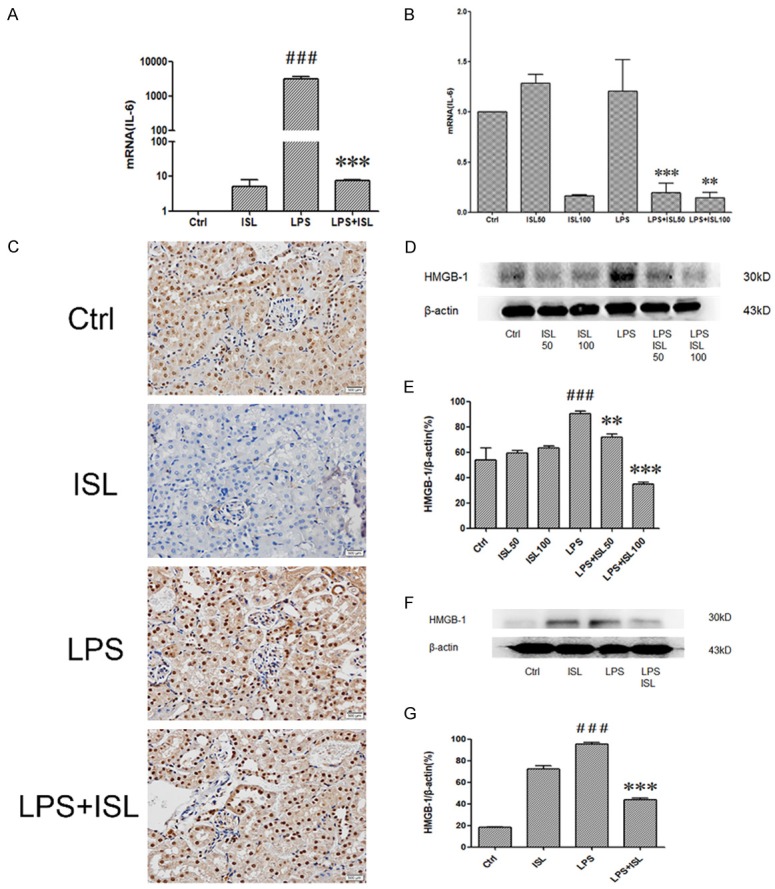
ISL downregulated expressions of IL-6 and HMGB-1 both in vivo and in vitro. A and B. The levels of mRNA of IL-6 were determined by quantitative Real-Time PCR. LPS increased IL-6 significantly while ISL downregulated the levels of IL-6. The samples were respectively isolated from cells and mouse. C. Immunohistochemistry was performed to detect HMGB-1 in mouse. LPS activated HMGB-1 expression in glomerulus and kidney tubules. ISL reduced the expression of HMGB-1 especially in renal tubular epithelial cells. (magnification, ×400). D, E. Total protein of cells was tested for HMGB-1 by western blot. Quantification of western blotting gel images was presented in graphical form. ISL with two concentrations in cells both can decrease expression of HMGB-1. F, G. Total kidney tissue protein of mouse was tested for HMGB1 by western blot. ISL reduced HMGB-1 expression after LPS-induced overexpression. **P < 0.01 vs. the LPS group, ***P < 0.001 vs. the LPS group, ###P < 0.001 vs. the Ctrl group.
ISL inhibited phosphorylation of IκB-α following LPS stimulation
To explore the mechanism of ISL, we detected phosphorylation of IκB-α by western blotting. LPS caused phosphorylation of IκB-α both in vitro and in vivo. However, ISL (50 μM/100 μM) inhibited phosphorylation of IκB-α after LPS induction both in vitro and in vivo (Figure 4A and 4C). The density analyzed according to the western blotting results also showed the anti-phosphorylation IκB-α role in LPS induction both in vitro and in vivo (Figure 4B and 4D).
Figure 4.
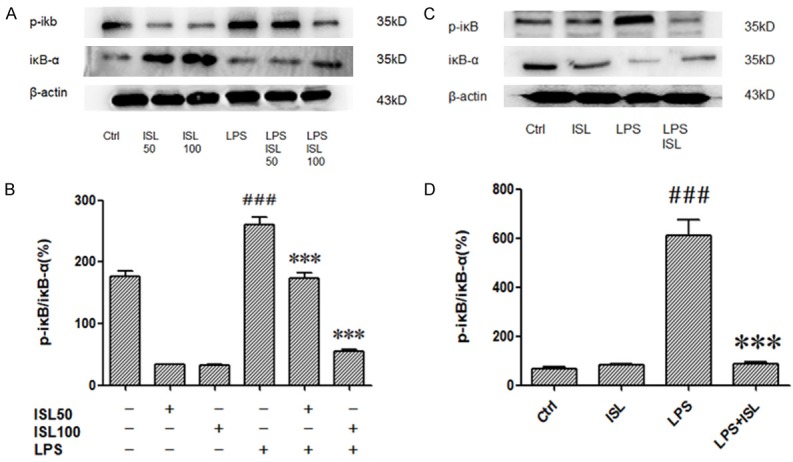
ISL inhibited phosphorylation of IκB-α following LPS stimulation. The expressions of IκB-α and p-IκB were measured by western blot. A and C. The samples were harvested from cells and mice. The results showed that ISL could effectively decreased protein levels of p-IκB in HK2 of the LPS-induced mice but increased IκB-α. B and D. Graphical forms show quantification of Western blot analysis images was performed using Image J software. ***P < 0.001 vs. the LPS group, ###P < 0.001 vs. the Ctrl group.
ISL inhibited phosphorylation of NF-κB p65 upon LPS administration
We measured the level of phosphorylation of NF-κB p65 in the whole cell through western blotting. Then we observed that LPS induction could increase phosphorylation of NF-κB p65 both in human renal tubular cells and murine renal tissue, whereas ISL inhibited phosphorylation of NF-κB p65 upon LPS stimulation (Figure 5A and 5C). The density measurement about phosphorylation of NF-κB p65 in the whole cell lysis also identified the same phenomena (Figure 5B and 5D). As the pivotal role of NF-κB p65 translocation, we further detected the level of phosphorylation of NF-κB p65 in the nuclear then we found that LPS induction could increase phosphorylation of NF-κB p65 in the nuclear extract both in human renal tubular cells and murine renal tissue, whereas ISL significantly inhibited phosphorylation of NF-κB p65 upon LPS stimulation (Figure 5E and 5G). The density measurement about phosphorylation of NF-κB p65 in the nuclear extract also identified the same phenomena (Figure 5F and 5H).
Figure 5.
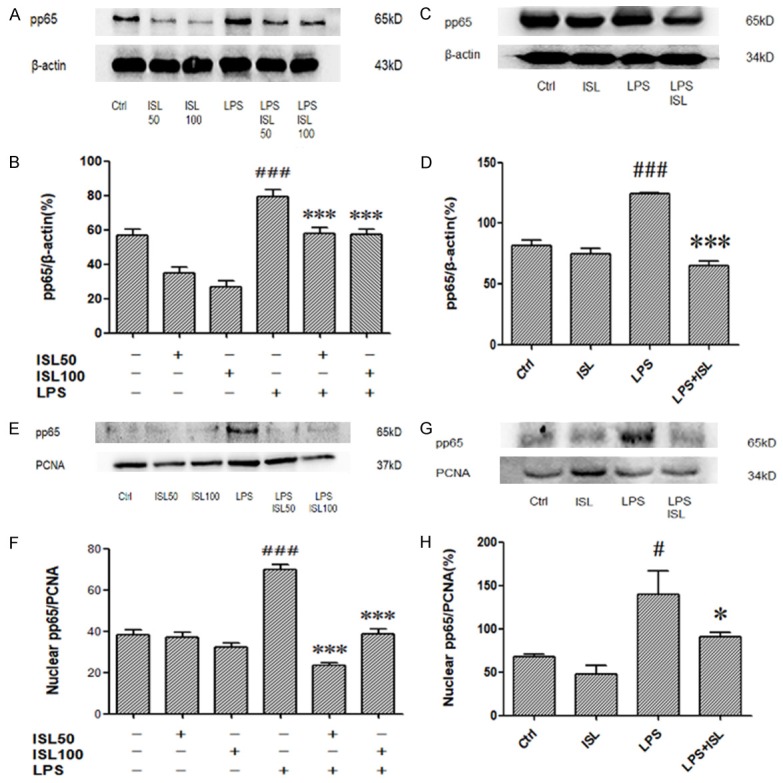
ISL reduced phosphorylation of NF-κB p65 upon LPS administration. A-D. Effects of ISL on pp65 in total were measured by western blot. LPS increased the expression of pp65 in total. ISL treatment can decrease pp65 both in mice and cells. E-H. Effects of ISL on pp65 in nuclear were measured by western blot. There is no significant difference between ISL group and Ctrl group. But ISL can decrease pp65 in nuclear on LPS-induced mouse model. *P < 0.05 vs. the LPS group, ***P < 0.001 vs. the LPS group, #P < 0.001 vs. the Ctrl group, ###P < 0.001 vs. the Ctrl group.
ISL did not induce pathological injury
We also treated mice with 50 mg/kg ISL alone. Then we did not observe significantly pathological difference of heart, lung, liver, spleen and kidney between mice in ISL treated alone group and normal control group (Figure 6A).
Figure 6.
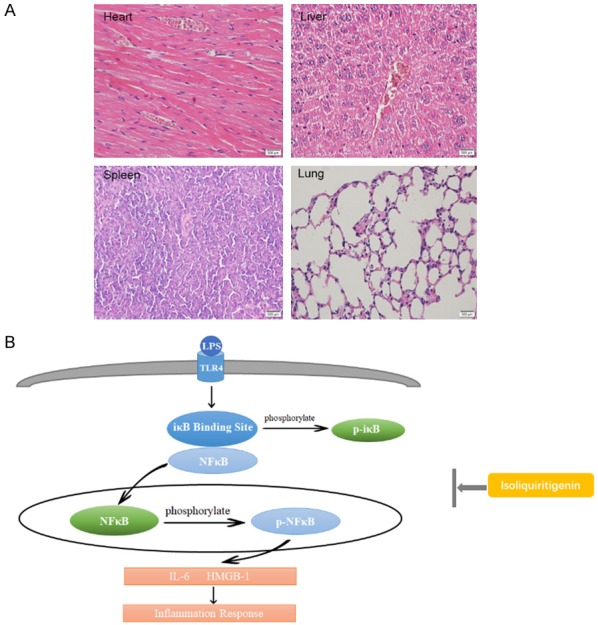
A. The HE staining of heart, liver, spleen and lung. The main organs of mice have no appearance of injury. ISL did not lead to pathological injury. The sample was from mice with ISL given alone. B. The diagram of mechanism we explored in this study. When LPS induce inflammation, iκB and NF-κB (p65) departs each other. IκB is phosphorylated in cytoplasm and p65 translocate into nuclear to be phosphorylated. At the same time, various pro-inflammatory cytokines are produced, including IL-6, HMGB-1. The possible mechanism of ISL is inhibit the translocation of NF-κB p65 and the phosphorylation of IκB and pp65.
Discussion
To demonstrate the effects of ISL on AKI induced by sepsis, we established murine AKI model by LPS injection which used in many researches [18-20]. In pathologic process of LPS-induced AKI, vacuolization could be found in renal tubular cells, indicating the moderate/severe renal injury. LPS injection also significantly increased the level of serum CREA and BUN. However, we observed that ISL treatment ameliorated renal dysfunction through reducing to the level of serum CREA and BUN. It also significantly attenuated the renal tubular injury following LPS induction. These results suggested the potent role of ISL against septic AKI.
The NF-κB pathway plays an essential role in the expression of pro-inflammatory cytokines in innate immunity [21]. The transcription factors NF-κB are the homodimers or heterodimers of those subunits. The NF-κB family has 5 subunits: p65 (Rel A), Rel B, c-Rel, p50 and p52 [22]. Among them, p65 is a central issue. In unstimulated status, NF-κB p65 locates in the cytoplasm and binds to the IκB-α which prevents the translocation of NF-κB p65 into the nuclear [23-25]. When stimulated by LPS, IκB-α is phosphorylated and the IκB-α departs from NF-κB p65. The free NF-κB p65 translocate into the nu-clear and then induced the expression of pro-inflammatory cytokines and chemokines [26,27]. Current study shows that LPS increased the level of phosphorylated IκB-α after LPS stimulation in both mu-rine renal tissue and HK2 human renal tubular cell line, whereas ISL inhibits the phosphorylation of IκB-α upon LPS induction both in vivo and in vitro. LPS increased the expression of NF-κB p65 in the nuclear in both murine renal tissue and human renal tubular cells, whereas ISL re-duced the level of NF-κB p65 in the nuclear following LPS treatment in both murine renal tissue and human renal tubular cells. The results illustrate that ISL could inhibit the phosphorylation of IκB-α and translocation of NF-κB p65 in septic AKI.
Once NF-κB p65 translocated into the nuclear, it triggers macrophage polarization, neutro-phil activation and pro-inflammatory cytokines secretion, especially HMGB-1 and IL-6 [28-31]. We observed that LPS injection increased the expression of CD68 and iNOS then reduced the expression of CD206 in murine kidney, suggesting rise of M1 type macrophage and reduction of M2 type macrophage. LPS injection also increased the expression of Myeloperoxidase (MPO) in murine renal tissue, indicating activation of neutrophil induced by LPS. However, ISL inhibited the macrophage polarization to M1 type macrophage by reduction of CD68 and iNOS then increasing of CD206. ISL also inactivated neutrophil activation led by LPS injection. Further, as reported by the protective role of its homologous derivative glycyrrhizin against HMGB-1 and IL-6 secretion in kidney [32], ISL decreased the expression of HMGB-1 and IL-6 after LPS treatment in both murine renal tissue and human renal tubular cells. This elucidates the protective role of ISL against macrophage polarization, neutrophil activation and pro-inflammatory cytokines secretion triggered by NF-κB p65 translocation. High dosage of ISL might induce apoptosis in human bladder cancer cells, indicating the possible toxicity of ISL [33]. Previous studies also use different dose of ISL in other aspects, such as psoriasis, breast cancer and COPD [9,34-36]. The delivery way and dosage of ISL depends on the particular cases. In our study, we use 50 mg/kg for oral administration to mice and 50 μM/100 μM to HK2 cells. The dosage was according to our cytotoxicity test by CCK-8 and we found the dosage was safe (Figure S1). We used 50 mg/kg ISL for once oral administration to mice and 50 μM/100 μM ISL to treat HK2 cells for 5 hrs. The use of ISL was not frequent and the time is not long. Maybe that is why the high dosage of ISL was safe and effective for septic AKI. Furthermore, we observed that the oral use of 50 mg/kg ISL did not impair the essential organs in mice including the heart, liver, spleen and lung (Figure 6A). Accordingly, the dosage of ISL we used was reasonable and safe.
Consequently, our study reveals that ISL alleviated the outcome of LPS-induced AKI such as the aggravated the inflammatory response (Figure 6B). In particular, we revealed protective role of ISL against NF-κB p65 translocation in LPS induced acute renal inflammation.
Acknowledgements
Authors thank Prof. Shaoping Deng and Prof. Zhenglin Yang in University of Electronic Science and Technology, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital for generously providing research platforms and technical support. This work was supported by National Natural Science Foundation of China (81770742, 81700607 and 81800613), Youth Science and Technology Creative Research Groups of Sichuan Province (2015TD0013), PHD Foundation of Sichuan Academy of Sciences & Sichuan Provincial People’s Hospital (2015BS05), China Postdoctoral Science Foundation Funded Project (2016M592672), Foundation of Health and family planning commission in Sichuan (16PJ424 and 17PJ060). Central university fund from UESTC (ZYGX2016J176) and Technology Supportive Plan of Sichuan Province (2015SZ0245). Sichuan Science and Technology Innovation Miaozi Project (2016063).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2017;14:121. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 2.Fani F, Regolisti G, Delsante M, Cantaluppi V, Castellano G, Gesualdo L, Villa G, Fiaccadori E. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J Nephrol. 2018;31:351–359. doi: 10.1007/s40620-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 3.Zarbock A, Gómez H, Kellum J. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20:588–95. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Yang R, Yuan B, Liu Y, Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B. 2015;5:310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng F, Du Q, Peng C, Wang N, Tang H, Xie X, Shen J, Chen J. A review: the pharmacology of isoliquiritigenin. Phytother Res. 2015;29:969–977. doi: 10.1002/ptr.5348. [DOI] [PubMed] [Google Scholar]
- 6.Gaur R, Yadav KS, Verma RK, Yadav NP, Bhakuni RS. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine. 2014;21:415–422. doi: 10.1016/j.phymed.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura S, Watanabe T, Tanigawa T, Shimada S, Nadatani Y, Miyazaki T, Iimuro M, Fujiwara Y. Isoliquiritigenin ameliorates indomethacin-induced small intestinal damage by inhibiting NOD-like receptor family, pyrin domain-containing 3 inflammasome activation. Pharmacology. 2018;101:236–245. doi: 10.1159/000486599. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Wang G, Zhou S. Protective effects of isoliquiritigenin on LPS-induced acute lung injury by activating PPAR-gamma. Inflammation. 2018;41:1290–1296. doi: 10.1007/s10753-018-0777-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Lv H, Wen Z, Ci X, Peng L. Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-kappaB pathway in macrophages and in acute lung injury. Front Immunol. 2017;8:1518. doi: 10.3389/fimmu.2017.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou Z, Chen L, Fang P, Cai H, Tang H, Peng Y, Deng Y, Cao L, Li H, Zhang B, Yan M. Mechanisms of triptolide-induced hepatotoxicity and protective effect of combined use of isoliquiritigenin: possible roles of nrf2 and hepatic transporters. Front Pharmacol. 2018;9:226. doi: 10.3389/fphar.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji B, Guo W, Ma H, Xu B, Mu W, Zhang Z, Amat A, Cao L. Isoliquiritigenin suppresses IL-1β induced apoptosis and inflammation in chondrocyte-like ATDC5 cells by inhibiting NF-κB and exerts chondroprotective effects on a mouse model of anterior cruciate ligament transection. Int J Mol Med. 2017;40:1709–1718. doi: 10.3892/ijmm.2017.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao Z, Zhang N, Xu N, Pan Y, Xiao M, Wu J, Zhou H, Yang S, Chen Y. Corrigendum: 1,25-dihydroxyvitamin D inhibits LPS-induced high-mobility group box 1 (HMGB1) secretion via targeting the NF-E2-related factor 2-hemeoxygenase-1-HMGB1 pathway in macrophages. Front Immunol. 2018;9:357. doi: 10.3389/fimmu.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280:74–82. doi: 10.1111/imr.12601. [DOI] [PubMed] [Google Scholar]
- 14.Richard SA, Jiang Y, Xiang LH, Zhou S, Wang J, Su Z, Xu H. Post-translational modifications of high mobility group box 1 and cancer. Am J Transl Res. 2017;9:5181–5196. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang R, Zou X, Tenhunen J, Tønnessen T. HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure. Mediators Inflamm. 2017;2017:5928078. doi: 10.1155/2017/5928078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi JH, Seo GS, Cheon JH, Lee SH. Isoliquiritigenin inhibits TNF-alpha-induced release of high-mobility group box 1 through activation of HDAC in human intestinal epithelial HT-29 cells. Eur J Pharmacol. 2017;796:101–109. doi: 10.1016/j.ejphar.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Oh SM, Park G, Lee SH, Seo CS, Shin HK, Oh DS. Assessing the recovery from prerenal and renal acute kidney injury after treatment with single herbal medicine via activity of the biomarkers HMGB1, NGAL and KIM-1 in kidney proximal tubular cells treated by cisplatin with different doses and exposure times. BMC Complement Altern Med. 2017;17:544. doi: 10.1186/s12906-017-2055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi M, Zeng X, Guo F, Huang R, Feng Y, Ma L, Zhou L, Fu P. Anti-inflammatory pyranochalcone derivative attenuates LPS-induced acute kidney injury via inhibiting TLR4/NF-kappaB pathway. Molecules. 2017;22 doi: 10.3390/molecules22101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu D, Chen M, Ren X, Ren X, Wu Y. Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia. 2014;97:148–155. doi: 10.1016/j.fitote.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Sun D, Bao Y, Shi Y, Cui Y, Guo M. Nerolidol protects against LPS-induced acute kidney injury via inhibiting TLR4/NF-kappaB signaling. Phytother Res. 2017;31:459–465. doi: 10.1002/ptr.5770. [DOI] [PubMed] [Google Scholar]
- 21.Manthiram K, Zhou Q, Aksentijevich I, Kastner DL. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol. 2017;18:832. doi: 10.1038/ni.3777. [DOI] [PubMed] [Google Scholar]
- 22.Solt LA, Madge LA, May MJ. NEMO-binding domains of both IKKalpha and IKKbeta regulate IkappaB kinase complex assembly and classical NF-kappaB activation. J Biol Chem. 2009;284:27596–27608. doi: 10.1074/jbc.M109.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 24.Kanai Y, Endou H. Functional properties of multispecific amino acid transporters and their implications to transporter-mediated toxicity. J Toxicol Sci. 2003;28:1–17. doi: 10.2131/jts.28.1. [DOI] [PubMed] [Google Scholar]
- 25.Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Chang MD. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang WC, Hung MC. Beyond NF-kappaB activation: nuclear functions of IkappaB kinase alpha. J Biomed Sci. 2013;20:67–72. doi: 10.1186/1423-0127-20-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsokos GC, Wong HK, Enyedy EJ, Nambiar MP. Immune cell signaling in lupus. Curr Opin Rheumatol. 2000;12:355–363. doi: 10.1097/00002281-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Xin X, Fang L, Jiang H, Xu X, Su X, Shi Y. HMGB1 mediates aspergillus fumigatus-induced inflammatory response in alveolar macrophages of COPD mice via activating MyD88/NF-kappaB and syk/PI3K signalings. Int Immunopharmacol. 2017;53:125–132. doi: 10.1016/j.intimp.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Kang OH, Li Z, Mun SH, Seo YS, Kong R, Tian Z, Liu X, Kwon DY. Antiinflammatory effects of Ciwujianoside C3, extracted from the leaves of acanthopanax henryi (Oliv. ) Harms, on LPSstimulated RAW 264.7 cells. Mol Med Rep. 2016;14:3749–3758. doi: 10.3892/mmr.2016.5710. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, Tian J, Gu X, Wang H, Tian J, Yu B. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao H, Li N, Zhang Z, Mu H, Meng C, Xia H, Fu L, Xu Y, Zhang S. Erlotinib protects LPS-induced acute lung injury in mice by inhibiting EGFR/TLR4 signaling pathway. Shock. 2019;51:131–138. doi: 10.1097/SHK.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 32.Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011;339:93–98. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- 33.Si L, Yang X, Yan X, Wang Y, Zheng Q. Isoliquiritigenin induces apoptosis of human bladder cancer T24 cells via a cyclin-dependent kinase-independent mechanism. Oncol Lett. 2017;14:241–249. doi: 10.3892/ol.2017.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Wang N, Han S, Wang D, Mo S, Yu L, Huang H, Tsui K, Shen J, Chen J. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One. 2013;8:e68566. doi: 10.1371/journal.pone.0068566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao L, Liu H, Lam DS, Yam GH, Pang CP. In vitro screening for angiostatic potential of herbal chemicals. Invest Ophthalmol Vis Sci. 2010;51:6658–6664. doi: 10.1167/iovs.10-5524. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33–40. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


