Abstract
A role of microRNA-4262 (miR-4262) in the carcinogenesis of colon cancer remains undetermined. In this study, we studied the effects and mechanisms of miR-4262 to the colon cancer cell proliferation and apoptosis. We found that the levels of miR-4262 significantly down-regulated in colon cancer tissue, compared to the paired adjacent non-tumor colon tissue. The miR-4262 levels in colon cancer cell lines were significantly lower than those in control normal colon tissues. Transfection with the miR-4262 mimic decreased the cell proliferation and increased cell apoptosis in colon cancer cells, while transfection with the antisense of miR-4262 (as-miR-4262) increased cell proliferation and suppressed cell apoptosis in colon cancer cells. Bioinformatics analyses showed that GALNT4 was a potential target gene of miR-4262. The luciferase activities assay and Western blot verified that miR-4262 targeted GALNT4 mRNA to modulate its protein levels. When we treated cells with miR-4262 and GALN4 siRNA, the cell viability was significantly decreased. Together, our study suggests that aberrantly expressed miR-4262 may affect cell apoptosis and proliferation of human colon cancer cells via GALNT4, which appears to be a promising therapeutic target for colon cancer.
Keywords: Colon cancer, miR-4262, GALNT4, proliferation, apoptosis
Introduction
Colon cancer is one of the common malignant tumors in human body. It is the secondary frequent gastroenteric tumor with a high morbidity in China, Europe and America [1]. Each year, more than one million new colon cancer patients are diagnosed in the world [2,3]. In China, with the development of economic and the improvement of people’s living standards and the change of lifestyles and living conditions, the morbidity, the prevalence rate and mortality rates of colorectal cancer are increasing in recent years [4]. The morbidity of colon cancer in China with 4.2% is higher than the international standard of 2% [5]. Nowadays, the combination of operation and chemotherapy is the main treatment of colon cancer. Although the 5 years survival rate of colon cancer of early stage exceed 70-90%, the curative effect is still not satisfying to advanced colon cancer [6]. Only palliative surgery or chemotherapy could be performed to these patients because of the metastasis and extensive invasion of cancer at the time of the diagnosis, even though the comprehensive treatment of operation and chemotherapy is still suitable to the middle and advanced stage of colon cancer patients. However, the clinical effect of this combinative therapy is not satisfying because of the anti-drug sensitivity from cancer cells or serious adverse reactions from chemotherapy.
The miRNAs are evolutionarily conserved short (approximately 18-22 nucleotides) noncoding single-stranded RNA molecules that act as posttranscriptional gene factors [7]. Initially transcribed in the nucleus by RNA polymerase II or III as long primary transcripts (pri-miRNAs), miRNAs are subsequently processed into 70-to 100-nt precursor RNAs (pre-miRNAs) by the microprocessor complex, consisting of the RNase III enzyme Drosha and its interacting partner DGCR8 [8]. This initial cleavage is followed by Exportin-5/RanGTP-mediated pre-miRNA translocation to the cytoplasm for further processing into a 19- to 25-nt duplex by the RNase III endonuclease Dicer and TRBP [9]. The final processing by Dicer is likely to culminate in the assembly of the two strands into the RNA-induced silencing complex (RISC); the key component of the RISC complex is an Argonaute protein [10].
Recently, miR-4262 has been shown to mediate the effects of Angiotensin-Converting Enzyme 2 on the induction of apoptosis of pulmonary endothelial cells during acute lung injury [11]. Interestingly, although miRNAs have been shown to regulate GALNT4 in some cancers [12,13], the relationship between miR-4262 and GALNT4 has not been reported yet. GALNT4, a member of the family of N-acetyl galactosaminyl transferases, can catalyze the transfer of GalNAc to Serine or Threonine residues in the initial step of mucin-type O-linked protein glycosylation [14-16]. This glycosylation type is the most complex post-translational modification of proteins, playing critical roles during cellular proliferation, differentiation and other pathological disorders. It has been shown that GALNTs can be targeted by a miRNA cluster, resulting in increased tumor invasion [17,18]. Furthermore, GALNTs was deregulated in colon cancer and other neoplasms [17].
Hence, in the present study, we studied the effects and mechanisms of miR-4262 to the colon cancer cell proliferation and apoptosis. The level of miR-4262 was measured in colon cancer tissue and colon cancer cell lines. Effect of transfection with the miR-4262 mimic or antisense of miR-4262on cell proliferation and cell apoptosis in colon cancer cells was also studied. The relationship between GALNT4 and miR-4262 was explored using bioinformatics analyses, luciferase activities assay and Western blot method. We also treated cells with miR-4262 and GALN4 siRNA and measured the cell viability.
Materials and methods
Clinical specimens
Total 32 colon cancer and adjacent normal colon tissues (CTL) were from Shanghai Jiao Tong University School of Medicine from 2012 to 2014. All the experiments were performed in the central laboratory of Shanghai Jiao Tong University School of Medicine. The Ethics Committees of Shanghai Jiao Tong University School of Medicine approved this study (approval number: 201106-5842), and the patient’s permission for this study was obtained. After surgery, the tissue samples were stored at -80°C, after which the pathological information was obtained.
Cell culture
Normal colon cells NCM460 were used as Control. NCM460 and the human CRC cell lines HT-29, SW620, SW480 and Caco-2 were all purchased from American Type Culture Collection (ATCC, Rockville, MD, USA), and were cultured in RPMI1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA) in a humidified chamber with 5% CO2 at 37°C.
Quantitative real time-PCR (RT-qPCR)
Total RNA of resected specimens from the patients or from cultured cells was extracted using miRNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Briefly, cell samples are homogenized in QIAzol Lysis Reagent. After addition of chloroform, the homogenate is separated into aqueous and organic phases by centrifugation. RNA partitions to the upper, aqueous phase, while DNA partitions to the interphase and proteins to the lower, organic phase or the interphase. The upper, aqueous phase is extracted, and ethanol is added to provide appropriate binding conditions for all RNA molecules from 18 nucleotides (nt) upwards. The sample is then applied to the RNeasy Mini spin column, where the total RNA binds to the membrane and phenol and other contaminants are efficiently washed away. Highquality RNA is then eluted in RNase-free water. Complementary DNA (cDNA) was randomly primed from 2 μg of total RNA using the Omniscript reverse transcription kit (Qiagen, Hilden, Germany). Primers of GALNT4 and miR-4262 were obtained from Qiagen (Hilden, Germany). The forward primer and reverse primer of GALNT4 were GGCCTATATCTTCGTGGAGCTC and CCTGCGGAGGCATGAAAA, respectively. The sequence of the primer of miR-4262 was CGACATTCAGACTACCTGAAAAA. Real-time quantitative PCR (RT-qPCR) was subsequently performed in triplicate with a 1:4 dilution of cDNA with QuantiTect SYBR Green PCR Kit (Qiagen, Hilden, Germany) in a Quantitect SyBr green PCR system (Qiagen, Hilden, Germany). All primers were purchased from Qiagen. A 2-ΔΔCt method was used for quantification of the relative mRNA expression levels. Values of genes were first normalized against β-actin, and then compared to controls.
Transfection
The specific miRNA mimic or antisense and a control null plasmid were synthesized by Genepharma (Shanghai, China). The sequences of MiR-4262 were as follows: forward: 5’-GCUGCAGGU G CUGA UGUU G-3’ and reverse: 5’-CGACGUCCA C GACU ACAG G-3’. They were transfected into colon cancer cells. After 48 hours, the transfection was evaluated. Cells at 80% concentration were seeded into 6-well plates before transfection. The miRNAs (Life techonologies, CA, Carlsbad, USA) were transfected to cells with LipofectamineTM 3000 Reagent (Invitrogen, USA), the final concentration miRNAs were 50 nmol/l. After 4 hours, cells were cultured with normal media, and harvested 48 hours later.
MTT assay
The MTT assay was performed following the manufacture’s instruction. At 48 h after transfection, cells were seeded into 96-well plates at a density of 5000 cells/well and incubated with MTT solution (5 mg/ml, 10 μl, bought from Beyotime, Shanghai, China) for 4 h in a cell incubator under normal condition. Afterwards, the supernatant was removed before 100 μl DMSO was added into each well. Finally, the absorbance at 570 nm was measured with a Microplate reader (Bio-Rad, CA, USA). Each experiment was repeated three times.
Apoptosis assay
Apoptosis rate was examined with dual-color flow cytometric method. Cells were harvested and detected apoptosis level with Annexin V-FITC apoptosis detection kit (KeyGEN, China) following the manufacturer’s protocol. The flow cytometry (Becton-Dickinson Biosciences, San Jose, CA, USA) and Flowjo software (Flowjo LLC, Ashland, OR, USA) were applied to obtain and analyze apoptosis data. Each experiment was repeated three times.
MicroRNA targets prediction and dual-luciferase reporter assay
The target gene of miR-4262 was predicted by TargetScan (http://www.targetscan.org). The dual-luciferase reporter plasmids, p3’-UTR-GALNT4 (containing the wild-type GALNT4 3’UTR binding site in luciferase reporter plasmid (RiboBio Co. Ltd., China) and p3’-UTR-GALNT4-mut (containing the mutant GALNT4 3’UTR; mut) were constructed. For the luciferase assay, the constructed plasmid (500 ng) and miR-4262 (mir-4262) (100 nmol/l) were co-transfected into colon cancer cells using LipofectamineTM 3000 Reagent (Invitrogen, USA). Then the luciferase activity was detected with the dual-luciferase reporter assay system (Promega, USA) after co-transfection cells for 48 hours, following the manufacturer’s protocol.
Western blot analysis
Cells were washed twice with phosphate buffered saline (PBS) and lysed with cold RIPA lysis buffer in the presence of 1 mM of phenylmethylsulphonyl fluoride. Afterwards, cell lysates were collected with a cell scraper. Protein was collected by centrifugation at 13,000 g and 4°C for 15 min. Protein concentrations were determined by the Enhanced BCA Protein Assay Kit (Beyotime, Shanghai, China). Total 20 μg of protein was used for western blotting. After SDS-PAGE gels electrophoresis, samples were transferred to PVDF membranes. After blocked, membranes were incubated with primary polyclonal against GALNT4 or β-actin (Cell Signaling, San Jose, CA, USA) and then secondary anti-rabbit antibody (Cell signaling). Protein signals were enhanced by chemiluminescence detection kit. The protein quantification was performed using Image J software (NIH, Bethesda, MA, USA).
Tumor xenograft study
Twenty Six-week-old male nude mice were housed under standard laboratory conditions and randomly divided into two groups (ten mice in each group). They were allowed to standard rodent diet and water ad libitum. When SW480-null cells or SW480-miR-4262 cells have been exponentially growing, they were subcutaneously injected into the right flank of nude mice (4 × 106 cells/mice). Body weight and diet consumption were recorded twice weekly. Eight weeks later, mice were sacrificed and the tumor of every mouse was dissected out. Their sizes were measured using digital vernier caliper. Tumor size was calculated by using the formula 0.5236 × L1 × (L2)2 (L1: long axis of the tumor; L2: short axis of the tumor) [19].
Statistical analysis
All statistical analysis was performed using SPSS 16.0 software. The data were presented as mean ± SD of three independent experiments and compared using one-way ANOVA and Student’s t-test. P<0.05 was considered to be statistically significant and indicated by (*).
Results
MiR-4262 is down-regulated in colon cancer tissues and cell lines
To research potential effects about miR-4262 in colon cancer, the miR-4262 levels were estimated in colon cancer tissues and cells specimens. Lower mRNA levels of miR-4262 were observed in colon cancer tissues compared with the paired CTL (Figure 1A). Moreover, MiR-4262 levels in colon cancer cell lines HT-29, SW620, SW480 and Caco-2 were all much lower than that in CTL (Figure 1B).
Figure 1.
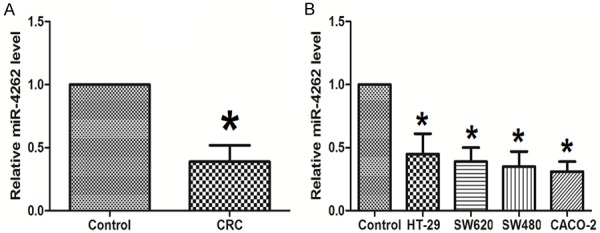
MiR-4262 down-regulates in colon cancer tissues and cell lines. A. To research potential effects about miR-4262 in colon cancer (CRC), the miR-4262 levels were estimated in colon cancer tissues and cells specimens. Lower mRNA levels of miR-4262 were observed in colon cancer tissues compared with Control (paired normal colon tissue). N=32. B. MiR-4262 levels in colon cancer cell lines were all much lower than that in Control (NCM460 cells). N=5. *P<0.05 compared to Control.
MiR-4262 has anti-cancer effects on colon cancer cells
We investigated whether miR-4262 influences the apoptosis and proliferation using colon cancer cell line, SW480. As-miR-4262 or miR-4262 mimic was transfected into SW480 cells to inhibit or increase the miR-4262 expression, respectively (Figure 2). In comparison to control cells, the cell viability of miR-4262-depleted colon cancer cells was markedly increased in MTT assay and in CCK-8 assay at the presence of 5-FU, while the cell viability of miR-4262-overexpressing colon cancer cells was markedly decreased (Figure 3A, 3B). Moreover, the apoptosis of colon cancer cells with miR-4262 depletion significantly decreased, and the apoptosis of colon cancer cells with miR-4262 overexpression significantly increased (Figure 3C).
Figure 2.

Modification of miR-4262 levels in colon cancer cells. We investigated whether miR-4262 influences the apoptosis and proliferation using colon cancer cell line, SW480. As-miR-4262 or miR-4262 mimic was transfected into SW480 cells to inhibit or increase the miR-4262 expression, respectively. RT-qPCR for miR-4262 was performed. *P<0.05 compared to null. N=5.
Figure 3.

MiR-4262 decreases cell proliferation and increases cell apoptosis in colon cancer cells. In comparison to control cells, the cell viability of miR-4262-depleted colon cancer cells was markedly increased in MTT assay (A) and in CCK-8 assay at presence of 5-FU (B), while the cell viability of miR-4262-overexpressing colon cancer cells was markedly decreased. (C) The apoptosis of colon cancer cells with miR-4262 depletion significantly decreased, and the apoptosis of colon cancer cells with miR-4262 overexpression significantly increased. *P<0.05 compared to null. n=5.
MiR-4262 functionally targets GALNT4 in colon cancer cell lines
Due to the important function of miR-4262 gene in colon cancer, we studied the underlying mechanisms. Using the publicly available databases TargetScan, we found a conserved miR-4262 binding site in the 3’-UTR of GALNT4 (Figure 4A). The GALNT4 levels were up-regulated significantly in colon cancer tissues, compared to CTL (Figure 4B). Furthermore, the levels of miR-4262 and GALNT4 inversely correlated in colon cancer tissue (Figure 4C).
Figure 4.
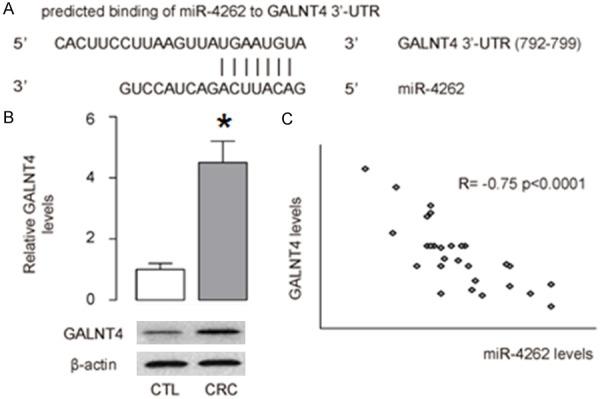
MiR-4262 and GALNT4 inversely correlate in colon cancer. A. Using the publicly available databases TargetScan, we found a conserved miR-4262 binding site in the 3’-UTR of GALNT4. B. The GALNT4 levels were up-regulated significantly in CRC (colon cancer tissues), compared to CTL (normal colon tissue). C. The levels of miR-4262 and GALNT4 inversely correlated. N=32. *P<0.05 compared to Control.
To further investigate whether GALNT4 was a target gene of miR-4262, the luciferase reporter plasmids containing either wild-type or mutant 3’-UTRs of GALNT4 were constructed. The luciferase reporter assay was set up to identify the direct miR4262-GALNT4 interaction. The relative luciferase activity was significantly lower in cells after 48 hours co-transfection with miR-4262-modified plasmids and p3’-UTR-GALNT4 or miR-4262 (Figure 5). There was a statistically difference between cells co-transfected with p3’-UTR-mut and cells co-transfected with the miR-4262 (Figure 5). Those consequences indicated that miR-4262 could specifically bind to seed zone of GALNT4 3’UTR to inhibit its expression, while mut vector could not combine with miR-4262 to decrease the relative luciferase activity. GALNT4 is thus a specific and direct target gene of miR-4262.
Figure 5.
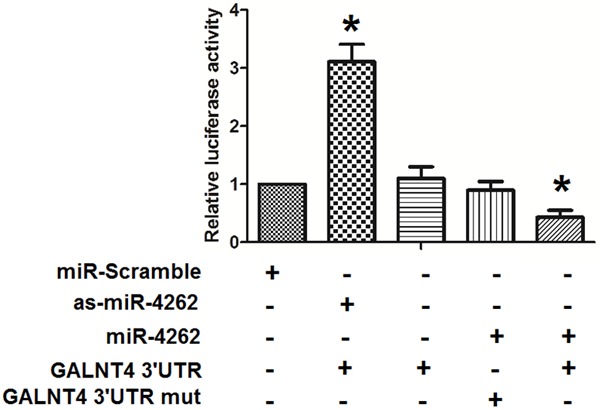
MiR-4262 functionally targets GALNT4 in colon cancer cells. To further investigate whether GALNT4 may be a target gene of miR-4262, the luciferase reporter plasmids containing either wild-type or mutant 3’-UTRs of GALNT4 were constructed. The luciferase reporter assay was set up to identify the direct miR4262-GALNT4 interaction. The relative luciferase activity was significantly lower in cells after 48 hours co-transfection with miR-4262-modified plasmids and p3’-UTR-GALNT4 or miR-4262. There was a statistically difference between cells co-transfected with p3’-UTR-GALNT4-mut and cells co-transfected with the pre-miR-4262. Those consequences indicated that miR-4262 could specifically bind to seed zone of GALNT4 3’-UTR to inhibit its expression, while mut vector could not combined with miR-4262 to decrease the relative luciferase activity. *P<0.05 compared to miR-scramble. NS: non-significant. N=5.
In order to confirm the regulative effects of miR-4262 on endogenous GALNT4 expression, we examined GALNT4 levels in miR-4262-modified SW480 cells. The western result showed that compared with control, the GALNT4 protein level was significant suppressed with miR-4262 enhancement and was significantly increased in the examined colon cancer cells with miR-4262 silence (Figure 6A), whereas the RT-qPCR result showed that endogenous GALNT4 mRNA levels were not significant altered in these cells (Figure 6B). Together, miR-4262 negatively regulates endogenous GALNT4 expression at post-translational level.
Figure 6.
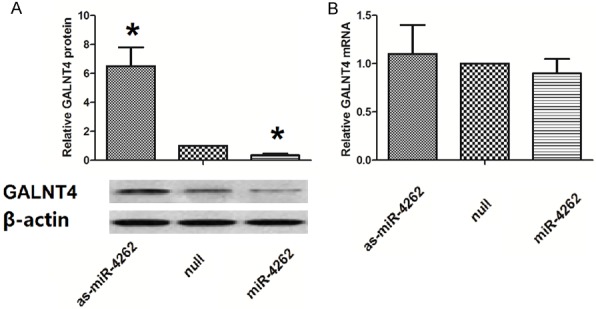
MiR-4262 inhibits GALNT4 translation in colon cancer cells. A. The western result showed that compared with control, the GALNT4 protein level was significant suppressed with miR-4262 enhancement and was up-regulated significantly in the examined colon cancer cells with miR-4262 silence. B. The RT-qPCR result showed that endogenous GALNT4 mRNA levels were not significant altered in these cells. *P<0.05 compared to null. NS: non-significant. N=5.
GALN4 siRNA decreases cell proliferation of miR-4262 treated cells
To confirm the role of GALN4 in the action of miR-4262 on cell proliferation, we treated cells with miR-4262 and siRNA non-specific control (NC) or GALN4 siRNA (si-GALN4), then measured the cell viability using CCK-8 assay. It showed that the cell viability in NC group was not significantly changed, but cell viability in si-GALN4 group was significantly decreased (Figure 7).
Figure 7.
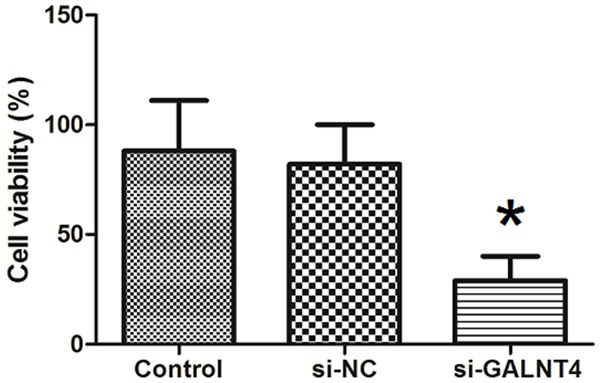
GALN4 siRNA decreases cell proliferation of miR-4262 treated cells. To confirm the role of GALN4 in the action of miR-4262 on cell proliferation, we treated cells with miR-4262 and siRNA non-specific control (NC) or GALN4 siRNA (si-GALN4), then measured the cell viability using CCK-8 assay. It showed that the cell viability in NC group was not significantly changed, but cell viability in si-GALN4 group was significantly decreased. *P<0.05 compared to Control. n=5.
MiR-4262 suppresses growth of colon cancer cells in vivo
Finally, same number of SW480-null cells or SW480-miR-4262 cells was implanted subcutaneously into nude mice and the tumor was dissected out after 8 weeks. The tumor by SW480-miR-4262 cells was significantly smaller than control (Figure 8A and 8B). These data suggest that miR-4262 suppresses growth of colon cancer cells in vivo.
Figure 8.
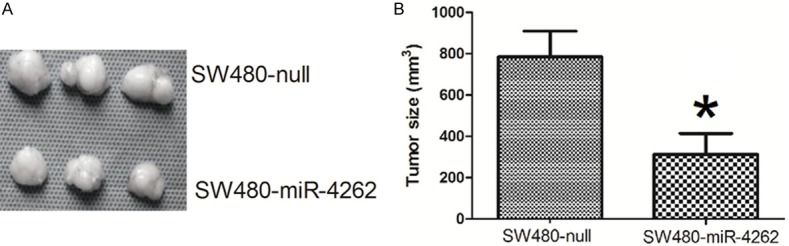
MiR-4262 suppresses growth of colon cancer cells in vivo. Same number of SW480-null cells or SW480-miR-4262 cells was implanted subcutaneously into nude mice and the tumor was dissected out after 8 weeks. The tumor by SW480-miR-4262 cells was significantly smaller than SW480-null. These data suggest that miR-4262 suppresses growth of colon cancer cells in vivo. *P<0.05 compared to SW480-null. n=10.
Discussion
Colon cancer cells have the vigorous proliferation ability and low apoptosis rate, and are strongly resistant to the chemotherapy, radiation and biological treatment, resulting in malignant tumor growth and high recurrence after resection [20]. It is well-established that aberrant miRNA expression regulates critical biological behaviors, such as cell apoptosis and proliferation. In this study, we studied the effects and mechanisms of miR-4262 to the colon cancer cell proliferation and apoptosis. We found that the levels of miR-4262 significantly down-regulated in colon cancer tissue, compared to the paired adjacent non-tumor colon tissue. The miR-4262 levels in colon cancer cell lines were significantly lower than those in control normal colon tissues. Transfection with the miR-4262 mimic decreased the cell proliferation and increased cell apoptosis in colon cancer cells, while transfection with the antisense of miR-4262 (as-miR-4262) increased cell proliferation and suppressed cell apoptosis in colon cancer cells. Bioinformatics analyses showed that GALNT4 was a potential target gene of miR-4262. The luciferase activities assay and Western blot verified that miR-4262 targeted GALNT4 mRNA to modulate its protein levels. When we treated cells with miR-4262 and GALN4 siRNA, the cell viability was significantly decreased. Together, our study suggests that aberrantly expressed miR-4262 may affect cell apoptosis and proliferation of human colon cancer cells via GALNT4, which appears to be a promising therapeutic target for colon cancer.
It is generally thought that the occurrence of CRC is a multifactor and multi step process, it’s also the result of the internal factor interact with many external factors (such as biological, physical, chemical and immune factors) [21]. The anti-drug mechanisms of tumor cells is very complicated, which includes three pathways, cytokinetics, biochemistry and pharmacological drug resistance [22]. The relation between cell apoptosis and tumor become the focus of scientific research. Since Kerr, Currie and Wyllie proposed the concept of apoptosis in 1972 [23]. It is thought that tumor occurrence is the result of the process of losing balance of cell proliferation and apoptosis. It should be great clinical significant if the clinical therapeutic efficacy be executed with the increase of drug sensitivity and the decrease of dosage and chemotherapy correlated adverse reactions.
Within RISC, miRNAs negatively regulate translation of target mRNAs by altering their stability either through the binding to their 30 untranslated regions (UTR), or, to a lesser extent, to the 50 UTR or to the coding sequence [9]. As a result, the miRNAs can directly target mRNA to degradation in the presence of perfect complementarity or induce translational repression through different mechanisms. It has also been demonstrated that miRNAs not only repress, but in some cases also may be able to activate gene expression directly or indirectly through the interaction with micro-ribonucleo-proteins [24]. Due to the complexity of these regulatory mechanisms, and because each miRNA can modulate the expression of multiple mRNAs and, furthermore, each mRNA may be targeted by several different miRNAs, it is not surprising that miRNAs have been shown to be involved in almost all key cellular processes, such as proliferation, differentiation, migration, apoptosis, and sternness maintenance [9].
In the present study, we first found that the level of miR-4262 was significantly down-regulated in colon cancer tissue, compared to the paired adjacent non-tumor colon tissue. The miR-4262 levels in colon cancer cell lines were significantly lower than those in control normal colon tissues. Transfection with the miR-4262 mimic decreased the cell proliferation and increased cell apoptosis in colon cancer cells, while transfection with the antisense of miR-4262 (as-miR-4262) increased cell proliferation and suppressed cell apoptosis in colon cancer cells. These evidences clearly suggest that miR-4262 plays an essential role in the proliferation and apoptosis of colon cancer cells.
GALNT4 has been shown to preferentially O-glycosylate the threonine residues at position 44 and 57 of the P-selectin glycoprotein ligand (PSGL-1) [25]. P-selectin (CD62P) was described as a key factor in the hematogenous dissemination of tumor cells, especially facilitating growth and metastasis of tumor progression of colon carcinomas [26]. Since knockdown of miR-4262 induced apoptosis and inhibited proliferation in colon cancer cells, and GALNT4 is a direct and pivotal target gene of miR-4262, we hypothesized that miR-4262 might regulate apoptosis and proliferation through directly down-regulating GALNT4. To confirm our hypothesis, we identified GALNT4 as a direct functional target of miR-4262 using a prediction program. Computational analysis revealed that there were binding sites for miR-4262 seed sequence at 3’-UTR of GALNT4. Furthermore, restoration of miR-4262 expression led to decreased luciferase activity of wild-type GALNT4 3’UTR whereas the site-directed mutation abrogated miR-4262 regulation. In addition, results from the RT-qPCR and protein expression analysis indicated that the ectopic expression of miR-4262 suppressed protein levels of GALNT4. Taken together, these results suggest that miR-4262 regulated the expression of GALNT4 by directly targeting 3’-UTR of GALNT4 in Colon cancer.
Our data from gain-of-function approaches confirmed that GALNT4 was a miR-4262’s direct target gene. First, GALNT4 gene was down-regulated in colon cancer tissues, and its expression showed negative relationship with the expression of miR-4262. Second, luciferase reporter assay verified the specific and direct combination between miR-4262 and GALNT4 mRNA. Third, over-expression of miR-4262 inhibits the expression of GALNT4 protein at post-translational level, and knockdown of miR-4262 could show contrary effects. Next, the siRNA-GALNT4 was transfected to down-regulate the expression of GALNT4 which was up-regulated in colon cancer cells with miR-4262 silence. We found that silence of GALNT4 could inhibit the apoptosis and increase the cell proliferation ability of colon cancer cells. The regulation effects of anti-miR-4262 on apoptosis and proliferation can be reversed by the knockdown of GALNT4.
In summary, aberrantly expressed miR-4262 regulates human colon cancer cells apoptosis and proliferation partly through directly down-regulating GALNT4 protein expression and this might offer a new potential therapeutic stratagem for colon cancer. Nevertheless, sequential research is essential to identify the detailed molecular mechanism about miR-4262 in the genesis and development of colon cancer.
Acknowledgements
The work was supported by the Tongren Hospital Medical Research Funding Program and the Key Medical Specialty Project of Shanghai (ZK2015A26).
Disclosure of conflict of interest
None.
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Tao K, Yang J, Guo Z, Hu Y, Sheng H, Gao H, Yu H. Prognostic value of mir-221-3p, mir-342-3p and mir-491-5p expression in colon cancer. Am J Transl Res. 2014;6:391–401. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim TD, Fuchs JR, Schwartz E, Abdelhamid D, Etter J, Berry WL, Li C, Ihnat MA, Li PK, Janknecht R. Pro-growth role of the jmjd2c histone demethylase in hct-116 colon cancer cells and identification of curcuminoids as jmjd2 inhibitors. Am J Transl Res. 2014;6:236–247. [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685–8. doi: 10.3748/wjg.v11.i30.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng S. Recent study on colorectal cancer in China: early detection and novel related gene. Chin Med J (Engl) 1997;110:309–10. [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67:129–39. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–60. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabris L, Ceder Y, Chinnaiyan AM, Jenster GW, Sorensen KD, Tomlins S, Visakorpi T, Calin GA. The potential of microRNAs as prostate cancer biomarkers. Eur Urol. 2016;70:312–22. doi: 10.1016/j.eururo.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 11.Bao H, Gao F, Xie G, Liu Z. Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cells during acute lung injury through suppressing mir-4262. Cell Physiol Biochem. 2015;37:759–767. doi: 10.1159/000430393. [DOI] [PubMed] [Google Scholar]
- 12.Liang HL, Hu AP, Li SL, Xie JP, Ma QZ, Liu JY. Mir-454 prompts cell proliferation of human colorectal cancer cells by repressing cyld expression. Asian Pac J Cancer Prev. 2015;16:2397–2402. doi: 10.7314/apjcp.2015.16.6.2397. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Jian W, Wei C, Song H, Gu Y, Luo Y, Fang L. Down-regulation of mir-181b promotes apoptosis by targeting cyld in thyroid papillary cancer. Int J Clin Exp Pathol. 2014;7:7672–7680. [PMC free article] [PubMed] [Google Scholar]
- 14.Niang B, Jin L, Chen X, Guo X, Zhang H, Wu Q, Padhiar AA, Xiao M, Fang D, Zhang J. Galnac-t4 putatively modulates the estrogen regulatory network through foxa1 glycosylation in human breast cancer cells. Mol Cell Biochem. 2016;411:393–402. doi: 10.1007/s11010-015-2601-1. [DOI] [PubMed] [Google Scholar]
- 15.Erbilgin A, Civelek M, Romanoski CE, Pan C, Hagopian R, Berliner JA, Lusis AJ. Identification of cad candidate genes in gwas loci and their expression in vascular cells. J Lipid Res. 2013;54:1894–1905. doi: 10.1194/jlr.M037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommer P, Napier HR, Hogan BL, Kidson SH. Identification of tgf beta1i4 as a downstream target of foxc1. Dev Growth Differ. 2006;48:297–308. doi: 10.1111/j.1440-169X.2006.00866.x. [DOI] [PubMed] [Google Scholar]
- 17.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaziel-Sovran A, Hernando E. miRNA-mediated GALNT modulation of invasion and immune suppression: a sweet deal for metastatic cells. Oncoimmunology. 2012;1:746–748. doi: 10.4161/onci.19535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rana S, Anil T, Sivanandhan D, Rajesh A, Chapla A. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 20.Selzner M, Bielawska A, Morse MA, Rüdiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–40. [PubMed] [Google Scholar]
- 21.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 22.Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–16. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- 23.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su J, Liang H, Yao W, Wang N, Zhang S, Yan X, Feng H, Pang W, Wang Y, Wang X, Fu Z, Liu Y, Zhao C, Zhang J, Zhang CY, Zen K, Chen X, Wang Y. MiR-143 and MiR-145 regulate IGF1R to suppress cell proliferation in colorectal cancer. PLoS One. 2014;9:e114420. doi: 10.1371/journal.pone.0114420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett EP, Hassan H, Mandel U, Mirgorodskaya E, Roepstorff P, Burchell J, Taylor-Papadimitriou J, Hollingsworth MA, Merkx G, van Kessel AG, Eiberg H, Steffensen R, Clausen H. Cloning of a human udp-n-acetyl-alpha-d-galactosamine: Polypeptide n-acetylgalactosaminyltransferase that complements other galnac-transferases in complete o-glycosylation of the muc1 tandem repeat. J Biol Chem. 1998;273:30472–30481. doi: 10.1074/jbc.273.46.30472. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Short HJ, Qian KX, Elhammer AP, Geng JG. Characterization of glycoprotein ligands for p-selectin on a human small cell lung cancer cell line nci-h345. Biochem Biophys Res Commun. 2001;288:637–644. doi: 10.1006/bbrc.2001.5806. [DOI] [PubMed] [Google Scholar]


