Abstract
This study was undertaken to investigate the cytoprotective role of transient receptor potential ankyrin 1 (TRPA1) in sepsis-induced kidney injury. The Cecal ligation and puncture (CLP) was employed to induce septic kidney injury in C57BL/6 mice. Six hours before CLP or a sham procedure, mice were injected intraperitoneally with 10 mg/kg hemin or 30 mg/kg of the TRPA1 antagonist A-967079. Our study showed that mice treated with A-967079 exhibited less sepsis-induced mortality and kidney injury compared with those in the sham group. Moreover, A-967079 prevented multiple organ dysfunction, pathological changes, and increased secretion of in proinflammatory cytokines. In addition, A-967079 decreased the levels of mitochondrial lipid peroxidation and mitochondrial dysfunction in kidney tissues. The protein levels of mitochondrial biogenesis markers, including Sirt1, nuclear respiratory factor 1, and mitochondrial transcription factor A, were decreased in the A-967079 treatment group. Additionally, A-967079 treatment attenuated mitochondrial mitophagy. The levels of PTEN-induced putative kinase 1 increased and parkin levels decreased compared to the untreated CLP group. Our findings suggest that TRPA1 prevents septic injury by modulating mitochondrial biogenesis and mitophagy.
Keywords: Transient receptor potential ankyrin 1 (TRPA1), septic injury, mitochondrial biogenesis, mitophagy, mortality
Introduction
Sepsis is a complex disease caused by uncontrollable inflammatory responses, which lead to serious adverse consequences, such as shock, organ dysfunction, and even death [1]. Research on sepsis has been carried out for more than 30 years, but its pathophysiology has not yet been elucidated. The hospitalization and mortality rates of patients with sepsis are often significantly elevated; previous studies found that 25% of septic patients in the intensive care unit had a mortality rate of about 50% [2,3]. Therefore, it is urgent to find reliable markers of sepsis for its early diagnosis and treatment, and to analyze its pathophysiology.
For septic patients, restoring the functions of cells and organs is critical to maintain optimal function of the mitochondria [4], as recent studies have found that mitochondrial quality control is significantly associated with certain inflammatory and neurodegenerative diseases [5]. The mitochondrial quality control network is responsible for maintaining mitochondrial homeostasis, including mitochondrial biosynthesis (mitochondrial formation), mitochondrial dynamic balance, and autophagy [6,7]. Excessive numbers of dysfunctional mitochondria, increases in mitochondrial fission, and impaired mitochondrial fusion are the main characteristics of many neurodegenerative diseases [8].
Transient receptor potential ankyrin 1 (TRPA1) is a receptor-activated, nonselective cation channel that can be activated by a variety of toxic and irritating substances. The sensitivity of transgenic mice lacking TRPA1 to cold, mechanical stimulation, and TNFα-induced mechanical pain was decreased, compared to TRPA1-expressing mice [9-11]. Thus, TRPA1 is able to mediate both acute and inflammatory pain [12]. In addition, TRPA1 is an oxygen sensor, which plays an important role in histamine-independent pruritus [13]. The cytoprotective ability of TRPA1 is closely related to mitochondrial function. Researchers are currently exploring the specific molecular mechanisms by which TRPA1 exhibits its cytoprotective effects. In this study, we analyzed the molecular mechanism by which TRPA1 protects kidney cells from septic injury.
Materials and methods
Animals
All the protocols were approved by the Institutional Animal Care and Use Committee of First People’s Hospital of Wenling, in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (approval ID: SYXK20140026). Male C57BL/6 mice (10-14 weeks of age; body weight, 23-25 g) were provided by the Shanghai Lab Animal Research Center. The mice were maintained in cages at 22±2°C, with an artificial 12-h light-dark cycle using 12/12 h occulting photos and allowed unlimited food and water intake.
Experimental procedure
A mouse model of sepsis was established using cecal ligation and puncture (CLP), as previously reported [14]. Mice were randomly divided into six groups: sham, hemin, A-967079, Sepsis, hemin + Sepsis, and A-967079 + Sepsis. Six hours before CLP or a sham procedure, mice were intraperitoneally injected with saline (control), 10 mg/kg hemin (Sigma-Aldrich, St Louis, MO, USA), or 30 mg/kg A-967079 (Sigma-Aldrich, St Louis, MO, USA). Mortality rates were recorded seven days after CLP, and survival was ensured for three weeks (n=10). For histological analyses, 6 mice were used from each group. Blood samples were collected from the inferior vena cava and multiple tissues of anesthetized mice six hours after CLP, and quickly stored at -80°C.
Isolation of mitochondrial fragments
As previously described, kidney mitochondrial fragments were isolated [15]. A BCA protein extraction kit (Thermo Scientific, Rockford, USA) was used to measure the protein concentration of the mitochondrial fragments.
Western blot analysis
Kidney tissues were homogenized in RIPA buffer containing PMSF (Beyotime Institute of Biotechnology, Shanghai, China) and centrifuged at 12,000 × g at 4°C for 15 min. Protein concentrations were measured using the Bradford method. Protein samples (20 μg) were loaded onto a 15% polyacrylamide gel, separated by SDS-polyacrylamide gel electrophoresis, and transferred to a PVDF membrane (Millipore, Shanghai, China). The membrane was blocked with 5% (w/v) skim milk (dissolved in Tris buffer containing 0.1% Tween-20) at room temperature and then incubated at 4°C overnight with primary antibodies. Primary antibodies included: anti-TRPA1 (1:2,500; Abcam, MA, USA), anti-cytochrome c oxidase IV, anti-superoxide dismutase 2 (SOD2), anti-NRFI, anti-mitochondrial transcription factor A (TFAM), anti-parkin, anti-dynamin-related protein 1 (DRP1; 1:2,500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-MR (1:2,500, Abcam, Cambridge, MA, USA), anti-BCL2/adenovirus E1B (19 kD)-interacting protein 3 (BNIP3), anti-mitochondrial fusion protein (MFN2; 1:2,500; Abcam, Cambridge, UK), anti-PGC1α, anti-phosphatase and tension homologue-induced kinase 1 (PINK1), and anti-β-actin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Anti-rabbitand anti-goat secondary antibodies (1:5,000, LI-COR Biosciences, USA) were added to the membrane and incubated for 1.5 h at room temperature. Each band shown represents the best result from three independent experiments. Each band was analyzed bythe Odyssey Infrared Imaging System (Li-COR Biosciences, USA). Data were expressed as the relative fold change based on the unprocessed value of the control group.
Histopathological analysis
The tissues were fixed with 10% neutral formalin, coated with paraffin, cut into 5-μm-thick slices, and stained with hematoxylin and eosin. Histological changes were observed in random fields during evaluations of morphology (Olympus Optical Co., Tokyo, Japan).
ALT and AST assays
Changes in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen (BUN), creatinine, and lactate dehydrogenase (LDH) levels were measured using specific test kits (Jiancheng Bioengineering Institute of Nanjing), according to the manufacturer’s protocols.
Serum cytokine detection
Serum levels of IL-6 and IL-1β were quantitatively analyzed by enzyme-linked immunosorbent assays (ELISAs) using IL-6 and IL-1β ELISA kits (BD Biosciences, San Jose, CA, USA), according to the manufacturer’s protocol.
Mitochondrial swelling
Rates of mitochondrial swelling represent the extent of the mitochondrial permeability transition (MPT). As previously described [16], MPT can be detected by the absorbance of a mitochondrial suspension at 520 nm.
Mitochondrial lipid peroxidation
Using spectrophotometry at 535 nm [13], the levels of malondialdehyde (MDA) in kidney mitochondrial fragments were determined as the amount of MDA that reacts with thiobarbituric acid.
Serum glutamic dehydrogenase activity
As previously described [17], the activity of glutamic dehydrogenase (GDH) in serum was determined by spectrophotometry.
Statistical analyses
All statistical analyses were carried out using GraphPad Prism (GraphPad Software, San Diego, CA, USA). The data were expressed as the mean ± standard deviation (SEM). Survival data were analyzed using the Kaplan-Meier curve and log-rank test. Other data were analyzed by one-way ANOVA, and multiple comparisons were analyzed by the Bonferroni test. A P value less than 0.05 was considered a statistically significant difference between groups.
Results
TRPA1 prevents sepsis-induced organ damage and death
To explore the role of TRPA1 in CLP-induced sepsis and subsequent death, we monitored survival for seven consecutive days. In the CLP group, the survival rate was 75% on the first day; survival remained stable at 25% from the fourth day after CLP and beyond. Compared with the CLP group, the hemin + CLP group had a higher survival rate (P=0.0352), whereas the A-967079 + CLP group had a lower survival rate (Figure 1A). We then analyzed whether TRPA1 affected the extent of organ damage in CLP-induced septic mice. In the sham group, serum ALT and AST levels were 28.7±1.5 U/L and 57.4±3.3 U/L, respectively. Compared with the sham group, CLP significantly increased serum ALT and AST levels. While the serum LDH level in the sham group was 885.2±93.7 U/L, LDH was significantly increased by 4.6 folds in the CLP group. Serum BUN and creatinine levels in the sham group were 22.6±2.5 U/L and 0.39±0.01 U/L, respectively. Compared with the sham group, CLP significantly increased serum BUN and creatinine levels. Hemin blocked these increases in the levels of ALT, AST, LDH, and creatinine (Table 1). Additionally, hemin was shown by a histological examination to prevent CLP-induced cell death, whereas A-967079 enhanced CLP-induced cell death (Figure 1B).
Figure 1.
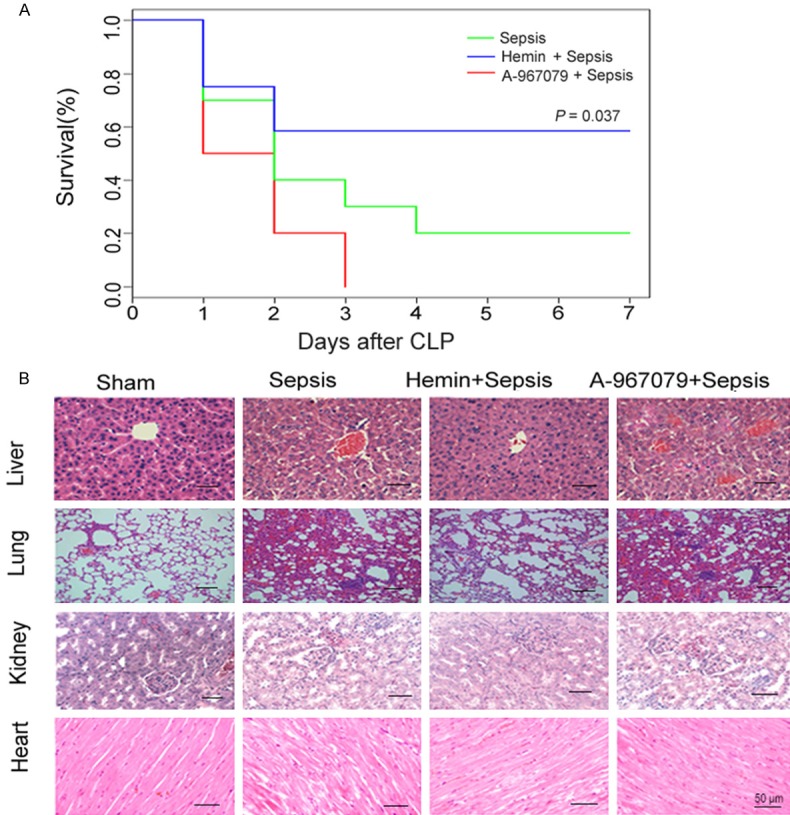
Effects of the TRPA1 antagonist (A-967079) on overall survival in mice with CLP-induced sepsis. A. Mice were monitored one week after CLP (n=10). B. Inflammation was detected in various tissues by histological examination. *P<0.05, significant versus sepsis group mice.
Table 1.
Effect of hemin or TRPA1 antagonist (A-967079) on multi-organ injury CLP-induced septic mice
| ALT (U/L) | AST (U/L) | LDH (U/L) | BUN (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|---|---|---|
| Sham | 28.7±1.5 | 57.2±2.4 | 885.1±95.6 | 22.8±2.1 | 0.39±0.01 |
| CLP | 78.6±3.3** | 271.8±11.5** | 4213.5±126.4** | 31.6±1.5** | 0.64±0.02* |
| Hemin + CLP | 57.2±1.7**,## | 250.4±8.6**,# | 3520.5±185.3**,# | 28.7±1.6 | 0.43±0.02# |
| A-967079 + CLP | 80.3±2.9** | 309.5±10.7** | 4465.2±174.6** | 39.1±2.4** | 0.75±0.03**,# |
CLP, cecal ligation and puncture; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase.
P<0.05, significant versus the sham group;
P<0.01, significant versus the sham group.
P<0.05, significant versus the sepsis group;
P<0.01, significant versus the sepsis group.
CLP increases TRPA1 protein expression in the kidney and mitochondria
We measured TRPA1 protein levels in septic mice. Compared with the sham group, CLP increased TRPA1 protein expression in kidney microsomal extracts by 1.3 and 12.8 folds, respectively. Hemin + CLP further increased TRPA1 protein expression, whereas A-967079 + CLP reduced TRPA1 protein expression, compared to CLP alone (Figure 2A). CLP also significantly increased TRPA1 protein expression in kidney mitochondria compared with the sham group. Hemin further increased this expression, whereas A-967079 abrogated the increase (Figure 2B).
Figure 2.
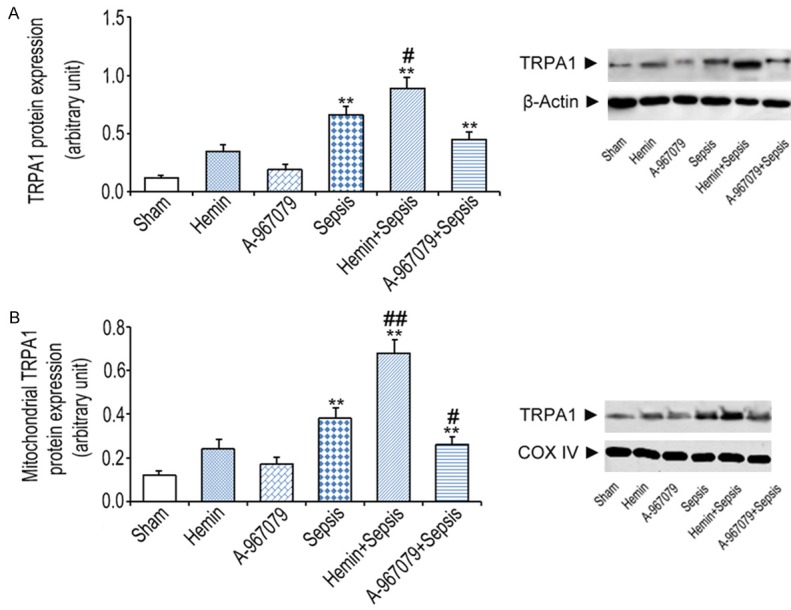
Effects of the TRPA1 antagonist (A-967079) on microsomal and mitochondrial TRPA1 protein expression six hours after CLP (n=10). A. Microsomal TRPA1 protein expression measured in kidney extracts. B. Mitochondrial TRPA1 protein expression in kidney mitochondria detected by western blot (n=10). *P<0.05 and **P<0.01, significant versus the sham group, #P<0.05 and ##P<0.01, significant versus the sepsis group.
TRPA1 downregulates the expression of proinflammatory cytokines induced by CLP
To analyze the role of TRPA1 in inflammatory responses, we measured the expression of serum inflammatory cytokines. Compared with the sham group, CLP increased serum IL-6 and IL-1β levels by 203.6 and 40.9 folds, respectively. Hemin blocked the CLP-induced increases in these levels, whereas A-967079 enhanced the expression of CLP-induced IL-6 and IL-1β levels (Figure 3A and 3B).
Figure 3.
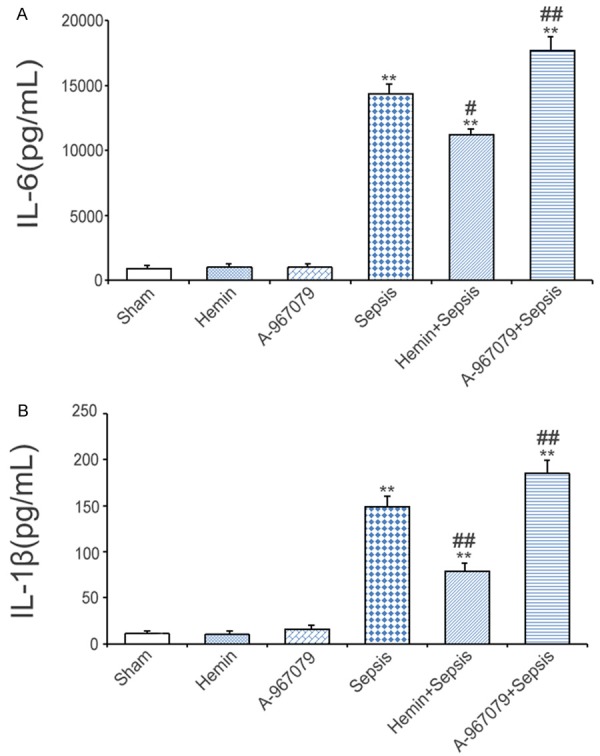
Effects of the TRPA1 antagonist (A-967079) on inflammatory cytokines in mice with CLP-induced sepsis. Serum IL-6 (A) and IL-1β (B) levels were detected six hours after CLP (n=10). *P<0.05 and **P<0.01, significant versus the sham group, #P<0.05 and ##P<0.01, significant versus the sepsis group.
TRPA1 reduces CLP-induced oxidative stress in mitochondria
The level of MDA in kidney mitochondria in the sham group was 0.31±0.06 nmol/mg. Compared with the sham group, CLP significantly increased MDA levels by 2.6 folds, whereas hemin + CLP blocked the increase in these levels (Figure 4A). CLP reduced SOD2 protein levels to 86.7% of sham group levels. Hemin + CLP attenuated this decrease (Figure 4B). To analyze changes in the mitochondrial membrane potential, we measured the rate of mitochondrial swelling. The swelling rate of mitochondria in the sham group was 0.004±0.001 (ΔA × 10-2/min·mg of protein), whereas CLP increased this rate by 7.2 folds. Hemin + CLP blocked the increase induced by CLP alone (Figure 4C). To examine the role of TRPA1 in mitochondrial dysfunction, we measured serum GDH activity and kidney ATP levels. The activity of serum GDH in the sham group was 4.9±0.3 U/L, whereas CLP significantly increased GDH activity by 5.3 folds, and hemin + CLP blocked this change (Figure 4D). Kidney ATP levels were significantly decreased to 76.5% of sham group levels by CLP. Hemin + CLP negated the effects of CLP alone, whereas A-967079 had no effect on CLP-induced mitochondrial swelling, kidney mitochondrial MDA, SOD2, and kidney ATP levels, or serum GDH activity. The addition of either hemin or A-967079 alone had no impact on the extent of mitochondrial oxidative damage or dysfunction (Figure 4E).
Figure 4.
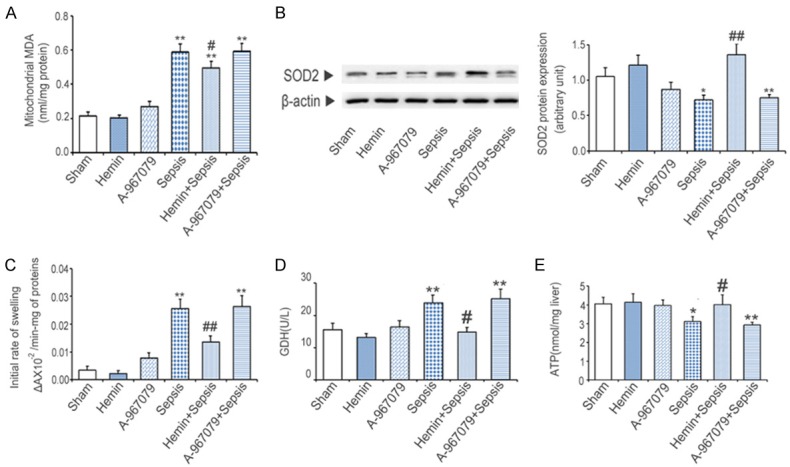
Effects of the TRPA1 antagonist (A-967079) on mitochondrial damage in mice with CLP-induced sepsis. A. Kidney mitochondrial MDA levels. B. Kidney mitochondrial SOD2 protein levels. C. Mitochondrial swelling was detected six hours after CLP. D. Kidney ATP levels were measured six hours after CLP. E. Serum GDH activity was measured six hours after CLP. *P<0.05 and **P<0.01, significant versus the sham group, #P<0.05 and ##P<0.01, significant versus the sepsis group.
TRPA1 enhances the homeostasis of mitochondria
To determine whether TRPA1 could play a protective role in mitochondrial homeostasis, we first measured the expression levels of biomarkers related to mitochondrial biosynthesis. Compared with the sham group, CLP reduced kidney PGC1α and TFAM protein levels by 68.3% and 53.15%, respectively. Hemin + CLP prevented these decreases. However, A-967079 had no effect on the CLP-induced effects on mitochondrial biosynthesis (Figure 5A). Furthermore, compared with the sham group, CLP increased PINK1 protein levels by 1.7 folds. Hemin + CLP blocked this increase, whereas A-967079 + CLP further enhanced this increase. CLP reduced parkin and BNIP3 protein levels to 77.6% and 75.4% of sham group levels, respectively. Hemin + CLP prevented these decreases, whereas A-967079 + CLP had no effects on either parkin or BNIP3 protein levels compared to CLP alone (Figure 5B).
Figure 5.
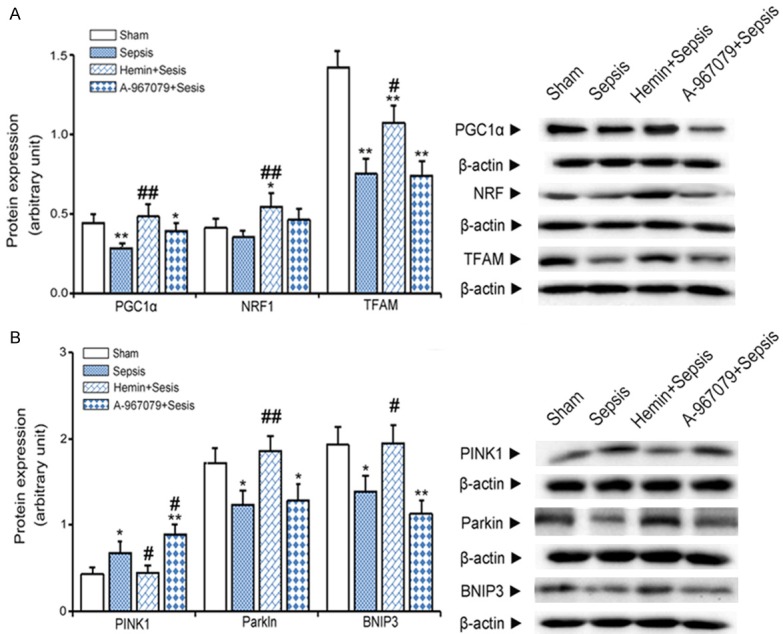
Effects of the TRPA1 antagonist (A-967079) on mitochondrial biogenesis and mitophagy in mice with CLP-induced sepsis. A. Protein expression levels of mitochondrial biogenesis markers detected in kidney extracts six hours after CLP by western blot (n=10). B. Protein expression levels of mitochondrial mitophagy biomarkers in kidney extracts six hours after CLP by western blot (n=10). *P<0.05 and **P<0.01, significant versus the sham group, #P<0.05 and ##P<0.01, significant versus the sepsis group.
TRPA1 inhibits mitochondrial mitosis and promotes fusion
Compared with the sham group, CLP increased kidney DRP1 protein levels by 1.4 folds. Hemin + CLP blocked this increase. CLP reduced the expression of MFN2 to 75.3% of sham group levels, whereas hemin + CLP prevented this decrease (Figure 6).
Figure 6.
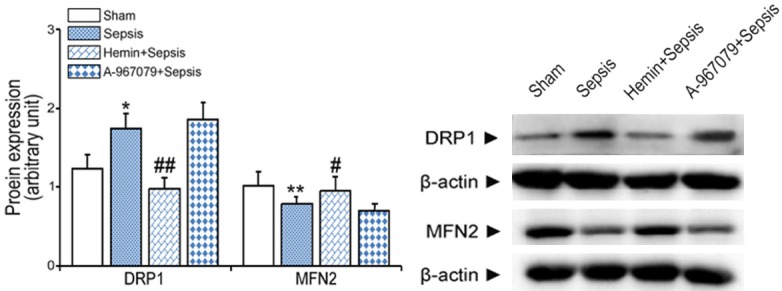
Effects of the TRPA1 antagonist (A-967079) on mitochondrial dynamics in mice with CLP-induced sepsis. Expression levels of DRP1 and MFN2 proteins were measured in kidney extracts six hours after CLP by western blot. *P<0.05 and **P<0.01, significant versus the sham group, #P<0.05 and ##P<0.01, significant versus the sepsis group.
Discussion
TRPA1 plays an important role in the sensory conduction of nerves, respiration, digestion, and other systems, typically as a mechanical, pain, or cold sensor [18]. TRPA1 can be translocated to mitochondria under stressful conditions, such as inflammation and oxidative stress [19]. TRPV1 can be activated by capsaicin, protons, high temperature, and inflammatory mediators [20]. In indomethacin-induced gastritis, mitochondrial TRPA1 can alleviate mitochondrial oxidative stress and subsequent damage to the gastric mucosa [21].
Previous studies have shown that pretreatment with hemin can effectively reduce mortality in models of sepsis [22]. Although physicians typically focus on treatment rather than prevention, it is clinically important to prevent sepsis by hemin pretreatment in hospitalized patients after major surgery and in elderly patients with a high risk of sepsis [23,24]. To verify the role of TRPA1 in sepsis, we compared the expression of TRPA1 in animals treated with either hemin or A-967079. We found that TRPA1 could significantly prevent inflammatory reactions and kidney injury, whereas inhibition of TRPA1 expression aggravated these changes. These results are in accordance with our histological findings. Our results suggest that the upregulation of TRPA1 levels may prevent inflammatory responses in organs and improve the survival of septic mice.
Mitochondrial dysfunction is the main cause of sepsis-induced organ failure, which is closely related to mortality. Mitochondria are the main source of ROS and the target of oxidative stress. Many targeting mechanisms are involved in the mitochondrial degradation of autophagosomes. Mitochondrial autophagy induced by PINK/parkin, and dependent on BNIP3 and NIX, is well known. Many studies have explored the key role for mitochondrial dysfunction in sepsis, finding that specific targeted antioxidant therapy for mitochondrial damage could relieve sepsis-related damage in experimental models [25]. In this study, CLP significantly promoted mitochondrial swelling and lipid peroxidation, and reduced SOD2 protein expression. Hemin prevented these changes.CLP increased serum GDH activity, whereas hemin blocked this change. Kidney ATP levels can be used as a marker of mitochondrial function. After hemin treatment, ATP levels in the kidney were higher than those in the CLP group, suggesting that the upregulation of TRPA1 can prevent mitochondrial oxidative damage and improve mitochondrial dysfunction during sepsis.
Mitochondrial biosynthesis is regulated by various transcription factors in mammalian cells. PGC1α is the major transcriptional regulator of mitochondrial biogenesis via NRF1 activation. PGC1α and NRF1 can activate TFAM, thereby regulating mitochondrial DNA replication and maintaining mitochondrial density. Recent studies have shown that the overexpression of tissue-specific TRPA1 can induce mitochondrial biosynthesis, thereby protecting against adriamycin-induced dilated cardiomyopathy [26]. Our study showed that CLP reduced the expression of PGC1α and TFAM levels, whereas hemin blocked these decreases, indicating that TRPA1 can activate mitochondrial biosynthesis.
Mitochondrial autophagy plays a vital role in the clearance of dysfunctional mitochondria. Deficiencies in mitochondrial autophagy have been shown to cause the accumulation of damaged mitochondria in chronic obstructive pulmonary disease [27]. In most mammalian cells, PINK1 and parkin play important roles in mediating the classical pathway of mitochondrial autophagy [28]. PINK1 is a sensor of mitochondrial damage, which accumulates in the outer membrane of damaged mitochondrial, and induces parkin translocation from the cytoplasm to mitochondria [29]. Compared with a nonseptic control group, PINK1 protein expression was found to be upregulated in septic patients [30]. A PARK2 deletion was shown to cause abnormal mitochondrial metabolic functions and reduce myocardial contractility in a model of LPS-induced sepsis [31]. BNIP3 is a protein related to Bcl2 with an atypical Bcl2 homologous domain 3 that is located in mitochondria. Mitochondrial BNIP3 induces mitochondrial autophagy by interfering with the interaction between Beclin1 and Bcl-xL. Recent studies have shown that BNIP3 is able to interact with PINK1, inhibit PINK1’s shear, and promote both parkin recruitment and clearance of mitochondria [32]. Our study found that CLP increased the expression of kidney PINK1, but downregulated the expression levels of parkin and BNIP3 in the kidney, whereas hemin + CLP prevented these changes. These results indicate that TRPA1 can enhance parkin- and BNIP3-mediated mitochondrial autophagy.
Mitochondrial fission and fusion are the main processes by which mitochondrial morphology is maintained. An imbalance in mitochondrial fission and fusion can promote the development of experimental sepsis. DRP1-mediated mitochondrial fission was shown to inhibit the activity of the super complex in the mitochondrial electron transport chain and induce lung injury in rats injected with LPS [33]. In this study, CLP significantly increased DRP1 protein expression and downregulated MFN2 levels. Hemin blocked these changes. Our results indicate that TRPA1 can regulate mitochondrial homeostasis by inhibiting mitochondrial fission and promoting mitochondrial fusion.
In conclusion, the upregulation of TRPA1 can protect kidneys from sepsis-related injury by promoting mitochondrial biosynthesis and dynamic balance. Induction of TRPA1 expression may alleviate such injuries. The regulation of mitochondrial function may also become a new therapeutic strategy to treat inflammatory diseases.
Acknowledgements
This work was supported by the Foundation of Zhejiang Medical and Health Science and Technology Project (No. 2017KY169), and the Scientific Research Foundation of Taizhou Science and Technology Bureau, Taizhou city, Zhejiang Province, China (No. 1601KY45).
Disclosure of conflict of interest
None.
References
- 1.Asgar J, Zhang Y, Saloman JL, Wang S, Chung MK, Ro JY. The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience. 2015;310:206–215. doi: 10.1016/j.neuroscience.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barriere DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin MA, Chapuis L, Salles J, Dubray C, Morio B. Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain. 2012;153:553–561. doi: 10.1016/j.pain.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: a gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnham JP, Lane MA, Kollef MH. Impact of sepsis classification and multidrug-resistance status on outcome among patients treated with appropriate therapy. Crit Care Med. 2015;43:1580–1586. doi: 10.1097/CCM.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudry IH, Wichterman KA, Baue AE. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. [PubMed] [Google Scholar]
- 6.De I, Dogra N, Singh S. The mitochondrial unfolded protein response: role in cellular homeostasis and disease. Curr Mol Med. 2017;17:587–597. doi: 10.2174/1566524018666180308110130. [DOI] [PubMed] [Google Scholar]
- 7.Elimadi A, Sapena R, Settaf A, Le Louet H, Tillement J, Morin D. Attenuation of liver normothermic ischemia--reperfusion injury by preservation of mitochondrial functions with S-15-176, a potent trimetazidine derivative. Biochem Pharmacol. 2001;62:509–516. doi: 10.1016/s0006-2952(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 8.Ellis G, Goldberg DM. Optimal conditions for the kinetic assay of serum glutamate dehydrogenase activity at 37 degrees C. Clin Chem. 1972;18:523–527. [PubMed] [Google Scholar]
- 9.Fei D, Meng X, Kang K, Nan C, Zhao M, Pan S, Gao M, Yang S, Zhao M. Heme oxygenase-1 modulates thrombomodulin and activated protein C levels to attenuate lung injury in cecal ligation and puncture-induced acute lung injury mice. Exp Lung Res. 2012;38:173–182. doi: 10.3109/01902148.2012.660559. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez AS, Elguero ME, Finocchietto P, Holod S, Romorini L, Miriuka SG, Peralta JG, Poderoso JJ, Carreras MC. Abnormal mitochondrial fusion-fission balance contributes to the progression of experimental sepsis. Free Radic Res. 2014;48:769–783. doi: 10.3109/10715762.2014.906592. [DOI] [PubMed] [Google Scholar]
- 11.Kasuya F, Igarashi K, Fukui M. Characterization of a renal medium chain acyl-CoA synthetase responsible for glycine conjugation in mouse kidney mitochondria. Chem Biol Interact. 1999;118:233–246. doi: 10.1016/s0009-2797(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Hwang SW. Emerging roles of TRPA1 in sensation of oxidative stress and its implications in defense and danger. Arch Pharm Res. 2013;36:783–791. doi: 10.1007/s12272-013-0098-2. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Pan CC, Shah N, Wheeler SE, Hoyt KR, Hempel N, Mythreye K, Lee NY. Activation of Mitofusin2 by Smad2-RIN1 complex during mitochondrial fusion. Mol Cell. 2016;62:520–531. doi: 10.1016/j.molcel.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Zhang C, Liu X. Role of glucose metabolism and ATP in maintaining PINK1 levels during Parkin-mediated mitochondrial damage responses. J Biol Chem. 2015;290:904–917. doi: 10.1074/jbc.M114.606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Tai Y, Caceres AI, Achanta S, Balakrishna S, Shao X, Fang J, Jordt SE. Oxidized phospholipid OxPAPC activates TRPA1 and contributes to chronic inflammatory pain in mice. PLoS One. 2016;11:e0165200. doi: 10.1371/journal.pone.0165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo Verso F, Carnio S, Vainshtein A, Sandri M. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity. Autophagy. 2014;10:1883–1894. doi: 10.4161/auto.32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 18.Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW. Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal. 2014;20:474–494. doi: 10.1089/ars.2013.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasa P, Juneja D, Singh O. Severe sepsis and septic shock in the elderly: an overview. World J Crit Care Med. 2012;1:23–30. doi: 10.5492/wjccm.v1.i1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naziroglu M. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res. 2012;32:134–141. doi: 10.3109/10799893.2012.672994. [DOI] [PubMed] [Google Scholar]
- 21.Piquereau J, Godin R, Deschenes S, Bessi VL, Mofarrahi M, Hussain SN, Burelle Y. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9:1837–1851. doi: 10.4161/auto.26502. [DOI] [PubMed] [Google Scholar]
- 22.Sarangi J, Coleby M, Trivella M, Reilly S. Prevention of post splenectomy sepsis: a population based approach. J Public Health Med. 1997;19:208–212. doi: 10.1093/oxfordjournals.pubmed.a024611. [DOI] [PubMed] [Google Scholar]
- 23.Shao Y, He J, Chen F, Cai Y, Zhao J, Lin Y, Yin Z, Tao H, Shao X, Huang P, Yin M, Zhang W, Liu Z, Cui L. Association study between promoter polymorphisms of ADAM17 and progression of sepsis. Cell Physiol Biochem. 2016;39:1247–1261. doi: 10.1159/000447830. [DOI] [PubMed] [Google Scholar]
- 24.Shiba-Fukushima K, Arano T, Matsumoto G, Inoshita T, Yoshida S, Ishihama Y, Ryu KY, Nukina N, Hattori N, Imai Y. Phosphorylation of mitochondrial polyubiquitin by PINK1 promotes Parkin mitochondrial tethering. PLoS Genet. 2014;10:e1004861. doi: 10.1371/journal.pgen.1004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siavashi V, Asadian S, Taheri-Asl M, Keshavarz S, Zamani-Ahmadmahmudi M, Nassiri SM. Endothelial progenitor cell mobilization in preterm infants with sepsis is associated with improved survival. J Cell Biochem. 2017;118:3299–3307. doi: 10.1002/jcb.25981. [DOI] [PubMed] [Google Scholar]
- 26.Smutzer G, Devassy RK. Integrating TRPV1 receptor function with capsaicin psychophysics. Adv Pharmacol Sci. 2016;2016:1512457. doi: 10.1155/2016/1512457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian L, Fan T, Zhou N, Guo H, Zhang W. Role of PAR2 in regulating oxaliplatin-induced neuropathic pain via TRPA1. Transl Neurosci. 2015;6:111–116. doi: 10.1515/tnsci-2015-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Guo J, Fei E, Mu Y, He S, Che X, Tan J, Xia K, Zhang Z, Wang G, Tang B. BAG5 protects against mitochondrial oxidative damage through regulating PINK1 degradation. PLoS One. 2014;9:e86276. doi: 10.1371/journal.pone.0086276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron. 2015;85:833–846. doi: 10.1016/j.neuron.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W, Cui Z, Cui T, Wang XL, Shen YH. PINK1-parkin-mediated mitophagy protects mitochondrial integrity and prevents metabolic stress-induced endothelial injury. PLoS One. 2015;10:e0132499. doi: 10.1371/journal.pone.0132499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Xue L, Li L, Tang C, Wan Z, Wang R, Tan J, Tan Y, Han H, Tian R, Billiar TR, Tao WA, Zhang Z. BNIP3 protein suppresses PINK1 kinase proteolytic cleavage to promote mitophagy. J Biol Chem. 2016;291:21616–21629. doi: 10.1074/jbc.M116.733410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Duan G, Lang L, Liu Y, Zhu J, Wang H, Liu Y. The bacterial component flagellin induces anti-sepsis protection through TLR-5, IL-1RN and VCAN during polymicrobial sepsis in mice. Cell Physiol Biochem. 2015;36:446–456. doi: 10.1159/000430111. [DOI] [PubMed] [Google Scholar]


