Abstract
Muscone is the main active monomer of traditional Chinese medicine musk. Previous studies have reported a variety of beneficial effects of muscone. However, the effects of muscone on chronic inflammation after myocardial infarction (MI) are rarely reported. This study evaluated the anti-inflammatory effects of muscone on myocardial infarction by establishing a MI model in mice. We found that muscone remarkably decreased the levels of inflammatory cytokines (IL-1β, TNF-α and IL-6), and ultimately improved cardiac function and survival rate. Furthermore, the main anti-inflammatory effect of muscone was alleviating cardiac macrophage-mediated inflammatory response in heart tissues after MI. Bone marrow-derived macrophages (BMDMs) induced with lipopolysaccharide (LPS) were used as an in vitro inflammation model to further clarify anti-inflammatory mechanisms of muscone. Muscone significantly downregulated the levels of LPS-induced inflammatory cytokines and inhibited NF-κB and NLRP3 inflammasome activation in BMDMs. Moreover, ROS and antioxidant indices in LPS-induced BMDMs were also ameliorated after muscone treatment. To sum up, our study found that muscone alleviated cardiac macrophage-mediated chronic inflammation by inhibiting NF-κB and NLRP3 inflammasome activation, thereby improving cardiac function in MI mice. Besides, the inhibitory effect of muscone on inflammation may be related to the scavenging of ROS. It is suggested that muscone may serve as a promising and effective drug for post-MI treatment.
Keywords: Muscone, anti-inflammation, myocardial infarction, macrophage, NF-κB, NLRP3 inflammasome
Introduction
Myocardial infarction (MI) is one of the primary causes of hospitalization and death worldwide. Although timely reperfusion therapy and active drug therapy contribute to reduce infarct size and mortality, adverse ventricular remodeling after MI is still the leading cause of heart failure [1,2]. Therefore, it is particularly urgent to develop new treatments to ameliorate adverse ventricular remodeling and post-MI heart failure. The latest CANTOS clinical trial published in New England Journal demonstrated that interleukin-1 beta (IL-1β) monoclonal antibody Canakinumab could remarkably decrease the incidence of recurrent cardiovascular events in MI patients [3]. This makes the coronary atherosclerosis inflammation hypothesis more scientific, and anti-inflammatory treatment after MI has once again become a research hotspot.
Inflammatory response can be immediately triggered after MI [4]. The inflammatory response exerts a two-way regulation in myocardial injury and repair after MI. Studies have shown that moderate inflammation in the early stage of MI may be beneficial to promote myocardial repair. However, overactive and persistent chronic inflammation after MI results in accentuated ventricular remodeling and poor cardiac function [5-7]. The inflammatory response after MI is primarily regulated by macrophages [8,9]. Meanwhile, macrophage-mediated chronic inflammation contributes to the development of fibrosis and dysfunction in multiple organs, including the heart [10].
Nuclear Factor-κB (NF-κB) is one of the critical transcription factors that are capable of regulating the inflammatory gene expressions. Necrotic cardiomyocytes after MI generate alarm signals known as danger-associated molecular patterns (DAMPs), further leading to the activation of toll-like receptor 4 (TLR4)/NF-κB signaling pathway. Multiple inflammatory cytokines and chemokines are subsequently released. Among them, IL-1β, tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are reported to be the most important inflammatory cytokines involved in chronic inflammation during ventricular remodeling [11,12]. Nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome participates in the post-MI inflammation as well. NLRP3 inflammasome contains a NLRP3 protein that interacts with its adapter apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC) to recruit and activate caspase-1, which processes pro-IL-1β to mature IL-1β [13,14].
Active monomers in traditional Chinese medicine have obtained great concerns in recent years, and they are expected to be novel clinical therapeutic drugs. Musk, a high-value traditional Chinese medicine, has been widely applied to alleviate symptoms of myocardial ischemia in clinic [15,16]. Moreover, Heart-Protecting Musk Pill exerts a significant effect on inflammation inhibition [17,18]. As the main active monomer of musk, muscone has been reported to ameliorate ventricular remodeling after MI and exert anti-inflammatory effects on several chronic diseases such as intervertebral disc degeneration [19,20]. However, to the best of our knowledge, whether the protective effect of muscone on myocardial infarction is associated with inhibition of chronic inflammation after MI is rarely reported.
In this study, we aimed to investigate the effects of muscone on chronic inflammation after MI and its underlying mechanisms using a MI model in mice and an inflammation model in BMDMs. We demonstrated that muscone efficiently inhibited NF-κB and NLRP3 inflammasome activation in macrophages, thereby alleviating inflammatory response of macrophages in heart tissues and improving cardiac function in MI mice.
Materials and methods
Antibodies and drugs
Primary antibodies against β-actin (ab8226), IL-1β (ab9722), F4/80 (ab6640), phospho-NF-κB p65 (ab28856), NLRP3 (ab214185) and SOD1 (ab16831) were purchased from Abcam (USA); GAPDH (#2118), NF-κB p65 (#8242), IκB-α (#9242) and phospho-IκB-α (#9246) were purchased from Cell Signaling Technology (USA); IL-6 (A0286) was purchased from AB-clonal (USA); TNF-α (GTX110520) was obtained from GeneTex (USA); SOD2 (NB100-1992) was obtained from Novus (USA); TLR4 (19811-1-AP) and caspase1 (22915-1-AP) were obtained from Proteintech (USA). Horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson ImmunoResearch (USA). Alexa 488 or CY3-conjugated secondary antibodies were purchased from Servicebio (China). Muscone was purchased from Nanjing Zelang Biological Technology Company (China). Muscone was diluted with normal saline to 0.5 mg/ml for in vivo study and diluted with RPMI 1640 medium to 6 μg/ml for in vitro study. Lipopolysaccharide (LPS) from Escherichia coli O55:B5 was purchased from Sigma (USA) and diluted with RPMI 1640 medium to 1 μg/ml for in vitro study.
Animals and experimental procedure
C57BL/6J mice (male, 7 to 8-week-old) were purchased from the Model Animal Research Center of Nanjing University. Mice had free access to food and water and were maintained in a 12 h/12 h light-dark cycle with a room temperature of 23±2°C and a relative humidity of 45±10%. All animal experiments were carried out according to the guidelines for animal care set by the Institute for Laboratory Animal Research of Nanjing Medical University. This study was approved by Animal Ethical and Welfare Committee of Nanjing Medical University (Approval No. IACUC-1709001).
Mice were randomly divided into sham group (n = 12), MI group (n = 24) and MI+muscone group (n = 24). Myocardial infarction was established by ligation of left anterior descending coronary artery permanently referring to previous description [21]. Briefly, mice were anesthetized by the intraperitoneal injection of pentobarbital sodium (50 mg/kg) and then placed in a supine position. After endotracheal intubation, artificial mechanical ventilation was performed (tidal volume of 1.8 ml, an inspiratory and expiratory ration of 2:1, and a respiratory rate of 130 breaths per min). After the heart exposure, an 8-0 nylon suture was passed approximately 2-3 mm below the tip of the left auricle for permanent ligation of left anterior descending coronary artery. It was observed that the color of the infarcted zone was changed from red to white. Mice in sham group received the same procedure except for ligation. Muscone was administrated once a day at a dose of 2 mg/kg by gavage according to previous study [19]. To avoid interference with the acute inflammatory phase, mice received administration of muscone (MI+muscone group) or equivalent volume of saline (sham and MI groups) at the 7th day after MI for consecutive 3 weeks. The survival rate was measured by Kaplan-Meier survival curve analysis. All mice were sacrificed by carbon dioxide at the 4th week after MI.
Echocardiography
Cardiac function was evaluated at the 4th week after MI by the Vevo 2100 ultrasound system (Visual Sonic, Canada) equipped with a 30-MHz transducer as previously described [21]. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) of each mouse were calculated.
Histopathology of myocardium
Mouse hearts from each group were harvested and then fixed in 10% buffered neutral formalin solution. After standard paraffin embedding, heart tissues were cut into sequential sections in 4 μm thickness and then stained with hematoxylin-eosin (HE). The representative histopathologic images were captured by a light microscopy (Nikon, Japan) and analyzed using Image Pro Plus software (version 6.0).
Measurement of antioxidant enzymes activity and lipid peroxidation
Myocardial tissues from left ventricle were homogenized in pre-cold phosphate buffered saline (PBS) to prepare for a 10% homogenate, followed by centrifugation at 3000 rpm at 4°C for 15 min. Malondialdehyde (MDA, a product of lipid peroxidation) and antioxidant enzymes such as total superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px) in the supernatant were measured by relative assay kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China).
Immunohistochemistry
Paraffin sections were deparaffinized, hydrated in alcohol, and washed in tap water. Antigen retrieval was carried out in 10 mM sodium citrate buffer (pH 6.0) for 20 min using a microwave oven. After blocking, sections were incubated with the primary antibodies overnight at 4°C. A two-step technique (Maixin Biotech, China) was used for visualization, with DAB as a chromogen. Finally, sections were counterstained with hematoxylin and sealed with neutral balsam. Primary antibodies for immunohistochemistry were as follows: IL-1β, TNF-α, IL-6, NLRP3 and p-p65.
Immunofluorescence
A double-staining procedure for F4/80 (macrophage marker) and inflammatory cytokine (IL-1β, TNF-α or IL-6) was conducted as briefly described below. After deparaffinization, hydration and washing, slides were boiled in Tris-EDTA retrieval solution (pH 8.0) (Servicebio, China) for 20 min using a microwave oven for antigen retrieval. After incubation with tissue spontaneous fluorescence quenching agent (Servicebio, China), sections were blocked by bovine serum albumin (BSA) (Servicebio, China). Subsequently, sections were incubated with mixed primary antibodies overnight at 4°C. On the next day, sections were incubated with Cy3-conjugated AffiniPure Goat Anti-Rat IgG (H+L) and Alexa Fluor® 488-conjugated Affini-Pure Goat Anti-Rabbit IgG (H+L) secondary antibodies at room temperature for 1 h. Slides were washed three times with PBS and sealed with DAPI Fluoromount-G® mounting medium (Southern Biotech, USA).
Cell culture and treatment
Bone marrow-derived macrophages (BMDMs) were isolated from femurs and tibias of 3 to 4-week-old C57BL/6J mice. After detached, washed and counted, cells were seeded in 6-well plates at 1 × 106 cells/well. Cells were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin/streptomycin (Gibco, USA) in presence of 20 ng/ml recombinant murine macrophage colony-stimulating factor(M-CSF) (Sigma, USA). The culture medium was exchanged every 3 days and nonadherent cells were discarded on the 3rd day. Adherent cells used for subsequent experiments as BMDMs were obtained on the 7th day. Then the culture medium was replaced by RPMI 1640 medium supplemented with 1% FBS for a 12-hour starvation treatment. Afterwards, inflammation was induced with 1 μg/ml LPS in the absence or presence of muscone for 24 h as LPS group or LPS+muscone group, respectively. Untreated BMDMs were served as control group.
Cell viability assays
To evaluate the possible toxicity of muscone towards BMDMs, the Enhanced Cell Counting Kit-8 (Beyotime, China) was used according to the manufacturer’s instructions. BMDMs were seeded in a 96-well plate at 2 × 104 cells/well and cultured to adherence. Then the culture medium was replaced by RPMI 1640 medium alone and BMDMs were treated with muscone in triplicate at each concentration (0, 1.5, 3, 6, 12 and 24 μg/ml) for 24 h. Subsequently, 10 μl enhanced CCK-8 solution was added to each well and the absorbance at 450 nm was measured 2 h after incubation.
Intracellular ROS analysis
Intracellular reactive oxygen species (ROS) in BMDMs was assessed using 2’,7’-dichlorofluorescin diacetate (DCFH-DA) (Sigma, USA). BMDMs were cultured in 6-well plates at 1 × 106 cells/well. After starvation treatment and inflammation induction, BMDMs were incubated with 10 μM DCFH-DA detection reagent in the dark at 37°C for 30 min and then washed twice with PBS. Oxidized DCF fluorescence (representing ROS level) was detected immediately by flow cytometry analysis in a FACS Calibur flow cytometer (Becton Dickinson, Germany) and fluorescent microscope (Mshot, China).
Enzyme-linked immunosorbent assay (ELISA)
Serum samples of mice from each group were collected at the 4th week after MI. Cell culture supernatants from each group were collected and concentrated using centrifugal filter units to remove cellular debris after 24 h-treatment. IL-1β in cell culture supernatants and serum level of brain natriuretic peptide (BNP) in mice were quantified using ELISA kits (YIFEIXUE BIO TECH, China) according to the manufacturer’s instructions.
Western blotting
Total protein was obtained from left ventricular myocardial tissues in mice or BMDMs by sonication, centrifugation and heat denaturation. Protein concentrations were measured by BCA Protein Assay Kit (KeyGEN BioTECH, Nanjing, China). Equal amount (30 µg/lane) of protein was subjected to 8-12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). After blocking with 5% skim milk in TBST at room temperature for 2 h, membranes were incubated with primary antibodies at 4°C overnight. On the next day, membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. Finally, the membranes were exposed using the ECL system (Tanon, China). The immunoreactivity was quantitated using Image J software (version 1.44). Primary antibodies used in the study were as follows: GAPDH, β-actin, TLR4, IL-1β, TNF-α, IL-6, NLRP3, caspase1, p-IκB-a, IκB-a, p-p65, p65, SOD1 and SOD2.
RNA preparation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted left ventricular myocardial tissues or BMDMs using Trizol reagent (Invitrogen, USA). Reverse transcription was performed using 5 × All-In-One RT MasterMix (Applied Biological Materials Inc., Canada) referring to the manufacturer’s instructions. qRT-PCR was performed using Hieff™ qPCR SYBR® Green Master Mix (Yeasen, China) on StepOne-Plus Real-Time PCR system (Applied Biosystems, USA) according to the manufacturer’s protocol. Relative gene expressions were processed using the 2-ΔΔCt method and normalized to GAPDH as an endogenous control for the mRNA detection. Primer sequences are shown in Table 1.
Table 1.
Primer sequences used for quantitative real-time polymerase chain reaction
| Targets | Primer sequences (sense) | Primer sequences (antisense) |
|---|---|---|
| GAPDH | 5’-CCCTTAAGAGGGATGCTGCC-3’ | 5’-TACGGCCAAATCCGTTCACA-3’ |
| IL-1β | 5’-TGCCACCTTTTGACAGTGATG-3’ | 5’-TGATGTGCTGCTGCGAGATT-3’ |
| IL-6 | 5’-CCCCAATTTCCAATGCTCTCC-3’ | 5’-CGCACTAGGTTTGCCGAGTA-3’ |
| TNF-α | 5’-GATCGGTCCCCAAAGGGATG-3’ | 5’-TTTGCTACGACGTGGGCTAC-3’ |
| SOD1 | 5’-ATTGGCCGTACAATGGTGGT-3’ | 5’-AGACTCAGACCACACAGGGA-3’ |
| SOD2 | 5’-GTAGGGCCTGTCCGATGATG-3’ | 5’-CGCTACTGAGAAAGGTGCCA-3’ |
| Gpx1 | 5’-TGCAATCAGTTCGGACACCA-3’ | 5’-GTAAAGAGCGGGTGAGCCTT-3’ |
| CAT | 5’-CACTGACGAGATGGCACACT-3’ | 5’-TGTGGAGAATCGAACGGCAA-3’ |
Statistical analysis
Data from at least three independent experiments were expressed as mean ± standard deviation (SD). Kaplan-Meier survival analysis was performed to evaluate the survival rate of mice after MI, followed by comparison using log-rank test. Statistical analyses between groups were performed by unpaired Student’s t-test or one-way ANOVA. A two-sided value of P < 0.05 was considered to be statistically significant. All statistical analyses were conducted using SPSS software (version 22.0) or GraphPad Prism software (version 6.02).
Results
Muscone improved survival rate and cardiac function in MI mice
No significant differences in preoperative body weight, heart rate and LVEF were found between the groups (Table 2). At the 4th week after surgery, there was no death in sham group, and the survival rate in MI+muscone group was higher than that in MI group (Figure 1A). At the 4th week after MI, we found abnormal left ventricular anterior wall motion and decreased LVEF and LVFS in MI group and MI+muscone group when compared with sham group. Motion range of left ventricle anterior wall, LVEF and LVFS were remarkably improved in MI+muscone group than those in MI group (Figure 1B-D). Meanwhile, muscone treatment significantly reduced serum levels of BNP in MI mice (Figure 1E). Taken together, these results indicated that muscone could improve survival rate and cardiac function in mice at the 4th week after MI.
Table 2.
Basic parameters of the mice. Data are expressed as mean ± SD
| Basic parameters | Sham (n = 12) | MI (n = 24) | MI+muscone (n = 24) | P value |
|---|---|---|---|---|
| Body mass (g) | 23.83±0.77 | 23.70±1.02 | 23.98±1.09 | 0.62 |
| Heart rate (per min) | 473.25±56.07 | 460.75±46.12 | 481.25±67.14 | 0.47 |
| LVEF (%) | 58.48±3.07 | 58.16±3.20 | 57.73±2.73 | 0.76 |
Figure 1.
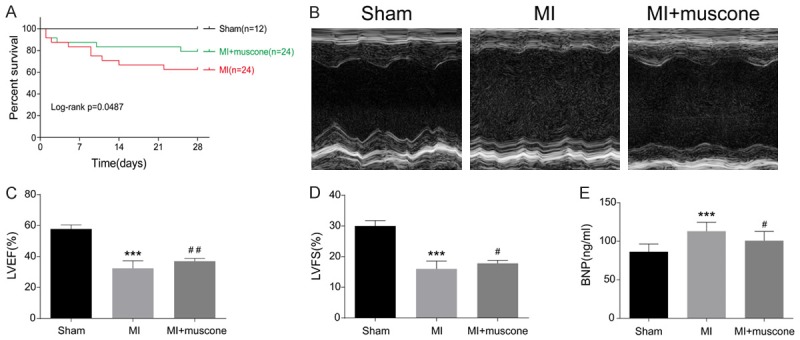
Muscone improved survival rate and cardiac function in MI mice. (A) Survival rate of mice at the 4th week after MI. Kaplan-Meier analysis showed lower mortality in mice of MI+muscone group compared with those in MI group (log-rank: P = 0.0457). (B) Representative M-mode echocardiograms of mice at the 4th week after MI. LVEF (C) and LVFS (D) were measured by echocardiography. Data are expressed as mean ± SD (n = 12 per group). ***P < 0.001 versus sham group, #P < 0.05, ##P < 0.01 versus MI group. (E) Serum levels of BNP in mice at the 4th week after MI were measured by ELISA. Data are expressed as mean ± SD (n = 12 per group). ***P < 0.001 versus sham group, #P < 0.05 versus MI group.
Muscone inhibited cardiac macrophage-mediated chronic inflammation in MI mice
Chronic inflammation plays a crucial role in post-MI ventricular remodeling. At the 4th week after MI, HE staining revealed that muscone reduced the infiltration of inflammatory cells after MI (Figure 2A). Immunohistochemical staining showed that inflammatory cytokines (IL-1β, TNF-α and IL-6) were mainly distributed in myocardial interstitium and the positive expressions of them could be reduced by muscone treatment (Figure 2B-D). qRT-PCR results showed that mRNA levels of inflammatory cytokines were lower in MI+muscone group than those in MI group (Figure 2E). Macrophages are the main immune cells involved in chronic inflammation in the late stage of MI. To further clarify the inhibitory effect of muscone on post-MI inflammation, we examined the colocalization of these inflammatory cytokines with macrophage surface marker F4/80. We found that IL-1β, TNF-α and IL-6 were mainly expressed in macrophages at the 4th week after MI, which were remarkably reduced after muscone treatment (Figure 2F-H). The above results demonstrated that muscone exerted its anti-inflammatory effects after MI mainly by inhibiting cardiac macrophage-mediated inflammatory response.
Figure 2.
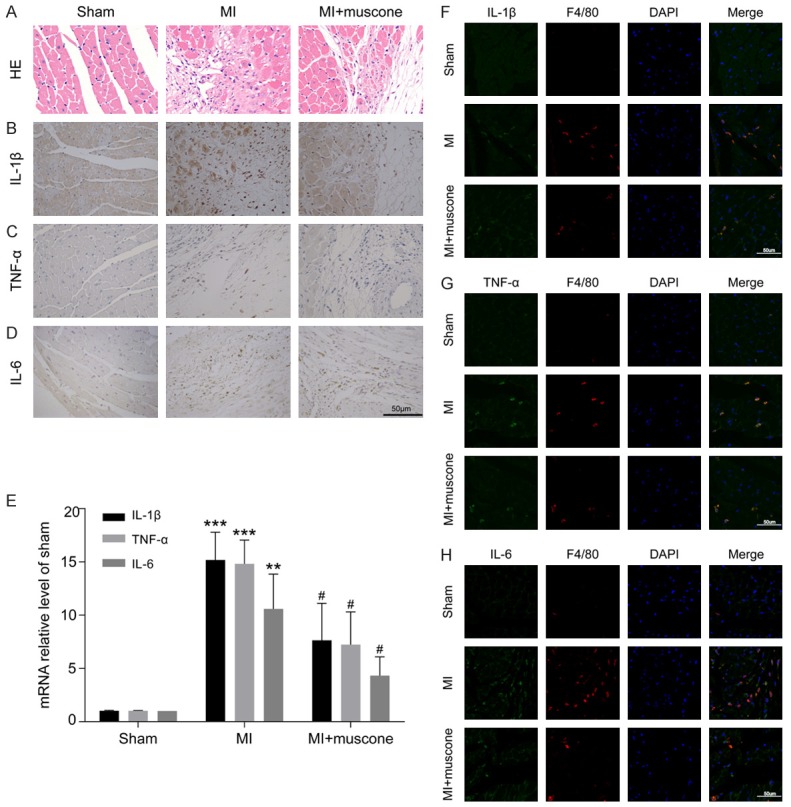
Muscone inhibited cardiac macrophage-mediated chronic inflammation in MI mice. Representative photomicrographs of HE staining (A) and immunohistochemical staining for IL-1β, TNF-α and IL-6 (B-D) in paraffin embedded heart sections at the 4th week after MI (bar = 50 μm). (E) The mRNA levels of IL-1β, TNF-α and IL-6 were determined by qRT-PCR, which were calculated as a ratio to the mRNA level of GAPDH and expressed relative to sham group. The results are representative of three independent experiments and shown as mean ± SD. **P < 0.01, ***P < 0.001 versus sham group, #P < 0.05 versus MI group. (F-H) The colocalization of IL-1β, TNF-α or IL-6 with F4/80 was detected by immunofluorescence staining (bar = 50 μm). Green represented IL-1β, TNF-α or IL-6; Red represented F4/80; Blue represented nuclei.
Muscone reduced LPS-induced inflammatory cytokines in BMDMs
To evaluate the possible cytotoxicity of muscone, BMDMs were treated with different doses of muscone for 24 h. CCK-8 results elucidated that no cytotoxicity to BMDMs was found even muscone dose was up to 24 μg/ml (Figure 3A). BMDMs induced with LPS are considered as a classical inflammation model in vitro. The inhibitory effect of muscone on LPS-induced IL-1β production in BMDMs presented a dose-dependent manner. Moreover, 6 μg/ml muscone itself did not affect IL-1β production in BMDMs (Figure 3B and 3C). Hence, we selected 6 μg/ml muscone as the treatment dose for the following experiments. After LPS induction for 24 h, protein and mRNA levels of IL-1β, TNF-α and IL-6 in BMDMs were obviously increased, which were remarkably suppressed by muscone (Figure 3D and 3E). Meanwhile, muscone reduced IL-1β production in culture supernatants induced with LPS (Figure 3F). All results indicated that muscone could remarkably alleviate inflammatory response in macrophages.
Figure 3.
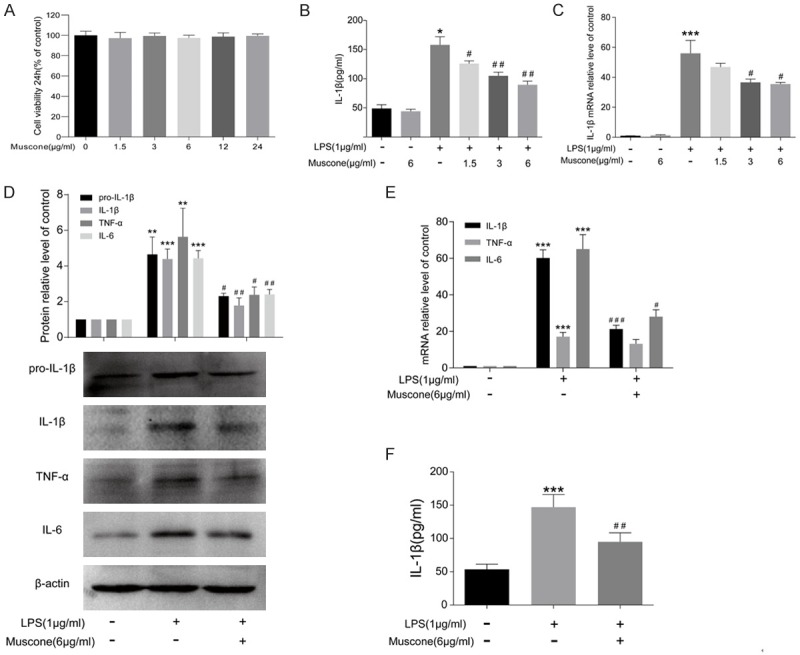
Muscone reduced LPS-induced inflammatory cytokines production in BMDMs. A. BMDMs were treated with muscone at increasing concentrations (0, 1.5, 3, 6, 12 and 24 μg/ml) for 24 h. Cell viability was measured using CCK-8 assay. B. IL-1β concentrations in culture supernatants were measured by ELISA following treatment with 1 μg/ml LPS and 1.5, 3 or 6 μg/ml muscone for 24 h. C. The mRNA level of IL-1β in BMDMs was measured by qRT-PCR following treatment with 1 µg/ml LPS and 1.5, 3 or 6 µg/ml muscone for 24 h. D. Western blot analysis of pro-IL-1β, IL-1β, TNF-α and IL-6 in BMDMs after being incubated for 24 h. β-actin was used as a loading control. E. The mRNA levels of IL-1β, IL-6 and TNF-α in BMDMs after being incubated for 24 h. F. IL-1β concentrations in culture supernatants were measured by ELISA after being incubated for 24 h. All results are representative of three independent experiments and shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group, #P < 0.05, ##P < 0.01, ###P < 0.001 versus LPS group.
Muscone suppressed inflammatory response by downregulating NF-κB and NLRP3 inflammasome both in vivo and in vitro
To further elucidate the detailed mechanisms, we investigated the expressions of TLR4, NF-κB and NLRP3 inflammasome, all of which were well documented to be involved in inflammatory response. Our results found that muscone treatment did not influence TLR4 expression in LPS-induced BMDMs (Figure 4A). Similarly, in heart tissue harvested from MI mice, muscone also had no significant effect on the expression of TLR4 (Figure 4B). As shown in Figure 4C, NF-κB and NLRP3 inflammasome pathway in BMDMs were activated after LPS induction for 24 h, manifesting as downregulated IκB-a and upregulated p-IκB-a, p-p65, NLRP3 and caspase-1. However, muscone treatment markedly alleviated the above changes. We also detected NF-κB and NLRP3 inflammasome in heart tissues of MI mice by western blot and immunohistochemistry, which were consistent with the results in vitro (Figure 4D and 4E).
Figure 4.
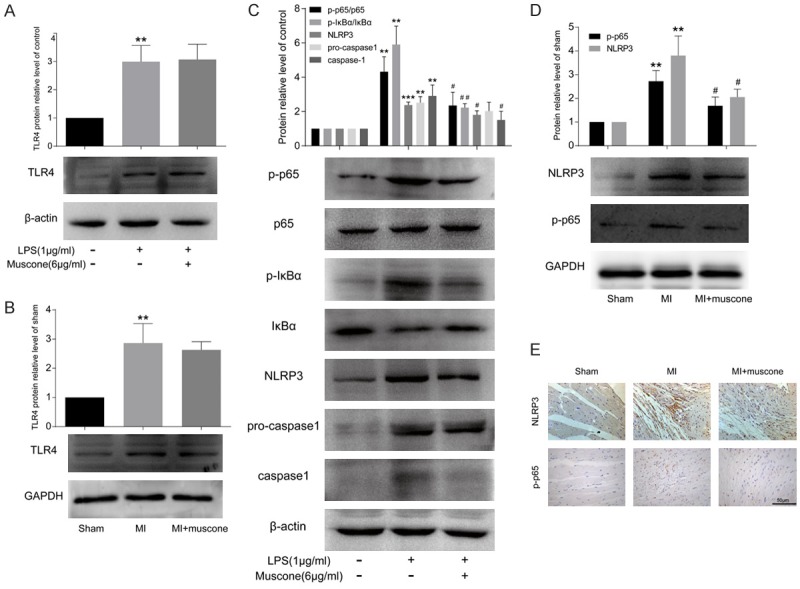
Muscone exerted anti-inflammatory effects by inhibiting NF-κB and NLRP3 inflammasome activation both in vivo and in vitro. A. After being incubated for 24 h, the protein level of TLR4 in BMDMs was measured by western blot. B. The protein level of TLR4 in mouse hearts at the 4th week after MI was measured by western blot. C. Western blot analysis of p-p65, p65, p-IκB-a, IκB-a, NLRP3, pro-caspase1and caspase1 in BMDMs after being incubated for 24 h. β-actin was used as a loading control. D. Western blot analysis of p-p65 and NLRP3 in mouse hearts at the 4th week after MI. GAPDH was used as a loading control. E. Representative photomicrographs of immunohistochemical staining for p-p65 and NLRP3 in paraffin embedded heart sections at the 4th week after MI (bar = 50 μm). All results are representative of three independent experiments and shown as mean ± SD. **P < 0.01, ***P < 0.001 versus control or sham group, #P < 0.05, ##P < 0.01versus LPS or MI group.
Muscone exhibited antioxidant effects both in vivo and in vitro
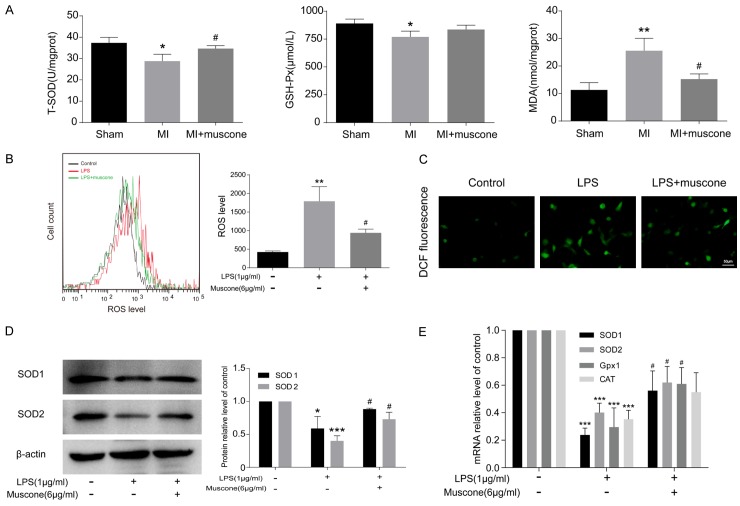
The levels of oxidative stress were measured in cardiac tissue homogenates of mice from each group at the 4th week after surgery. Compared with sham group, heart tissues from MI mice presented decreased T-SOD and GSH-Px, as well as increased MDA at the 4th week after MI. Mice in MI+muscone group showed higher T-SOD and GSH-Px, but lower MDA than those in MI group (Figure 5A). In vitro, experiments demonstrated higher ROS level in LPS-induced BMDMs than control cells, which decreased after muscone treatment (Figure 5B and 5C). Protein and mRNA levels of antioxidant enzymes were increased in LPS+muscone group when compared with LPS group (Figure 5D and 5E). Taken together, muscone could reduce the production of ROS and exhibit antioxidant effects in macrophages after MI.
Figure 5.
Muscone exhibited antioxidant effects both in vivo and in vitro. (A) Concentrations of total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) in cardiac tissue homogenates were determined by spectrophotometry. BMDMs were incubated for 24 h and then reacted with 10 mM DCFH-DA for 30 min. ROS was detected by flow cytometry (B) and fluorescence microscope (C). (D) Western blot analysis of SOD1 and SOD2 in BMDMs after being incubated for 24 h. β-actin was used as a loading control. (E) The mRNA levels of SOD1, SOD2, Gpx1 and CAT in BMDMs after being incubated for 24 h. All results are representative of three independent experiments and shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control or sham group, #P < 0.05 versus LPS or MI group.
Discussion
Myocardial infarction has long been considered as an inflammatory disease. The occurrence, development and prognosis of MI are closely related to the overexpression of inflammatory cytokines. Anti-inflammatory therapy is now expected to become a novel target for MI intervention [22-24]. In this study, our experimental data found that muscone inhibited cardiac macrophage-mediated chronic inflammation in MI mice, thereby improving cardiac function. In vitro, our study showed that muscone reduced the expressions of LPS-induced inflammatory cytokines in BMDMs.
Inflammatory response in macrophages induced with LPS is typically initiated through TLR4, which consequently promotes the downstream events [25]. Besides, studies have shown that the inhibition of TLR4 signaling pathway is beneficial for alleviating inflammatory response and improving cardiac function [26,27]. However, our study found that muscone treatment had no significant effects on the expression of TLR4. These results indicated that the anti-inflammatory effects of muscone on macrophages are not directly dependent on the inhibition of TLR4 but may be related to the inhibition of its downstream genes.
NF-κB is involved in the generation of inflammatory cytokines when LPS is recognized by TLR4 in macrophages [28]. NLRP3 inflammasome is another important factor in regulating macrophage inflammatory response. It has been found that caspase-1 activation mediated by NLRP3 inflammasome is necessary for conversion of pro-IL-1β into a mature active form IL-1β [29,30]. NLRP3 expression is upregulated in mice after MI. Knockdown of NLRP3 expression can reduce post-MI inflammatory response and improve ventricular remodeling [31,32]. Our study found that muscone effectively inhibited the activation of NF-κB and NLRP3 inflammasome both in vivo and in vitro. Therefore, we concluded that the anti-inflammatory effects of muscone may be achieved by inhibiting NF-κB and NLRP3 inflammasome activation in macrophages.
In our study, muscone increased expression of antioxidant enzymes and decreased MDA production in MI mice. Besides, muscone also reduced ROS level and increased antioxidant enzymes in LPS-induced BMDMs. It is generally believed that ischemic myocardium produces ROS after MI, and ROS can promote inflammatory response [33]. Previous studies have found that ROS produced by macrophages can regulate the NF-κB pathway and trigger the activation of NLRP3 inflammasome, thereafter producing a series of inflammatory cytokines [34-36]. Meanwhile, the scavenging of ROS in macrophages is reported to be capable of inhibiting NF-κB and NLRP3 inflammasome activation, and reducing the production of inflammatory cytokines, such as IL-1β [37-39]. Taken together, the inhibition of NF-κB and NLRP3 inflammasome activation after muscone treatment may be attributed to the scavenging of ROS. However, the specific interaction still needs to be further studied.
In conclusion, our study firstly found that muscone could improve cardiac function in MI mice by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome activation. Besides, the anti-inflammatory effect of muscone on macrophages may be related to the scavenging of ROS. Therefore, muscone may become a promising and effective drug for post-MI treatment.
Acknowledgements
This study was funded by the National High Technology Research and Development Program of China (863 Program), the National Natural Science Foundation of China (No. 81170102 & No. 81441011 & No. 81670328), the Doctoral Scientific Fund Project of the Ministry of Education of China (20123234110015), the Fourth Period Project “333” of Jiangsu Province (BRA2012207), the Chinese Medical Association of the Sunlight Foundation (SCRFCMDA201217), and the Collaborative Innovation Center of Nanjing Medical University.
Disclosure of conflict of interest
None.
References
- 1.Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 2.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein J, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi P, Troquay R, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 4.Gombozhapova A, Rogovskaya Y, Shurupov V, Rebenkova M, Kzhyshkowska J, Popov SV, Karpov RS, Ryabov V. Macrophage activation and polarization in post-infarction cardiac remodeling. J Biomed Sci. 2017;24:13. doi: 10.1186/s12929-017-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Guo Z, Ding Z, Mehta JL. Inflammation, autophagy, and apoptosis after myocardial infarction. J Am Heart Assoc. 2018;7:e008024. doi: 10.1161/JAHA.117.008024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167:152–166. doi: 10.1016/j.trsl.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, Kachel E, Holbova R, Mardor Y, Daniels D, Ocherashvilli A, Orenstein A, Danon D. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114:I94–I100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 10.Zhou D, Yang K, Chen L, Wang Y, Zhang W, Xu Z, Zuo J, Jiang H, Luan J. Macrophage polarization and function: new prospects for fibrotic disease. Immunol Cell Biol. 2017;95:864–869. doi: 10.1038/icb.2017.64. [DOI] [PubMed] [Google Scholar]
- 11.Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol. 2014;63:185–195. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 13.Fang L, Moore XL, Dart AM, Wang LM. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol. 2015;12:305–312. doi: 10.11909/j.issn.1671-5411.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 15.Wang SH, Chu L, Xu Z, Zhou HL, Chen JF, Ning HF. Effect of Shexiang Tongxin dropping pills on the immediate blood flow of patients with coronary slow flow. Chin J Integr Med. 2018 doi: 10.1007/s11655-018-2559-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Shen W, Yu L, Xu C, Wu Q. A Chinese patent medicine, Shexiang Baoxin Pill, for Non-ST-elevation acute coronary syndromes: a systematic review. J Ethnopharmacol. 2016;194:1130–1139. doi: 10.1016/j.jep.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Cen W, Chen Z, Gu N, Hoppe R. Prevention of AMI induced ventricular remodeling: inhibitory effects of heart-protecting musk pill on IL-6 and TNF-Alpha. Evid Based Complement Alternat Med. 2017;2017:3217395. doi: 10.1155/2017/3217395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei D, Zheng N, Zheng L, Wang L, Song L, Sun L. Shexiang Baoxin pill corrects metabolic disorders in a rat model of metabolic syndrome by targeting mitochondria. Front Pharmacol. 2018;9:137. doi: 10.3389/fphar.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Meng H, Chen P, Yang N, Lu X, Wang ZM, Gao W, Zhou N, Zhang M, Xu Z, Chen B, Tao Z, Wang L, Yang Z, Zhu T. Beneficial effects of muscone on cardiac remodeling in a mouse model of myocardial infarction. Int J Mol Med. 2014;34:103–111. doi: 10.3892/ijmm.2014.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang QQ, Zhang M, Zhou Q, Shi Q, Wang YJ. Muscone protects vertebral end-plate degeneration by antiinflammatory property. Clin Orthop Relat Res. 2010;468:1600–1610. doi: 10.1007/s11999-009-1079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng W, Wu P, Du Y, Wang Y, Zhou N, Ge Y, Yang Z. Puerarin improves cardiac function through regulation of energy metabolism in Streptozotocin-Nicotinamide induced diabetic mice after myocardial infarction. Biochem Biophys Res Commun. 2015;463:1108–1114. doi: 10.1016/j.bbrc.2015.06.067. [DOI] [PubMed] [Google Scholar]
- 22.Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orn S, Ueland T, Manhenke C, Sandanger O, Godang K, Yndestad A, Mollnes TE, Dickstein K, Aukrust P. Increased interleukin-1beta levels are associated with left ventricular hypertrophy and remodelling following acute ST segment elevation myocardial infarction treated by primary percutaneous coronary intervention. J Intern Med. 2012;272:267–276. doi: 10.1111/j.1365-2796.2012.02517.x. [DOI] [PubMed] [Google Scholar]
- 24.Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: from bench to bedside. Biodrugs. 2018;32:111–118. doi: 10.1007/s40259-018-0274-5. [DOI] [PubMed] [Google Scholar]
- 25.Byun EB, Sung NY, Park JN, Yang MS, Park SH, Byun EH. Gamma-irradiated resveratrol negatively regulates LPS-induced MAPK and NF-kappaB signaling through TLR4 in macrophages. Int Immunopharmacol. 2015;25:249–259. doi: 10.1016/j.intimp.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Wang Y, Cao ZY, Wang MM, Liu XM, Gao T, Hu QK, Yuan WJ, Lin L. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J Cell Mol Med. 2015;19:2728–2740. doi: 10.1111/jcmm.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soraya H, Clanachan AS, Rameshrad M, Maleki-Dizaji N, Ghazi-Khansari M, Garjani A. Chronic treatment with metformin suppresses toll-like receptor 4 signaling and attenuates left ventricular dysfunction following myocardial infarction. Eur J Pharmacol. 2014;737:77–84. doi: 10.1016/j.ejphar.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Wu XL, Liou CJ, Li ZY, Lai XY, Fang LW, Huang WC. Sesamol suppresses the inflammatory response by inhibiting NF-kappaB/MAPK activation and upregulating AMP kinase signaling in RAW 264.7 macrophages. Inflamm Res. 2015;64:577–588. doi: 10.1007/s00011-015-0836-7. [DOI] [PubMed] [Google Scholar]
- 29.Di A, Xiong S, Ye Z, Malireddi R, Kometani S, Zhong M, Mittal M, Hong Z, Kanneganti TD, Rehman J, Malik AB. The TWIK2 potassium efflux channel in macrophages mediates nlrp3 inflammasome-induced inflammation. Immunity. 2018;49:56–65. doi: 10.1016/j.immuni.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang TC, Chang PY, Lu SC. L5-LDL from ST-elevation myocardial infarction patients induces IL-1beta production via LOX-1 and NLRP3 inflammasome activation in macrophages. Am J Physiol Heart Circ Physiol. 2017;312:H265–H274. doi: 10.1152/ajpheart.00509.2016. [DOI] [PubMed] [Google Scholar]
- 31.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisue K, Sugamura K, Kurokawa H, Matsubara J, Ishii M, Izumiya Y, Kaikita K, Sugiyama S. Colchicine improves survival, left ventricular remodeling, and chronic cardiac function after acute myocardial infarction. Circ J. 2017;81:1174–1182. doi: 10.1253/circj.CJ-16-0949. [DOI] [PubMed] [Google Scholar]
- 33.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 34.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Guo Y, Zhao J, Wang N, Ding J, Liu Q. Hydroxysafflor yellow a inhibits LPS-induced NLRP3 inflammasome activation via binding to xanthine oxidase in mouse RAW264.7 macrophages. Mediators Inflamm. 2016;2016:8172706. doi: 10.1155/2016/8172706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao LK, Lin CH, Chiu HW, Wong WT, Chiu HW, Tasi YL, Kuo YH, Chiu YC, Liu ML, Ho CL, Hua KF. Peroxyauraptenol inhibits inflammation and NLRP3 inflammasome activation by inhibiting reactive oxygen species generation and preserving mitochondrial integrity. J Agric Food Chem. 2015;63:1210–1219. doi: 10.1021/jf5054436. [DOI] [PubMed] [Google Scholar]
- 38.Ka SM, Kuoping CL, Lin JC, Chen ST, Li WT, Lin CN, Cheng JC, Jheng HL, Chen A, Hua KF. A low toxicity synthetic cinnamaldehyde derivative ameliorates renal inflammation in mice by inhibiting NLRP3 inflammasome and its related signaling pathways. Free Radic Biol Med. 2016;91:10–24. doi: 10.1016/j.freeradbiomed.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Ren JD, Wu XB, Jiang R, Hao DP, Liu Y. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochim Biophys Acta. 2016;1863:50–55. doi: 10.1016/j.bbamcr.2015.10.012. [DOI] [PubMed] [Google Scholar]