Abstract
This study was designed to explore the effects of long-term renal denervation (RDN) on blood pressure and renal function in spontaneously hypertensive rats (SHR). RDN was performed in bilateral renal arteries with 10% phenol in absolute ethanol in SHR and Wistar-Kyoto rats (WKY) at 13 weeks. Age-matched SHR and WKY served as controls. Blood pressure was measured. Plasma, urine and kidneys were collected 8 months after the RDN operation. Plasma renin activity (PRA), aldosterone levels, reactive oxidative stress, renal function and structural remodeling were assessed. RDN-treated SHR demonstrated a lower spontaneous rise in systolic blood pressure than rats in the SHR-Sham group (P < 0.01, at 20, 27, 34 and 41 weeks), except at 48 weeks (198.2 ± 12.9 vs 209.4 ± 11.9 mmHg, P = 0.145). WKY were not affected by RDN. Renal tissue norepinephrine was decreased by RDN in both SHR and WKY. Plasma PRA activity, aldosterone levels, and NAD(P)H oxidase activity were reduced by the RDN in SHR. Plasma eNOS and NO were increased by RDN only in SHR. The renal nerve was destroyed by RDN with no regeneration after 8 months. The progression of renal dysfunction associated with urinary protein excretion, glomerular sclerosis, and tubulointerstitial fibrosis was attenuated by RDN only in SHR through downregulation of the ACE/Ang II/AT1R axis and upregulation of the ACE2/Ang-(1-7)/MasR axis in the kidney. Thus, RDN delays the onset of hypertension and ameliorates glomerular sclerosis and tubulointerstitial fibrosis in SHR.
Keywords: Renal sympathetic denervation, hypertension, glomerular sclerosis, renal fibrosis
Introduction
Hypertension is a common cardiovascular disease worldwide and is a major factor involved in coronary heart disease, chronic heart failure, stroke, chronic kidney disease and even death [1]. It has been estimated that 972 million adults suffered hypertension in 2000 and that this number will increase to 1.56 billion in 2025 [2]. In addition, it has been reported that 8.9% of the population of the United States cannot control their blood pressure (BP > 140/90 mmHg) after taking of a minimum of 3 antihypertensive agents [3].
Sympathetic nerve activity (SNA) plays a distinct role in the development of hypertension. It can regulate hypertension through the innervation of the kidney, which enhances the renal SNA in different pathways including renin secretion and sodium reabsorption and reducing renal blood flow (RBF) [4]. Studies have indicated that most hypertension patients and animal models are characterized by sympathetic hyperactivity [5,6]. Renal SNA is doubled in hypertensive patients compared with that of normotensive individuals [7]. However, Gattone VH et al. proved that inhibition of sympathetic function ameliorates renal damage independently of systemic hypertension [8].
Recently, renal sympathetic denervation (RDN) has become a promising strategy for the treatment of hypertension [9-13]. In 2009, Krum et al. found that percutaneous renal artery ablation can effectively control refractory hypertension [9]. The Symplicity Hypertension 2 (HTN-2) trial furtherly confirmed the safety and effectiveness of RDN [10]. However, the negative results of HTN-3 challenged the feasibility of RDN [14], which was a disappointing outcome. However, the benefits of RDN have not yet been ruled out. Instead, more comprehensive mechanistic studies should be performed to explore the effects of RDN in the treatment of hypertension.
Spontaneously hypertensive rats (SHR), which compose a rat model of hypertension, develop high blood pressure spontaneously with age, as is observed in humans. The change in BP and the resulting end-organ damage in SHR are similar to those reported in patients selected for in clinical RDN. Additionally, an increase in renal sympathetic activity has been observed in this model [15]. Previous studies have indicated that RDN performed in young SHR delays or attenuates the development of hypertension [16-19]. Furthermore, RDN performed in the established phase of hypertension can lead to a significant and sustained reduction in BP [20-23]. Nevertheless, the effect of long-term RDN on BP in SHR has not yet been investigated yet. Luippold G et al. showed that chronic RDN could improve renal function in diabetic rats [24]. Hence, the present study was undertaken to evaluate the effects of long-term RDN on BP and renal function in SHR.
Methods and materials
Animals
Male SHR and Wistar-Kyoto rats (WKY) were obtained from Vital River Laboratory Animal Technology Company (Beijing, China) at the age of 12 weeks. Animals were maintained in standard laboratory animal housing conditions under a 12 h light/dark cycle and were given normal laboratory rat chow and water. These protocols completely conformed to the relevant ethical standards of the National Institutes of Health (NIH Publication no. 85-23, revised 1996) and were approved by the committees on animal research of Huadong Hospital affiliated with Fudan University.
Experimental design
To evaluate the effects of RDN on hypertension and renal function, all rats were followed up for 8 months after the treatments, which included bilateral RDN (SHR-RDN group, n = 10; WKY-RDN group, n = 10) and a sham operation (SHR-Sham group, n = 10; WKY-Sham group, n = 10). The RDN surgery was performed at the age of 13 weeks after acclimatization for one week, as described below in detail. Blood pressure (BP) was monitored every 7 weeks after the surgery. The 24-hour urine samples were collected at the age of 48 weeks. Then, plasma was collected by cardiac puncture under anesthetization. Kidneys and renal nerves were excised for histological identification.
Renal denervation and sham operation
Animals were fasted for one night before the surgery. A ventral midline abdominal incision was made under sodium pentobarbital anesthesia (30 mg/kg i.p.). Then, the renal arteries and veins of each kidney were identified. All of visible nerves, adipose tissue and surrounding connective tissue from the origin of the renal vessels at the abdominal aorta to the renal hilus were carefully stripped. Furthermore, the bilateral renal arteries were painted with a 10% phenol/ethanol solution for 2 minutes to ensure the destruction of any remaining renal sympathetic nerves, while the renal vessels of the sham group were only briefly exposed and painted with saline. The incision was finally closed with silk suture [24,25].
Measurement of blood pressure
Blood pressure (BP) was determined at baseline and every 7 weeks after the RDN or sham treatment with the tail-cuff plethysmography method in conscious rats (BP-2000 Blood Pressure Analysis System II, USA).
Measurement of biochemical parameters
Blood samples were collected by cardiac puncture. Plasma renin activity (PRA) and the levels of aldosterone (ALD), NAD(P)H oxidase, nitric oxide (NO), endothelial nitric oxide synthase (eNOS), creatinine, and renal norepinephrine (NE) were examined by ELISA (Beijing FuRuiZe Biological and Technology Company, China). Urinary protein excretion was determined via Bradford protein assay with a commercially available assay kit (Beijing FuRuiZe Biological and Technology Company, China). The analyses were conducted according to a previously reported method [26].
Histological examination
All rats were euthanized and immediately perfused transcardially with 300 ml of ice-cold 0.9% saline followed by 300 ml of 4% paraformaldehyde in saline. The kidneys were removed and stored overnight in a 4% paraformaldehyde solution. Paraffin-embedded kidneys were sectioned into 5 µm slices and subjected to hematoxylin-eosin (HE), Periodic acid-Schiff (PAS) and Masson staining along with immunohistochemical staining for tyrosine hydroxylase (TH, Abcam, Cambridge, UK). Two pathologists semiquantitatively analyzed the PAS-stained sections in a blinded manner. The severity of glomerular injury was quantified by the glomerulosclerosis (GS) score. The GS index ranged from 0 to 4 and was assessed as follows: 0 points, normal appearance; 1 point, minimal or segmental sclerotic changes in less than 25% of the entire area in an isolated glomerulus; 2 points, mild sclerotic changes in 25% to 50% of the area; 3 points, moderate sclerotic changes in 50% to 75% of the area; and 4 points, severe sclerotic changes in over 75% of the area. At least 50 glomeruli were randomly selected from each kidney, and the mean score was calculated per animal [27,28]. Tubulointerstitial fibrosis was assessed by the % fibrotic area in 30 randomly selected fields per section of kidney using Image-Pro Plus 6.0 [29].
Western blot analysis
Western blotting was carried out as described previously [30]. Kidneys were excised, frozen in liquid nitrogen and stored for later protein evaluation. The tissues were homogenized on ice and lysed in RIPA buffer containing a protease inhibitor cocktail. Total protein concentration was measured using a Bradford assay with BSA as a standard. Samples (60 µg) were subjected to 10% SDS-PAGE and transferred to a PVDF membrane (Millipore). Blot membranes were blocked at room temperature with 5% nonfat milk in TBST. Then, membranes were incubated overnight at 4°C with primary antibodies including rabbit monoclonal anti-ACE (1:1000 dilution; Abcam), rabbit monoclonal anti-AT1R (1:800 dilution; Abcam), rabbit monoclonal anti-ACE2 (1:1000 dilution; Abcam), rabbit monoclonal anti-Mas receptor (1:200 dilution; Alomone Labs), and rabbit monoclonal anti-rat β-actin antibody (1:1000 dilution; Abcam). The membranes were washed three times with TBST. Subsequently, the membranes were incubated with a secondary antibody (goat anti-rabbit IgG-horseradish peroxidase conjugate, 1:2000 dilution; Sangon Biotech, Shanghai, China) for 1 h at room temperature. Immunoreactive bands were visualized using enhanced chemiluminescence (Thermo Scientific), band intensities were quantified using an image analyzer (BioRad, Hercules, CA, USA) and intensity analysis was performed with a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
All data are expressed as the means ± standard deviation and were assessed by analysis of variance (ANOVA) followed by Bonferroni’s post hoc test using SPSS version 22.0 software. All P < 0.05 was considered to represent a statistically significant difference.
Results
RDN attenuates the increase in blood pressure in SHRs
RDN in SHR significantly restrained a spontaneous rise in systolic blood pressure (SBP) after the 8 months after surgery. RDN-treated SHR demonstrated a lower spontaneous rise in SBP than the rats of the SHR-Sham group (P < 0.01, at 20, 27, 34 and 41 weeks), but there was no significant difference between the SHR-RDN and SHR-Sham groups at the 48 weeks (198.2 ± 12.9 vs 209.4 ± 11.9 mmHg, P = 0.145). The SBP of WKY, however, was not affected by RDN at the 8-months follow-up (Figure 1).
Figure 1.
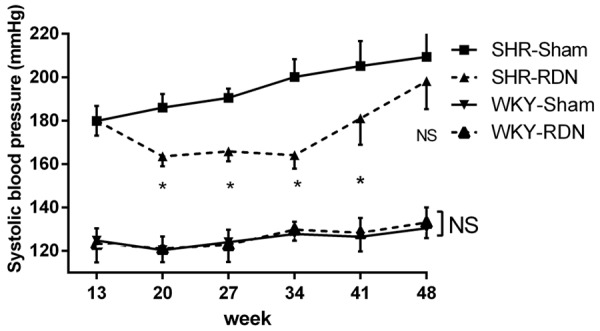
Systolic blood pressure measured by tail-cuff plethysmography 35 weeks after RDN performed at the age of 13 weeks. Asterisk indicates statistical significance (P < 0.01) vs sham operated rats; NS, not significant; Values are mean ± standard deviation; N = 10 in each group; WKY-Sham indicates sham-operated Wistar-Kyoto rats; WKY-RDN, Wistar-Kyoto rats subjected to renal denervation; SHR-Sham, sham-operated spontaneously hypertensive rats, SHR-RDN, spontaneously hypertensive rats subjected to renal denervation.
Effect of RDN on renal tissue norepinephrine
Renal tissue norepinephrine was significantly decreased by RDN in both SHR (197.5 ± 35.27 vs 50.5 ± 31.01 ng/g, P < 0.01) and WKY (153.6 ± 28.61 vs 54.5 ± 29.57 ng/g, P < 0.01). The SHR-Sham group showed higher renal tissue norepinephrine content than the WKY-Sham group (197.5 ± 35.27 vs 153.6 ± 28.61 ng/g, P = 0.02). However, no difference was existed between the SHR-RDN and WKY-RDN groups (50.5 ± 31.01 vs 54.5 ± 29.57 ng/g, P = 1.00, Figure 2).
Figure 2.
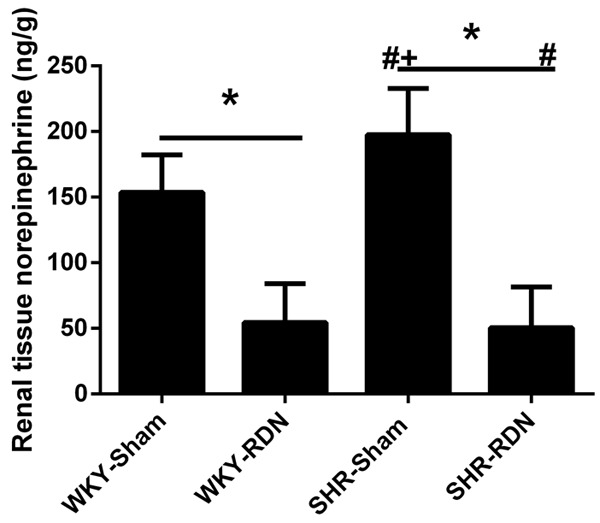
Effect of RDN on renal tissue norepinephrine at the age of 48 weeks. Asterisk indicates statistical significance (P < 0.01) vs sham operated rats; NS, not significant; the statistical significance is indicated by the sharp (P < 0.01 vs WKY-Sham) and dagger (P < 0.01 vs WKY-RDN). Values are mean ± standard deviation; N = 10 in each group; WKY-Sham indicates sham-operated Wistar-Kyoto rats; WKY-RDN, Wistar-Kyoto rats subjected to renal denervation; SHR-Sham, sham-operated spontaneously hypertensive rats, SHR-RDN, spontaneously hypertensive rats subjected to renal denervation.
Effect of RDN on plasma renin activity (PRA) and aldosterone
PRA was significantly decreased by the RDN in SHR (17.9 ± 4.79 vs 7.7 ± 4.66 ng/ml/hr, P < 0.01). The SHR-Sham group demonstrated a higher PRA than the WKY-Sham group (17.9 ± 4.79 vs 10.8 ± 3.11 ng/ml/hr, P < 0.01), while there was no difference between the SHR-RDN and WKY-RDN groups (7.7 ± 4.66 vs 11.0 ± 3.10 ng/ml/hr, P = 0.44, Figure 3A). The change in the level of plasma aldosterone was similar to that of PRA. The SHR-sham group displayed a progressive increase in aldosterone compared to the levels in the WKY-Sham group 35 weeks after RDN or sham operation that was significantly attenuated by RDN (1.31 ± 0.47 vs 0.78 ± 0.37 ng/ml, P = 0.02, Figure 3B).
Figure 3.
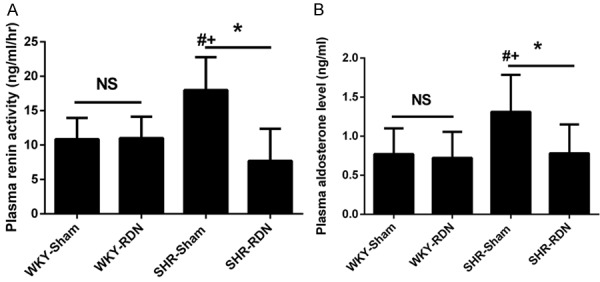
Effect of RDN on plasma renin activity (A) and aldosterone (B) at the age of 48 weeks. Asterisk indicates statistical significance (P < 0.01) vs sham operated rats; NS, not significant; the statistical significance is indicated by the sharp (P < 0.01 vs WKY-Sham) and dagger (P < 0.01 vs WKY-RDN). Values are mean ± standard deviation; N = 10 in each group; WKY-Sham indicates sham-operated Wistar-Kyoto rats; WKY-RDN, Wistar-Kyoto rats subjected to renal denervation; SHR-Sham, sham-operated spontaneously hypertensive rats, SHR-RDN, spontaneously hypertensive rats subjected to renal denervation.
Changes in the production of the reactive oxygen species
Plasma NAD(P)H oxidase, eNOS and NO levels were measured to show the activity of the ROS. RDN resulted in a significant decrease in plasma NADPH oxidase and an increase in eNOS and NO levels in SHR (171.7 ± 4.32 vs 152.4 ± 4.23 nmol/ml, P < 0.01; 2.41 ± 0.59 vs 3.16 ± 0.47 U/ml, P = 0.03; 51.7 ± 6.87 vs 60.9 ± 5.30 μmol/l, P = 0.03). The NAD(P)H oxidase, eNOS and NO levels WKY, however, were not affected by the RDN (Figure 4).
Figure 4.
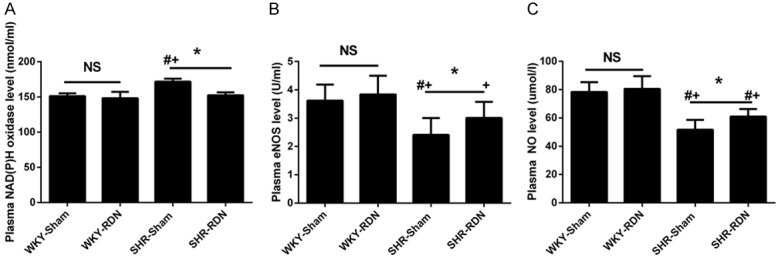
Effect of RDN on plasma NAD(P)H oxidase (A), Enos (B) and NO (C) level. Asterisk indicates statistical significance (P < 0.01) vs sham operated rats; NS, not significant; the statistical significance is indicated by the sharp (P < 0.01 vs WKY-Sham) and dagger (P < 0.01 vs WKY-RDN). Values are mean ± standard deviation; N = 10 in each group; WKY-Sham indicates sham-operated Wistar-Kyoto rats; WKY-RDN, Wistar-Kyoto rats subjected to renal denervation; SHR-Sham, sham-operated spontaneously hypertensive rats, SHR-RDN, spontaneously hypertensive rats subjected to renal denervation.
Histological analysis of renal sympathetic nerve fascicles
Compared with that of the sham-treated SHR and WKY, the renal nerve bundle showed conspicuous changes under routine staining (HE, Masson, PAS) in the RDN-treated rats. There were notable changes in the perineurium including perineuronal inflammation and fibrosis and even the loss of normal perineurial sheath. In the endoneurium, there were a variety of pyknotic nuclei, digestion chambers and swelling nerve nuclei along with fragmented and unclear nucleoli. More importantly, mononuclear inflammatory cell response, rare hemorrhage and segmental demyelination of myelin were observed in endoneurium injury. In contrast, the renal nerve bundle was surrounded by a thin fibrotic connective tissue sheath in both the SHR-Sham group and the WKY-Sham group. There was slight injury in the renal nerve in these two groups. Additionally, immunohistochemistry morphology of the nerve bundles revealed a significantly higher expression of TH in both the SHR-Sham group and the WKY-Sham group than that in the SHR-RDN and WKY-RDN groups (Figure 5).
Figure 5.
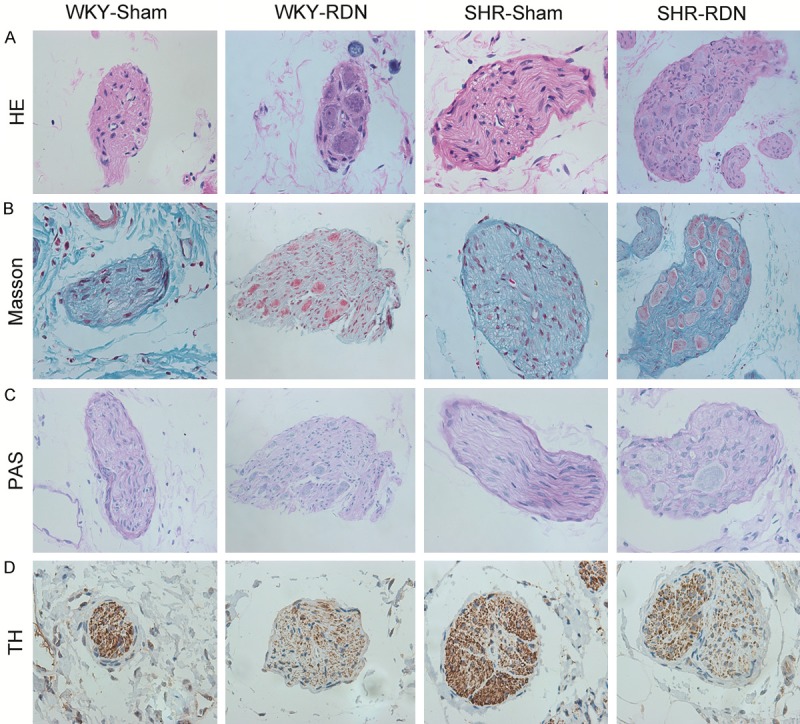
Representative images of renal nerves with HE (A), Masson (B), PAS (C), TH-antibody staining (D). Magnification, × 400. Strong positive reaction to TH-antibody staining was observed in Sham-operated rats, whereas weaker reaction in RDN-operated rats. WKY-Sham indicates sham-operated Wistar-Kyoto rats; WKY-RDN, Wistar-Kyoto rats subjected to renal denervation; SHR-Sham, sham-operated spontaneously hypertensive rats, SHR-RDN, spontaneously hypertensive rats subjected to renal denervation.
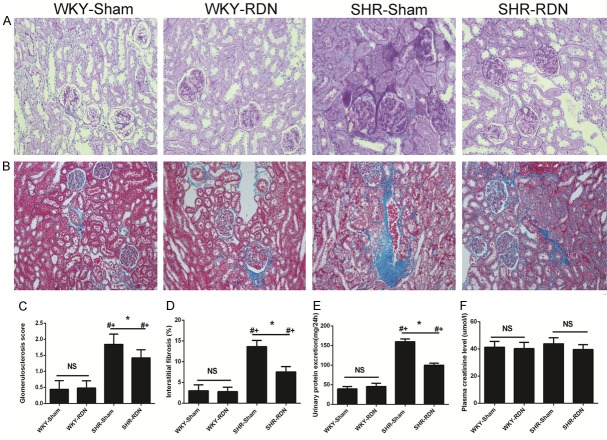
Kidney remodeling
The histological findings of the kidney sections from PAS staining are depicted in Figure 6A. The kidneys of the SHR-Sham group displayed moderate to severe segmental sclerosis and proliferation of the mesangial area in the glomeruli compared those of the WKY-Sham group with normal blood pressure, while the renal damage was attenuated following RDN treatment in SHR-RDN. The GS score was significantly decreased in the SHR-RDN group compared with that in the SHR-Sham group (1.42 ± 0.25 vs 1.84 ± 0.32, P < 0.01). The kidneys of the SHR-RDN group showed more severe segmental sclerosis than that of the WKY-RDN group (1.42 ± 0.25 vs 0.48 ± 0.27, P < 0.01, Figure 6C). Representative Masson staining of the tubulointerstitium is shown in Figure 6B. The SHR-Sham group exhibited mild interstitial fibrosis along with slight tubular changes, whereas the SHR-RDN group showed lower interstitial fibrosis under the operation after 8 months after the surgery. No differences in interstitial fibrosis were observed between the WKY-Sham and WKY-RDN group. The interstitial fibrosis score (IF score) was markedly lower in the SHR-RDN group than in the SHR-Sham group (7.52 ± 1.29 vs 13.63 ± 1.51, P < 0.01, Figure 6D). These data indicate that RDN ameliorates glomerular and tubulointerstitial damage.
Figure 6.
Effect of RDN on renal structural remodeling and function: semiquantitative glomerular scoring (A, C, Magnification, × 200), quantification of interstitial kidney fibrosis (B, D, Magnification, × 100) with representative histological pictures, urinary protein excretion (E), plasma creatinine (F). Asterisk indicates statistical significance (P < 0.01) vs sham operated rats; NS, not significant; the statistical significance is indicated by the sharp (P < 0.01 vs WKY-Sham) and dagger (P < 0.01 vs WKY-RDN). Values are mean ± standard deviation; N = 10 in each group; WKY-Sham indicates sham-operated Wistar-Kyoto rats; WKY-RDN, Wistar-Kyoto rats subjected to renal denervation; SHR-Sham, sham-operated spontaneously hypertensive rats, SHR-RDN, spontaneously hypertensive rats subjected to renal denervation.
Effects of RDN on plasma creatinine and urinary protein excretion
Urinary protein excretion in the SHR-Sham group was significantly higher than that in the WKY-Sham group (159.9 ± 6.92 vs 39.4 ± 5.97 mg/24 h, P < 0.01). However, RDN led to a significant decrease in 24 h urinary protein excretion in SHR 8 months after surgery (159.9 ± 6.92 vs 99.9 ± 5.36, mg/24 h, P < 0.01). There was also a remarkable difference in 24 h urinary protein excretion between the SHR-RDN and WKY-RDN groups (99.9 ± 5.36 vs 45.6 ± 8.17 mg/24 h, P < 0.01, Figure 6E). The RDN treatment did not change the level of plasma creatinine in either SHR or WKY (Figure 6F).
Effect of RDN on renal ACE, AT1R, ACE2, and MasR expression
RDN caused a significant decrease in the renal expression of ACE and AT1R and a marked increase in the renal expression of ACE2 and MasR in RDN-treated SHR compared to the expression in sham-operated SHR. However, the renal expression of ACE, AT1R, ACE2, and MasR was not affected by RDN in WKY. This result indicated that RDN can result in downregulation of renal ACE and AT1R expression and the upregulation of ACE2 and MasR expression in SHR, but not WKY (Figure 7).
Figure 7.
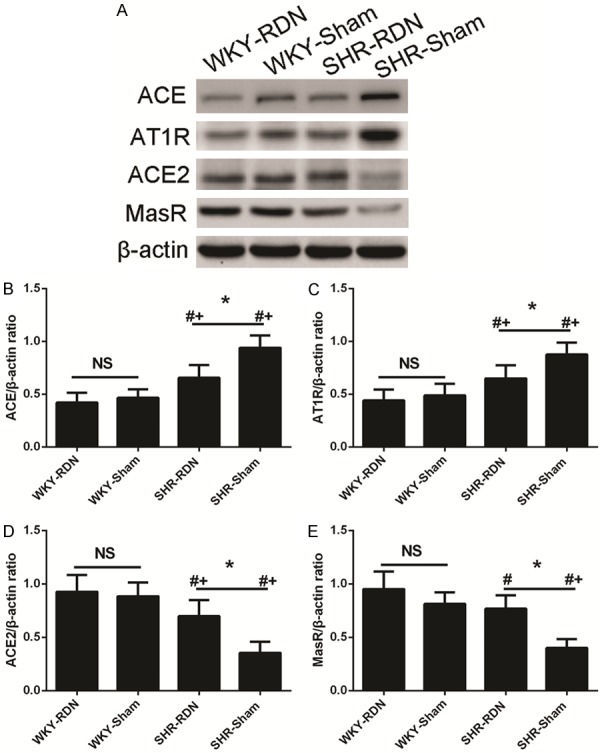
RDN result in downregulation of renal ACE, AT1R expression, and upregulation of ACE2, MasR expression in SHR. Upper panels show representative Western blots (A). Each protein value in individual rats was normalized to β-actin. Lower panels show western blot analyses (B-E). Asterisk indicates statistical significance (P < 0.01) vs sham operated rats; NS, not significant; the statistical significance is indicated by the sharp (P < 0.01 vs WKY-Sham) and dagger (P < 0.01 vs WKY-RDN). Values are mean ± standard deviation; N = 10 in each group; WKY-Sham indicates sham-operated Wistar-Kyoto rats; WKY-RDN, Wistar-Kyoto rats subjected to renal denervation; SHR-Sham, sham-operated spontaneously hypertensive rats, SHR-RDN, spontaneously hypertensive rats subjected to renal denervation.
Discussion
We obtained three major findings from the present study: 1) Long-term RDN significantly attenuated SBP only in SHR. 2) RDN significantly reduced renal tissue norepinephrine with histological damage to the renal nerve. 3) RDN attenuated glomerulosclerosis and tubulointerstitial fibrosis; moreover, it had a strong anti-proteinuric effects in SHR through downregulation of the ACE/Ang II/AT1R axis and upregulation of the ACE2/Ang-(1-7)/MasR axis in the kidney.
It has become controversial whether RDN can help reduce blood pressure since the confirmed result of the symplicity HTN-3 trial, which proved to be a failure for RDN [14]. However, multiple lines of studies, including our previous experiment, demonstrated that RDN can effectively lead to a significant decrease in blood pressure in hypertensive animals, including SHRs, dogs, pigs, and sheep et al. [11-13,17,19,25,31-34]. Our findings were similar to those of the other previous studies. The considerable discrepancy between findings of studies and the Symplicity HTN-3 trial can be attributed to several factors. On the one hand, it is difficult to determine the certain numbers of lesions with a single-tip catheter in a 3-dimensional artery; on the other hand, no accurate intraprocedural makers of success have been identified as of yet [35]. These issues may result in incomplete denervation. A more important fact is that the achievement of RDN in the Symplicity HTN-3 trial was mainly evaluated by the reduction in norepinephrine in plasma or renal tissues compared with levels the control group; the trial lacked histological analysis of the renal nerve. The extent of renal nerve ablation remained unknown. Recently, Rousselle SD et al. found poorly organized neuromatous regeneration after 90 days of RDN in swine [36]. Thus, the lack of histological analysis of the renal nerve in the Symplicity HTN-3 trial can partly explain these inconsistent results.
In our study, we denervated the adventitia of the renal artery with 10% phenol in absolute ethanol [23] and estimated the effect of RDN through norepinephrine content in renal tissue combined with the morphology of the renal nerve. We successfully established an in vivo model of RDN in rats with the striking decreases in SBP and renal tissue norepinephrine concentrations. Additionally, notable histological changes without regeneration of the renal nerve were observed in this study. Early studies suggested that definite renal nerve injury after radiofrequency ablation was observed at the site of ablation [36-38], which further supports our findings. However, the SBP of the SHR-RDN group increased gradually begining 21 weeks after RDN treatment and reached levels equivalent of those of the SHR-Sham group at the age of 48 weeks. This result indicated that RDN cannot completely prevent the progressive increase in blood pressure in SHR. Renal nerves did not play a significant role in the maintenance of increased BP in stabled hypertension. Early intervention by RDN may contribute to a delay in the development of hypertension [39].
A large amount of evidence has indicated that sympathetic nervous system overactivity plays a crucial role in the development of hypertension [5,40-42]. Recently, studies have indicated that tyrosine hydroxylase (TH) is the rate-limiting enzyme in the synthesis of the catecholamine pathway and that TH activity can represent the ability to produce norepinephrine. Hence, TH immunoreactivity could be used as an appropriate indicator to evaluate the level of regional sympathetic nerve activity and reflect changes in sympathetic nerve activity. Burgi et al. explored the relationship between TH immunoreactivity (THir) and sympathetic activity in SHRs and used THir as an indicator of sympathetic activity. Their findings showed increased TH activity in the heart and kidney in the SHRs than in WKY rats [42]. We also found a strong positive reaction to TH-antibody staining in the renal nerve bundle in sham-operated rats, and this reaction was inhibited by RDN (Figure 5), a finding that was consistent with the renal tissue norepinephrine concentrations (Figure 2). Furthermore, renal norepinephrine content has been used to measure the sympathetic activity [17].
It has been proven that increased sympathetic activity plays an important role in the pathophysiology of the kidney [44,45]. The renal artery adventitia is richly filled with nerves, including afferent and efferent renal sympathetic nerves that innerve the kidney. Possible mechanisms for sympathetic hyperactivity involved in renal damage are as follow: 1) the initiation of ROS and oxidative stress; 2) abnormal interglomerular pressure and renal hemodynamic changes through regulating of the glomerular afferent and efferent arteries with renal sympathetic nerve [25,46]. Therefore, reduced SNA could prevent the progressive renal damage and have a reno-protective effect.
It is well accepted that activation of renin-angiotensin-aldosterone (RAS), especially intrarenal RAS, plays a central role in the pathogenesis of hypertension and many types of kidney damage, including diabetic nephropathy and chronic kidney disease. More importantly, the ACE/Ang II/AT1R axis is known to contribute to chronic renal injury. Ang II is associated with perpetuating glomerular injury in the kidney through the modulation of nephrin signaling, the integrity of which is crucial for the glomerular filtration barrier [47]. However, ACE2, a newly discovered homolog of ACE, is a monocarboxypeptidase capable of processing angiotensin peptides, cleaving Ang II to generate Ang-(1-7). Generally, ACE2 counteracts many functions of the conventional ACE/Ang II/AT1R axis. It degrades Ang I into the inactive Ang-(1-9) and degrades Ang II into Ang-(1-7). Furthermore, Ang-(1-7) exerts antioxidant, antifibrotic properties through its receptor, Mas (MasR) [48,49]. Recently, Berger RC et al. found that a high-salt diet increased the ACE/ACE2 ratio while causing glomerular hypertrophy, loss of podocyte foot processes, and proteinuria in SHR [50]. Similarly, the work of Jin HY et al. showed that ACE2 deficiency enhances renal fibrosis and exacerbates the progression of chronic kidney disease [51]. Indeed, clinical evidence has demonstrated that the blockade of RAS by ACE inhibitors or AT1 receptor blockers alleviates renal injury, improves renal function and reduces renal events in patients with chronic kidney disease and end-stage renal disease [52,53]. These data indicate that the relative decrease in renal activity of the classic ACE/Ang II/AT1R pathway in comparison to that of the ACE2/Ang-(1-7)/Mas pathway is key to protection against renal injury. In our study, increased renal ACE2 and MasR expression along with decreased renal ACE and AT1R expression were observed only in SHR treated with RDN and were related to the amelioration of renal injury.
In conclusion, RDN delays the onset of hypertension and mitigates the progression of hypertension in SHR. Furthermore, RDN has a protective effect against glomerulosclerosis, interstitial fibrosis and urinary protein excretion through downregulation of the ACE/Ang II/AT1R axis and upregulation of the ACE2/Ang-(1-7)/MasR axis in the kidney.
Study limitation
The mechanism BP regulation includes not only the sympathetic nervous system but also the renin-angiotensin system, renal sodium handling, reactive oxygen species, endothelin activity and the carotid barore-flex system. Thus, further studies are needed to distinguish the precise mechanisms responsible for the anti-hypertensive and renoprotective effects of RDN in SHR [17,54,55].
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (81370361), Science and Technology Commission of Shanghai Municipality (12140902800), Research Fund for the Scientific and Technical Project of Shanghai Chest Hospital (2014YZDH20300).
Disclosure of conflict of interest
None.
References
- 1.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 4.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 5.Lundin S, Ricksten SE, Thoren P. Renal sympathetic activity in spontaneously hypertensive rats and normotensive controls, as studied by three different methods. Acta Physiol Scand. 1984;120:265–272. doi: 10.1111/j.1748-1716.1984.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 6.Funder JW. Aldosterone and mineralocorticoid receptors in the cardiovascular system. Prog Cardiovasc Dis. 2010;52:393–400. doi: 10.1016/j.pcad.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99s–105s. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 8.Gattone VH 2nd, Evan AP, Overhage JM, Severs WB. Developing renal innervation in the spontaneously hypertensive rat: evidence for a role of the sympathetic nervous system in renal damage. J Hypertens. 1990;8:423–428. doi: 10.1097/00004872-199005000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 10.Symplicity HTN-2 Investigators. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity HTN-2 trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 11.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 12.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, shamcontrolled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 13.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Bohm M SPYRAL HTN-OFF MED trial investigators. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 15.Sun ZJ, Zhang ZE. Historic perspectives and recent advances in major animal models of hypertension. Acta Pharmacol Sin. 2005;26:295–301. doi: 10.1111/j.1745-7254.2005.00054.x. [DOI] [PubMed] [Google Scholar]
- 16.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731–767. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 17.Katayama T, Sueta D, Kataoka K, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, Mingjie M, Nakagawa T, Maeda M, Ogawa H, Kim-Mitsuyama S. Long-term renal denervation normalizes disrupted blood pressure circadian rhythm and ameliorates cardiovascular injury in a rat model of metabolic syndrome. J Am Heart Assoc. 2013;2:e000197. doi: 10.1161/JAHA.113.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulla MH, Sattar MA, Salman IM, Abdullah NA, Ameer OZ, Khan MA, Johns EJ. Effect of acute unilateral renal denervation on renal hemodynamics in spontaneously hypertensive rats. Auton Autacoid Pharmacol. 2008;28:87–94. doi: 10.1111/j.1474-8673.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T, Hasegawa Y, Uekawa K, Ma M, Katayama T, Sueta D, Toyama K, Kataoka K, Koibuchi N, Maeda M, Kuratsu J, Kim-Mitsuyama S. Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J Am Heart Assoc. 2013;2:e000375. doi: 10.1161/JAHA.113.000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maranon RO, Reckelhoff JF. Mechanisms responsible for postmenopausal hypertension in a rat model: roles of the renal sympathetic nervous system and the renin-angiotensin system. Physiol Rep. 2016;4:e12669. doi: 10.14814/phy2.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart EC, McBryde FD, Burchell AE, Ratcliffe LE, Stewart LQ, Baumbach A, Nightingale A, Paton JF. Translational examination of changes in baroreflex function after renal denervation in hypertensive rats and humans. Hypertension. 2013;62:533–541. doi: 10.1161/HYPERTENSIONAHA.113.01261. [DOI] [PubMed] [Google Scholar]
- 22.Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol. 2006;290:R341–344. doi: 10.1152/ajpregu.00035.2005. [DOI] [PubMed] [Google Scholar]
- 23.Maranon RO, Lima R, Mathbout M, do Carmo JM, Hall JE, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of the sympathetic nervous system in an animal model. Am J Physiol Regul Integr Comp Physiol. 2014;306:R248–256. doi: 10.1152/ajpregu.00490.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luippold G, Beilharz M, Muhlbauer B. Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant. 2004;19:342–347. doi: 10.1093/ndt/gfg584. [DOI] [PubMed] [Google Scholar]
- 25.Linz D, Hohl M, Schutze J, Mahfoud F, Speer T, Linz B, Hubschle T, Juretschke HP, Dechend R, Geisel J, Rutten H, Bohm M. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: the role of renal sympathetic innervation. Am J Hypertens. 2015;28:256–265. doi: 10.1093/ajh/hpu123. [DOI] [PubMed] [Google Scholar]
- 26.Han W, Fang W, Gan Q, Guan S, Li Y, Wang M, Gong K, Qu X. Low-dose sustained-release deoxycorticosterone acetate-induced hypertension in Bama miniature pigs for renal sympathetic nerve denervation. J Am Soc Hypertens. 2017;11:314–320. doi: 10.1016/j.jash.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Namikoshi T, Tomita N, Fujimoto S, Haruna Y, Ohzeki M, Komai N, Sasaki T, Yoshida A, Kashihara N. Isohumulones derived from hops ameliorate renal injury via an anti-oxidative effect in Dahl salt-sensitive rats. Hypertens Res. 2007;30:175–184. doi: 10.1291/hypres.30.175. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe H, Iwanaga Y, Miyaji Y, Yamamoto H, Miyazaki S. Renal denervation mitigates cardiac remodeling and renal damage in Dahl rats: a comparison with beta-receptor blockade. Hypertens Res. 2016;39:217–226. doi: 10.1038/hr.2015.133. [DOI] [PubMed] [Google Scholar]
- 29.Sugama I, Kohagura K, Yamazato M, Nakamura T, Shinzato T, Ohya Y. Superoxide dismutase mimetic, tempol, aggravates renal injury in advanced-stage stroke-prone spontaneously hypertensive rats. J Hypertens. 2014;32:534–541. doi: 10.1097/HJH.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YJ, Wang B, Fu XL, Guan SF, Han WZ, Zhang J, Gan QA, Fang WY, Ying WH, Qu XK. Exogenous NAD(+) administration significantly protects against myocardial ischemia/reperfusion injury in rat model. Am J Transl Res. 2016;8:3342–3350. [PMC free article] [PubMed] [Google Scholar]
- 31.Henegar JR, Zhang Y, De Rama R, Hata C, Hall ME, Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27:1285–1292. doi: 10.1093/ajh/hpu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan Q, Qu XK, Gong KZ, Guan SF, Han WZ, Dai JJ, Li RG, Zhang M, Liu H, Xu YJ, Zhang YJ, Fang WY. Efficacy and safety of a novel multi-electrode radiofrequency ablation catheter for renal sympathetic denervation in pigs. J Geriatr Cardiol. 2015;12:618–625. doi: 10.11909/j.issn.1671-5411.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension. 2015;65:393–400. doi: 10.1161/HYPERTENSIONAHA.114.04176. [DOI] [PubMed] [Google Scholar]
- 34.Thomsen LL, Mikkelsen RT, Derejko M, Schroder HD, Licht PB. Sympathetic block by metal clips may be a reversible operation. Interact Cardiovasc Thorac Surg. 2014;19:908–913. doi: 10.1093/icvts/ivu311. [DOI] [PubMed] [Google Scholar]
- 35.Papademetriou V, Tsioufis C, Doumas M. Renal denervation and symplicity HTN-3: “Dubium sapientiae initium” (doubt is the beginning of wisdom) Circ Res. 2014;115:211–214. doi: 10.1161/CIRCRESAHA.115.304099. [DOI] [PubMed] [Google Scholar]
- 36.Rousselle SD, Brants IK, Sakaoka A, Hubbard B, Jackson ND, Wicks JR, Dillon KN, Naiche LA, Hart R, Garza JA, Tellez A. Neuromatous regeneration as a nerve response after catheter-based renal denervation therapy in a large animal model: immunohistochemical study. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002293. [DOI] [PubMed] [Google Scholar]
- 37.Sakakura K, Tunev S, Yahagi K, O’Brien AJ, Ladich E, Kolodgie FD, Melder RJ, Joner M, Virmani R. Comparison of histopathologic analysis following renal sympathetic denervation over multiple time points. Circ Cardiovasc Interv. 2015;8:e001813. doi: 10.1161/CIRCINTERVENTIONS.114.001813. [DOI] [PubMed] [Google Scholar]
- 38.Sakakura K, Ladich E, Edelman ER, Markham P, Stanley JR, Keating J, Kolodgie FD, Virmani R, Joner M. Methodological standardization for the pre-clinical evaluation of renal sympathetic denervation. JACC Cardiovasc Interv. 2014;7:1184–1193. doi: 10.1016/j.jcin.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machino T, Murakoshi N, Sato A, Xu D, Hoshi T, Kimura T, Aonuma K. Anti-hypertensive effect of radiofrequency renal denervation in spontaneously hypertensive rats. Life Sci. 2014;110:86–92. doi: 10.1016/j.lfs.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Schlaich MP, Hering D, Sobotka P, Krum H, Lambert GW, Lambert E, Esler MD. Effects of renal denervation on sympathetic activation, blood pressure, and glucose metabolism in patients with resistant hypertension. Front Physiol. 2012;3:10. doi: 10.3389/fphys.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertog SC, Sobotka PA, Sievert H. Renal denervation for hypertension. JACC Cardiovasc Interv. 2012;5:249–258. doi: 10.1016/j.jcin.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Lee YH, Kim YG, Moon JY, Kim JS, Jeong KH, Lee TW, Ihm CG, Lee SH. Genetic variations of tyrosine hydroxylase in the pathogenesis of hypertension. Electrolyte Blood Press. 2016;14:21–26. doi: 10.5049/EBP.2016.14.2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burgi K, Cavalleri MT, Alves AS, Britto LR, Antunes VR, Michelini LC. Tyrosine hydroxylase immunoreactivity as indicator of sympathetic activity: simultaneous evaluation in different tissues of hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R264–271. doi: 10.1152/ajpregu.00687.2009. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013;24:229–242. doi: 10.1681/ASN.2012070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT Jr, Oakes R, Lukas MA, Anderson KM, Bell DS GEMINI Investigators. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 46.Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Sakuta T, Tomita N, Kashihara N. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transplant. 2010;25:2889–2898. doi: 10.1093/ndt/gfq139. [DOI] [PubMed] [Google Scholar]
- 47.Candido R, Jandeleit-Dahm KA, Cao Z, Nesteroff SP, Burns WC, Twigg SM, Dilley RJ, Cooper ME, Allen TJ. Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation. 2002;106:246–253. doi: 10.1161/01.cir.0000021122.63813.32. [DOI] [PubMed] [Google Scholar]
- 48.Padda RS, Shi Y, Lo CS, Zhang SL, Chan JS. Angiotensin-(1-7): a novel peptide to treat hypertension and nephropathy in diabetes? J Diabetes Metab. 2015;6:6. doi: 10.4172/2155-6156.1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Y, Lo CS, Padda R, Abdo S, Chenier I, Filep Janos G, Ingelfinger Julie R, Zhang SL, Chan John SD. Angiotensin-(1-7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci. 2015;128:649–63. doi: 10.1042/CS20140329. [DOI] [PubMed] [Google Scholar]
- 50.Berger RC, Vassallo PF, Crajoinas Rde O, Oliveira ML, Martins FL, Nogueira BV, Motta-Santos D, Araujo IB, Forechi L, Girardi AC, Santos RA, Mill JG. Renal effects and underlying molecular mechanisms of long-term salt content diets in spontaneously hypertensive rats. PLoS One. 2015;10:e0141288. doi: 10.1371/journal.pone.0141288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin HY, Chen LJ, Zhang ZZ, Xu YL, Song B, Xu R, Oudit GY, Gao PJ, Zhu DL, Zhong JC. Deletion of angiotensin-converting enzyme 2 exacerbates renal inflammation and injury in apolipoprotein E-deficient mice through modulation of the nephrin and TNF-alpha-TNFRSF1A signaling. J Transl Med. 2015;13:255. doi: 10.1186/s12967-015-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marquez E, Riera M, Pascual J, Soler MJ. Renin-angiotensin system within the diabetic podocyte. Am J Physiol Renal Physiol. 2015;308:F1–10. doi: 10.1152/ajprenal.00531.2013. [DOI] [PubMed] [Google Scholar]
- 53.Torres VE, Abebe KZ, Chapman AB, Schrier RW, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Moore CG, Perrone RD HALT-PKD Trial Investigators. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2267–2276. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moorhouse RC, Webb DJ, Kluth DC, Dhaun N. Endothelin antagonism and its role in the treatment of hypertension. Curr Hypertens Rep. 2013;15:489–496. doi: 10.1007/s11906-013-0380-1. [DOI] [PubMed] [Google Scholar]