Abstract
Androgen deprivation therapy (ADT) was an important management for metastatic prostate cancer. However, patients would finally progress to the metastatic castration-resistant prostate cancer (mCRPC) and lose sensitivity to ADT. In addition to lower testosterone level, ADT could cause anemia, which might impair the chemotherapy efficiency and worsen the outcomes of cancer patients. However, inconsistent results were found between anemia and mCRPC prognosis. Our study was the first systematic review to evaluate the influence of anemia in mCRPC prognosis. Thirteen studies with 6,484 samples were involved in this meta-analysis. We found anemia would worsen the Overall survival (OS) of mCRPC patients in both prognostic designed studies (HR = 1.55, 95% CI = 1.24-1.94) and retrospective designed studies (HR = 1.82, 95% CI = 1.52-2.18). Prognostic analyses also demonstrated that anemia associated with poor Progression free survival (PFS) (HR = 1.47, 95% CI = 1.22-1.75). In conclusion, we found that anemia was significantly associated with poor OS and PFS of mCRPC patients. Larger RCTs are needed for future study, especially for the evaluation of treatment value for anti-anemia in mCRPC.
Keywords: Systematic review, meta-analysis, metastatic castration-resistant prostate cancer, anemia, androgen deprivation therapy
Introduction
Anemia is one of the most common demonstrations of cancer, according to World Health Organization (WHO), hemoglobin <13 g/dL in males was defined as anemia. Almost 40% of cancer patients presented anemia, the proportion raised to 90% when patients were treated with chemotherapy [1]. The anemia was associated with shorter PFS and survival. It was also suggested to be a worse prognostic factor in many cancers, including prostate cancer [2,3]. The deterioration of cancer caused by anemia may result from hypoxia. Low hemoglobin level would cause hypoxia [4], which contributes to chemotherapy and radiotherapy resistance [5]. As shown in the Figure 1, hypoxia could influence the chemotherapy by reducing the formation of reactive O2 species and slowing down the cell cycle [6]. The hypoxia also induces the hypoxia-inducible factor 1 alpha (HIF-1α), which could dimerize with HIF-1β to activate the transcription of multiple oncogenic genes such as vascular endothelial growth factor, glycolytic enzymes, and glucose transporters [7].
Figure 1.
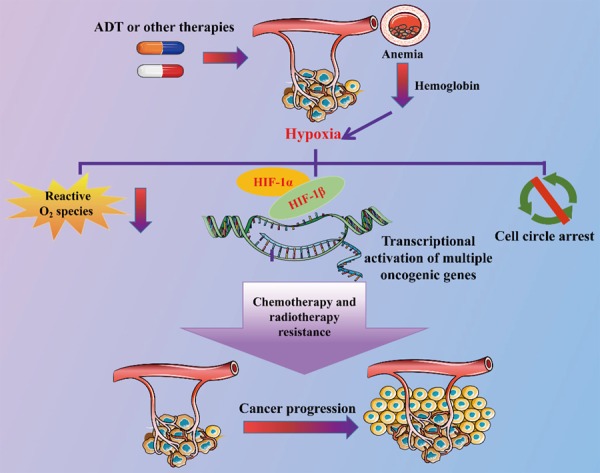
The mechanism of anemia promotes cancer progression. Androgen deprivation therapy and other therapies (such as chemotherapy) will cause hemoglobin decline which may finally lead to anemia and cause hypoxia in prostate cancer. Hypoxia can reduce the formation of active O2 species and slow the cell cycle. Hypoxia also induces the dimerization of hypoxia-inducible factor 1 alpha (HIF-1 alpha) and HIF-1 beta to activate transcription of various oncogenes that finally leads to chemotherapy and radiotherapy resistance and cancer progression.
Prostate cancer is the fourth most commonly diagnosed cancer and the second most commonly diagnosed cancer in men, with 1.1 million new cases per year worldwide [8]. The five-year relative survival rates dropped sharply when prostate cancer spreads to other organs such as bones [8]. Increasing incidence of metastatic prostate cancer was found recently [9]. The 5 year survival rate of metastatic prostate cancers is about 30% [10]. Since almost 75% of metastatic prostate cancers are hormone sensitive which make androgen deprivation therapy (ADT) established as a standard care for patients have metastatic prostate cancers. ADT refers to a variety of medical and surgical treatments such as bilateral orchiectomy and injections of estrogen that result in a reduction of androgens, or male sex hormones [11]. Up to nearly 90% of patients with metastatic prostate cancers witnessed serum prostate-Specific Antigen (PSA) level decrease after the using of ADT [12]. ADT could contribute to tumor regrssion and extend overall survival (OS) [13]. However, the average time for ADT therapy response is about 18 months, then the patients progress to the metastatic castration-resistant prostate cancer (mCRPC) [14,15]. mCRPC patients have a poor prognosis with a fewer than 2 years survival from the initial time of progression [16,17] (Figure 2).
Figure 2.
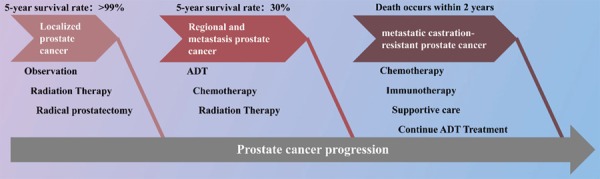
Brief introduction of treatment and prognosis of prostate cancer at different stages. ADT: Androgen deprivation therapy.
ADT has several side effects such as adverse bone health, metabolic disorder, sexual dysfunction, cognitive effects, fatique and anemia [18-22]. In prostate cancer, ADT could cause anemia and anemia related fatique, reducing the quality of life (QoL) of patients, and this was more frequently and more severely occurred in metastatic prostate cancer patients [23]. A network meta-analysis showed that compared with other therapies, ADT had relative high surface under the cumulative ranking curve (SUCRA) value (82.8%) to induce anemia in the treatment of metastatic prostate cancer [24]. Furthermore, the ADT continued when metastatic prostate cancer patients progressed to mCRPC. The effects of anemia on mCRPC prognosis remains inconsistent, the majority of the studies showed that anemia could worsen the prognosis of mCRPC [25-34], yet others found that anemia was not associated with the prognosis of mCRPC [35-37].
Although the ADT was found to be a cause of anemia in prostate cancer, and inconsistent results were obtained when evaluated the prognostic value of anemia in the pretreatment mCRPC patients [25-37], there was no systematic review on the association of anemia with mCRPC prognosis. Therefore, we decide to perform this systematic review and meta-analysis to explore the association between anemia and mCRPC prognosis.
Methods
Data collection
This systematic review was conducted following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [38] (Data not shown). We collected studies from online database PubMed without time limitation, only studies in English language were involved. The searching keywords were “((((((((Anemia[mesh]) OR Anemias)) OR ((Ferrous Hemoglobin) OR Hemoglobin))) AND ((((((((((Prostate Cancer[mesh]) OR Cancer of Prostate) OR Cancers, Prostatic) OR Prostatic Cancer) OR Cancer of the Prostate) OR Prostate Cancers) OR Neoplasms, Prostatic) OR Prostatic Neoplasm) OR Prostate Neoplasm) OR Prostate Neoplasms)) AND ((((prognosis[mesh]) OR Factors, Prognostic) OR Prognostic Factors) OR Prognoses))) NOT (((animals[mh] NOT humans[mh])”. The searching was updated until March 5, 2018. The included studies should meet the follow items: (1) it’s a cohort study that involve mCRPC patients; (2) it contains definition of anemia by dimidiating the hemoglobin level; (3) it has sufficient information to calculate the Hazard Ratio (HR) and 95% Confidence Interval (95% CI) for outcomes such as OS, progression free survival (PFS). We chose HR as outcome data because of its time-independent nature. The study quality was assessed by two separate tools. The risk of bias was evaluated by Cochrane Risk of Bias Tool for randomized controlled trials (RCTs) [39]. In addition, the Newcastle-Ottawa Quality Assessment Form for Cohort Studies (NOS) was used to evaluate the quality of each cohort study. The searching, study selection and study quality assessment were performed by two independent reviewers (LL and YW). If disagreements occur, decisions would be made by discussions and subsequent consensus. For the studies involved duplicate cohorts, the most recent, largest, or best-quality one would be selected.
Data extraction
Data extraction was performed by two independent investigators (DD and YG). The extractions included the name of first author, the year of publication, the NOS score, the region of where the cohort from, ethnicity of cohort, type of the study (prognostic or retrospective cohort), outcome types, sample size, definition of anemia, median follow-up time for outcomes, and the result that whether the anemia is a significant risk factor for metastatic prostate cancer prognosis.
Data analyses
All the analyses were performed by Stata software 11.0 [40]. The pooled HR and 95% CI were calculated for OS and PFS. Only the multivariate results were pooled. I2 test [41] was used to calculate statistical heterogeneity. The random effect model was used in current meta-analyses for random and fix model present similar results when heterogeneity is low [42]. Funnel plots, Begg and Egger were performed to evaluate potential publication bias [43,44]. Subgroup stratification and sensitive analysis would be performed if any heterogeneity occurred. A p value of less than 0.05 was considered significant.
Results
Study selection
As shown in Figure 3, we collected 424 studies from PubMed, after reading titles and abstracts, sixty full-texts were obtained for further selections. We further excluded 2 studies that are not on anemias, 3 non-English language studie. 29 studies without HR and 95% CI value, 7 studies without anemia definition, and 6 studies not involving mCRPC patients. Finally, thirteen studies with 6,484 samples were involved in final meta-analysis (Table 1). Among them, there were 5 prognostic cohorts and 8 retrospective cohorts, different population were selected that including Caucasians (n = 5), Asians (n = 4), and mixed population (n = 4). And there were 10 studies [25-34] showed that the anemia significantly associated with a worse prognosis of mCRPC and 3 studies [35-37] showed that anemia had no association with mCRPC. Relative high quality of involved studies were observed by Cochrane Risk of Bias Tool and NOS scale (over 5 stars) (Tables 2, 3).
Figure 3.
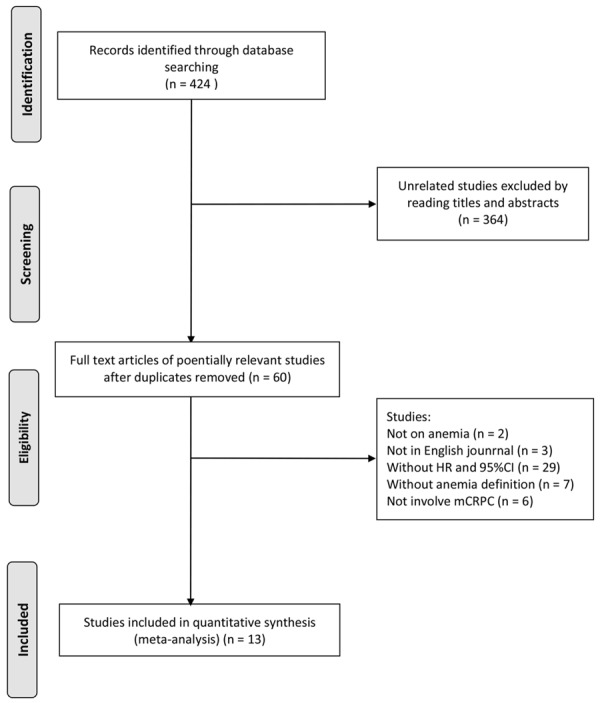
Flowchart of selection process in the meta-analyses.
Table 1.
The characteristic of each involved study
| Author/Year | Ethnicity | Type | Outcomes | Sample size | Definition of anemia (g/dL) | Median follow-up time (months) | Results |
|---|---|---|---|---|---|---|---|
| [25] C Ryan/2017 | Mixed | Prognostic | PFS | 1088 | <12.7 | 28 | S |
| [26] C Praet/2017 | Caucasian | Retrospective | OS | 368 | <12 | 14 | S |
| [35] C Buttigliero/2016 | Caucasian | Retrospective | OS | 179 | <13 | 32 | N.S |
| [27] O Caffo/2014 | Caucasian | Retrospective | OS | 260 | <11 | 11 | S |
| [28] K Fizazi/2014 | Mixed | Prognostic | OS | 1901 | <12.8 | 20 | S |
| [36] H Matsuyama/2014 | Asian | Retrospective | OS | 279 | <11.3 | 26 | N.S |
| [37] N Kamiya/2014 | Asian | Retrospective | CSS | 145 | <12.2 | 16 | N.S |
| [29] Y Qu/2012 | Asian | Retrospective | OS | 115 | <11 | 17 | S |
| [30] M Ito/2011 | Asian | Retrospective | OS | 80 | <11 | 15 | S |
| [31] J Shamash/2011 | Caucasian | Prognostic | OS | 270 | <11 | 19 | S |
| [32] A Armstrong/2009 | Mixed | Prognostic | OS | 1006 | <13 | 15 | S |
| [33] R Wyatt/2004 | Mixed | Retrospective | OS | 379 | <12 | 14 | S |
| [34] R Abratt/2004 | Caucasian | Prognostic | PFS | 414 | <13 | 4 | S |
OS, Overall survival; PFS, Progression free survival; S, significant; N.S, Non-significant.
Table 2.
RCTs were evaluated by the Cochrane Risk of Bias assessment tool
Table 3.
NOS scale for cohort studies
| Author/Year | Selection | Comparability | Outcomes | NOS score |
|---|---|---|---|---|
| [26] C Praet/2017 | 4 | 0 | 2 | 6 |
| [35] C Buttigliero/2016 | 4 | 1 | 2 | 7 |
| [27] O Caffo/2014 | 4 | 0 | 2 | 6 |
| [36] H Matsuyama/2014 | 4 | 0 | 2 | 6 |
| [37] N Kamiya/2014 | 4 | 0 | 2 | 6 |
| [29] Y Qu/2012 | 4 | 1 | 2 | 7 |
| [30] M Ito/2011 | 4 | 0 | 2 | 6 |
| [33] R Wyatt/2004 | 4 | 1 | 2 | 7 |
NOS, Newcastle-Ottawa Quality Assessment Form for Cohort Studies.
Anemia is a risk factor for prostate cancer prognosis
Considering that the mix of prognostic and retrospective cohorts might cause heterogeneity, we separately calculated the pooled HR and 95% CI for different study types. Meta-analyses based on prognostic studies showed that the anemia significantly lead to a worse prognostic for both OS (HR = 1.55, 95% CI = 1.24-1.94, I2 = 60.6%, Table 4; Figure 4) and PFS (HR = 1.47, 95% CI = 1.22-1.75, I2 = 0, Table 4; Figure 4). Meta-analyses based on retrospective studies also showed that the anemia promote prostate cancer progression (HR = 1.63, 95% CI = 1.25-2.13, I2 = 75%, Table 4). However, the heterogeneity was relatively high in this analysis. Since different populations were involved in current meta-analyses, we further performed meta-analyses in different populations. Surprisingly, no heterogeneity was found in sub-group population-based meta-analyses, and consistently, the meta-analyses showed that the anemia lead to a worse prognosis in Caucasians (HR = 2.00, 95% CI = 1.56-2.56, I2 = 0, Table 4; Figure 5) and Asians (HR = 1.64, 95% CI = 1.27-2.13, I2 = 0, Table 4; Figure 5). Meanwhile, sensitivity analysis was performed. After excluding one potential heterogeneity-causing study [33], no heterogeneity was found in the new meta-analysis and the results showed the anemia still promote the prostate cancer progression (HR = 1.82, 95% CI = 1.52-2.18, I2 = 0, Table 4; Figure 5). We speculated the heterogeneity might result from the population, as the excluding study was the only study involving African-Americans and Hispanics patients. Besides, one study [37] with cancer specific survival (CSS) endpoint showed no significant association between anemia and mCRPC prognosis (Table 4).
Table 4.
The meta-analyses of associations between anemia and mCRPC
| Group | Subgroup | Studies number | Sample size | HR (95% CI) | I2 (%) | Begg | Egger |
|---|---|---|---|---|---|---|---|
| Prognostic (OS) | NA | 3 | 3177 | 1.55 (1.24, 1.94) | 60.6 | 0.456 | 0.792 |
| Prognostic (PFS) | NA | 2 | 1502 | 1.47 (1.22, 1.75) | 0 | NA | NA |
| Retrospective (OS) Before sensitivity analysis | Total | 7 | 1666 | 1.63 (1.25, 2.13) | 75 | 1 | 0.011 |
| Caucasian | 3 | 807 | 2.00 (1.56, 2.56) | 0 | 1 | 0.31 | |
| Asian | 3 | 474 | 1.64 (1.27, 2.13) | 0 | 1 | 0.768 | |
| Mixed | 1 | 379 | 1.15 (1.07, 1.24) | NA | NA | NA | |
| Retrospective (OS) after sensitivity analysis | NA | 6 | 1287 | 1.82 (1.52, 2.18) | 0 | 1 | 0.653 |
| Retrospective (CSS) | NA | 1 | 145 | 1.47 (0.55, 3.99) | NA | NA | NA |
HR, hazard ratio; OS, Overall survival ; PFS, Progression free survival; CSS, Cause-Specific Survival; NA, Not available.
Figure 4.
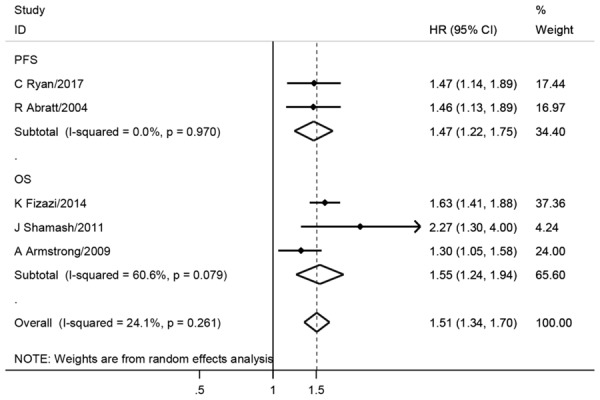
Forest plots of anemia with mCRPC outcomes among prognostic studies. The large diamond at the bottle of the table represents the pooled hazard ratio of all studies. The width of the diamond represents with 95% CI.
Figure 5.
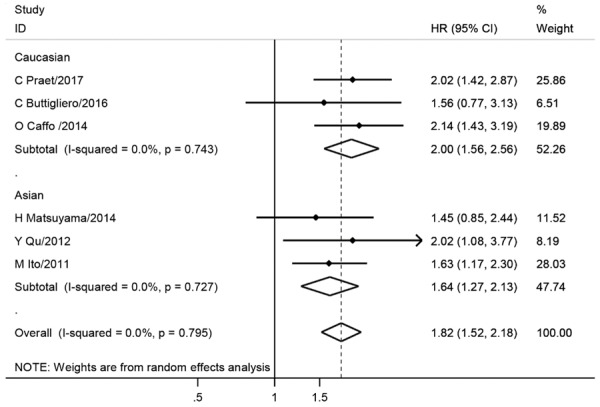
Forest plots of anemia with mCRPC OS among retrospective studies after sensitivity analysis. The large diamond at the bottle of the table represents the pooled hazard ratio of all studies. The width of the diamond represents with 95% CI.
Publications bias
As shown in Table 4, after sensitivity analysis of retrospective group, the Begg and Egger text showed no publication bias in all analyses (Begg >0.05, Egger >0.05). The funnel plots also showed symmetrical shapes that suggested no publication bias (Figure 6).
Figure 6.
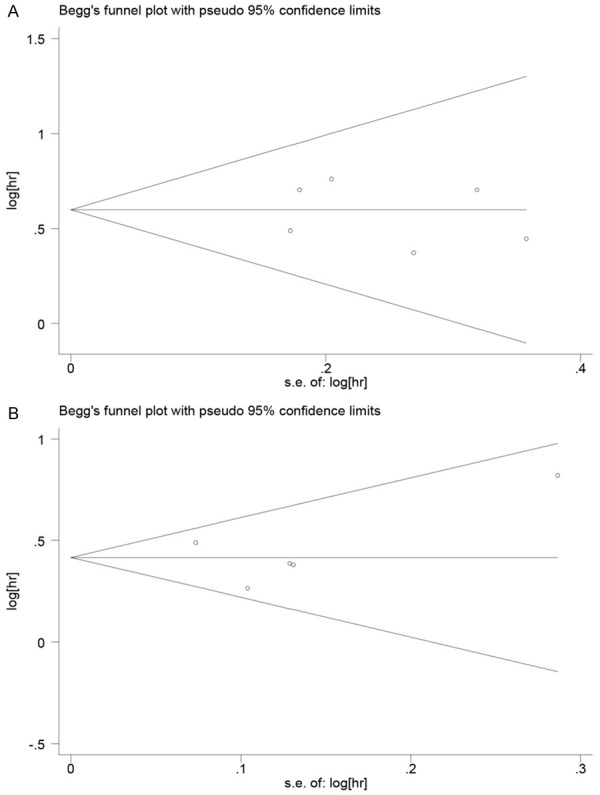
Funnel plots of anemia with mCRPC. A: Funnel plots of association between anemia and mCRPC OS among retrospective studies after sensitivity analysis; B: Funnel plots of association between anemia and mCRPC OS among prognostic studies. Hr: hazard ratio; SE, standard error; One cycle represents one individual study.
Discussion
Our meta-analysis showed that the anemia was significantly associated with a worse prognosis in mCRPC, and sub-group analyses by ethnicity found the anemia was a hazard factor both in Asians and Caucasians. Both prognostic and retrospective studies were involved in current meta-analysis. And the positive result was obtained from both types of studies.
Anemia frequently occurs in advanced prostate cancer [45]. ADT is the most common reason for anemia in advanced prostate cancer [23]. Testosterone could promote the generation of renal erythropoietin which could promote the differentiation of bone marrow erythroid stem cells to erythrocytes. Men with untreated hypogonadism commonly have mild anemia [46]. With no doubt, ADT would lower the level of testosterone and therefore impair the erythropoiesis. In non-metastatic prostate cancer patients without anemia, the use of gonadotropin-releasing hormone agonist therapy or orchiectomy would lead to a 1-2 g/dl fall in hemoglobin, which could cause a mild normochromic and normocytic anemia that was often not associated with bad clinical consequence. However, in metastatic prostate cancer patients, the use of ADT was more likely to lead to a more severe anemia, and the duration of ADT use was correlated with the severity of anemia [23]. Among the 15 studies included in current study, there were 12 studies [25-34,47,48] showed that the anemia was significantly associated with worse outcome of mCRPC while 3 studies [35-37] found no significant association between mCRPC prognosis and anemia. And our meta-analysis finally showed that the anemia indeed could promote the worse outcomes of mCRPC.
Most prostate cancer patients with ADT-induced anemia do not need anti-anemia treatment. However, treatments for symptomatic patients with more severe anemia are required (Figure 7). Any deficiencies in vitamin B12, folate or iron should be corrected. Erythropoiesis stimulating agents (ESAs) are effective in managing anemia and were demonstrated to reduce transfusion requirements and improve QoL in cancer patients with symptomatic anemia. However, the use of ESAs in cancer related anemia was controversial, as the ESAs could also promote angiogenesis that might stimulate cancer growth. In addition, ESAs might increase the risk of thrombotic events [49], despite the influence of ESAs in OS of cancer patients was uncertain [50-54]. Low doses of oral dexamethasone were found to decrease the severity of anemia for hormone-refratcory prostate carcinoma [55]. For the prostate cancer patients with limited bone marrow reserve, or symptomatic patients with less than 10 g/dl Hb, or asymptomatic patients with comorbidities such as congestive heart failure, blood transfusions may be the only effective treatment [56,57]. The treatments for ADT-induced anemia still need further studies to confirm their impacts on QoL and survival [23].
Figure 7.
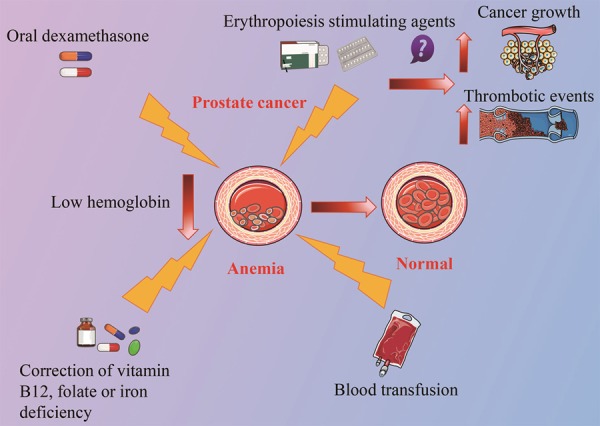
Treatment for anemia caused by ADT. For prostate cancer patients with symptoms of severe anemia, corrections of vitamin B12, folic acid or iron deficiency is needed. Erythropoiesis stimulants agents are also effective in controlling anemia. However, it could promote angiogenesis that might stimulate cancer growth and it might also cause thrombotic events. For anemia in hormone-refractory prostate cancer, low dose oral dexamethasone is found to be effective. For patients with more severe conditions, blood transfusion may be the only effective treatment.
There were some limitations in this systematic review. First, certain heterogeneity was found in prognostic group, while the sensitivity analysis failed to identify the potential heterogeneity (data not shown). We speculated that the heterogeneity was from the different diagnostic criteria of anemia and the ethnic diversity. Second, effects of confounding factors could not be assessed, which might influence the reliability of study. Third, no record was found about the treatment of anemia for mCRPC, considering the impacts of ADT on anemia incidence and the relationship of anemia and worse outcome of mCRPC, more studies on the anti-anemia treatments in mCRPC patients are required. Fourth, only one study involved CSS endpoint and showed no significant result, which indicated that anemia might be the direct cause for death, more studies are needed to draw a more solid result. On the other hand, focusing on OS is more important for the effect of anemia in mCRPC patients.
In conclusion, we found the anemia played a hazard role in mCRPC patients’ prognosis. We speculated that anemia was likely to be caused by ADT treatment. Larger RCTs are required to evaluate the effect of anti-anemia treatment on mCRPC patients.
Acknowledgements
This grant was supported by National Natural Science Foundation of China (81372178; 81502386; 81772944) and Zhejiang Provincial Program for High-level Innovative Healthcare talents.
Disclosure of conflict of interest
None.
References
- 1.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–21. [PubMed] [Google Scholar]
- 3.Beer TM, Tangen CM, Bland LB, Thompson IM, Crawford ED. Prognostic value of anemia in newly diagnosed metastatic prostate cancer: a multivariate analysis of southwest oncology group study 8894. J Urol. 2004;172:2213–2217. doi: 10.1097/01.ju.0000147771.92104.83. [DOI] [PubMed] [Google Scholar]
- 4.Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63:25–36. doi: 10.1016/j.ijrobp.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9(Suppl 5):31–40. doi: 10.1634/theoncologist.9-90005-31. [DOI] [PubMed] [Google Scholar]
- 6.Green SL, Giaccia AJ. Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy. Cancer J Sci Am. 1998;4:218–223. [PubMed] [Google Scholar]
- 7.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 8.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 9.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004-2013) Prostate Cancer Prostatic Dis. 2016;19:395–397. doi: 10.1038/pcan.2016.30. [DOI] [PubMed] [Google Scholar]
- 10.Noone AM HNKM. SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute; https://seer.cancer.gov/csr/1975_2015/based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 11.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 12.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Vogelzang NJ. Second-line hormonal therapy for advanced prostate cancer: a shifting paradigm. J. Clin. Oncol. 1997;15:382–388. doi: 10.1200/JCO.1997.15.1.382. [DOI] [PubMed] [Google Scholar]
- 15.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Semin Surg Oncol. 2000;18:52–74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Chau CH, Figg WD. Challenges to improved therapeutics for metastatic castrate resistant prostate cancer: from recent successes and failures. J Hematol Oncol. 2012;5:35. doi: 10.1186/1756-8722-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frieling JS, Basanta D, Lynch CC. Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control. 2015;22:109–120. doi: 10.1177/107327481502200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alibhai SM, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol. 2006;60:201–215. doi: 10.1016/j.critrevonc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Wrobel J, Millen J, Sredy J, Dietrich A, Kelly JM, Gorham BJ, Sestanj K. Orally active aldose reductase inhibitors derived from bioisosteric substitutions on tolrestat. J Med Chem. 1989;32:2493–2500. doi: 10.1021/jm00131a012. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadi H, Daneshmand S. Androgen deprivation therapy: evidence-based management of side effects. BJU Int. 2013;111:543–8. doi: 10.1111/j.1464-410X.2012.11774.x. [DOI] [PubMed] [Google Scholar]
- 21.Nelson AM, Gonzalez BD, Jim HS, Cessna JM, Sutton SK, Small BJ, Fishman MN, Zachariah B, Jacobsen PB. Characteristics and predictors of fatigue among men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer. 2016;24:4159–4166. doi: 10.1007/s00520-016-3241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis KK, Pruthi RK, Fonseca R, Gornet MK. Transfusion-dependent anemia after initiation of androgen deprivation therapy for metastatic prostate cancer. Urology. 2007;70:811–815. doi: 10.1016/j.urology.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Grossmann M, Zajac JD. Hematological changes during androgen deprivation therapy. Asian J Androl. 2012;14:187–192. doi: 10.1038/aja.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Chen WK, Zhang W, Zhang JS, Liu JH, Jiang YM, Fang KW. Network meta-analysis of the efficacy and adverse effects of several treatments for advanced/metastatic prostate cancer. Oncotarget. 2017;8:59709–59719. doi: 10.18632/oncotarget.19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan CJ, Kheoh T, Li J, Molina A, De Porre P, Carles J, Efstathiou E, Kantoff PW, Mulders P, Saad F, Chi KN. Prognostic index model for progression-free survival in chemotherapy-naive metastatic castration-resistant prostate cancer treated with abiraterone acetate plus prednisone. Clin Genitourin Cancer. 2017 doi: 10.1016/j.clgc.2017.07.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Praet C, Rottey S, Van Hende F, Pelgrims G, Demey W, Van Aelst F, Wynendaele W, Gil T, Schatteman P, Filleul B, Schallier D, Machiels JP, Schrijvers D, Everaert E, D’Hondt L, Werbrouck P, Vermeij J, Mebis J, Clausse M, Rasschaert M, Van Erps J, Verheezen J, Van Haverbeke J, Goeminne JC, Lumen N. Which factors predict overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate post-docetaxel? Clin Genitourin Cancer. 2017;15:502–508. doi: 10.1016/j.clgc.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Caffo O, De Giorgi U, Fratino L, Alesini D, Zagonel V, Facchini G, Gasparro D, Ortega C, Tucci M, Verderame F, Campadelli E, Lo RG, Procopio G, Sabbatini R, Donini M, Morelli F, Sartori D, Zucali P, Carrozza F, D’Angelo A, Vicario G, Massari F, Santini D, Sava T, Messina C, Fornarini G, La Torre L, Ricotta R, Aieta M, Mucciarini C, Zustovich F, Macrini S, Burgio SL, Santarossa S, D’Aniello C, Basso U, Tarasconi S, Cortesi E, Buttigliero C, Ruatta F, Veccia A, Conteduca V, Maines F, Galligioni E. Clinical outcomes of castration-resistant prostate cancer treatments administered as third or fourth line following failure of docetaxel and other second-line treatment: results of an Italian multicentre study. Eur Urol. 2015;68:147–153. doi: 10.1016/j.eururo.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Fizazi K, Massard C, Smith M, Rader M, Brown J, Milecki P, Shore N, Oudard S, Karsh L, Carducci M, Damiao R, Wang H, Ying W, Goessl C. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol. 2015;68:42–50. doi: 10.1016/j.eururo.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Qu YY, Dai B, Kong YY, Ye DW, Yao XD, Zhang SL, Zhang HL, Ma CG, Yang WY. Prognostic factors in Chinese patients with metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Asian J Androl. 2013;15:110–115. doi: 10.1038/aja.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito M, Saito K, Yasuda Y, Sukegawa G, Kubo Y, Numao N, Kitsukawa S, Urakami S, Yuasa T, Yamamoto S, Yonese J, Fukui I. Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology. 2011;78:1131–1135. doi: 10.1016/j.urology.2011.07.1416. [DOI] [PubMed] [Google Scholar]
- 31.Shamash J, Powles T, Sarker SJ, Protheroe A, Mithal N, Mills R, Beard R, Wilson P, Tranter N, O’Brien N, McFaul S, Oliver T. A multi-centre randomised phase III trial of dexamethasone vs dexamethasone and diethylstilbestrol in castration-resistant prostate cancer: immediate vs deferred diethylstilbestrol. Br J Cancer. 2011;104:620–628. doi: 10.1038/bjc.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–211. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt RB, Sanchez-Ortiz RF, Wood CG, Ramirez E, Logothetis C, Pettaway CA. Prognostic factors for survival among Caucasian, African-American and Hispanic men with androgen-independent prostate cancer. J Natl Med Assoc. 2004;96:1587–1593. [PMC free article] [PubMed] [Google Scholar]
- 34.Abratt RP, Brune D, Dimopoulos MA, Kliment J, Breza J, Selvaggi FP, Beuzeboc P, Demkow T, Oudard S. Randomised phase III study of intravenous vinorelbine plus hormone therapy versus hormone therapy alone in hormone-refractory prostate cancer. Ann Oncol. 2004;15:1613–1621. doi: 10.1093/annonc/mdh429. [DOI] [PubMed] [Google Scholar]
- 35.Buttigliero C, Pisano C, Tucci M, Vignani F, Bertaglia V, Iaconis D, Guglielmini P, Numico G, Scagliotti GV, Di Maio M. Prognostic impact of pretreatment neutrophil-to-lymphocyte ratio in castration-resistant prostate cancer patients treated with first-line docetaxel. Acta Oncol. 2017;56:555–562. doi: 10.1080/0284186X.2016.1260772. [DOI] [PubMed] [Google Scholar]
- 36.Matsuyama H, Shimabukuro T, Hara I, Kohjimoto Y, Suzuki K, Koike H, Uemura H, Hayashi T, Ueno M, Kodaira K, Tomita Y, Sakurai T, Shimizu N. Combination of hemoglobin, alkaline phosphatase, and age predicts optimal docetaxel regimen for patients with castration-resistant prostate cancer. Int J Clin Oncol. 2014;19:946–954. doi: 10.1007/s10147-013-0638-2. [DOI] [PubMed] [Google Scholar]
- 37.Kamiya N, Suzuki H, Ueda T, Sato N, Nakatsu H, Mikami K, Sato N, Nomura K, Akakura K, Okano T, Ooki T, Naya Y, Ota S, Masai M, Ichikawa T. Clinical outcomes by relative docetaxel dose and dose intensity as chemotherapy for Japanese patients with castration-resistant prostate cancer: a retrospective multi-institutional collaborative study. Int J Clin Oncol. 2014;19:157–164. doi: 10.1007/s10147-012-0510-9. [DOI] [PubMed] [Google Scholar]
- 38.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jamshidi AM, Moosazadeh M, Feizi MM, Feizi MM, Kiani A, Fakhri M. Prevalence of smoking in 15-64 years old population of north of Iran: meta-analysis of the results of non-communicable diseases risk factors surveillance system. Acta Med Iran. 2013;51:494–500. [PubMed] [Google Scholar]
- 41.Coory MD. Comment on: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39:932. doi: 10.1093/ije/dyp157. author reply 933. [DOI] [PubMed] [Google Scholar]
- 42.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805–3820. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 44.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nalesnik JG, Mysliwiec AG, Canby-Hagino E. Anemia in men with advanced prostate cancer: incidence, etiology, and treatment. Rev Urol. 2004;6:1–4. [PMC free article] [PubMed] [Google Scholar]
- 46.Ellegala DB, Alden TD, Couture DE, Vance ML, Maartens NF, Laws EJ. Anemia, testosterone, and pituitary adenoma in men. J Neurosurg. 2003;98:974–977. doi: 10.3171/jns.2003.98.5.0974. [DOI] [PubMed] [Google Scholar]
- 47.Yang YJ, Lin GW, Li GX, Dai B, Ye DW, Wu JL, Xie HY, Zhu Y. External validation and newly development of a nomogram to predict overall survival of abiraterone-treated, castration-resistant patients with metastatic prostate cancer. Asian J Androl. 2018;20:184–188. doi: 10.4103/aja.aja_39_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SH, Suh YS, Lee DE, Park B, Joo J, Joung JY, Seo HK, Lee KH, Chung J. A retrospective comparative study of progression-free survival and overall survival between metachronous and synchronous metastatic renal cell carcinoma in intermediate- or poor-risk patients treated with VEGF-targeted therapy. Oncotarget. 2017;8:93633–93643. doi: 10.18632/oncotarget.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glaspy J, Crawford J, Vansteenkiste J, Henry D, Rao S, Bowers P, Berlin JA, Tomita D, Bridges K, Ludwig H. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102:301–315. doi: 10.1038/sj.bjc.6605498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamson JW, Spivak JL. Physiologic basis for the pharmacologic use of recombinant human erythropoietin in surgery and cancer treatment. Surgery. 1994;115:7–15. [PubMed] [Google Scholar]
- 51.Alghamdi AA, Albanna MJ, Guru V, Brister SJ. Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. J Card Surg. 2006;21:320–326. doi: 10.1111/j.1540-8191.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 52.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 53.Vansteenkiste J, Glaspy J, Henry D, Ludwig H, Pirker R, Tomita D, Collins H, Crawford J. Benefits and risks of using erythropoiesis-stimulating agents (ESAs) in lung cancer patients: study-level and patient-level meta-analyses. Lung Cancer. 2012;76:478–485. doi: 10.1016/j.lungcan.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Smith SW, Sato M, Gore SD, Baer MR, Ke X, McNally D, Davidoff A. Erythropoiesis-stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica. 2012;97:15–20. doi: 10.3324/haematol.2011.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishimura K, Nonomura N, Yasunaga Y, Takaha N, Inoue H, Sugao H, Yamaguchi S, Ukimura O, Miki T, Okuyama A. Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer. 2000;89:2570–6. doi: 10.1002/1097-0142(20001215)89:12<2570::aid-cncr9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 56.Nalesnik JG, Mysliwiec AG, Canby-Hagino E. Anemia in men with advanced prostate cancer: incidence, etiology, and treatment. Rev Urol. 2004;6:1–4. [PMC free article] [PubMed] [Google Scholar]
- 57.MRCP JMWM and MRCP CAMC. Management of erectile dysfunction in men treated with androgen deprivation therapy. Trends in Urology Gynaecology & Sexual Health. 2013;4:13–18. [Google Scholar]