Abstract
Inflammatory bowel diseases (IBD) such as ulcerative colitis and Crohn’s disease are characterized by chronic inflammation of the gastrointestinal system. There is no permanent cure from IBD except constant medication or surgery to keep the disease in remission. In the present study, the effect of menthol, a major ingredient of peppermint has been investigated in acetic acid-induced colitis model in Wistar rats. Menthol (50 mg/kg/day) was orally administered for either 3 days before or 30 min after IBD induction for 7 days. The changes in body weight, macroscopic and microscopic analysis of the colon of rats of different experimental groups were observed on day 0, 2, 4 and 7. Acetic acid caused a significant reduction in mean body weight and induced macroscopic and microscopic ulceration along with a significant decline of glutathione (GSH) levels, an antioxidant substrate concomitant to increased malondialdehyde (MDA) level, a marker of lipid peroxidation and raised myeloperoxidase (MPO) activity, itself a marker for neutrophil activation. Acetic acid also induced the release of pro-inflammatory cytokines. Furthermore, acetic acid also raised the levels of calprotectin, a protein released by neutrophils under inflammatory conditions of the gastrointestinal tract. Treatment with menthol significantly improved IBD-induced reduction in mean body weight and mean macroscopic and microscopic ulcer scores and reduced activities of MPO and levels of MDA with concomitant increase in GSH level. Additionally, menthol treatment significantly reduced the levels of pro-inflammatory cytokines such as interleukin-1, interleukin-23 and tumor necrosis factor-α with no significant change in interleukin-6 levels. The data indicate that menthol improved body weight gain, mean macroscopic and microscopic ulcer scores, attenuated lipid peroxidation, oxidative stress and inflammation in the IBD rat mucosa.
Keywords: Menthol, inflammation, oxidative stress, rats, acetic acid, colitis
Introduction
Inflammatory bowel diseases (IBD) which includes ulcerative colitis and Crohn’s disease have been characterized by low grade sustained chronic inflammation of the gastrointestinal system [1]. In IBD the mucosal tissue damage is initiated and perpetuated by a dysregulated immune response. This also involves several intra- and extra-intestinal manifestations with an autoimmune component involving interaction between genetic, environmental and immunological factors [1]. The accumulation of neutrophils, macrophages and lymphocytes triggers pro-inflammatory mediators such as cytokines, eicosanoids, reactive oxygen species and neutrophil infiltration that culminate in inflammation of the intestine [2]. The management of IBD involves either medical or surgical approaches [3]. Currently available agents for IBD including corticosteroids, thiopurines, 5-aminosalicylates and immunosuppressants are not entirely effective and also exhibit numerous adverse effects which limit their long term use [3]. As a permanent cure from IBD is still not possible, most patients require constant medication or surgery to keep the disease in remission. However, response to these drugs is variable and can diminish over time [3]. Despite controlling the remission and diseases flares the onset and complications appear perplexing and far from satisfaction [3,4]. This imperfection points to the need for newer therapeutic agents that have potential to target intimately linked cascade; oxidative stress-inflammatory cytokine signaling [3,4]. As current therapeutic strategies are risky or ineffective for long-term use, new therapies for prevention of IBD remain a priority to reduce the burden of this disease. Thus, it is imperative to consider novel therapeutic modalities to overcome the debilitating clinical conditions of IBD. In search of novel therapeutic agents, medicinal plants have been studied extensively in the past few years and are considered as a major source of drug discovery as many reports have shown that about 80% of drug molecules are either natural products or the phytochemicals derived from plants. Also, since ancient time, botanical and ethno-botanical researches have focused for decades on the search for a single active principle in plants, based on the assumption that plants have one or few ingredients that determine therapeutic effects. Plants have shown promising results in the treatment of IBD in several earlier studies [5-7]. Among the natural compounds that have been investigated in recent studies, numerous plants containing essential oils including mentha have been shown to exert gastroprotective effects [8-10]. Therefore, the present investigation focuses on the actions of menthol, the main active constituent of peppermint plant. Menthol, a monocyclic monoterpene has been shown to exhibit significant therapeutic actions in various pathological conditions of gastrointestinal system [10,11]. Both peppermint and menthol are effective in vomiting and nausea [12], gastrointestinal spasm [13,14], dyspepsia [10,11,15], irritable bowel syndrome [9,16] and gastric ulcers [17,18].
In IBD, experimental models such as acetic acid-induced IBD model have proven to be important tools for detecting potential therapeutic agents and for investigating the mechanisms of pathogenesis. In the present study, the effects of menthol on macroscopic and microscopic pathologies of the colon were investigated. Furthermore, the activities of the enzymes involved in free radical formation and the levels of pro-inflammatory cytokines and calprotectin were investigated in acetic acid-induced IBD in rat model.
Materials and methods
The experiment was done with male albino Wistar rats weighing 225-240 g acquired from the local experimental animal breeding facility of the College of Medicine & Health Sciences (CMHS), United Arab Emirates University. Rats were housed in polypropylene cages (47 × 34 × 20 cm) lined with husk (replaced every 24 h) in a 12 h light/dark cycle at around 22°C. Rats were fed on standard diet and water ad libitum. After overnight fasting, the rats were lightly anaesthetized with ether and IBD was induced by intrarectal administration of 1 ml of 4% acetic acid at 8 cm proximal to the anus for 30 s. To flush the colon, 1 ml of phosphate buffered saline (PBS) was similarly administered. The experiments were carried out according to the Ethical Guidelines on Animal Studies of the United Arab Emirates University (NP-14-45).
Experimental design
Menthol was applied under two different application regimes, (1) where the effect of menthol was tested for 1 week after the induction of IBD, (2) prophylactic, where menthol was applied 3 days before the induction of IBD and continued for 7 days after IBD induction. In summary, for each application modality, rats were divided into pre IBD menthol treated (n = 18) and post IBD menthol treated groups (n = 18). Menthol 50 mg/kg in 1% carboxymethyl cellulose (CMC) was administered orally 30 min after the induction of IBD (post treated groups) daily. Control animals received 1% CMC alone using the same technique. To study the protective role of menthol on IBD, rats were administered menthol treatment 3 days before IBD induction (pretreated groups). The dose of 50 mg/kg was selected based on a dose-response pilot study in our laboratory and the results of published studies [18]. The animals were weighed at 0, 2, 4 and 7 days after IBD and colon samples from control, post and pretreatment groups were collected in liquid nitrogen and 4% neutral buffered formalin.
Assessment of macroscopic ulcer score
To examine the extent of colonic inflammation by macroscopic and microscopic analysis, histological samples were collected at selective time points (0 day and after 2, 4 and 7 days of IBD with or without menthol treatment). On 7th day after IBD, the rats were euthanized by cervical dislocation and the colons were excised 2 cm above the anal margin, opened longitudinally and washed with saline. Macroscopic damage was assessed by the scoring system of Wallace and Keenan [19], which takes into account the area of inflammation and the presence or absence of ulcers. The criteria for assessing macroscopic damage was based on a semi quantitative scoring system where features are graded as follows: 0, no ulcer, no inflammation; 1, no ulcer, local hyperemia; 2, ulceration without hyperemia; 3, ulceration and inflammation at one site only; 4, two or more sites of ulceration and inflammation; 5, ulceration extending more than 2 cm.
Assessment of microscopic ulcer score
After macroscopic observation, samples of the colon were subsequently excised for microscopic observation. The colon was fixed in 4% formalin in phosphate buffered saline (PBS) for 1 week after which it was washed under running tap water for 2 h. The samples were then dehydrated in graded ethanol and then embedded in paraffin wax. Thereafter, 5 µM sections were deparaffinized with xylene, stained with haematoxylin-eosin and were viewed microscopically. Microscopic analysis was based on the scoring system of Neurath et al. [20].
Preparation of colon tissue homogenate
Samples of 8 cm portion of distal colon were cut longitudinally to open, washed with ice-cold PBS. The tissue was weighed and homogenized with 10 volumes of in ice-cold high KCl lysis buffer (10 mM Tris-HCl, pH 8.0, 140 mM NaCl, 300 mM KCl, 1 mM EDTA, 0.5% Triton X-100 and 0.5% sodium deoxycholate) with complete protease inhibitor cocktail through 28 mm ceramic beads by using bead raptor 4 homogenizer (Omni international, United States). The resulting homogenates, after 30 min incubation on ice, were centrifuged at 15000 rpm for 30 min at 4°C. The resulting supernatant was stored at -40°C until ELISA. Protein concentration was determined in each sample by the BCA method based commercial kit.
Determination of myeloperoxidase (MPO)
Myeloperoxidase (MPO) was measured by sandwich ELISA according to manufacturer’s protocols. Briefly, 100 μl of the sample and standards were added to 96 well microtiter plate, coated with antibodies recognizing rat MPO, for 1 h at room temperature. After washing, 100 μl/well biotinylated trace antibody was added for 1 h at room temperature. After washing, Streptavidin-peroxidase conjugate was added to bind with biotinylated trace antibody for 1 h at room temperature. The TMB-ELISA substrate was added for 30 min at room temperature, after washing the plate. The enzyme reaction was stopped by oxalic acid. The absorbance was read at 450 nm, after adding stop solution, with a microplate reader (Tecan Group Ltd., Männedorf, Switzerland). MPO levels were expressed as ng per milligram of protein.
Determination of calprotectin
Enzyme immunoassay of calprotectin, in distal colon protein samples was performed by using commercial MyBioSource sandwich ELISA kit. Briefly, 50 µl sample and standards with 100 µl HRP were added to the coated 96 well micro titer plate for 1 h at 37°C. After washing, TMB-ELISA substrate was added at 37°C. The absorbance was read at 450 nm, after adding stop solution, with a microplate reader (Tecan Group Ltd., Mannedorf, Switzerland). Calprotectin levels were expressed as ng per milligram of protein.
Measurements of reduced glutathione (GSH)
The GSH content in colon homogenate was estimated following manufacturer protocol of the assay kit. Briefly, the measurement of GSH used a kinetic assay in which catalytic amounts (nmoles) of GSH cause a continuous reduction of 5,5-dithiobis (2-nitrobenzoic acid) to nitrobenzoic acid (TNB), and the glutathione disulfide (GSSG) formed was recycled by glutathione reductase and NADPH. The yellow color product, 5-thio-2-TNB, was measured spectrophotometrically at 412 within 5 min of 5,5-dithio-bis (2-nitrobenzoic acid) addition, against a blank with no homogenate. GSH concentration was expressed as μM of GSH per milligram of tissue.
Measurement of malondialdehyde (MDA)
The lipid peroxidation product, MDA, in the colon homogenate from each group was measured using MDA assay kit. Briefly, the assay is based on the reaction of MDA with thiobarbituric acid (TBA) to form a MDA-TBA adduct that absorbs strongly at 532 nm. Briefly, the deproteinated tissue sample was added to 1 M phosphoric acid and butylated hydroxytoluene in ethanol and then the mixture was heated at 60°C for 60 min. The suspension was cooled to room temperature and centrifuged at 10000 × g for 2-3 min and the pink colored supernatant was taken for spectroscopic measurements at 532 nm for the assay of MDA. The concentration of MDA was expressed as μM per 10 mg of tissue.
Determination of inflammatory cytokines TNFα, IL-1β, IL-6, and IL-23
Enzyme immunoassay of TNFα, IL-1β, IL-6 and IL-23 in colon homogenate was performed by using commercial sandwich Duoset ELISA kit (R&D Systems Inc. Minneapolis, MN, USA). In brief, the wells of a microtiter plate were coated with respective primary antibody in phosphate buffer saline (PBS), (100 µL/well) overnight at room temperature, washed with phosphate-buffered saline containing 0.05% Tween (PBST) and then blocked with 1% albumin bovine serum in PBS for one hour. After washing, the plates were incubated with serum, kidney homogenates and respective standards for 2 hours. After washing with PBST, a detection antibody was added for 2 hours. After washing, 100 µl of HRP was added and kept it for 30 minutes. Then, TMB-ELISA from (Sigma Chemical Co., St. Louis, MO, USA) was added and the color intensity was read at 450 nm with a Tecan microplate reader (Tecan Group Ltd, Männedorf, Switzerland). Cytokines levels were expressed as pg per milligram of protein. Enzyme immunoassay of IL-23, in distal colon protein samples was performed by using commercial MyBioSource sandwich ELISA kit. Briefly, sample and standards were added to the coated 96 well micro titer plates for 2 h at 37°C. After removal of samples, detection reagent A was added and incubated for 1 h at 37°C. After washing, detection reagent B was added and incubated for 1 h at 37°C. The TMB-ELISA substrate was added after washing. The absorbance was read at 450 nm, after adding a stop solution, with a microplate reader (Tecan Group Ltd., Männedorf, Switzerland). IL-23 levels were expressed as pg per milligram of protein.
Chemicals and kits
Acetic acid was purchased from BDH Prolabo (Johannesburg, South Africa). Menthol, Sodium deoxycholate, GSH enzyme kinetic assay kit and all other chemicals, if not specified were purchased from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO, USA). BCA Protein Assay kit and complete protease inhibitor cocktail were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Rat MPO sandwich ELISA kit was purchased from Hycult Biotech (Uden, The Netherlands). Cytokines duo set ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA). Rat IL-23 and Calprotectin ELISA kit was purchased from MyBioSource, Inc. (San Diego, CA, USA). Malondialdehyde Assay kit was purchased from Northwest Life Science Specialties, (Vancouver, WA, USA).
Statistical analysis
Data were analyzed statistically using SPSS 23.0 software. The mean of the data is presented with the standard error mean (SEM). The data were analyzed using independent t-test to determine the significance of the mean between the groups. Values of P < 0.05 were considered significant.
Results
Effect of menthol on body weight in acetic acid-induced IBD
The mean body weight (MBW) in controlled rats (before IBD) was 233.89 ± 3.03 g (n = 9, Table 1). IBD significantly reduced (P < 0.001) the MBW to 194.14 ± 9.51 g (n = 6). After 4 and 7 days of IBD, the MBW of the untreated rats recovered to 239.67 ± 11.15 (n = 9) and 231.38 ± 7.18 g (n = 10), respectively. Menthol, administered after the induction of IBD and continued for 7 days, at a dose of 50 mg/kg significantly (P < 0.05) increased the MBW to 220 ± 6.76 g 2 days after the onset of IBD (n = 6), but non-significantly to 223.40 ± 3.66 g on day 4 of IBD (n = 6) when compared to treated IBD rats (Table 1) and significantly (P < 0.05) to 245.57 ± 4.37 g when compared to untreated control. The body weight of rats pre-treated with menthol by gastric gavage at doses of 50 mg/kg per day for 3 days before the induction of IBD is presented in Table 1. The MBW of control rats was significantly (P < 0.01, n = 6) increased from 194.14 ± 9.51 g (n = 6) to 215 ± 7.59 on day 2 of IBD to 252.71 ± 3.66 g (P < 0.05, n = 6) on day 7 of IBD. However, a non-significant effect was seen in MBW on day 4 of IBD (244.50 ± 9.80 g, n = 6) compared to control (239.67 ± 11.15 g). In addition, the MBW of menthol-pretreated rats with administered at 50 mg/kg, increased significantly (P < 001, n = 6) on day 7 after the onset of IBD from 194.14 ± 9.51 g (n = 6) (untreated rats) to 252.71 ± 3.66 g.
Table 1.
Effect of menthol on mean body weight in rat model of IBD
| Time point | Control, no menthol | Post treatment | Pre treatment |
|---|---|---|---|
| Day 0 control | 233.89 ± 3.03 | ||
| Menthol + 2 Days IBD | 194.14 ± 9.51*** | 220.00 ± 6.76# | 215.00 ± 7.59## |
| Menthol + 4 Days IBD | 239.67 ± 11.15 | 223.40 ± 3.66 | 244.50 ± 9.80 |
| Menthol + 7 Days IBD | 231.38 ± 7.18 | 245.57 ± 4.37 | 252.71 ± 3.66# |
Values are expressed as mean ± SEM;
P < 0.001 vs. non IBD control group.
P < 0.05 vs. relative IBD control group;
P < 0.01 vs. relative IBD control group.
Effect of menthol on mean macroscopic ulcer score (MMaUS) in acetic acid-induced IBD
The effect of menthol on MMaUS in rats when given orally immediately after the induction of IBD is depicted in Figure 1A. The mucosa of the colon of untreated rats is hyperemic and ulcerative. Scoring of the lesions of the hyperemia and ulcer of the colon of acetic acid-induced IBD rats showed significant (P < 0.001) increase in MMaUS from 0 (control, no IBD) to 4.9 ± 0.1, 4 ± 0.4, and 0.8 ± 0.2 after 2, 4 and days 7, respectively. Menthol at a dose of 50 mg/kg, caused a significant (P < 0.05) decrease in MMaUS administered either before (3.4 ± 0.6, n = 6) or after (3.9 ± 0.4, n = 6) 2 days of IBD when compared to untreated groups, 4.9 ± 0.1 (IBD, no menthol, n = 6). Similarly, menthol administered orally before and after IBD at a dose of 50 mg/kg caused significant (P < 0.05-0.001) reduction on MMaUS after 4 and 7 days of IBD when compared to control (IBD, no menthol). The MMaUS was reduced from 4.0 ± 0.4 to 2.1 ± 0.3 (pretreatment) and to 1.4 ± 0.2 (post-treatment) after 4 days of IBD and from 0.75 ± 0.2 (control, no menthol) to 0.0 ± 0.0 (pretreatment) and to 0.2 ± 0.2 (post-treatment) on day 7 of IBD.
Figure 1.
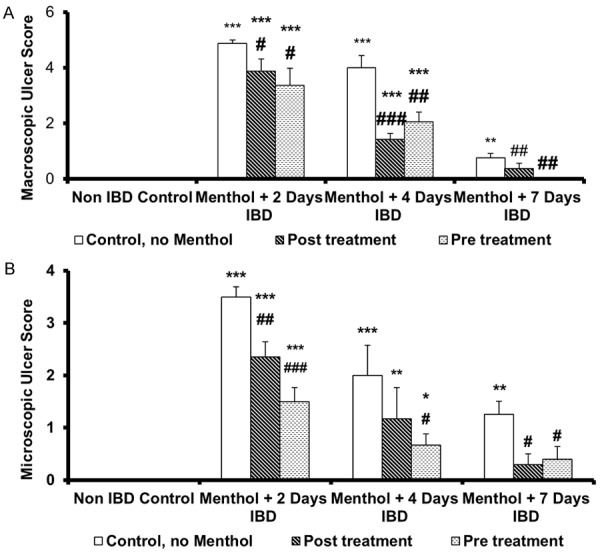
Effect of menthol on ulcer score in rat model of IBD. A. Colon macroscopic ulcer score; B. Colon microscopic ulcer score. Values are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. non IBD control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. relative IBD control group.
Effect of menthol on mean microscopic ulcer score (MMiUS) in acetic acid-induced IBD
In histologic analysis, massive epithelial loss, severe inflammatory cell infiltration, vasculitis and submucosal edema were evident in the colitis group. The effects of menthol on microscopic structure in the mucosa of the colon of control, untreated, and treated rats are presented in Figures 1B and 2. IBD caused a significant (P < .001) increase in MMiUS from 0 (untreated) to 3.5 ± 0.2 (n = 6), 2.7 ± 0.3, (n = 6) and 1.4 ± 0.2 at days 2, 4 and 7 of IBD induction, respectively (Figure 2Ba-Da). Menthol administered 3 days before IBD at a dose of 50 mg/kg to IBD rats, resulted in a significant (P < 0.05, n = 6) reduction in MMiUS from 3.5 ± 0.2, (IBD, no menthol) to 1.5 ± 0.3 at day 4, from 2.7 ± 0.3 to 0.6 ± 0.3 and from 1.4 ± 0.2 to 0.4 ± 0.2 at days 2, 4 and 7, respectively (Figure 2Bc-Dc). Administration of menthol also reduced lymphatic infiltration in the colon of rats treated with menthol after the induction of IBD (Figure 2Bb-Db).
Figure 2.
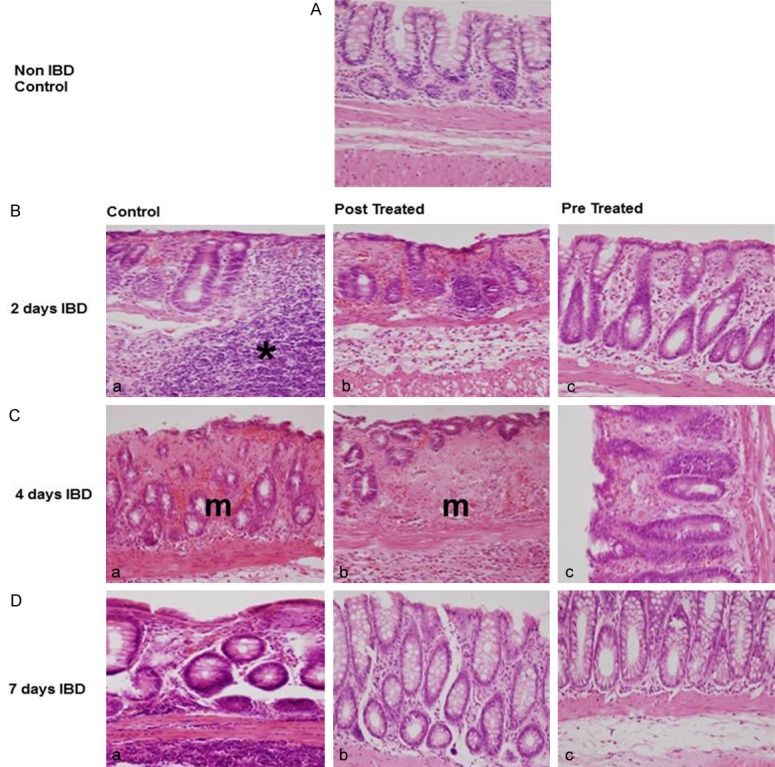
Photomicrographs representing the effects of menthol on the histopathological changes on descending colon in rat model of IBD. A: Non IBD control; B: 2 days IBD; C: 4-day IBD; D: 7-day IBD, (a) Control, no menthol, (b) Post IBD menthol treated (c) Pre IBD menthol treated. Note a large degree of lymphatic infiltration in B(a). In addition, the mucosal layer of C(a) and C(b) are highly compromised. *Lymphatic infiltration. m = mucosa.
Effect of menthol on mean colon myeloperoxidase levels in acetic acid-induced IBD
Mean colon myeloperoxidase (MCMPO) activity, the indicator of neutrophil migration to injured tissue, showed a marked increase in colitis group compared to control group (P < 0.001). The effect of menthol on MCMPO levels in rats administered orally for 3 days before or 30 min after the induction of IBD was measured to determine the extent of inflammation (Figure 3A). MCMPO level in control (no menthol, no IBD) was 0 ng/mg protein. IBD significantly (P < 0.05) increased MCMPO levels to 514 ± 44 ng/mg protein in untreated group (IBD, no menthol) after 2 days of IBD. However, menthol administered post-IBD, significantly (P < 0.001, n = 6) reduced MCMPO levels from 514 ± 44 (no menthol) to 176 ± 17 ng/mg of protein from 405 ± 122 (no menthol) to 79 ± 20 ng/mg of protein and from 67 ± 25 ng/mg of protein to 7 ± 2 (n = 6) ng/mg of protein at 2, 4 and 7 days of IBD, respectively. When menthol was administered 3 days before IBD, MCMPO levels were significantly (P < 0.05, P < 0.001, n = 6) reduced from 514 ± 44 ng/mg of protein in untreated group (IBD, no menthol) to 146 ± 20 ng/ml of protein, from 405 ± 51 ng/mg protein to 52 ± 6 of protein, and from 67 ± 25 to 9 ± 2 ng/mg protein at 2, 4 and 7 days of IBD, respectively.
Figure 3.
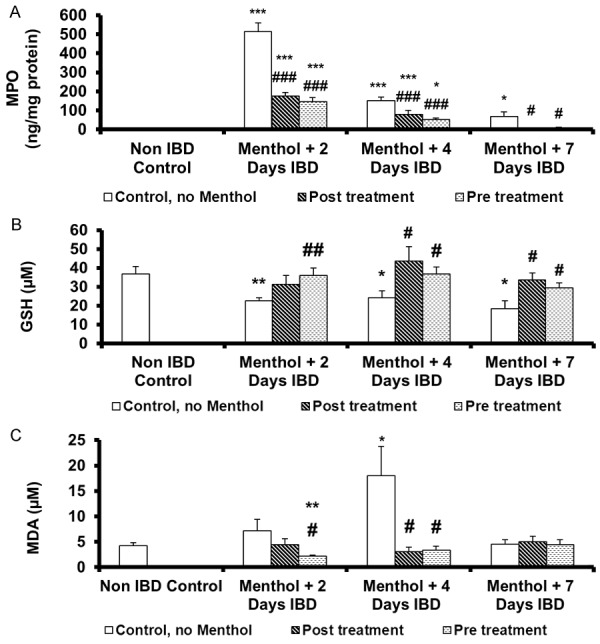
Effect of menthol on the markers of oxidative stress in colonic tissues of rat model of IBD. A. Activities of myeloperoxidase (MPO); B. Glutathione (GSH) level; C. Malondialdehyde (MDA) level. Values are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. non IBD control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. relative IBD control group.
Effect of menthol on the mean colon glutathione (MCGSH) levels in acetic acid-induced IBD
The effect of menthol on MCGSH levels in rats when administered orally after the induction of IBD is shown in Figure 3B. MCGSH in control (no IBD, no menthol) was 37 ± 3.9 μM (n = 9). MCGSH levels decreased significantly (P < 0.05, P < 0.01, n = 6) on days 2, 4 and 7 of IBD. The MCGSH levels were 23 ± 1.7 µM, 24 ± 3.6 μM and 18 ± 4.1 μM, respectively. When menthol was administered post-IBD, it significantly (P < 0.05) increased MSGSH levels from 24 ± 3.6 µM to 44 ± 7.6 µM and from 18 ± 4.1 to 30 ± 3.6 µM on days 4 and 7 of IBD, respectively. At day 2 of IBD, there was a non-significant increase from 23 ± 1.7 µM to 31 ± 4.6 µM. Menthol significantly (P < 0.05) increased MCGSH levels, when given orally for 3 days before the induction of IBD. MCGSH increased from 23 ± 1.7 μM to 36 ± 4.6 µM, from 24 ± 3.6 µM to 37 ± 7.6 µM and from 18 ± 4.1 µM to 29 ± 3.6 µM on days 2, 4 and 7, respectively.
Effect of menthol on mean colonic malondialdehyde levels in acetic acid-induced IBD
Mean colonic malondialdehyde (MCMDA), another mediator of inflammation, was measured to examine the effects of menthol administered either 3 days before or 30 min after the induction of IBD in rats is shown in Figure 3C. IBD non-significantly increased MCMDA levels from 4 ± 0.5 µM to 7 ± 2.2 µM on day 2 and from 4 ± 0.5 µM to 5 ± 0.9 µM on day 7. IBD significantly (P < 0.05, n = 6) increased MCMDA levels from 4 ± 0.5 µM to 18 ± 6.0 µM on day 4 of IBD. Menthol administered 30 min after IBD, significantly (P < 0.05, n = 6) deceased MCMDA levels from 18 ± 6.0 µM to 3 ± 0.9 µM after 4 days of IBD. Menthol had no effect on MCMDA at day 2 and day 7 of IBD. Menthol, administered pre-IBD, significantly (P < 0.05) decreased MCMDA levels from 7 ± 2.2 µM to 2 ± 0.3 µM and from 18 ± 6.0 µM to 3 ± 0.9 µM on day 7 of IBD. Menthol, administered either before or after IBD had no effect on MCMDA on day 7. Menthol also significantly (P < 0.01) reduced MCMDA levels when administered before IBD and compared to control (non-IBD, no menthol).
Effect of menthol on mean colon calprotectin levels in acetic acid-induced IBD
Mean colonic calprotectin, a marker of bowel inflammation, was measured to examine the effects of menthol administered either 3 days before or 30 min after the induction of IBD in rats is shown in Figure 4. IBD caused a significant (P < 0.05) increase in mean colonic calprotectin levels from 11 ± 1.6 ng/mg protein (control, no IBD), to 16 ± 1.0 ng/mg protein after 2 days of IBD. IBD had no effect on the mean colonic calprotectin after 4 and 7 days of IBD. Menthol, administered 30 min after IBD significantly (P < 0.001) from 15 ± 1.0 ng/mg protein to 10 ± 0.3 ng/mg protein and from 13 ± 0.5 ng/mg protein to 9 ± 0.4 ng/mg protein on days 2 and 4 of IBD, respectively. Menthol administered 3 days before IBD, MCMDA levels significantly (P < 0.001) decreased from 16 ± 1.0 ng/mg protein to 9 ± 0.4 ng/mg protein and from 13 ± 0.5 ng/mg protein on days 4 and 7 of IBD, respectively No effect was seen on MCMDA levels on day 7 of IBD whether administered 3 days before or 30 min after IBD.
Figure 4.
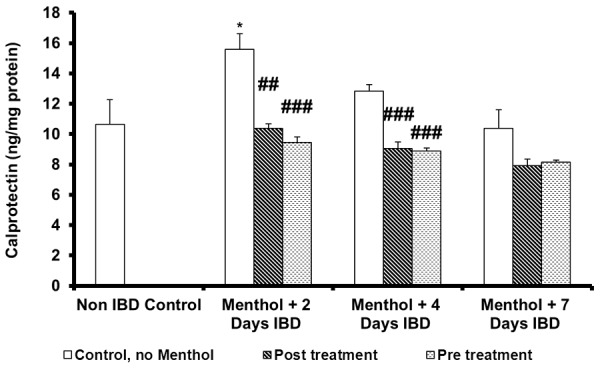
Effect of menthol on calprotectin level in colonic tissues of rat model of IBD. Values are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. non IBD control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. relative IBD control group.
Effect of menthol on mean colon interleukin-1 (IL-1) levels in acetic acid-induced IBD
Colonic interleukin-1 (IL-1), a marker of inflammation, was measured to examine the effect of menthol administered either before or after the induction of IBD in rats (Figure 5A). Mean colonic IL-1 level before IBD in the control group (no IBD, no menthol) was 85 ± 6 pg/mg of protein. IBD significantly (P < 0.001, n = 6) increased mean colonic IL-1 levels to 454 ± 10 (IBD, no menthol), and 207 ± 29 pg/gm of protein, on days 2 and 7 of IBD, respectively. No effect was observed on the mean colonic IL-1 levels on day 7 of IBD. However, menthol administered post-IBD, significantly (P < 0.05, P < 0.01, n = 6) reduced IL-1 levels from 454 ± 10 (no menthol) to 221 ± 30 ng/mg of protein, and from 207 ± 28 (no menthol) to 139 ± 18 ng/mg of protein and from 108 ± 14 ng/mg of protein to 57 ± 12 (n = 6) ng/mg of protein at 2, 4 and 7 days of IBD, respectively. When menthol was administered 3 days before IBD, menthol significantly (P < 0.05, P< 0.01, n = 6) reduced the IL-1 levels from 454 ± 10 ng/mg of protein in untreated group (IBD, no menthol) to 170 ± 15 ng/ml of protein, from 207 ± 29 ng/mg protein to 128 ± 16 of protein, and from 108 ± 25 to 62 ± 11 ng/mg protein at 2, 4 and 7 days of IBD, respectively.
Figure 5.
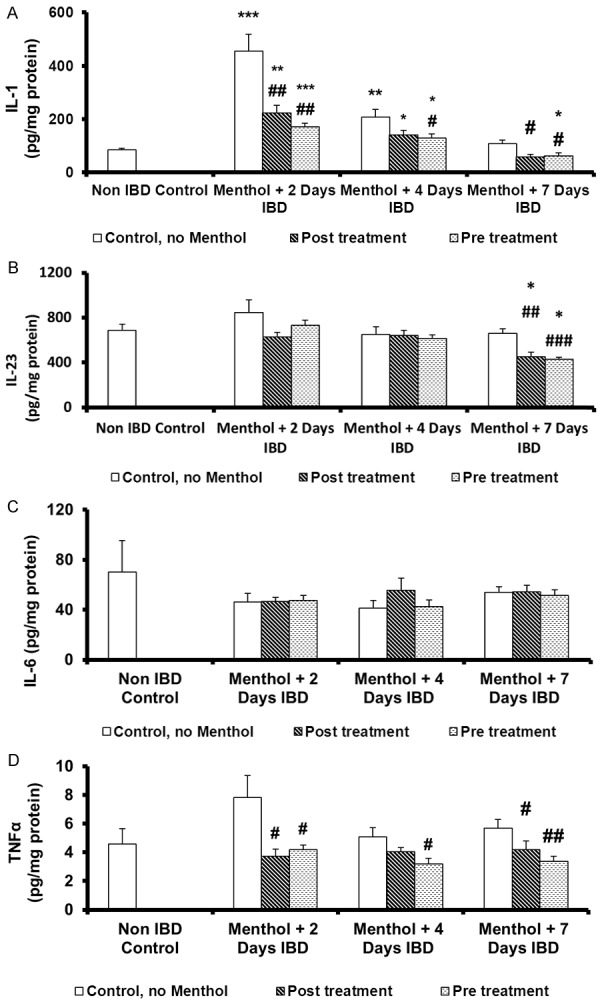
Effect of menthol on level of pro-inflammatory cytokines in colonic tissues of rat model of IBD. A. Interleukin-1 (IL-1); B. Interleukin-23 (IL-23); C. Interleukin-6 (IL-6); D. Tumor necrosis factor -α (TNFα). Values are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. non IBD control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. relative IBD control group.
Effect of menthol on mean colon interleukin-23 levels in acetic acid-induced IBD
Colonic interleukin-23 (IL-23), a mediator of inflammation, was measured to examine the effect of menthol before and after the induction of IBD (Figure 5B). Mean colonic IL-23 level before IBD in the control group (no IBD, no menthol) was 684 ± 58 pg/mg protein. IBD had no effect on colonic IL-23 levels but a non-significant increase was seen on day 2 of IBD. Post-treatment with menthol had a significant (P < 0.01) effect on mean colonic IL-23 levels only on day 7 of IBD. Mean colonic IL-23 levels decreased from 659 ± 40 pg/mg of protein to 452 ± 38 pg/mg protein. Similarly, when menthol was administered 3 days before IBD, it significantly (P < 0.001, n = 6) reduced the mean colonic IL-23 levels from 659 ± 40 (no menthol) to 430 ± 18 pg/mg of protein (n = 6) after 7 days. Menthol, whether administered before or after IBD, significantly (P < 0.05) reduced mean colonic IL-23 when compared to control group (No IBD, no menthol).
Effect of menthol on mean colon interleukin-6 levels in acetic acid-induced IBD
Colonic interleukin-6 (IL-6), another mediator of inflammation, was measured to examine the effect of menthol administered either 3 days before or 30 min after the induction of IBD (Figure 5C). Mean colonic IL-1 level before IBD in the control group (no IBD, no menthol) was 70 ± 25 pg/mg of protein. IBD and menthol treated groups had no effect on colonic IL-6 levels whether administered before or after IBD.
Effect of menthol on mean colon tumor necrosis factor-α levels in acetic acid-induced IBD
Colonic tumor necrosis factor-α (TNF-α), another mediator of inflammation, was measured to examine the effect of menthol administered either 3 days before or 30 min after the induction of IBD in rats (Figure 5D). Mean colonic TNF-α level before IBD in the control group (no IBD, no menthol) was 4.6 ± 1.0 pg/mg of protein. IBD non-significantly (n = 6) increased mean colonic TNF-α levels to 7.8 ± 10 pg/mg protein (IBD, no menthol) on day 2 of IBD but had no effect on TNF-α levels on days 4 and 7 of IBD. Menthol administered post-IBD, significantly (P < 0.05, n = 6) reduced mean colonic TNF-α levels from 7.8 ± 10 (no menthol) to 3.7 ± 0.5 pg/mg of protein, and from 5.0 ± 0.6 (no menthol) to 4.0 ± 0.3 pg/mg of protein and from 6.0 ± 0.6 pg/mg of protein to 3.4 ± 0.3 (n = 6) pg/mg of protein at 2, 4 and 7 days of IBD, respectively. When menthol was administered 3 days before IBD, menthol significantly (P < 0.05, n = 6) reduced the TNF-α levels from 7.8 ± 1.5 ng/mg of protein to 4.0 ± 0.3 ng/ml of protein, from 5.0 ± 0.6 ng/mg protein to 3.0 ± 0.4 of protein, and from 6.0 ± 0.6 to 3.4 ± 0.3 ng/mg protein at 2, 4 and 7 days of IBD, respectively.
Discussion
Ulcerative colitis and Crohn’s disease are the two main types of IBD. Patients with IBD can be become severely wasted during an acute attack of the disease [21] and generally the chronic nature of the disease causes debilitating health problems. Medications such as corticosteroids, thiopurines, and immunomodulators are commonly used in the treatment of IBD. However, response to these drugs is variable and can diminish over time. Therefore, it is imperative to consider novel therapeutic modalities to overcome devastating chronic conditions of IBD. Among new treatment modalities, the use of herbal therapy for IBD is increasing worldwide [5-7]. Among the natural compounds that have been investigated in recent studies, several monoterpenes including menthol have shown therapeutic effects in gastrointestinal diseases [5-9]. This study focuses on the actions of menthol, as the main active constituent of peppermint plant. Menthol belongs to monoterpene class of a structurally diverse group of phytochemicals found in plant-derived ‘essential oils’. Monoterpenes such as carvedilol [22], geraniol [23], carvacrol [24], and eucalyptol [25] have also been shown to suppress inflammatory process in earlier studies. Menthol has been shown to have beneficial effects in various pathological conditions of gastrointestinal system [5,6]. Both peppermint and menthol have been proposed to have therapeutic effects in preventing vomiting and nausea [5-7], gastrointestinal spasm [8,9], dyspepsia [5,6,10], irritable bowel syndrome [11,12], and in treatment of gastric ulcers [13,14]. Although sites of actions of menthol currently remain largely unknown. The functions of various ion channels [26,27] and enzymes [28,29] have been shown to be modulated by menthol.
The rat model of colonic inflammation was first developed by MacPherson and Pfeiffer in 1978 [30] using intra-luminal instillation of acetic acid. The initial injury in this model was a significant epithelial necrosis and edema that variably extended into the lamina propria, submucosa, or external muscle layers, depending of the concentrations and length of exposure to acetic acid [1]. Epithelial injuries were a relatively specific reaction to organic acids because HCl at a similar pH did not induce a similar injury [1]. Acetic acid-induced colitis is an easily inducible model of IBD, and the similarity of the profile of inflammatory mediators to IBD suggests that the inflammatory phase bears some resemblance to acute human intestinal inflammation [1,30,31]. In the present study 1 ml of 4% acetic acid, a known inducer of colonic inflammation, was used. The results showed that IBD decreases body weight in rats, a similar trend seen in clinical studies [21] and menthol caused a significant reduction of weight loss observed in acetic acid model of IBD. The etiology of weight loss and malnutrition in IBD are multifactorial, and the nutritional status is the result of complex pathophysiological processes [32,33]; these include postprandial pain, diarrhea or anorexia, malabsorption and maldigestion due to either the active disease, protein loss through the bowel or metabolic stress associated with inflammation and steroid therapy [34,35]. Diarrhea was observed in all animals tested and 50% of the IBD rats had blood in their stool, which is in line with the reports of other investigators [33,36].
In addition to weight loss and diarrhea, both microscopic and macroscopic pathologies induced by acetic acid were significantly reduced in menthol administered treatment groups. Histopathological examination of colon tissue indicated that increased microscopic and macroscopic damage scores in control IBD group were significantly reduced in menthol treated groups. In line with histopathological findings, biochemical analysis of colonic tissue also indicated that activity of MPO which is directly proportional to quantity of neutrophils in inflamed tissue, and the level of malondialdehyde, an end product of lipid peroxidation, was significantly reduced in menthol-treated groups. Similarly, levels of glutathione, an antioxidant molecule that is oxidized with peroxidase enzyme and eliminates hydrogen peroxide radical, were significantly enhanced in menthol-treated groups. Since, neutrophil infiltration, free radical formation and increased oxidative stress are among the established causal factors in IBD [37], some of the beneficial effects of menthol can be due to decrease of oxidative stress in inflamed colonic tissue. In line with our findings, antioxidant actions of menthol and menthol derivatives have been shown in earlier investigations [18,38-40].
Calprotectin is a protein occurring in the cytosol of inflammatory cells and is released by the activation of leukocytes, and increased tissue levels of calprotectin has been shown to be associated with progression of IBD [41]. In the present study, increased calprotectin levels were significantly reduced in menthol-treated groups further indicating that menthol suppresses inflammatory process in colonic tissue. In addition to calprotectin, production of pro-inflammatory cytokines such as IL-1, IL-6, IL-23, and TNF-α in inflamed colonic tissue was also significantly reduced in menthol-treated groups. In earlier studies, menthol has been shown to decrease inflammatory cytokine levels in gastric tissue [18] and monocytes [42,43].
In earlier studies, treatment with antioxidant drugs [44,45] has also been shown to improve the macroscopic and microscopic scores in acetic acid-induction model of IBD. In line with the results of these reports, the present study indicates that menthol has a protective effect on IBD by reducing the formation of free radicals. This is the first report of its kind on the effect of menthol on experimental colitis model in rats. Currently, there is an intensive search for a new drugs derived from medicinal plants. The new compound should be accessible, safe, and gastroprotective, and it should effectively heal the IBD, thus avoiding its recurrence. Our results indicate that menthol significantly reduces pathological and biochemical parameters of IBD in rat acetic acid model and suggest that this compound can be used in treatment of IBD.
Conclusions
The findings of the present study indicate that menthol, a cyclic monoterpene and the main compound of the essential oil of the genus Mentha has a significant anti-inflammatory effect in acetic acid-induced IBD model. Suppression of the inflammatory process by menthol is associated with decreased oxidative stress and reduced levels of pro-inflammatory cytokines in colonic tissues.
Acknowledgements
The authors gratefully acknowledge the financial support to the investigators from the United Arab Emirates University for providing facilities to conduct the experiments. Special thanks to Ms. Noora Al-Shehhi and Ms. Hessa Abdulla Al Sharqi for providing part of the technical help. The authors, SB, EA, NA and MO have made substantial contributions to the conception and design of the study (SB, EA, SO, MO), carrying out the experiments, analysis and interpretation of data (NA), drafting or revising the article critically for important intellectual content, and of course the final approval of the version to be submitted SB, EA, SO, MO.
Disclosure of conflict of interest
None.
References
- 1.Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sales-Campos H, Basso PJ, Alves VB, Fonseca MT, Bonfá G, Nardini V, Cardoso CR. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96–107. doi: 10.1590/1414-431X20143774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean LP, Cross RK. Adverse events in IBD: to stop or continue immune suppressant and biologic treatment. Expert Rev Gastroenterol Hepatol. 2014;8:223–40. doi: 10.1586/17474124.2014.881715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esters P, Dignass A. Complementary therapies in inflammatory bowel diseases. Curr Drug Targets. 2014;15:1079–1088. doi: 10.2174/1389450115666140903112802. [DOI] [PubMed] [Google Scholar]
- 6.Gilardi D, Fiorino G, Genua M, Allocca M, Danese S. Complementary and alternative medicine in inflammatory bowel diseases: what is the future in the field of herbal medicine? Exp Rev Gastroenterol Hepatol. 2014;8:835–846. doi: 10.1586/17474124.2014.917954. [DOI] [PubMed] [Google Scholar]
- 7.Triantafillidis JK, Triantafyllidi A, Vagianos C, Papalois A. Favorable results from the use of herbal and plant products in inflammatory bowel disease: evidence from experimental animal studies. Ann Gastroenterol. 2016;29:268–281. doi: 10.20524/aog.2016.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker J, Brown K, Rajendiran E, Yip A, De Coffe D, Dai C, Molcan E, Chittick SA, Ghosh S, Mahmoud S, Gibson DL. Medicinal lavender modulates the enteric microbiota to protect against Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G825–G836. doi: 10.1152/ajpgi.00327.2011. [DOI] [PubMed] [Google Scholar]
- 9.Nolen HW 3rd, Friend DR. Menthol-beta-Dglucuronide: a potential prodrug for treatment of the irritable bowel syndrome. Pharmaceut Res. 1994;11:1707–1711. doi: 10.1023/a:1018950930134. [DOI] [PubMed] [Google Scholar]
- 10.Grigoleit HG, Grigoleit P. Gastrointestinal clinical pharmacology of peppermint oil. Phytomedicine. 2005;12:607–611. doi: 10.1016/j.phymed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Grigoleit HG, Grigoleit P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine. 2005;12:612–616. doi: 10.1016/j.phymed.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Tate S. Peppermint oil: a treatment for postoperative nausea. J Adv Nurs. 1997;26:543–549. doi: 10.1046/j.1365-2648.1997.t01-15-00999.x. [DOI] [PubMed] [Google Scholar]
- 13.Micklefield GH, Greving I, May B. Effects of peppermint oil and caraway oil on gastroduodenal motility. Phytother Res. 2000;14:20–23. doi: 10.1002/(sici)1099-1573(200002)14:1<20::aid-ptr542>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Hiki N, Kaminishi M, Yasuda K, Uedo N, Kobari M, Sakai T, Hiratsuka T, Ohno K, Honjo H, Nomura S, Yahagi N, Tajiri H, Suzuki H. Multicenter phase II randomized study evaluating dose-response of antiperistaltic effect of L-menthol sprayed onto the gastric mucosa for upper gastrointestinal endoscopy. Dig Endosc. 2012;24:79–86. doi: 10.1111/j.1443-1661.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 15.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L. ) Phytother Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 16.Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. Effect of fiber, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozza AL, Hiruma-Lima CA, Takahira RK, Padovani CR, Pellizzon CH. Effect of menthol in experimentally induced ulcers: pathways of gastroprotection. Chem Biol Interact. 2013;206:272–278. doi: 10.1016/j.cbi.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Rozza AL, Meira de Faria F, Souza Brito AR, Pellizzon CH. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One. 2014;9:e86686. doi: 10.1371/journal.pone.0086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 1990;258:G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- 20.Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silk DB, Payne-James J. Inflammatory bowel disease: nutritional implications and treatment. Proc Nutr Soc. 1989;48:355–361. doi: 10.1079/pns19890051. [DOI] [PubMed] [Google Scholar]
- 22.Fatani AJ, Al Hosaini KA, Ahmed MM, Abuohashish HM, Parmar MY, Al Rejaie SS. Carvedilol attenuates inflammatory biomarkers and oxidative stress in a rat model of ulcerative colitis. Drug Develop Res. 2015;76:204–214. doi: 10.1002/ddr.21256. [DOI] [PubMed] [Google Scholar]
- 23.Medicherla K, Sahu BD, Kuncha M, Kumar JM, Sudhakar G, Sistla R. Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct. 2015;6:2984–2995. doi: 10.1039/c5fo00405e. [DOI] [PubMed] [Google Scholar]
- 24.Arigesavan K, Sudhandiran G. Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1, 2-dimethyl hydrazine plus dextran sodium sulfate induced inflammation associated carcinogenicity in the colon of Fischer 344 rats. Biochem Biophys Res Commun. 2015;461:314–320. doi: 10.1016/j.bbrc.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Santos FA, Silva RM, Campos AR, De Araujo RP, Lima Júnior RC, Rao VS. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem Toxicol. 2004;42:579–584. doi: 10.1016/j.fct.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Ashoor A, Nordman JC, Veltri D, Yang KH, Al Kury L, Shuba Y, Mahgoub M, Howarth FC, Sadek B, Shehu A, Kabbani N, Oz M. Menthol binding and inhibition of α7-nicotinic acetylcholine receptors. PLoS One. 2013;8:e67674. doi: 10.1371/journal.pone.0067674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashoor A, Nordman JC, Veltri D, Yang KH, Shuba Y, Al Kury L, Sadek B, Howarth FC, Shehu A, Kabbani N, Oz M. Menthol inhibits 5-HT3 receptor-mediated currents. J Pharmacol Exp Therpeut. 2013;347:398–409. doi: 10.1124/jpet.113.203976. [DOI] [PubMed] [Google Scholar]
- 28.Riachi LG, De Maria CA. Peppermint antioxidants revisited. Food Chem. 2015;176:72–81. doi: 10.1016/j.foodchem.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Oz M, Lozon Y, Sultan A, Yang KH, Galadari S. Effects of monoterpenes on ion channels of excitable cells. Pharmacol Therpeut. 2015;152:83–97. doi: 10.1016/j.pharmthera.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 30.MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135–150. doi: 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- 31.Sartor RB. How relevant to human inflammatory bowel disease are current animal models of intestinal inflammation? Alim Pharmacol Therapeut. 1997;11:89–96. doi: 10.1111/j.1365-2036.1997.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 32.Jeejeebhoy KN. Clinical nutrition: management of nutritional problems in of patients with Crohn’s disease. Canadian Med Ass J. 2002;166:913–918. [PMC free article] [PubMed] [Google Scholar]
- 33.Goh J, O’Morain CA. Nutrition and adult inflammatory bowel disease. Alimentary Pharmacol Therapeut. 2003;17:307–320. doi: 10.1046/j.1365-2036.2003.01482.x. [DOI] [PubMed] [Google Scholar]
- 34.Klein S, Meyers S, O’Sullivan P, Barton D, Leleiko N, Janovitz HD. The metabolic impact of active ulcerative colitis. Energy expenditure and nitrogen balance. J Clin Gastroenterol. 1988;10:34–40. doi: 10.1097/00004836-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Gassull MA. Nutritional consideration in inflammatory bowel disease: its relation to pathophysiology, outcome and therapy. Dig Dis. 2003;21:220–227. doi: 10.1159/000073339. [DOI] [PubMed] [Google Scholar]
- 36.Bastaki SM, Al Ahmed MM, Al Zaabi A, Amir N, Adeghate E. Effect of turmeric on colon histology, body weight, ulcer, IL-23, MPO and glutathione in acetic-acid-induced inflammatory bowel disease in rats. BMC Complement Altern Med. 2016;16:72. doi: 10.1186/s12906-016-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, Kumar SS. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. 2016;2016:5276130. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang SA, Jeon SK, Lee EJ, Shim CH, Lee IS. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat Prod Res. 2010;24:140–151. doi: 10.1080/14786410802496598. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Shen C, Tao Y, Wang S, Wei Z, Cao Y, Wu H, Fan F, Lin C, Shan Y, Zhu P, Sun L, Chen C, Wang A, Zheng S, Lu Y. Chemopreventive efficacy of menthol on carcinogen-induced cutaneous carcinoma through inhibition of inflammation and oxidative stress in mice. Food Chem Toxicol. 2015;82:12–18. doi: 10.1016/j.fct.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Mimica-Dukic N, Bozin B, Sokovic M, Mihajlovic B, Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Medica. 2003;69:413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 41.Chatzikonstantinou M, Konstantopoulos P, Stergiopoulos S, Kontzoglou K, Verikokos C, Perrea D, Dimitroulis D. Calprotectin as a diagnostic tool for infl ammatory bowel diseases. Biomed Rep. 2016;5:403–407. doi: 10.3892/br.2016.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juergens UR, Stober M, Vetter H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: a novel perspective for its therapeutic use in inflammatory diseases. Eur J Med Res. 1998;3:539–545. [PubMed] [Google Scholar]
- 43.Marcuzzi A, Tommasini A, Crovella S, Pontillo A. Natural isoprenoids inhibit LPS-induced production of cytokines and nitric oxide in aminobisphosphonate-treated monocytes. Int Immunopharmacol. 2010;10:639–642. doi: 10.1016/j.intimp.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, Panetta J, Morris CJ, Blake DR. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–415. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choudhary S, Keshavarzian A, Young M, Wade M, Bocckino S, Day BJ, Banan A. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Dig Dis Sci. 2001;46:2222–2230. doi: 10.1023/a:1011975218006. [DOI] [PubMed] [Google Scholar]


