Abstract
Skin wound healing is complex and involves the processes of many factors, among which angiogenesis and inflammatory responses play important roles. New blood vessels provide nutrition and oxygen for skin wound repair. Cytokines in skin wounds, which include pro-inflammatory and anti-inflammatory factors, can modulate the inflammatory response. Therefore, treatment strategies that promote angiogenesis and modulate the inflammatory response in skin wounds can accelerate skin wound healing. This study explored the effects of peptide Ser-Ile-Lys-Val-Ala-Val (SIKVAV)-modified chitosan hydrogels in skin wound healing. General observation demonstrated that SIKVAV-modified chitosan hydrogels promoted the contraction of skin wounds compared with the negative and positive controls. Masson’s trichrome staining indicated that peptide-modified chitosan hydrogels accelerated the deposition of more collagen fibers in the skin wounds compared with the negative and positive controls. Immunohistochemistry assays showed that more myofibroblasts were deposited and more angiogenesis was found in skin wounds treated with peptide-modified chitosan hydrogels compared with the negative and positive controls. In addition, qRT-PCR assays showed that peptide-modified chitosan hydrogels promoted the expression of TGF-β1 (transforming growth factor-β1) mRNA and inhibited the expression of TNF-α (tumor necrosis factor-α) mRNA and IL-1β (Interleukin-1β) mRNA and IL-6 (Interleukin-6) mRNA in skin wounds. Taken together, these results indicate the potential of SIKVAV-modified chitosan hydrogels in skin wound healing as complex biomaterials.
Keywords: SIKVAV, chitosan hydrogel, skin wound healing, angiogenesis, cytokine
Introduction
Cutaneous wound healing is a very complex process comprising hemostasis, inflammation, angiogenesis, re-epithelialization, extracellular matrix (ECM) deposition, and tissue remodeling in addition to the formation of granulation tissue [1]. Many factors can interfere with one or more phases of this process, affecting skin wound healing. Severe skin wounds can lead to disability and even death [2]. The current treatment strategies are composed of autograft, allograft, xenograft, and bioengineered skin substitutes and cytokine application [3]. In recent years, biomaterial-based skin wound dressings have drawn extensive attention due to their non-toxicity, degradability, and good tissue compatibility.
Angiogenesis plays a critical role in the proliferation and differentiation of cells in addition to granulation formation and tissue remodeling [4]. New blood vessels in the skin wounds provide nutrients and oxygen for skin wound healing. Angiogenesis is regulated by more factors such as growth factors, which include VEGF (vascular endothelial growth factor), bFGF (basic fibroblast growth factor), PDGF (platelet-derived growth factor) and TGF-β1 [5], scaffold [6], and the peptide SIKVAV, which is the functional domain in the α-peptide chain of laminin [7]. Studies have demonstrated that SIKVAV accelerates endothelial cells for blood vessel adhesion, migration and invasion [8], which are important in the formation of blood vessels in vivo. In addition, Kibbey MC et al. demonstrated that SIKVAV accelerates tumor cell growth and angiogenesis [9].
There are a variety of cytokines involved in the inflammation stage in skin wounds [10,11], including pro-inflammatory factors and anti-inflammatory factors. IL-1β, TNF-α and IL-6 are pro-inflammatory cytokines, and IL-4 (Interleukin-4), IL-10 (Interleukin-10), and TGF-β1 are anti-inflammatory factors. Pro-inflammatory factors promote the production of nitric oxide, arachidonic acid, histamine, etc., activate the complement system, and form a cytokine network of the cascade, leading to increased inflammation detriment in skin wound healing. Anti-inflammatory factors inhibit the production of pro-inflammatory cytokines and the activity of T cells and B cells, affect the ability of monocyte antigen presentation, and maintain the balance of the body’s cytokine network [12]. Excessive inflammatory reactions can inhibit wound healing [12]. Therefore, therapeutic strategies that modulate the inflammatory response would promote skin wound healing.
Chitosan is a deacetylated compound of chitin, which is a significant component in the shells/exoskeletons of crustaceans and is one of the most abundant polysaccharides in nature [13]. Chitosan contains a large number of hydroxyl and primary amino functional groups [14], which shows that it has many applications in drug delivery and pharmaceuticals, biotechnology, agricultural and environmental protection due to its excellent adsorption, and carrier and antibacterial capabilities. Chitosan has already been proposed as a biomaterial because of various characteristics, including its biocompatibility, non-toxicity and biodegradability [15]. Due to its good antimicrobial and hemostatic activities, chitosan has also been extensively explored for skin wound dressing [15]. In addition, studies has demonstrated that chitosan promotes the formation of granulation tissue in skin wounds by accelerating the functions of inflammatory cells, including polymorphonuclear leukocytes and macrophages [16].
In this study, we synthesized a composite hydrogel sheet composed of chitosan and SIKVAV and assessed the in vivo effect of the hydrogel in skin wound healing in full-thickness wounds in a mouse skin defect model. Our previous studies [17] showed that peptide-modified chitosan hydrogels promoted skin wound healing by modulating the function of macrophages. Considering the potential of the peptide-modified chitosan hydrogels in skin wound healing, the present study was pursued to evaluate the effect of skin wound healing and to explain the healing mechanism of the hydrogels in skin excision wounds in mice.
Materials and methods
Materials
Chitosan (85% deacetylation degree, molecular weight 100,000 Da) was obtained from Golden-Shell Pharmaceutical Co., Ltd. (Yuhuan, China). Methacrylic anhydride was purchased from APC Chemicals Company (Montreal, Canada). 3-(Maleimido) propionic acid n-hydroxysuccinimide ester (SMP; 97%) was purchased from Polysciences Corporation (Tamil Nadu, India). N,N,N,N-tetramethylethylenediamine (TEMED), ammonium persulfate (APS), and dimethylformamide (DMF) were purchased from Sigma-Aldrich (Guangzhou, China). The SIKVAV peptide was obtained from Peptide Biotech Co., Ltd.(Shanghai, China). Sodium pentobarbital was purchased from Aladdin (Guangzhou, China). Trizol reagent and cDNA reverse transcription kit were purchased from Invitrogen (Carlsbad, CA, USA). qRT-PCR test kits for TNF-α mRNA, IL-6 mRNA, IL-1 mRNA, TGF-β1 mRNA, and GAPDH were obtained from Shanghai Lichen Biotechnology Company (Shanghai, China). CD31 monoclonal antibody was purchased from Dako (Guangzhou, China). K6 polyclonal antibody was purchased from Convance (New York, USA). Alpha-smooth muscle actin (α-SMA) polyclonal antibody, biotinylated secondary antibody, and streptavidin-biotin complex (SABC) detection kits were purchased from Wuhan Boster Biological Engineering Co., Ltd. (Wuhan, China).
Synthesis of peptide-modified chitosan hydrogels
Peptide-modified chitosan hydrogels were prepared as described in our previous reports [18,19]. Briefly, methacrylic anhydride was added to the chitosan solution containing 3% glacial acetic acid and then dialyzed to obtain double-bond chitosan. The 3-maleimidopropionic acid-N-succinimide ester was dissolved in dimethylformamide (DMF), added to the double-bond chitosan solution, stirred at room temperature overnight, dialyzed, and freeze-dried to obtain SMP-modified double-bond chitosan. SIKVAV was added to the SMP-modified double-bond chitosan solution and stirred at room temperature under the protection of nitrogen for 24 hours, dialyzed and then lyophilized to obtain peptide SIKVAV-modified chitosan. The SIKVAV-modified chitosan solution was added to the centrifuge tube. Ammonium persulfate and then TEMED solution were added using a pipette gun to blow evenly. The solution was shaken to form a hydrogel. As a control, a double-bond chitosan hydrogel that did not contain SIKVAV was obtained using the same synthesis method as the SIKVAV-modified chitosan hydrogel.
In vivo studies of peptide-modified chitosan hydrogels on skin wound healing in mice
Animal experiments were performed at the Animal Experimental Center of Jiujiang University and approved by Jiujiang University Ethics Committee, which strictly conforms to the NIH action guidelines for laboratory animal management and safety. C57 mice were selected from 8 to 12 weeks, and 1% pentobarbital sodium was intraperitoneally injected at 0.01 body weight. After the mouse was anesthetized, its unilateral back hair was removed, and a 0.6-cm wound was made on the dorsal skin of the mouse with a perforator. The wound was fixed at the edge of the skin wound with plastic rings, which prevented shrinkage of the skin wound. After the trauma model was established, 72 mice were randomly divided into 4 groups, including the control group, SIKVAV group, chitosan hydrogel group, and SIKVAV + chitosan hydrogel group, and there were 18 mice in each group. Digital cameras were used to record the effect of skin wound healing on 3, 5, and 7 days after trauma, and the remaining area ratio of the skin wound was calculated using Eq. (1).
Remaining area ratio of wound (%) = St/So × 100%
So: the original area of the wound, St: the remaining area of the wound at different times.
Histological observations
On days 3, 5 and 7 after trauma, the wounds and 5 mm of normal skin tissue around the wounds were obtained, washed with PBS, fixed with 4% paraformaldehyde, washed in PBS, gradually dehydrated using 70% to 100% ethanol, and embedded in paraffin. A 5-μm paraffin section was obtained, and the tissue was stained according to the Masson trichrome staining procedure.
Immunohistochemistry assays
The 5 μm paraffin sections were deparaffinized by xylene, rehydrated and then neutralized with 0.1 M citrate buffer solution (pH 6.0). Next, 10% H2O2 was used to inactivate the endogenous enzymes for 10 min. The tissue sections were blocked with 5% BSA for 2 h and then incubated with monoclonal goat anti-mouse α-SMA antibody at 4°C overnight. The tissue sections were reacted with SABC for 20 min, colored with 3,30-diaminobenzidine (DAB), stained with hematoxylin, and dehydrated with a gradient ethanol series. The tissue sections were soaked with xylene and then sealed with resin. Each tissue section was observed with a microscope (400 ×) at five randomly selected fields from one section (three sections from three mice in each group). Image-Pro Plus was used to analyze the average optical density values for α-SMA expression. Five randomly selected fields of view were examined for each group at each time point and used to assess the average optical density value per unit area. Immunohistochemistry assays adopted to detect CD31 and K6 in the skin wound tissue samples were performed using the above methods.
mRNA expression studies
Total RNA of the skin wound tissue was extracted by Trizol reagent according to the manufacturer’s protocol. Reverse transcription was performed with 2 μg RNA using a high-capacity cDNA reverse transcription kit. The annealing of the primers was performed at 25°C for 10 min, followed by elongation at 37°C for 2 h and inactivation by the enzyme at 85°C for 5 min. The primers were synthesized by Sangon Biotech (Shanghai, China), and the sequences are as follows: IL-1β forward 5’-GCAACTGTTCCTGAACTCAACT-3’ and reverse 5’-ATCTTTTGGGGTCCGTCAACT-3’, IL-6 forward 5’-GAGGATACCACTCCCAACAGACC-3’ and reverse 5’-AAGTGCATCATCGTTGTTCATACA-3’, and TGF-β1 forward 5’-CGAGAAGCGGTACCTGAAC-3’ and reverse 5’-TGAGGTATCGCCAGGAATTGT-3’, TNF-α forward 5’-CATCTTCTCAAAATTCGAGTGACAA-3’ and reverse 5’-TGGGAGTAGACAAGGTACAACCC-3’. GAPDH forward 5’AGCAGTCCCGTACACTGGCAAAC-3’ and reverse 5’TCTGTGGTGATGTAAATGTCCTCT-3’.
PCR was performed using QuantiFast SYBR Green PCR Kit (Qiagen, Valencia, CA, USA). Taq polymerase was activated at 95°C for 5 min. The cycling parameters were denatured at 95°C for 30 s and extended at 60°C for 1 min (for 40 cycles). PCR was performed in triplicate in a model 7500 Real-time PCR system (Applied Biosystems, Foster City, Ca, USA). Relative gene quantitation was detected by the 2-ΔΔCT method. GAPDH was used as the endogenous control gene.
Statistical analysis
The measurement data are expressed as the mean ± standard deviation. For similar time points in the study, multiple samples from the four groups were compared by one-way analysis of variance, including statistical analysis of the remaining area of the skin wound, statistical analysis of α-SMA expression, statistical analysis of K6 expression, statistical analysis of the number of new blood capillaries, and statistical analysis of the expression of TNF-α mRNA and IL-6 mRNA and IL-1β mRNA and TGF-β1 mRNA. A p-value of less than 0.01 (**P < 0.01) indicates a statistically significant difference between the groups. The SPSS 20.0 software was used to analyze the data. All experiments were performed at least three times.
Results
Peptide-modified chitosan hydrogels accelerated contraction of skin wounds in mice
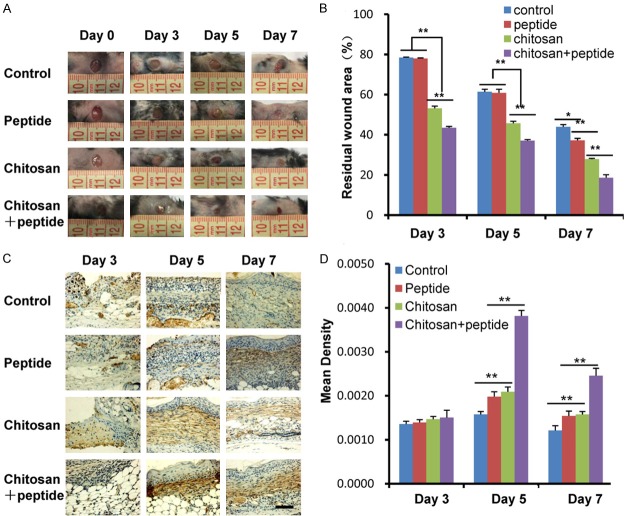
As shown in Figure 1A, general observation demonstrated that the skin wound healing effect of the SIKVAV + chitosan group was better than those of the control, SIKVAV, and chitosan groups. The statistical analysis of the residual area ratio of the skin wounds in all groups is shown in Figure 1B. The residual wound area ratio of the SIKVAV + chitosan group was significantly smaller than those of the control, SIKVAV, and chitosan groups 3, 5, and 7 days after trauma. General photomicrographs of the skin wound in mice show that the SIKVAV-modified chitosan hydrogels promote skin wound healing.
Figure 1.
Peptide-modified chitosan hydrogels promote wound contraction in skin wounds in mice. A. General observations of the skin wounds in mice showed that the skin wound healing effect of the SIKVAV + chitosan group was better than those of the control, peptide, and chitosan groups 3, 5, and 7 days after trauma. B. Statistical analysis showed that the remaining area of the skin wound of the SIKVAV + chitosan group was smaller than those of the control, peptide, and chitosan groups 3, 5, and 7 days after trauma. C. Detection of α-SMA expression in skin wound in mice by immunohistochemistry among the control, peptide, chitosan, and peptide + chitosan groups 3, 5, and 7 days after trauma (scale bar: 50 μm). D. Statistical analysis showed that α-SMA expression of skin wound of the SIKVAV + chitosan group was more than those of the control, peptide, and chitosan groups 3, 5, and 7 days after trauma the other groups.
The pulling of myofibroblasts in the skin wounds promotes wound contraction. During wound healing, fibroblasts transform into myofibroblasts, which express α-SMA. Therefore, the expression of α-SMA was detected by immunohistochemistry in skin wound tissue 3, 5, and 7 days after trauma. The results are shown in Figure 1C. The expression of α-SMA of the skin wound tissue in each group was weaker 3 days after trauma; it gradually increased 5 days after trauma and gradually decreased in each group 7 days after trauma. Quantitative analyses of the optical density of α-SMA in the skin wounds among the 4 groups 3, 5, and 7 days after trauma are shown in Figure 1D. There was no significant difference in the optical density of α-SMA among the 4 groups 3 days after trauma. The optical density of α-SMA in the skin wound tissue of the SIKVAV + chitosan group was significantly higher than those in the other groups 5 and 7 days after trauma.
Peptide-modified chitosan hydrogels accelerated the proliferation of keratinocytes in the skin wounds
K6 is a marker of the proliferation of keratinocytes during skin wound healing. To further verify that the peptide-modified chitosan hydrogels promote proliferation of keratinocytes in the skin wounds, immunohistochemistry was used to detect the expression of K6 of keratinocytes in the skin wounds. The results are shown in Figure 2A. The expression of K6 of keratinocytes in the skin wounds in each group was weaker 3 days after trauma; it gradually increased 5 days after trauma and gradually decreased in each group 7 days after trauma. Quantitative analyses of the optical density of K6 of keratinocytes in the skin wounds among the 4 groups 3, 5, and 7 days after trauma are shown in Figure 2B. There was no significant difference in the optical density of K6 of keratinocytes in the skin wounds among the 4 groups 3 days after trauma, and it was significantly higher in the SIKVAV + chitosan group than in the other groups 5 and 7 days after trauma. Then, there was no significant difference of the optical density of K6 of keratinocytes in the skin wounds between the SIKVAV and chitosan groups 5 and 7 days after trauma.
Figure 2.
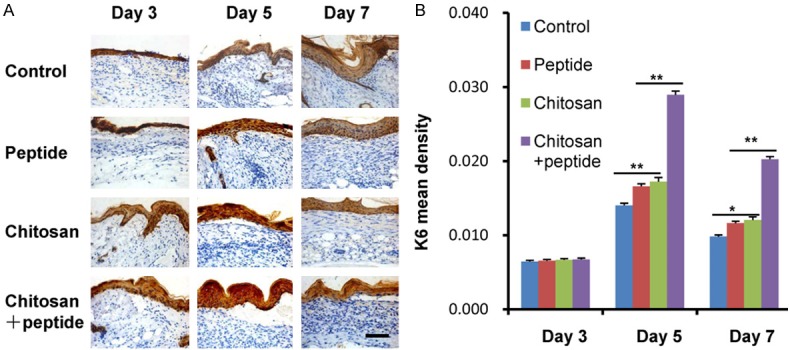
Peptide-modified chitosan hydrogels promote proliferation of keratinocytes in the skin wounds in mice. A. Detection of the K6 expression in skin wound in mice by immunohistochemistry among the control, peptide, chitosan, and peptide + chitosan groups 3, 5, and 7 days after trauma (scale bar: 50 μm). B. Statistical analysis showed that K6 expression of skin wound of the SIKVAV + chitosan group was more than those of the control, peptide, and chitosan groups 3, 5, and 7 days after trauma.
Peptide-modified chitosan hydrogels promoted angiogenesis of the skin wounds
New blood vessels in skin wounds provide nutrients for the formation of granulation tissue and proliferation of keratinocytes, which play an important role during skin wound healing. In this study, immunohistochemistry was used to mark the expression of CD31 in the endothelial cells of capillary in the skin wounds to observe angiogenesis. The results are shown in Figure 3A. The effect of angiogenesis in the skin wound in the SIKVAV + chitosan group was better than those among the control, SIKVAV and chitosan groups. Statistical analysis of the number of new blood capillaries in the skin wounds is shown in Figure 3B. The number of new blood capillaries in the skin wounds in the SIKVAV + chitosan group was significantly more than those in the control, SIKVAV and chitosan groups 5 and 7 days after trauma, and there was no difference between the SIKVAV group and chitosan group. This result fully demonstrates that SIKVAV-modified chitosan hydrogels promote angiogenesis in skin wounds in mice.
Figure 3.

Peptide-modified chitosan hydrogels accelerate angiogenesis in skin wounds in mice. A. Immunohistochemistry was used to detect the expression of CD31 in the vascular endothelium of skin wounds in mice among the control, peptide, chitosan, and peptide + chitosan groups 5 and 7 days after trauma (scale bar: 50 μm). B. Statistical analysis showed that the number of new blood capillaries in skin wounds in the SIKVAV + chitosan group was more than those in the control, peptide, and chitosan groups 5 and 7 days after trauma.
SIKVAV-modified chitosan hydrogels accelerated collagen synthesis in skin wounds
Collagen synthesis plays a critical role during skin wound healing by providing a scaffold for the proliferation of cells and regeneration of blood vessels. In this experiment, Masson’s trichrome staining was used to observe new collagen fibers in the skin wounds. As shown in Figure 4, on days 3, 5, and 7 after trauma, there were more collagen fibers in the granulation tissue of the skin wounds in the SIKVAV + chitosan group and fewer collagen fibers in the other three groups. On day 5 after trauma, the deposition of collagen fibers in the skin wounds in the SIKVAV + chitosan group increased, but there were fewer collagen fibers in the skin wounds among the control, SIKVAV and chitosan groups. On day 7 after trauma, a large amount of collagen fibers appeared in the skin wound in the SIKVAV + chitosan group. This result indicates that the SIKVAV-modified chitosan hydrogels promote the deposition of collagen fibers in the skin wounds in mice.
Figure 4.
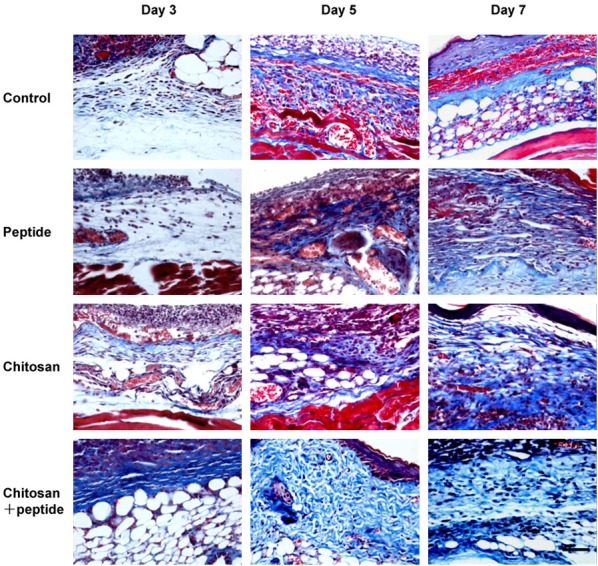
Masson’s trichrome staining showed that deposition of new collagen fibers in the skin wounds in the peptide + chitosan group was more than those in the control, peptide, and chitosan groups on days 3, 5, and 7 after trauma (scale bar: 50 μm).
Peptide-modified chitosan hydrogels modulated the secretion of cytokines in skin wounds
There is an inflammatory response involved in skin wounds with a variety of cytokines, including pro-inflammatory and anti-inflammatory cytokines. IL-1β, TNF-α and IL-6 are pro-inflammatory cytokines, and TGF-β1 is an anti-inflammatory factor. qRT-PCR was used to detect the expression of cytokines in the skin wounds. Figure 5A-D shows the expression of four important cytokines in the skin wounds in mice among the control, SIKVAV, chitosan, and peptide + chitosan groups 3, 5, and 7 days after trauma. At each time point, the expression level of TNF-α mRNA, IL-6 mRNA, and IL-1 mRNA in the skin wounds in the SIKVAV + chitosan group was significantly lower than those in the control and SIKVAV groups, and there was no difference from that of the chitosan group. However, at each time point, the expression level of TGF-β1 mRNA in the skin wounds in the SIKVAV + chitosan group was significantly higher than those in the control, SIKVAV and chitosan groups.
Figure 5.

Peptide-modified chitosan hydrogels inhibited the expression of pro-inflammatory cytokines, including TNF-α mRNA (A), IL-6 mRNA (B), and IL-1β mRNA (C) and promoted the expression of anti-inflammatory cytokines including TGF-β1 mRNA (D) by qRT-PCR assays among the control, peptide, chitosan, and peptide + chitosan groups 3, 5, and 7 days after trauma in the surfaces of skin wounds in mice.
Discussion
Skin wound healing is a complicated process that involves inflammation, cell proliferation, angiogenesis, granulation tissue formation, and tissue remodeling [1]. Less angiogenesis and severe inflammatory reactions can all delay skin wound healing. An ideal wound dressing should have many roles in promoting angiogenesis and inhibiting the inflammation reaction in addition to maintaining a moist environment at the skin wound bed and acting as a barrier to protect against bacterial invasion. It should also be nontoxic, non-allergenic, biodegradable, and an easily synthesized biomaterial that has antimicrobial properties and accelerates skin wound healing [20].
Angiogenesis shows a critical role in the proliferation and differentiation of cells, tissue formation and remodeling [21]. New blood vessels can provide nutrients and oxygen during skin wound healing. Angiogenesis is regulated by other growth factors such as VEGF, bFGF, PDGF, and TGF-β [22]. Studies [8,23,24] showed that SIKVAV had the property of a growth factor and promotes the adhesion, migration and invasion of endothelial cells; these physiological characteristics of endothelial cells are crucial in the formation of blood vessels in vivo. In vitro, SIKVAV also promotes vascular endothelial cells to form endothelial tubular structures [25] and promotes angiogenesis, which provides nutrition for post-traumatic tissue repair. The results of our study showed that peptide-modified chitosan hydrogels promoted skin wound healing (Figure 1A, 1B) and the proliferation of keratinocytes (Figure 2) in addition to the transformation (Figure 1C) of fibroblasts into myofibroblasts, which accelerated skin wound contraction (Figure 1A). Peptide-modified chitosan hydrogels significantly promoted angiogenesis (Figure 3) during skin wound healing, which allowed new blood vessels to supply sufficient oxygen and nutrition for keratinocytes to migrate and proliferate (Figure 2) and for fibroblasts to synthesize more collagen fibers (Figure 2). Therefore, peptide-modified chitosan hydrogels significantly promoted skin wound healing by angiogenesis and deposition of collagen.
The inflammatory response is an indispensable stage in skin wounds [26,27]. However, severe inflammation can delay skin wound healing. Therefore, therapeutic strategies that modulate the inflammatory response can promote skin wound healing. The biomaterial composition of the scaffolds may affect the host immune response, which benefits inflammatory cell infiltration and body reaction. Previous studies [11] indicated that peptide-modified chitosan hydrogels inhibited inflammation after application in the skin defects of mice. Macrophages are the major inflammatory cells present in skin wounds and have two phenotypes: M1 and M2 [28]. M2 macrophages have an important property for angiogenesis and wound healing compared with pro-inflammatory M1 macrophages. Cytokines include pro-inflammatory and anti-inflammatory factors. IL-1β, TNF-α and IL-6 are pro-inflammatory cytokines, and IL-4, IL-10, and TGF-β1 is an anti-inflammatory factor [12].
TGF-β1 is produced by M2 macrophages, fibroblasts, keratinocytes, and platelets. TGF-β1 accelerates fibroblasts proliferation and their transformation into myofibroblasts in addition to the formation of connective tissue and the re-epithelialization process and modulates the inflammation reaction [27,28]. Dermal collagen is primarily composed of collagen I and III. Newly formed collagen fibers provide a scaffold for cell proliferation and angiogenesis and facilitate epidermal cell migration in skin wounds. The results of our studies showed that the peptide-modified chitosan hydrogels promoted the expression of TGF-β1 in skin wounds (Figure 5D). Masson’s trichrome staining results showed that the mice treated with peptide-modified chitosan hydrogels had a greater deposition of new collagen fibers at the skin wound bed than the other groups (Figure 4).
Pro-inflammatory cytokines, which include IL-1β and IL-6 and TNF-α, are upregulated during the inflammatory phase in the skin wounds [12]. IL-1β is secreted by neutrophils, monocytes/macrophages, and keratinocytes [29]. IL-6 is produced by myofibroblasts, monocytes/macrophages, lymphocytes, epithelial cells, and keratinocytes [30]. The expression of IL-6 increases after trauma and persistently exists in chronic wounds. TNF-α is secreted by macrophages, CD4+ lymphocytes, NK cells, neutrophils, mast cells, and eosinophils [31]. TNF-α can inhibit the re-epithelialization process of keratinocytes in the skin wounds. However, at higher levels, especially when persisting for a long time, TNF-α has a detrimental effect on skin wound healing. TNF-α inhibits the synthesis of ECM proteins. In addition, high levels of IL-1β have a similar effect to that of TNF-α. Both TNF-α and IL-1β have been demonstrated to impact the others’ expression and accelerate this negative effect in skin wound healing [32].
Pro-inflammatory factors, which include IL-1β and IL-6 and TNF-α, promote the production of nitric oxide, arachidonic acid, histamine, etc., activate the complement system, and form the cytokine network of the cascade, which leads to increased inflammation detriments [12]. The results of our study showed that peptide-modified chitosan hydrogels decreased the expression of IL-1β and IL-6 and TNF-α in the skin wounds (Figure 5A-C). The expression levels of TNF-α and IL-1β significantly increased in chronic wounds. In addition, infection in chronic wounds can significantly prolong inflammation, which inhibits skin wound healing. At the same time, non-healing wounds exhibit high levels of interstitial collagenases, gelatinases, and stromelysins, which are secreted by inflammatory cells induced by TNFα and IL-1β [33].
Studies have shown that many cytokines and inflammatory factors are involved in vascular proliferation and immune regulation during skin wound healing [34]. Some cytokines are involved in vascular proliferation and participate in the inflammatory process. Some shortcomings in this study are that only four major inflammatory factors were studied, and other related inflammatory factors were not involved. Four inflammatory factors involved in skin wound healing are studied only at their genetic levels, and how these genes change at the protein level during skin wound healing is still unknown. Whether these changes in the inflammatory factor gene levels cause changes in their related proteins is unknown; thus, this will constitute the direction of our future work.
Conclusion
This study on skin wounds in mice indicated that SIKVAV-modified chitosan hydrogels promoted skin wound healing and the proliferation of keratinocytes in addition to collagen deposition and angiogenesis. Peptide-modified chitosan hydrogels accelerated skin wound healing in a variety of manners and modulated the secretion of cytokines in skin wounds in vivo. Therefore, these results showed that peptide-modified chitosan hydrogels are promising synthesized biomaterials for the treatment of skin wounds.
Acknowledgements
This work was supported by the Base and Talent Plan/Excellent Young Talent Funding Plan of the Jiujiang Science and Technology Bureau (Jiu Cai Jiao Zhi [2016]43-74) and the Jiujiang University Doctoral Fund.
Disclosure of conflict of interest
None.
References
- 1.Zhao X, Wu H, Guo BL, Dong RN, Qiu YS, Ma PX. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47. doi: 10.1016/j.biomaterials.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Curtis K, Lam M, Mitchell R, Black D, Taylor C, Dickson C, Jan S, Palmer CS, Langcake M, Myburgh J. Acute costs and predictors of higher treatment costs of trauma in New South Wales, Australia. Injury. 2014;45:279–284. doi: 10.1016/j.injury.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255. doi: 10.1002/14651858.CD011255.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CG, Wang QQ, Gao WD, Zhang ZJ, Lou YT, Jin HM, Chen XF, Lei B, Xu HZ, Mao C. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomaterialia. 2018;69:156–169. doi: 10.1016/j.actbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao WD, Jin WW, Li YN, Wan L, Wang CG, Lin C, Chen XF, Lei B, Mao C. A highly bioactive bone extracellular matrix-biomimetic nanofibrous system with rapid angiogenesis promotes diabetic wound healing. Journal of Materials Chemistry B. 2017;5:7285–7296. doi: 10.1039/c7tb01484h. [DOI] [PubMed] [Google Scholar]
- 7.Hejcl A, Ruzicka J, Proks V, Mackova H, Kubinova S, Tukmachev D, Cihlar J, Horak D, Jendelova P. Dynamics of tissue ingrowth in SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores after bridging a spinal cord transection. J Mater Sci Mater Med. 2018;29:89. doi: 10.1007/s10856-018-6100-2. [DOI] [PubMed] [Google Scholar]
- 8.Boccafoschi F, Fusaro L, Mosca C, Bosetti M, Chevallier P, Mantovani D, Cannas M. The biological response of poly(L-lactide) films modified by different biomolecules: role of the coating strategy. J Biomed Mater Res A. 2012;100:2373–2381. doi: 10.1002/jbm.a.34180. [DOI] [PubMed] [Google Scholar]
- 9.Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- 10.Dragostin OM, Samal SK, Dash M, Lupascu F, Panzariu A, Tuchilus C, Ghetu N, Danciu M, Dubruel P, Pieptu D, Vasile C, Tatia R, Profire L. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydrate Polymers. 2016;141:28–40. doi: 10.1016/j.carbpol.2015.12.078. [DOI] [PubMed] [Google Scholar]
- 11.Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219:172–178. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Munkholm K, Brauner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47:1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harkins AL, Duri S, Kloth LC, Tran CD. Chitosan-cellulose composite for wound dressing material. Part 2. Antimicrobial activity, blood absorption ability, and biocompatibility. J Biomed Mater Res B Appl Biomater. 2014;102:1199–1206. doi: 10.1002/jbm.b.33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Servat-Medina L, González-Gómez A, Reyes-Ortega F, Sousa IM, Queiroz Nde C, Zago PM, Jorge MP, Monteiro KM, de Carvalho JE, San Román J, Foglio MA. Chitosan-tripolyphosphate nanoparticles as arrabidaea chica standardized extract carrier: synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int J Nanomedicine. 2015;10:3897–3909. doi: 10.2147/IJN.S83705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, Blackstone B, Powell HM, Khanna S, Roy S. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun. 2018;9:936. doi: 10.1038/s41467-018-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Zhang M, Wang X, Chen Y, Yan Y, Zhang L. Peptide-modified chitosan hydrogels promote skin wound healing by enhancing wound angiogenesis and inhibiting inflammation. Am J Transl Res. 2017;9:2352–2362. [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SX, Zhang M, Shao XB, Wang X, Zhang L, Xu PC, Zhong W, Zhang L, Xing M, Zhang L. A laminin mimetic peptide SIKVAV-conjugated chitosan hydrogel promoting wound healing by enhancing angiogenesis, re-epithelialization and collagen deposition. Journal of Materials Chemistry B. 2015;3:6798–6804. doi: 10.1039/c5tb00842e. [DOI] [PubMed] [Google Scholar]
- 19.Chen XL, Zhang M, Chen SX, Wang XE, Tian ZH, Chen YH, Xu PC, Zhang L, Zhang L, Zhang L. Peptide-modified chitosan hydrogels accelerate skin wound healing by promoting fibroblast proliferation, migration, and secretion. Cell Transplantation. 2017;26:1331–1340. doi: 10.1177/0963689717721216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70:2059–81. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaves ME, Araujo AR, Piancastelli AC, Pinotti M. Effects of low-power light therapy on wound healing: LASER x LED. An Bras Dermatol. 2014;89:616–623. doi: 10.1590/abd1806-4841.20142519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muppala S, Xiao R, Krukovets I, Verbovetsky D, Yendamuri R, Habib N, Raman P, Plow E, Stenina-Adognravi O. Thrombospondin-4 mediates TGF-beta-induced angiogenesis. Oncogene. 2017;36:5189–5198. doi: 10.1038/onc.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davel LE, Puricelli LI, Del Carmen C Vidal M, De Lorenzo MS, Sacerdote de Lustig E, Bal de Kier Joffe ED. Soluble factors from the target organ enhance tumor cell angiogenesis: role of laminin SIKVAV sequence. Oncol Rep. 1999;6:907–911. doi: 10.3892/or.6.4.907. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto T, Suzuki Y, Tanihara M, Kakimaru Y, Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials. 2004;25:1407–1414. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Grant DS, Kinsella JL, Fridman R, Auerbach R, Piasecki BA, Yamada Y, Zain M, Kleinman HK. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol. 1992;153:614–625. doi: 10.1002/jcp.1041530324. [DOI] [PubMed] [Google Scholar]
- 26.Bikle D, Tu C, Oda Y. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol. 2015;135:S121–S121. doi: 10.1016/j.jsbmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol. 2010;19:777–783. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 28.Ballotta V, Driessen-Mol A, Bouten CV, Baaijens FP. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 2014;35:4919–4928. doi: 10.1016/j.biomaterials.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12:14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 30.Huang M, Yang D, Xiang M, Wang J. Role of interleukin-6 in regulation of immune responses to remodeling after myocardial infarction. Heart Fail Rev. 2015;20:25–38. doi: 10.1007/s10741-014-9431-1. [DOI] [PubMed] [Google Scholar]
- 31.Meraz IM, Hearnden CH, Liu X, Yang M, Williams L, Savage DJ, Gu J, Rhudy JR, Yokoi K, Lavelle EC, Serda RE. Multivalent presentation of MPL by porous silicon microparticles favors T helper 1 polarization enhancing the anti-tumor efficacy of doxorubicin nanoliposomes. PLoS One. 2014;9:e94703. doi: 10.1371/journal.pone.0094703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang LL, Zhao R, Li JY, Li SS, Liu M, Wang M, Zhang MZ, Dong WW, Jiang SK, Zhang M, Tian ZL, Liu CS, Guan DW. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur J Pharmacol. 2016;786:128–136. doi: 10.1016/j.ejphar.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Thandavarayan RA, Garikipati VN, Joladarashi D, Suresh Babu S, Jeyabal P, Verma SK, Mackie AR, Khan M, Arumugam S, Watanabe K, Kishore R, Krishnamurthy P. Sirtuin-6 deficiency exacerbates diabetes-induced impairment of wound healing. Exp Dermatol. 2015;24:773–778. doi: 10.1111/exd.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]