Abstract
Introduction:
Hepatitis B virus (HBV) infection acquired during pregnancy can pose a risk to the infant at birth that can lead to significant and lifelong morbidity. Hepatitis B vaccine (HepB) is recommended for anyone at increased risk for contracting HBV infection, including pregnant women. Limited data are available on the safety of HepB administration during pregnancy.
Objectives:
To assess the frequency of maternal HepB receipt among pregnant women and evaluate the potential association between maternal vaccination and pre-specified maternal and infant safety outcomes.
Methods:
We examined a retrospective cohort of pregnancies in the Vaccine Safety Datalink (VSD) resulting in live birth outcomes from 2004 through 2015. Eligible pregnancies in women aged 12–55 years who were continuously enrolled from 6 months pre-pregnancy to 6 weeks postpartum in VSD integrated health systems were included. We compared pregnancies with HepB exposure to those with other vaccine exposures, and to those with no vaccine exposures. High-risk conditions for contracting HBV infection were identified up to one-year prior to or during the pregnancy using ICD-9 codes. Maternal and fetal adverse events were also evaluated according to maternal HepB exposure status.
Results:
Among over 650,000 pregnancies in the study period, HepB was administered at a rate of 2.1 per 1000 pregnancies (n= 1399), commonly within the first 5 weeks of pregnancy. Less than 3% of the HepB-exposed group had a high-risk ICD-9 code indicating need for HepB; this was similar to the rate among HepB unvaccinated groups. There were no significant associations between HepB exposure during pregnancy and gestational hypertension, gestational diabetes, pre-eclampsia/eclampsia, cesarean delivery, pre-term delivery, low birthweight or small for gestational age infants.
Conclusions:
Most women who received maternal HepB did not have high-risk indications for vaccination. No increased risk for the adverse events that were examined were observed among women who received maternal HepB or their offspring.
Keywords: Hepatitis B vaccine, Vaccine safety, Pregnancy
1. Introduction
Hepatitis B virus (HBV) infection during pregnancy can pose a substantial risk to infants at birth. Perinatal transmission of HBV can occur if the mother acquired acute hepatitis B during late pregnancy or in the early postpartum period, or if the mother is chronically infected with HBV [1]. Vertical transmission from mothers who are positive for both HBV surface antigen (HBsAg) and HBV e-antigen (HBeAg) exceeds 90% and, in the absence of postexposure prophylaxis, between 70% and 90% of infected infants develop chronic infection by age 6 months, which increases the lifetime risk for cirrhosis and hepatocellular carcinoma [1–3].
The hepatitis B vaccine (HepB) is universally recommended as part of the routine immunization schedule for children [4]. To minimize the risk of maternal infection and vertical transmission, women who are susceptible (e.g., unvaccinated) to HBV and who have certain high-risk behaviors or conditions that increase their risk for contracting HBV infection are recommended to receive HepB during pregnancy. HepB administered as either a singleantigen (Recombivax HB® or Engerix-B®) or a combination vaccine with Hepatitis A (Twinrix®), is effective in reducing the risk of acquiring HBV infection [3,5]. Risk factors for contracting HBV infection include a range of behavioral (e.g., high-risk sexual activity, drug use), comorbid, occupational, household, and travel-related factors [6,7]. Although safety data are limited, HepB is considered safe and the benefits to protecting both the mother and the fetus from acquiring HBV infection are considered to outweigh any potential risks of vaccination during pregnancy [8,9].
Receipt of HepB during pregnancy may be intentional, prompted by a provider’s concern over the presence of one or more risk factors for contracting HBV. Maternal HepB administration may also occur inadvertently, when a woman is vaccinated without knowledge of pregnancy status. For these reasons, there is value in continuing to assess the safety of maternal HepB administration to understand how exposure affects pregnancy outcomes. However, there are limited studies on the safety of HepB administration during pregnancy. Two small studies evaluated maternal and fetal outcomes following maternal vaccination with HepB and found no evidence of associated adverse events, but the studies were underpowered to detect associations with potentially serious outcomes [1,10]. One additional descriptive study summarized reports to the Vaccine Adverse Events Reporting System following maternal receipt of HepB and did not find any concerning patterns of adverse events [11].
We evaluated 11 years of data from six integrated health systems, with the aim of describing vaccine administration patterns and evaluating the potential association between vaccination with HepB during pregnancy and pre-specified maternal and infant safety outcomes among women with live births.
2. Methods
2.1. Study design and population
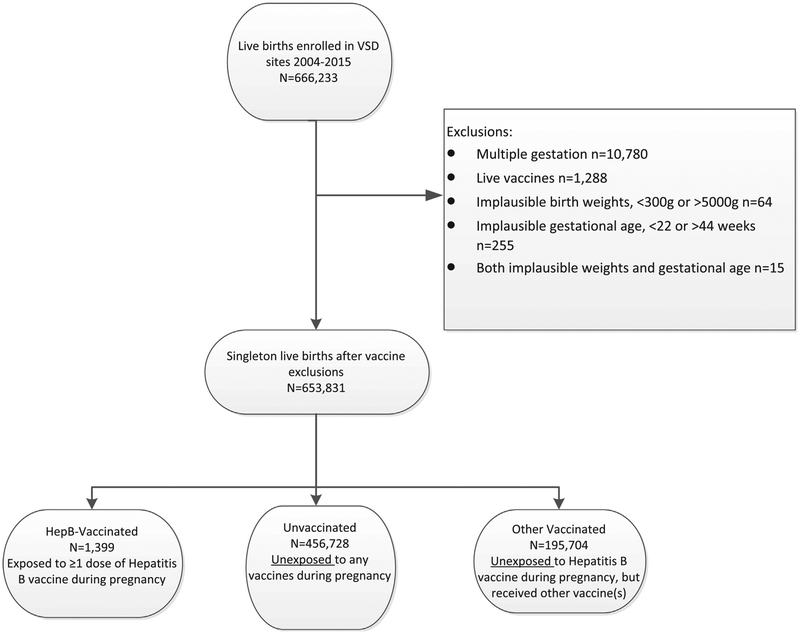
The study population was drawn from members of six integrated healthcare systems participating in the Vaccine Safety Datalink (VSD), a collaboration with the Centers for Disease Control and Prevention (CDC). The six participating sites from five states (California, Colorado, Oregon, Washington, and Wisconsin), provide comprehensive medical care to health plan members, and document patient encounters within electronic health record (EHR) systems. Data from the respective EHR systems were used to identify a retrospective cohort of eligible pregnancies with start dates from January 1, 2004 through December 31, 2014 among women aged 12–55 years who were continuously enrolled in the health plan from 6 months prior to pregnancy through 6 weeks after the pregnancy ended in a live birth. Pregnancies were identified using a validated algorithm based on administrative, EHR, and claims data. Comparisons to medical record abstraction have shown that the pregnancy episode algorithm has 99% agreement for live birth pregnancy designation, and 98% agreement for gestational age [12]. Exclusion criteria were established for multiple gestation pregnancies, pregnancies with exposure to live virus vaccines, and pregnancies with implausible gestational age (<22 or >44 weeks) or birthweight values (<300 g). Gestational age cutoffs were used to decrease errors in gestational age estimation and to remove infants with borderline viability who may be less likely to survive [13]. Women could experience multiple pregnancies over the study period and thus have more than one pregnancy included in the analysis (Fig. 1). The study protocol was reviewed and approved by Institutional Review Boards at each participating study site and the Centers for Disease Control and Prevention.
Fig. 1.
Study population of pregnancies with live birth outcomes in the VSD, 2004–2015.
2.2. Measurement of exposure and covariates
Eligible pregnancies were assigned to one of three groups: (1) pregnancies where at least one dose of HepB (Recombivax®, Engerix® or Twinrix®) was administered during the study period and during pregnancy (HepB vaccinated); (2) pregnancies with no vaccines administered during pregnancy (unvaccinated); and (3) pregnancies that were not exposed to HepB, but were exposed to at least one other inactivated vaccine (other vaccinated) [14]. To correct for potential misclassification due to uncertainty of the pregnancy onset date and to limit inclusion of postpartum administrations, we included vaccines administered ≥8 days after last menstrual period (LMP) through 7 days before pregnancy end date. Provider assessment of HBV-immune status was not evaluated for this study.
The three groups were compared for differences in baseline demographic characteristics (age, educational background, marital status, gravidity, enrollment site) and three pre-specified high-risk vaccine indications up to one year prior to and during pregnancy (chronic liver disease, injection drug use and/or outpatient visit to chemical dependency or methadone use, and sexually transmitted infections) (Table 1). Maternal use of alcohol and tobacco, in the 12 months prior to and during pregnancy were also assessed as behaviors that could impact maternal and fetal outcomes. All diagnoses were identified from International Classification of Diseases, Ninth Revision (1CD-9) codes assigned at inpatient, emergency department, or outpatient visits (Supplementary table).
Table 1.
High-risk indications for vaccination with HepB and corresponding ICD-9 codes.
| High-risk indication for HepB | ICD-9 code |
|---|---|
| Chronic liver disease | 571.x |
| Injection or non-injection drug use | 304.x, 305.2–305.9 |
| Evaluated/treated for STI | |
| Syphilis | 091.0 |
| Gonorrhea | 098.0, 098.1, 098.2, 098.3 |
| Chlamydia | 099.41, 099.5 |
| Trichomoniasis | 131.0 |
| Genital Herpes | 054.1x |
| Hepatitis C Virus | 070.51, 070.54, 070.70 |
| HIV (acute, chronic, unspecified) | 042.x |
| Hemodialysis patient | Not included* |
| Diabetes | Not included† |
| Developmentally disabled in LTCF | NA |
| Household contacts of HBV- chronically infected | NA |
| Occupational risk | NA |
| >1 sex partner in past 6 months | NA |
| Men who have sex with men | NA |
| Travel to endemic countries | NA |
Abbreviations: HepB, hepatitis b vaccine; ICD-9, International Classification of Dis- eases-9th edition, STI, sexually transmitted infection; HIV, Human immunodeficiency virus; LTCF, long term care facility; NA, Not available as ICD-9 code in EHR
Pregnancies where hemodialysis was occurring were not enumerated given the low likelihood of pregnancy among women undergoing hemodialysis.
Diabetes was not included as a high-risk indication for HEPB until 2012, at the very end of our study period.
2.3. Reasons for vaccination
Given that several HepB indications could not be identified through 1CD-9 codes documented in the EHR (e.g., occupational status, risk of exposure from HBV-positive household contact, travel to and from endemic areas, sexual activity), we conducted a single-site chart review of all HepB-exposed pregnancies to determine whether the presence of risk factors may have contributed to decisions for maternal HepB administration, and to explore the frequency of inadvertent maternal vaccination. This chart review was conducted by a single reviewer in a two-step process. First, we assessed whether providers were aware that the patient was pregnant at the time of maternal HepB; referred to as either ‘known’ or ‘unknown’ pregnancy status. Those with a positive pregnancy test prior to HepB receipt were defined as vaccination with ‘known’ pregnancy status. The second step of chart review involved reviewing provider notes in the EHR of each HepB-exposed pregnancy, detailing any explanation for administering HepB during pregnancy. The six categorial options for explanations included the following four high-risk indications: (1) work or school-related requirements (e.g., health care worker, student in health-related specialty); (2) travel to or from a country with endemic hepatitis B circulation; (3) risk of exposure due to a HBV-positive household member; (4) chemical dependence or methadone treatment; plus two categories for non high-risk factors: (5) catch-up vaccination for those who had not completed the 3-dose HepB series; and (6) unknown. Results from this review were used to highlight the potential for unmeasured confounding when using only ICD-9 codes to identify which individuals had one or more high-risk indications for vaccination.
2.4. Measurement of maternal and fetal outcomes
In the absence of specific safety concerns about maternal HepB, outcomes and exposure periods were selected a priori, as informed by previous safety studies of seasonal influenza and tetanus- diphtheria-acellular pertussis vaccination during pregnancy [14–16]. The adverse events of interest included the following common pregnancy-related complications: gestational hypertension, gestational diabetes, preeclampsia/eclampsia, and cesarean delivery. Fetal adverse outcomes among only infants of live births included pre-term birth (i.e., delivery before 37 weeks of completed gestation), low birth weight (<2500 g), and small for gestational age. Fetal outcomes were identified from the mother’s chart and included outcomes up to 6-weeks post-partum. As described in the supplementary table, each outcome had a pre-specified timeframe and setting (e.g., inpatient or outpatient) during the pregnancy or postpartum period. Small for gestational age (SGA) was determined by using a referent standard for expected weights according to gestational age at birth. Births less than the 10th percentile for the expected weight-to-gestational age were classified as SGA [17,18].
2.5. Analysis
Since pregnancies were clustered within each patient, General Estimating Equations (GEE) were used to first compare patient characteristics between the three study groups (except for pregnancy trimester at vaccination, which could only be compared between the HepB vaccinated and other vaccinated groups). To evaluate and compare maternal and fetal outcomes for HepB-exposed pregnancies and HepB non-exposed pregnancies, the unvaccinated and other vaccinated groups were then combined into a single unexposed comparison group. The GEE model was then used to evaluate potential associations between HepB and pre-specified study outcomes, controlling for patient characteristics and high- risk indications for vaccination, including: chronic liver disease, injection drug use and/or outpatient visit to chemical dependency or methadone use, and sexually transmitted infections.
A binomial model with a logit link function was used to model the binary outcomes and estimate the odds ratios (OR) and associated 95% confidence intervals (CI) before and after adjusting for potential confounding factors. Exchangeable covariance structure was specified to account for the correlation among multiple pregnancies by the same patient. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
A total of 1399 pregnancies resulting in live births were included in the HepB vaccinated group, 456,728 in the unvaccinated group, and 195,704 in the other vaccinated group (Fig. 1). While pregnancies with HepB exposure (<1% of the study population) were significantly different across most descriptive characteristics compared to pregnancies in either comparison group, the absolute percentage differences between groups are generally small (<3%) (Table 2). Women with a HepB-exposed pregnancy tended to be slightly younger and experiencing their first pregnancy. In the HepB vaccinated cohort, 71% of HepB exposures occurred in the first trimester of pregnancy, 51% of which occurred within the first 5 weeks of pregnancy (data not shown), whereas for pregnancies with exposure to other vaccines (primarily influenza and Tdap vaccines) vaccinations were more evenly distributed across trimesters (30%, 35%, and 35% for 1st, 2nd, and 3rd trimesters, respectively).
Table 2.
Maternal characteristics based on vaccination status.
| Characteristics | Group 1. HepB- vaccinated (n = 1399) % | Group 2. Unvaccinated* (N = 456,728) % | Group 3. Other vaccinated† (n = 195,704) % |
|---|---|---|---|
| Mean age (SD), years | 29.9 (5.9) | 30.1 (5.6) | 30.2 (5.9) |
| Age | |||
| <18 | 2.1 | 1.9 | 1.2 |
| 18–25 | 20.4 | 19.9 | 19.1 |
| 26–35 | 59.7 | 59.8 | 62.4 |
| >35 | 17.7 | 18.4 | 17.3 |
| Marital status | |||
| Married | 57.5 | 61.0 | 59.4 |
| Single | 25.2 | 21.4 | 23.0 |
| Unknown | 17.2 | 17.6 | 17.6 |
| Study Site‡ | |||
| A | 5.4 | 5.4 | 4.8 |
| B | 3.2 | 5.4 | 5.6 |
| C | 1.9 | 2.0 | 1.9 |
| D | 47.0 | 40.0 | 40.5 |
| E | 5.6 | 5.5 | 4.8 |
| F | 36.9 | 41.7 | 42.4 |
| Education | |||
| <High school | 2.8 | 3.6 | 2.7 |
| High school graduate | 7.8 | 10.6 | 10.3 |
| >High school | 70.4 | 67.3 | 68.3 |
| Unknown | 19.0 | 18.5 | 18.8 |
| Gravidity | |||
| 1 | 37.3 | 29.3 | 35.7 |
| 2+ | 48.0 | 56.1 | 50.9 |
| Unknown | 14.7 | 14.6 | 13.4 |
| Trimester of vaccine exposure | |||
| 1st (0–13 weeks) | 71.0 | NA | 29.6 |
| 2nd (14–26 weeks) | 23.4 | NA | 35.4 |
| 3rd (27–42 weeks) | 5.6 | NA | 35.0 |
| Maternal vaccines received | |||
| Only HepB | 33.2 | NA | NA |
| 1 other vaccine | 43.7 | NA | 61.9 |
| 2+ other vaccines | 23.1 | NA | 38.1 |
| Any HBV risk factor§ | 4.4 | 3.4 | 3.1 |
| Chronic liver disease | 0.9 | 0.3 | 0.2 |
| Injection-drug use | 2.3 | 2.2 | 2.2 |
| Visit to chemical dependency | 2.2 | 1.7 | 1.6 |
| Any sexually-transmitted infection | 1.6 | 1.0 | 0.8 |
| HIV | 0.3 | <0.1 | <0.1 |
| Herpes Simplex Virus | 0.8 | 0.7 | 0.6 |
| Hepatitis C Virus | 0.4 | <0.1 | <0.1 |
| Maternal alcohol use | 11.8 | 13.1 | 17.9 |
| Maternal Smoking | 6.9 | 6.4 | 7.2 |
Bold face indicates high-risk indications for HepB vaccination.
At least 1 cell size too small (expected frequency < 5).
Abbreviations: HepB, hepatitis b vaccine; HBV, hepatitis B virus; HIV, Human immunodeficiency virus; SD, standard deviation.
Pregnancies with no vaccine exposure during pregnancy.
Pregnancies with no exposure to HepB during pregnancy, but with exposure to >1 other vaccine.
Sites were not identified for reasons of confidentiality.
Pregnancies with any of the individual high-risk indications, based on presence of ICD9 codes either during, or within 12 months preceding, the pregnancy.
In this analysis, we were able to examine only three of the high- risk indications specified for maternal administration of HepB: chronic liver disease, injection drug use, and STI diagnoses. The prevalence of other high-risk indications could not be assessed as there are not corresponding ICD-9 codes. The proportions of pregnancies with any of these high-risk indications, as documented in the 12 months preceding or during pregnancy, were very low (<1% with chronic liver disease; <3% with injection drug use or documented chemical dependency; and <2% with a documented STI) for all three groups (Table 2). Pregnancies with HepB exposure had significantly higher rates of chronic liver disease (0.9% vs. <0.4% in both comparison groups), and higher rates of STI diagnoses than the unvaccinated and other vaccinated groups, although these rates were all very low (1.6% in HepB vaccinated vs. 1.0% in unvaccinated and 0.8% in other vaccinated). Women with HepB-exposed pregnancies had lower rates of documented maternal alcohol use than did women in the other vaccinated comparison group (11.8% vs. 17.9%); rates of maternal smoking were similar across all groups.
3.1. Reasons for vaccination
Of 72 charts identified and available for review from one study site, we found that providers were not aware of the patient’s pregnancy status at the time of vaccination for 32% of HepB vaccinated pregnancies (23/72) (data not shown), meaning that there was no indication of a positive pregnancy test prior to vaccination. Where pregnancy status was known at the time of vaccination (n = 45 or 63% of cases), the most common reasons for vaccination were work- or school-related requirements (40%), followed by catch-up vaccination, either as initiating or completing the HepB vaccine series (38%), and travel (13%). In comparison, where pregnancy status was not known at the time of vaccination, the most common reasons for vaccination were catch-up vaccination (44%), travel (26%), and work- or school-related requirements (22%). Chemical dependency (known pregnancy status) or having a hepatitis B infected household contact (unknown pregnancy status) were noted as the primary reasons in only 2 of 72 (3%) HepB vaccinated cases. It was unclear if pregnancy status was known or unknown in 4/72 (6%) cases.
3.2. Maternal and fetal outcomes
Given minimal differences in population characteristics between the three study groups, the two unexposed groups (unvaccinated and other vaccinated) were combined into a single unexposed group for assessment of study outcomes. There were no significant associations between HepB exposure during pregnancy and gestational hypertension, gestational diabetes, pre-eclampsia/eclampsia, cesarean delivery, pre-term delivery, low birthweight or small-for-gestational age infants (Table 3).
Table 3.
Association between maternal vaccination with HepB and adverse maternal and fetal outcomes among pregnancies resulting in live births.
| Outcome | HepB-Vaccinated (n = 1399) % (n) | Not HepB-Vaccinated* (N = 652,432) % (n) | aOR (95% CI)† |
|---|---|---|---|
| Gestational hypertension‡ | |||
| No (ref) | 95.1 (1331) | 95.4 (622,327) | |
| Yes | 4.9 (68) | 4.6 (30,105) | 1.02 (0.80–1.30) |
| Gestational diabetes | |||
| No (ref) | 87.2 (1220) | 87.8 (572,621) | |
| Yes | 12.8 (179) | 12.2 (79,811) | 1.06 (0.91–1.23) |
| Pre-eclampsia/eclampsia | |||
| No (ref) | 95.0 (1329) | 95.5 (623,195) | |
| Yes | 5.0 (70) | 4.5 (29,237) | 1.07 (0.84–1.36) |
| Cesarean delivery§ | |||
| No (ref) | 70.0 (864) | 70.6 (408,463) | |
| Yes | 30.0 (370) | 29.4 (170,118) | 1.01 (0.91–1.13) |
| Pre-term birth (<37 wks)§ | |||
| No (ref) | 91.6 (1173) | 92.8 (550,816) | |
| Yes | 8.4 (107) | 7.2 (42,835) | 1.14 (0.94–1.39) |
| Low birth weight (<2500 g)§ | |||
| No (ref) | 93.9 (1200) | 95.0 (563,214) | |
| Yes | 6.1 (78) | 5.0 (29,573) | 1.21 (0.96–1.52) |
| Small for gestational age at birth§ | |||
| No (ref) | 90.6 (1156) | 91.7 (540,996) | |
| Yes | 9.4 (120) | 8.3 (49,277) | 1.13 (0.94–1.37) |
Abbreviations: HepB, hepatitis B vaccine; wks, weeks.
The two unexposed groups have been combined.
OR adjusted for demographics and presence of any high-risk condition(s).
Includes those diagnosed with ‘Gestational Hypertension’ and/or ‘Hypertension during Pregnancy’.
Pregnancies with missing outcome values were removed from Unexposed and Exposed denominators, including: Cesarean (Exposed N = 165, Unexposed N = 73,851); Gestational Age < 37 weeks (Exposed N = 119, Unexposed N = 58,781); Low birthweight (Exposed N = 121, Unexposed N = 59,645); Small for gestational age at birth (Exposed N = 123, Unexposed N = 62,159).
4. Discussion
This study represents the first large-scale evaluation of administration patterns and select safety outcomes following maternal HepB administration. Using a network of large, integrated healthcare systems, our study confirmed that maternal vaccination with HepB is uncommon; <1% of over 650,000 singleton pregnancies were exposed to HepB in an 11-year period. Since there is not specific maternal HepB safety concerns noted in the literature, we chose to examine a pre-specified set of commonly examined maternal and fetal outcomes. We found no significant associations between maternal HepB and hypertension, gestational diabetes, pre-eclampsia/eclampsia, cesarean delivery, pre-term birth, low birth weight, or small for gestational age.
Characteristics of exposed pregnancies resulting in live births were comparable to the unexposed; differences that were observed were statistically significant but quite small (data not shown). The low rate of EHR-documented high-risk indications for vaccination with HepB among our HepB vaccinated pregnancies (4.4%), implies that almost all maternal HepB was due to an indication that was not easily identified within the EHR (i.e., household risk, healthcare worker status, travel to or from an endemic area, catch-up vaccination, etc.), or may have been inadvertently timed during pregnancy. Most doses (71%) of HepB were administered during the first trimester and 50% were administered before the 6th week of pregnancy, which also supports the possibility that HepB administered during pregnancy often represents inadvertent administration.
The extent to which maternal vaccination with HepB in our study population occurred due to risks associated with occupational status, household risk, travel to- or from an endemic area, or was related to sexual activity, is unknown. Data from NHIS 2016 show that among individuals (both male and female) with a high-risk indication for HepB, those with occupational risk (i.e., health-care personnel) and travel-related risks have the highest HepB coverage (61% and 31%, respectively, in 2016) [19], which likely means some proportion of vaccinees in our study population also had these high-risk indications. It is possible health systems that serve populations from highly endemic countries (i.e. central Asian republics, Southeast Asia, etc.) may be more likely to vaccinate these women, regardless of pregnancy status, given that the majority of chronic HBV infections in the United States are among Asians and Pacific Islanders [5]. We did not include a racial/ethnic breakdown of our study population due to concerns around incomplete capture, however Asians are 15% of the total VSD study population (in comparison to 5% in the United States, overall) [20].
Findings from our single-site chart review revealed that 32% of maternal vaccination appeared to be inadvertent, as there was no mention of pregnancy found in the EHR at the time of vaccination. Chart review also determined that the most common risk factor among those intentionally vaccinated during pregnancy was occupational or student status (40%); a similar proportion had no high- risk indication for vaccination and were instead vaccinated as catch-up for routine vaccination (38%). Women who received HepB as part of routine vaccination had no other known or documented risk factors for HBV which suggests that providers are comfortable with maternal vaccination and may prefer to take opportunities to vaccinate even in the absence of a high-risk indication for vaccination.
Our study had several limitations. Even with an 11-year study period, we have small numbers of HepB exposed pregnancies. The maternal and fetal outcomes included in our analysis are not expected to be causally linked to maternal exposure to HepB vaccines. Study findings are intended to reassure providers and patients that common adverse pregnancy outcomes were not associated with vaccine exposure. We did not examine outcomes related to congenital anomalies, nor did we include pregnancies with non-live birth outcomes. Any possible association between maternal HepB exposure and non-live birth outcomes was not addressed in this study. Using ICD-9 codes to identify high-risk indications for vaccination limited us to identifying those behaviors or conditions that have corresponding ICD-9 codes; we were unable to identify or describe vaccine indications related to sexual exposure, or household or occupational exposure. Even those high- risk indications that we did attempt to identify in the EHR from one study site (e.g., injection drug use, STI infection) are poorly reported and documented and are likely an under-representation of the true extent of these high-risk indications in our study population. As such, there is the potential for unmeasured confounding in this analysis. Data have shown that 54% of newly acquired hepatitis B cases are associated with high-risk sexual activity (i.e., multiple partners), or injection-drug use [21]. Nevertheless, it is possible, that by including STI diagnoses, we were able to ascertain risks associated with sexual activity for some proportion of our vaccinated population. We included pregnant women that were undergoing dialysis but did not describe these pregnancies separately, even though hemodialysis is among the high-risk indications for vaccination with HepB. The rationale for its exclusion is that pregnancy is extremely uncommon among women with chronic kidney disease and, when it does occur, often results in significant adverse fetal outcomes [22]. We therefore anticipate that excluding this diagnosis does not impact our study findings.
This large, multisite study provides evidence that HepB administration during pregnancy does not increase risk for adverse events examined among pregnancies resulting in live births. Given low rates of known high-risk indications for maternal vaccination, it is expected that vaccination with HepB in early pregnancy is commonly occurring as part of routine vaccination and not necessarily due to a high-risk indication; this is true whether or not pregnancy status was known at the time of maternal vaccination. Regardless of the vaccination intent, our findings are consistent with those of previously published studies and provide added reassurance that it is safe to administer HepB to pregnant women both with and without high-risk indications for vaccination.
Supplementary Material
Acknowledgement
We would like to acknowledge the contributions of Project Managers and Data Managers from participating VSD sites. We would also like to extend our appreciation to Dr. Noele Nelson, the Acting Branch Chief for the Prevention Branch in CDC’s Division of Viral Hepatitis, for her review of, and contributions to, the final manuscript.
Footnotes
Conflict of interest statement
Ms. Groom has received research support from Merck & CO. Ms. Irving has received research support from Medimmune. Dr. Nale- way has received research support from Merck & CO, Pfizer, and Medimmune. Dr. Getahun has received research support from Bayer AG. Dr. Klein has received research support from GlaxoS- mithKlein, Merck & CO, Sanofi Pasteur, Pfizer, Medimmune, Protein Science (now Sanofi Pasteur), and Dynavax.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.08.074.
☆ Funding for this study was provided by the Centers for Disease Control and Prevention, Contract 200-2012-53584. Findings and conclusions of this study are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Levy M, Koren G. Hepatitis B vaccine in pregnancy: maternal and fetal safety. Am J Perinatol 1991;8(3):227–32. [DOI] [PubMed] [Google Scholar]
- [2].Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on immunization Practices (ACiP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(RR-16):1–31. [PubMed] [Google Scholar]
- [3].Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of Hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2018;67 (1):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention. Recommended immunization Schedule for Children and Adolescents Aged 18 Years or Younger, United States, 2018; 2018. [Google Scholar]
- [5].Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- [6].Swamy GK, Heine RP. Vaccinations for pregnant women. Obstet Gynecol 2015;125(1):212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2018; 2018. [02.12.18].
- [8].General CDC. recommendations on immunization -- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60(2):1–64. [PubMed] [Google Scholar]
- [9].Kroger AT, Duchin J, Vazquez M. General best practice guidelines for immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP); 2017. [02.12.18].
- [10].Ayoola EA, Johnson AO. Hepatitis B vaccine in pregnancy: immunogenicity, safety and transfer of antibodies to infants. Int J Gynaecol Obstet 1987;25 (4):297–301. [DOI] [PubMed] [Google Scholar]
- [11].Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the vaccine adverse event reporting system after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013;31(27):2898–903. [DOI] [PubMed] [Google Scholar]
- [13].Vazquez-Benitez G, Kharbanda EO, Naleway AL, Lipkind H, Sukumaran L, McCarthy NL, et al. Risk of preterm or small-for-gestational-age birth after influenza vaccination during pregnancy: caveats when conducting retrospective observational studies. Am J Epidemiol 2016;184(3):176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway AL, et al. Maternal Tdap vaccination: coverage and acute safety outcomes in the vaccine safety datalink, 2007–2013. Vaccine 2016;34 (7):968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sukumaran L, McCarthy NL, Kharbanda EO, Weintraub ES, Vazquez-Benitez G, McNeil MM, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol 2015;126(5):1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol 2013;122(3):659–67. [DOI] [PubMed] [Google Scholar]
- [17].Joseph KS, Kramer MS, Allen AC, Mery LS, Platt RW, Wen SW. Implausible birth weight for gestational age. Am J Epidemiol 2001;153(2):110–3. [DOI] [PubMed] [Google Scholar]
- [18].Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hung M, Williams W, Lu P, Kim DK, Grohskopf L, et al. Vaccination coverage among adults in the United States National Health Interview Survey, 2016. Centers for Disease Control and Prevention; 2017. [02.12.18]. [Google Scholar]
- [20].Sukumaran L, McCarthy NL, Li R, Weintraub ES, Jacobsen SJ, Hambidge SJ, et al. Demographic characteristics of members of the Vaccine Safety Datalink (VSD): A comparison with the United States population. Vaccine 2015;33 (36):4446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. In: Hamborsky J, Kroger A, Wolfe C, editors. [13th]. Washington, D.C.: Public Health Foundation; 2015. [Google Scholar]
- [22].Shemin D Dialysis in pregnant women with chronic kidney disease. Semin Dial 2003;16(5):379–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.