Abstract
BACKGROUND
Ibrutinib has been approved by the Food and Drug Administration for the treatment of patients with untreated chronic lymphocytic leukemia (CLL) since 2016 but has not been compared with chemoimmunotherapy. We conducted a phase 3 trial to evaluate the efficacy of ibrutinib, either alone or in combination with rituximab, relative to chemoimmunotherapy.
METHODS
Patients 65 years of age or older who had untreated CLL were randomly assigned to receive bendamustine plus rituximab, ibrutinib, or ibrutinib plus rituximab. The primary end point was progression-free survival. The Alliance Data and Safety Monitoring Board made the decision to release the data after the protocol-specified efficacy threshold had been met.
RESULTS
A total of 183 patients were assigned to receive bendamustine plus rituximab, 182 to receive ibrutinib, and 182 to receive ibrutinib plus rituximab. Median progression-free survival was reached only with bendamustine plus rituximab. The estimated percentage of patients with progression-free survival at 2 years was 74% with bendamustine plus rituximab and was higher with ibrutinib alone (87%; hazard ratio for disease progression or death, 0.39; 95% confidence interval [CI], 0.26 to 0.58; P<0.001) and with ibrutinib plus rituximab (88%; hazard ratio, 0.38; 95% CI, 0.25 to 0.59; P<0.001). There was no significant difference between the ibrutinib-plus-rituximab group and the ibrutinib group with regard to progression-free survival (hazard ratio, 1.00; 95% CI, 0.62 to 1.62; P=0.49). With a median follow-up of 38 months, there was no significant difference among the three treatment groups with regard to overall survival. The rate of grade 3, 4, or 5 hematologic adverse events was higher with bendamustine plus rituximab (61%) than with ibrutinib or ibrutinib plus rituximab (41% and 39%, respectively), whereas the rate of grade 3, 4, or 5 nonhematologic adverse events was lower with bendamustine plus rituximab (63%) than with the ibrutinib-containing regimens (74% with each regimen).
CONCLUSIONS
Among older patients with untreated CLL, treatment with ibrutinib was superior to treatment with bendamustine plus rituximab with regard to progression-free survival. There was no significant difference between ibrutinib and ibrutinib plus rituximab with regard to progression-free survival. (Funded by the National Cancer Institute and Pharmacyclics; ClinicalTrials.gov number, NCT01886872.)
CHRONIC LYMPHOCYTIC LEUKEMIA (CLL) is the most prevalent form of leukemia in adults and is incurable in most cases. Investigation into the pathogenesis of CLL has implicated B-cell receptor signaling as a central driver, and targeting of this pathway through inhibition of Bruton’s tyrosine kinase (BTK) has delayed and prevented the onset of disease in experimental systems.1,2
Among patients 65 years of age or older, chemoimmunotherapy with either chlorambucil plus obinutuzumab3 or bendamustine plus rituximab4 has shown efficacy and represents standard treatment, although the approach is often modified. Chemoimmunotherapy is associated with toxic effects in many patients, and the risk of toxic effects increases with age. Thus, a targeted oral therapy that is effective and is associated with an acceptable toxic-effect profile could be of value in patients with CLL.
Ibrutinib is an irreversible BTK inhibitor that abrogates CLL-related cell signaling, adhesion, proliferation, and homing in vitro and in vivo.5-11 Among patients with CLL, treatment with single-agent ibrutinib led to a median progression-free survival of 52 months among those who had relapsed or refractory disease12; among those who received ibrutinib as initial treatment, the percentage of patients who were alive and free from disease progression at 2 years was 89%.13 Ibrutinib has been widely used as an initial treatment for CLL since 2016, when it was approved by the Food and Drug Administration and by the European Medicines Agency for this indication, on the basis of its superiority to chlorambucil.14 The benefit of ibrutinib relative to standard chemoimmunotherapy remains a critical consideration.
The addition of rituximab or other CD20 antibodies to chemotherapy prolongs progression-free survival and overall survival,3,15 and such antibodies have been thought to be indispensable in the treatment of CLL. Whether the addition of rituximab to ibrutinib leads to increased efficacy is controversial. In this phase 3 trial (A041202), we address two main questions. First, among older patients with untreated CLL, is treatment with ibrutinib or ibrutinib plus rituximab superior to treatment with bendamustine plus rituximab? Second, does the addition of rituximab to single-agent ibrutinib lead to increased efficacy?
METHODS
PATIENTS
Eligible patients were 65 years of age or older and had untreated CLL for which treatment was indicated, as defined by International Workshop on CLL (IWCLL) criteria.16 The IWCLL criteria and a full list of eligibility criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
TRIAL OVERSIGHT
This phase 3 trial was coordinated by the Alliance for Clinical Trials in Oncology (Alliance) in collaboration with the National Cancer Institute Cancer Trials Support Unit and was approved by a central institutional review board, as well as local institutional review boards as required by participating institutions. The trial was conducted in accordance with the principles of the Declaration of Helsinki. The data were collected by the investigators and entered into an electronic database that was maintained by the Alliance Statistics and Data Center. To ensure data quality, a review of data was performed by the Alliance Statistics and Data Center and by the trial chairperson in accordance with Alliance policies. The trial was monitored at least twice annually by the Alliance Data and Safety Monitoring Board, a standing committee that was composed of persons from inside and outside the Alliance. All the authors reviewed and approved the manuscript and vouch for the completeness and accuracy of the data and the fidelity of the trial to the protocol, available at NEJM.org. No one who is not an author contributed to authorship of the manuscript. The National Cancer Institute was the trial sponsor and obtained ibrutinib under a cooperative research and development agreement with Pharmacyclics (a subsidiary of AbbVie). Pharmacyclics had no role in the design of the trial, collection or interpretation of the data, or authorship of the manuscript. Fluorescence in situ hybridization (FISH) probes were provided by Abbott Molecular and Leica Biosystems.
EVALUATION, RANDOMIZATION, AND TREATMENT
Before each patient underwent randomization, a blood sample was submitted for central testing for methylation at the promoter region of the ZAP70 gene (encoding zeta chain–associated protein kinase 70 [ZAP70]).17 Unmethylated ZAP70 correlates with expression of ZAP70, a finding that conveys a poor prognosis. Approximately 76% of CLL cells that express ZAP70 also contain unmutated IgVH (immunoglobulin variable heavy chain) genes, another predictor of poor prognosis. Sequencing of IgVH genes in CLL cells was not routinely performed in this trial. The following risk factors for CLL were used for stratification: ZAP70 methylation status on central testing (unmethylated [<20%] vs. methylated [≥20%]), risk category according to modified Rai stage (intermediate vs. high),18 and status with regard to del(17p13.1) or del(11q22.3) on local FISH analysis (absent vs. present).
Patients were randomly assigned, in a 1:1:1 ratio, to receive bendamustine plus rituximab, ibrutinib, or ibrutinib plus rituximab. A dynamic randomization method was used, with stratification according to risk factors for CLL.19 Treatment was administered in 28-day cycles. Bendamustine-plus-rituximab therapy consisted of six cycles of bendamustine (administered at a dose of 90 mg per square meter of body-surface area on days 1 and 2 of each cycle) plus rituximab (administered at a dose of 375 mg per square meter on the day before day 1 of cycle 1 and then at a dose of 500 mg per square meter on day 1 of cycles 2 through 6). At the investigator’s discretion, the cycle 1 dose of bendamustine could be 70 mg per square meter. Ibrutinib was administered at a dose of 420 mg daily until the patient had unacceptable toxic effects or disease progression. Ibrutinib-plusrituximab therapy consisted of ibrutinib (administered as described previously and given before rituximab on days when they were administered together) plus rituximab (administered at a dose of 375 mg per square meter weekly for 4 weeks starting on day 1 of cycle 2 and then on day 1 of cycles 3 through 6). Patients in the bendamustine-plus-rituximab group who had disease progression could cross over to receive ibrutinib within 1 year after progression. Details regarding treatment, including instructions for dose delays and modifications, are provided in the Methods section in the Supplementary Appendix.
END POINTS AND ASSESSMENTS
The primary end point was progression-free survival, which was defined as the time from the date of randomization until the earliest date on which disease progression (as defined by IWCLL criteria) or death from any cause was recorded. Data from patients who were alive and had not had disease progression were censored on the date of the last assessment. Data from patients who started a therapy for CLL that was not specified in the protocol or withdrew consent for further follow-up were also censored on the date of the last assessment.
A secondary end point was overall survival. Assessments of response and complete response were performed by means of computed tomography (CT) and physical examination. A central assessment of minimal residual disease in bone marrow was performed at cycle 9 with the use of a standard flow-based assay, which is capable of detecting 1 tumor cell in 10,000 cells. An adverse-event analysis was also performed.
For a correlative analysis, patients underwent a geriatric assessment and central laboratory studies before treatment. The geriatric assessment included an analysis of the score for activities of daily living (with scores ranging from 0 to 14 and higher scores indicating better performance) and of the number of coexisting conditions. Details regarding these assessments are provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
For the comparison of ibrutinib with bendamustine plus rituximab, we estimated that a sample of 332 patients, with an expected 159 events, would provide the trial with 90% power to detect a hazard ratio for disease progression or death of 0.586 (corresponding to an estimated percentage of patients with progression-free survival at 2 years of 61% with bendamustine plus rituximab and 75% with ibrutinib), at a one-sided significance level of 0.025 by a log-rank test. The same assumptions, sample, and power calculation applied for the comparison of ibrutinib plus rituximab with bendamustine plus rituximab. If ibrutinib and ibrutinib plus rituximab were each superior to bendamustine plus rituximab, then ibrutinib plus rituximab was to be compared with ibrutinib. For the comparison of ibrutinib plus rituximab with ibrutinib, we estimated that a sample of 332 patients, with an expected 119 events, would provide the trial with 90% power to detect a hazard ratio of 0.57 (corresponding to an estimated percentage of patients with progression-free survival at 2 years of 75% with ibrutinib and 85% with ibrutinib plus rituximab), at a one-sided significance level of 0.05 by a log-rank test. Thus, the total planned sample was 498 patients who could be evaluated, or 166 patients per group.
For the comparisons of ibrutinib and ibrutinib plus rituximab with bendamustine plus rituximab, three interim efficacy and futility analyses were planned. For the comparison of ibrutinib plus rituximab with ibrutinib, two interim efficacy and futility analyses were planned. In May 2018, the Alliance Data and Safety Monitoring Board made the decision to release these data on the basis of the results of the protocol-specified second interim analysis for the comparisons of the two ibrutinib-containing regimens with bendamustine plus rituximab and the protocol-specified first interim analysis for the comparison of ibrutinib plus rituximab with ibrutinib.
In accordance with the protocol, the primary analysis of progression-free survival included all patients who underwent randomization except those who, after randomization, were determined to have not met the eligibility criteria at screening. P values for the primary analysis are one-sided. Secondary analyses included all patients who underwent randomization, regardless of eligibility. P values for all secondary analyses are two-sided. Prespecified and exploratory subgroup analyses were also performed. All analyses were performed by the Alliance Statistics and Data Center with the use of SAS software, version 9.4. Data were locked for this analysis as of October 4, 2018.
RESULTS
PATIENT CHARACTERISTICS
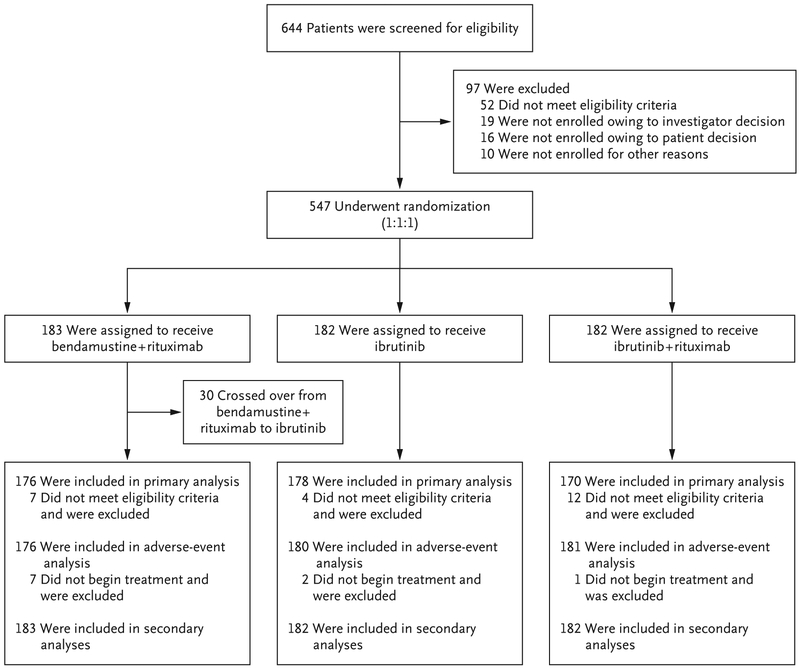
From December 2013 to May 2016, a total of 644 patients were preregistered and 547 were enrolled at 219 sites throughout the United States and Canada (Fig. 1, and see the Supplementary Appendix). Of the 547 patients who were enrolled in the trial, 183 were randomly assigned to receive bendamustine plus rituximab, 182 to receive ibrutinib, and 182 to receive ibrutinib plus rituximab. The characteristics of the patients were typical of a population with untreated CLL (Table 1, and Table S2 in the Supplementary Appendix); the median age was 71 years (range, 65 to 89), and 367 patients (67%) were men.
Figure 1. Screening, Randomization, and Analysis.
Of the 547 patients who underwent randomization, 23 (4%) were determined to have not met the eligibility criteria at screening and were excluded from the primary analysis, in accordance with the protocol. These patients were included in the intention-to-treat analysis.
Table 1.
Characteristics of the Patients at Baseline.
| Characteristic | All Patients (N = 547) |
Bendamustine+ Rituximab (N = 183) |
Ibrutinib (N = 182) |
Ibrutinib+ Rituximab (N = 182) |
P Value* |
|---|---|---|---|---|---|
| Age — yr | 0.53 | ||||
| Median | 71 | 70 | 71 | 71 | |
| Range | 65–89 | 65–86 | 65–89 | 65–86 | |
| Male sex — no. (%) | 367 (67) | 119 (65) | 123 (68) | 125 (69) | 0.75 |
| High-risk disease according to modified Rai stage — no. (%) | 296 (54) | 99 (54) | 99 (54) | 98 (54) | 0.99 |
| ECOG performance-status score — no. (%)† | 0.06 | ||||
| 0 | 271 (50) | 98 (54) | 87 (48) | 86 (47) | |
| 1 | 259 (47) | 75 (41) | 90 (49) | 94 (52) | |
| 2 | 17 (3) | 10 (5) | 5 (3) | 2 (1) | |
| FISH analysis according to hierarchical classification of Döhner et al. — no./total no. (%)‡ | 0.99 | ||||
| Del(17p13.1) | 34/542 (6) | 14/181 (8) | 9/181 (5) | 11/180 (6) | |
| Del(11q22.3) | 105/542 (19) | 33/181 (18) | 35/181 (19) | 37/180 (21) | |
| Trisomy 12 | 118/542 (22) | 40/181 (22) | 40/181 (22) | 38/180 (21) | |
| None | 90/542 (17) | 29/181 (16) | 32/181 (18) | 29/180 (16) | |
| Del(13q14.3) | 195/542 (36) | 65/181 (36) | 65/181 (36) | 65/180 (36) | |
| Mutated TP53 — no./total no. (%) | 51/510 (10) | 16/174 (9) | 15/168 (9) | 20/168 (12) | 0.60 |
| Complex karyotype — no./total no. (%)§ | 143/499 (29) | 44/166 (27) | 39/165 (24) | 60/168 (36) | 0.04 |
| Unmethylated ZAP70 — no./total no. (%) | 287/546 (53) | 95/182 (52) | 96/182 (53) | 96/182 (53) | 0.99 |
| Unmutated IgVH gene — no./total no. (%)¶ | 218/360 (61) | 71/123 (58) | 77/122 (63) | 70/115 (61) | 0.69 |
All P values are for comparisons across all three treatment groups and are two-sided. P values for continuous variables were calculated with the use of the Kruskal–Wallis test, and P values for categorical variables were calculated with the use of the chi-square test or Fisher’s exact test.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
Central fluorescence in situ hybridization (FISH) analysis was performed with the use of the hierarchical classification method established by Döhner et al.20
Complex karyotype was defined as the presence of at least three unrelated abnormalities as assessed by central review.
IgVH denotes immunoglobulin variable heavy chain.
With regard to risk factors for CLL, 46% of the patients had intermediate-risk disease and 54% had high-risk disease according to modified Rai stage, 53% had ZAP70-unmethylated disease on central testing (with ZAP70-unmethylated disease status used as a surrogate for IgVH-unmutated disease status), and 27% had disease associated with the presence of del(17p13.1) or del(11q22.3) on local FISH analysis. A separate, central FISH analysis performed with the use of the hierarchical classification method established by Döhner et al.20 revealed the presence of del(17p13.1) in 6% of the patients, del(11q22.3) in 19%, trisomy 12 in 22%, and del(13q14.3) in 36%, as well as the absence of all these abnormalities in 17%. In addition, 29% of the patients had a complex karyotype, with at least three unrelated cytogenetic abnormalities as assessed by central review,21 and 10% had a mutation in TP53 with a variant allele frequency of more than 10%. Of the 360 patients who underwent central sequencing of IgVH genes, 61% had IgVH-unmutated disease. There was no significant difference among the three treatment groups with regard to baseline characteristics, with the exception of a higher percentage of patients with a complex karyotype in the ibrutinib-plus-rituximab group than in the other two treatment groups (P=0.04).
Of the 524 patients who were enrolled in the trial and were determined to have met the eligibility criteria at screening, 389 patients (74%) consented to undergo the geriatric assessment for the correlative analysis, and 369 of those patients (95%) completed the assessment before treatment. The mean (±SD) score for activities of daily living was 13.7±0.8 (range, 9 to 14), and the mean number of coexisting conditions was 2.5±1.9 (range, 0 to 14) (Table S3 in the Supplementary Appendix). There was no significant difference among the three treatment groups with regard to results on the geriatric assessment.
PROGRESSION-FREE SURVIVAL AND OVERALL SURVIVAL
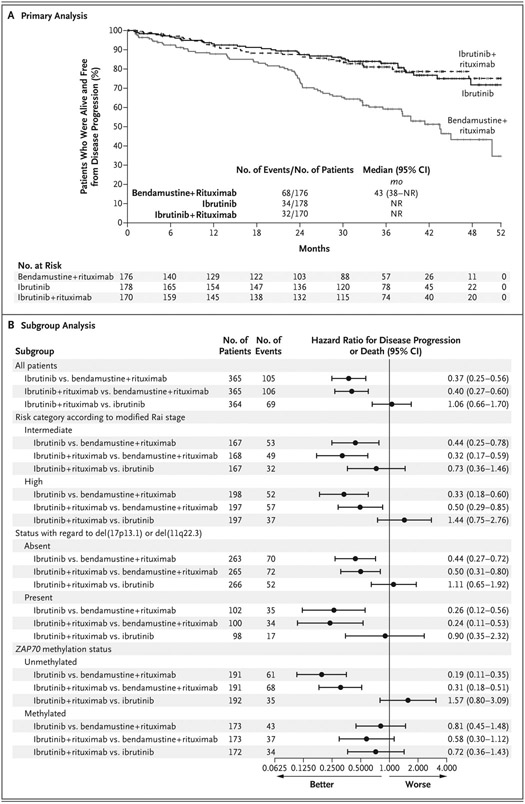
Of the 547 patients who underwent randomization, 524 (96%) were determined to have met the eligibility criteria at screening and were included in the primary analysis. Median progression-free survival was reached only with bendamustine plus rituximab. The estimated percentage of patients with progression-free survival at 2 years was 74% (95% confidence interval [CI], 66 to 80) with bendamustine plus rituximab, as compared with 87% (95% CI, 81 to 92) with ibrutinib and 88% (95% CI, 81 to 92) with ibrutinib plus rituximab (Fig. 2A). The hazard ratio for disease progression or death was 0.39 (95% CI, 0.26 to 0.58) for the comparison of ibrutinib with bendamustine plus rituximab (one-sided P<0.001) and 0.38 (95% CI, 0.25 to 0.59) for the comparison of ibrutinib plus rituximab with bendamustine plus rituximab (one-sided P<0.001). There was no significant difference between the ibrutinib-plus-rituximab group and the ibrutinib group with regard to progression-free survival (hazard ratio, 1.00; 95% CI, 0.62 to 1.62; one-sided P = 0.49) (Fig. 2A). In an intention-to-treat analysis, which included all patients who underwent randomization, the same conclusions were reached (Fig. S1 in the Supplementary Appendix).
Figure 2. Primary and Subgroup Analyses of Progression-free Survival.
Panel A shows Kaplan–Meier estimates of progression-free survival for each treatment group. The primary analysis included all patients who underwent randomization and were determined to have met the eligibility criteria at screening. Panel B shows hazard ratios for disease progression or death at the time of data cutoff, according to subgroups that were based on risk factors for chronic lymphocytic leukemia. The subgroup analysis was performed in the intention-to-treat population. Hazard ratios were calculated with univariable Cox proportional-hazards models. NR denotes not reached.
In analyses of subgroups that were defined according to risk factors for CLL, progression-free survival was longer with the ibrutinib-containing regimens than with bendamustine plus rituximab in all risk factor–related subgroups, but the difference was not significant among patients with ZAP70-methylated disease (Fig. 2B). In exploratory analyses of subgroups that were defined according to cytogenetic factors, there was an interaction between cytogenetics and the effect of treatment on progression-free survival. Progression-free survival was longer with the ibrutinib-containing regimens than with bendamustine plus rituximab in all cytogenetic factor–related subgroups, but the difference was greater among patients with del(17p13.1) (Fig. S2 in the Supplementary Appendix). In an additional analysis, progression-free survival was longer among patients with IgVH-mutated disease than among those with IgVH-unmutated disease (hazard ratio, 0.51; 95% CI, 0.32 to 0.81), but there was no significant interaction between IgVH mutation status and the effect of treatment on progression-free survival. Details regarding this analysis are provided in Tables S4 and S5 and Figure S3 in the Supplementary Appendix.
At the time of data cutoff, 66 deaths had occurred. The estimated percentage of patients with overall survival at 2 years was 95% (95% CI, 91 to 98) with bendamustine plus rituximab, 90% (95% CI, 85 to 94) with ibrutinib, and 94% (95% CI, 89 to 97) with ibrutinib plus rituximab. There was no significant difference among the three treatment groups with regard to overall survival (P≥0.65 for all pairwise comparisons) (Fig. S4 in the Supplementary Appendix).
TREATMENT AND RESPONSE
At the time of data cutoff, the median follow-up was 38 months among the 481 patients who were alive. A total of 114 of 182 patients (63%) in the ibrutinib group and 117 of 182 patients (64%) in the ibrutinib-plus-rituximab group were still receiving ibrutinib, and 88 of 183 patients (48%) in the bendamustine-plus-rituximab group were still in remission and undergoing surveillance in the trial after completion of treatment. In the bendamustine-plus-rituximab group, 67% of the patients received six cycles of treatment; the number of cycles received ranged from one to six, with a dose held in 67% of the patients and the dose reduced in 37%. In the ibrutinib group, the median duration of treatment at the time of data cutoff was 32 months (range, 0 to 51), with the dose reduced in 13% of the patients. In the ibrutinib-plus-rituximab group, the median duration of ibrutinib treatment at the time of data cutoff was 32 months (range, 0 to 52), with the dose reduced in 14% of the patients; 92% of the patients received all planned doses of rituximab.
The best response was determined by means of CT and physical examination in 504 patients (92%) and by means of physical examination alone in 18 (3%) and was not evaluated in 25 (5%). Among all the patients, the response rate was lower with bendamustine plus rituximab than with the ibrutinib-containing regimens: 81% (95% CI, 75 to 87) with bendamustine plus rituximab, as compared with 93% (95% CI, 88 to 96) with ibrutinib and 94% (95% CI, 89 to 97) with ibrutinib plus rituximab. However, the complete response rate was higher with bendamustine plus rituximab than with the ibrutinib-containing regimens: 26% (95% CI, 20 to 33), as compared with 7% (95% CI, 4 to 12) and 12% (95% CI, 8 to 18). The percentage of patients with undetectable minimal residual disease was significantly higher with bendamustine plus rituximab than with the ibrutinib-containing regimens: 8% (95% CI, 5 to 13), as compared with 1% (95% CI, <1 to 3) and 4% (95% CI, 2 to 8).
ADVERSE EVENTS
Because adverse events associated with these treatments have been reported extensively in the literature, we have focused on grade 3 or higher adverse events of special interest (Table 2). These adverse events are reported regardless of attribution and include events that occurred during treatment and follow-up, excluding events that occurred after crossover. The rate of grade 3, 4, or 5 hematologic adverse events was higher with bendamustine plus rituximab (61%) than with ibrutinib or ibrutinib plus rituximab (41% and 39%, respectively), whereas the rate of grade 3, 4, or 5 nonhematologic adverse events was lower with bendamustine plus rituximab (63%) than with the ibrutinib-containing regimens (74% with each regimen). Infections occurred in all three treatment groups, with respiratory tract infections, urinary tract infections, sepsis, and abdominal infections being the most common (Table 3, and Table S6 in the Supplementary Appendix). Atrial fibrillation of any grade occurred in 3% of the patients in the bendamustine-plus-rituximab group, 17% in the ibrutinib group, and 14% in the ibrutinib-plus-rituximab group. Grade 3 or higher hypertension occurred in 14%, 29%, and 34%, respectively. Summaries of all adverse events are provided in Tables S7 through S10 in the Supplementary Appendix.
Table 2.
Summary of Grade 3, 4, or 5 Adverse Events.*
| Adverse Event | Bendamustine+ Rituximab (N = 176) |
Ibrutinib (N = 180) |
Ibrutinib+ Rituximab (N = 181) |
P Value† |
|---|---|---|---|---|
| number of patients (percent) | ||||
| Hematologic | ||||
| Any | <0.001 | |||
| Grade 3 | 62 (35) | 59 (33) | 49 (27) | |
| Grade 4 | 45 (26) | 15 (8) | 21 (12) | |
| Anemia | 0.09 | |||
| Grade 3 | 22 (12) | 20 (11) | 11 (6) | |
| Grade 4 | 0 | 1 (1) | 0 | |
| Decreased neutrophil count | <0.001 | |||
| Grade 3 | 39 (22) | 15 (8) | 20 (11) | |
| Grade 4 | 32 (18) | 12 (7) | 19 (10) | |
| Decreased platelet count | 0.008 | |||
| Grade 3 | 16 (9) | 9 (5) | 8 (4) | |
| Grade 4 | 10 (6) | 3 (2) | 1 (1) | |
| Nonhematologic | ||||
| Any | 0.04 | |||
| Grade 3 | 76 (43) | 97 (54) | 100 (55) | |
| Grade 4 | 20 (11) | 12 (7) | 12 (7) | |
| Grade 5 | 15 (9) | 24 (13) | 22 (12) | |
| Bleeding‡ | 0.46 | |||
| Grade 3 | 0 | 2 (1) | 3 (2) | |
| Grade 4 | 0 | 1 (1) | 1 (1) | |
| Grade 5 | 0 | 0 | 1 (1) | |
| Infection§ | 0.62 | |||
| Grade 3 | 17 (10) | 29 (16) | 28 (15) | |
| Grade 4 | 6 (3) | 6 (3) | 7 (4) | |
| Grade 5 | 3 (2) | 2 (1) | 2 (1) | |
| Febrile neutropenia | <0.001 | |||
| Grade 3 | 13 (7) | 3 (2) | 1 (1) | |
| Atrial fibrillation | 0.05 | |||
| Grade 3 | 5 (3) | 15 (8) | 10 (6) | |
| Grade 4 | 0 | 2 (1) | 0 | |
| Hypertension | <0.001 | |||
| Grade 3 | 24 (14) | 53 (29) | 60 (33) | |
| Grade 4 | 1 (1) | 0 | 1 (1) | |
| Secondary cancer | 0.17 | |||
| Grade 3 | 6 (3) | 5 (3) | 13 (7) | |
| Grade 4 | 0 | 1 (1) | 1 (1) | |
| Grade 5 | 1 (1) | 4 (2) | 1 (1) | |
| Unexplained or unwitnessed death | 0.24 | |||
| Grade 5 | 2 (1) | 7 (4) | 4 (2) | |
Shown are adverse events that occurred during treatment or follow-up, excluding events that occurred after crossover. The adverse-event analysis included all patients who began the assigned treatment.
All P values are for comparisons across all three treatment groups and are two-sided. P values were calculated with the use of Fisher’s exact test.
Bleeding events included epistaxis (in three patients), epistaxis and oral hemorrhage (in one patient), and intracranial hemorrhage (in four patients, including one with a grade 5 event).
Details regarding infections are provided in Table 3.
Table 3.
Summary of Grade 3, 4, or 5 Infections.*
| Type of infection | Bendamustine+ Rituximab (N = 176) |
Ibrutinib (N = 180) |
Ibrutinib+ Rituximab (N = 181) |
|---|---|---|---|
| number of patients (percent) | |||
| Abdominal infection: appendicitis or enterocolitis | |||
| Grade 3 | 0 | 2 (1) | 2 (1) |
| Other infection or infestation: conjunctivitis, hepatitis virus infection, otitis media, or infection involving the bone, joint, lymph gland, scrotum, tooth, skin, or a wound | |||
| Grade 3 | 8 (5) | 14 (8) | 15 (8) |
| Respiratory tract infection: bronchial, lung, or upper respiratory infection | |||
| Grade 3 | 14 (8) | 11 (6) | 17 (9) |
| Grade 5 | 0 | 1 (1) | 0 |
| Central nervous system infection: encephalitis or meningitis | |||
| Grade 3 | 0 | 1 (1) | 1 (1) |
| Grade 4 | 0 | 1 (1) | 0 |
| Grade 5 | 1 (1) | 0 | 0 |
| Sepsis | |||
| Grade 4 | 6 (3) | 5 (3) | 7 (4) |
| Grade 5 | 2 (1) | 1 (1) | 2 (1) |
| Urinary tract infection, including bladder infection | |||
| Grade 3 | 3 (2) | 4 (2) | 5 (3) |
Shown are infections that occurred during treatment or follow-up, excluding events that occurred after crossover.
Death occurred during treatment or within 30 days after treatment discontinuation in 2 patients (1%) in the bendamustine-plus-rituximab group, 13 (7%) in the ibrutinib group, and 13 (7%) in the ibrutinib-plus-rituximab group. Death occurred within the first six cycles of treatment, within 30 days after the sixth cycle among those who completed six cycles, or within 30 days after treatment discontinuation among those who did not complete six cycles in 2 patients (1%) in the bendamustine-plus-rituximab group, 3 (2%) in the ibrutinib group, and 6 (3%) in the ibrutinib-plus-rituximab group. All causes of death are shown in Table S11 in the Supplementary Appendix.
Secondary cancers occurred in 13% of the patients in the bendamustine-plus-rituximab group (excluding events that occurred after crossover), 13% in the ibrutinib group, and 16% in the ibrutinib-plus-rituximab group. Richter’s transformation (CLL that evolved into an aggressive lymphoma) occurred in 1 patient in the bendamustine-plus-rituximab group and in 2 patients in the ibrutinib-plus-rituximab group.
DISCUSSION
In this phase 3 trial, we found that treatment with continuous ibrutinib, either alone or in combination with rituximab, was superior to treatment with six cycles of bendamustine plus rituximab with regard to progression-free survival. We also found that there was no significant difference between ibrutinib and ibrutinib plus rituximab with regard to progression-free survival. An ongoing National Clinical Trials Network (NCTN) trial (Clinicaltrials.gov number, NCT02048813) aims to evaluate whether treatment with ibrutinib plus rituximab is superior to chemoimmunotherapy among younger adults.
Improvement in overall survival is the ultimate goal of new therapies, and in this analysis, there was no significant difference among the three treatment groups with regard to overall survival, although the follow-up period was short for this disease. The rate of grade 5 adverse events was higher than expected with the ibrutinib-containing regimens, although this finding may be due to the crossover design and relatively short followup. At the time of this analysis, the most common causes of death associated with the ibrutinib-containing regimens, aside from CLL, included unexplained or unwitnessed death, infection, and secondary cancers. It is not clear that these events occur more frequently with ibrutinib than with bendamustine plus rituximab, but they will be monitored closely in long-term follow-up. Both atrial and ventricular arrhythmias are known complications of ibrutinib treatment22,23 that have potentially devastating consequences. The mechanism that underlies this association remains unclear but may be related to alternative targets of ibrutinib, since the incidence of these events is lower with the use of more specific BTK inhibitors.24,25 The patients who are at highest risk for these events and viable treatment options for those patients have not yet been identified.
Although this trial was not powered to detect differences among subgroups, the results of our subgroup analyses raise a few points. First, treatment with the ibrutinib-containing regimens, with ibrutinib administered continuously until disease progression, appeared to result in longer progression-free survival than treatment with six cycles of bendamustine plus rituximab in all cytogenetic factor–related subgroups, as well as among patients with IgVH-mutated and IgVH-unmutated disease. In addition, the presence of a complex karyotype, which has previously been shown to be an indicator of poor prognosis, did not appear to influence ibrutinib-induced progression-free survival. Most data regarding complex karyotype are from patients with relapsed CLL, so it is possible that the presence of a complex karyotype is biologically different in the absence of DNA-damaging therapy. During long-term follow-up, further study of complex karyotype in these patients is warranted.
The results of this analysis show the efficacy of treatment with continuous ibrutinib among patients with untreated CLL, but the results also raise the issue of whether indefinite therapy with a BTK inhibitor is needed. The significantly lower rates of undetectable minimal residual disease with the ibrutinib-containing regimens than with bendamustine plus rituximab suggest that treatment with single-agent ibrutinib must be continued indefinitely. Treatments with combined targeted therapies, with the goal of increasing the rate of undetectable minimal residual disease and ultimately discontinuing treatment, have shown promise in early clinical studies26,27 and will be evaluated in upcoming NCTN studies (NCT03737981 and NCT03701282), which may help to address this issue.
Supplementary Material
Acknowledgments
Supported by the following awards from the National Cancer Institute (NCI) of the National Institutes of Health: U10CA180821 and U10CA180882, to the Alliance for Clinical Trials in Oncology (Alliance); UG1CA189823, to the Alliance NCI Community Oncology Research Program Research Base; U10CA180790, U10CA180833, U10CA180836, U10CA180850, U10CA180857, U10CA180867, P30CA033572, UG1CA189858, U10CA180820, U10CA180790, and U10CA180888, to the Eastern Cooperative Oncology Group and Southwest Oncology Group; K23CA178183, to Dr. Woyach; R01CA197870, to Drs. Woyach and Byrd; and R35CA197734, to Dr. Byrd. The trial was supported in part by Pharmacyclics (a subsidiary of AbbVie).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank Susan Geyer, Ph.D., for assistance with the initial trial design; Samantha Sublett, Eva Hoke, and Luke Wilson for assistance with the protocol and data management; and Neil Kay and Tait Shanafelt for assistance with the conception and design of the trial.
Contributor Information
J.A. Woyach, Ohio State University Comprehensive Cancer Center, Columbus
A.S. Ruppert, Ohio State University Comprehensive Cancer Center, Columbus, Alliance Statistics and Data Center, Rochester, MN
N.A. Heerema, Ohio State University Comprehensive Cancer Center, Columbus
W. Zhao, Ohio State University Comprehensive Cancer Center, Columbus
A.M. Booth, Alliance Statistics and Data Center, Rochester, MN
W. Ding, Mayo Clinic, Rochester, MN
N.L. Bartlett, Washington University School of Medicine, St. Louis
D.M. Brander, Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina
P.M. Barr, University of Rochester Medical Center, Rochester, NY
K.A. Rogers, Ohio State University Comprehensive Cancer Center, Columbus
S.A. Parikh, Mayo Clinic, Rochester, MN
S. Coutre, Stanford University School of Medicine, Stanford, California
A. Hurria, City of Hope Comprehensive Cancer Center, Duarte, California.
J.R. Brown, Dana–Farber Partners CancerCare, Boston
G. Lozanski, Ohio State University Comprehensive Cancer Center, Columbus
J.S. Blachly, Ohio State University Comprehensive Cancer Center, Columbus
H.G. Ozer, Ohio State University Comprehensive Cancer Center, Columbus
B. Major-Elechi, Alliance Statistics and Data Center, Rochester, MN
B. Fruth, Alliance Statistics and Data Center, Rochester, MN
S. Nattam, Fort Wayne Medical Oncology and Hematology, Fort Wayne, IN
R.A. Larson, University of Chicago Comprehensive Cancer Center, Chicago
H. Erba, Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina
M. Litzow, Mayo Clinic, Rochester, MN
C. Owen, the University of Calgary, Tom Baker Cancer Centre, Calgary, AB, Canada
C. Kuzma, First Health of the Carolinas Cancer Center, Pinehurst, North Carolina
J.S. Abramson, the Massachusetts General Hospital Cancer Center, Boston
R.F. Little, the Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD
S.E. Smith, Loyola University Chicago, Chicago
R.M. Stone, Dana–Farber Partners CancerCare, Boston
S.J. Mandrekar, Alliance Statistics and Data Center, Rochester, MN
J.C. Byrd, Ohio State University Comprehensive Cancer Center, Columbus
REFERENCES
- 1.Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood 2014;123:1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kil LP, de Bruijn MJ, van Hulst JA, Langerak AW, Yuvaraj S, Hendriks RW. Bruton’s tyrosine kinase mediated signaling enhances leukemogenesis in a mouse model for chronic lymphocytic leukemia. Am J Blood Res 2013;3:71–83. [PMC free article] [PubMed] [Google Scholar]
- 3.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101–10. [DOI] [PubMed] [Google Scholar]
- 4.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17:928–42. [DOI] [PubMed] [Google Scholar]
- 5.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-κB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014;123:3286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman SE, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with ibrutinib inhibits BTK- and VLA-4-dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clin Cancer Res 2015;21:4642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood 2014;123:1810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gorter DJ, Beuling EA, Kersseboom R, et al. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity 2007;26:93–104. [DOI] [PubMed] [Google Scholar]
- 10.de RooiJ MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012;119:2590–4. [DOI] [PubMed] [Google Scholar]
- 11.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012;119:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood 2018;131:1910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr PM, Robak T, Owen C, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica 2018;103: 1502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–74. [DOI] [PubMed] [Google Scholar]
- 16.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claus R, Lucas DM, Stilgenbauer S, et al. Quantitative DNA methylation analysis identifies a single CpG dinucleotide important for ZAP-70 expression and predictive of prognosis in chronic lymphocytic leukemia. J Clin Oncol 2012;30:2483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai KR. A critical analysis of staging in CLL In: Gale RP, Rai KR, eds. Chronic lymphocytic leukemia: recent progression and future directions. New York: Liss, 1987:253–64. [Google Scholar]
- 19.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. [PubMed] [Google Scholar]
- 20.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000;343:1910–6. [DOI] [PubMed] [Google Scholar]
- 21.Haferlach C, Dicker F, Schnittger S, Kern W, Haferlach T. Comprehensive genetic characterization of CLL: a study on 506 cases analysed with chromosome banding analysis, interphase FISH, IgV(H) status and immunophenotyping. Leukemia 2007;21:2442–51. [DOI] [PubMed] [Google Scholar]
- 22.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood 2016;128:138–40. [DOI] [PubMed] [Google Scholar]
- 23.Guha A, Derbala MH, Zhao Q, et al. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol 2018;72:697–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam C, Grigg AP, Opat S, et al. The BTK inhibitor, Bgb-3111, is safe, tolerable, and highly active in patients with relapsed/refractory B-cell malignancies: initial report of a phase 1 first-in-human trial. Blood 2015;126:832. abstract. [Google Scholar]
- 26.Rogers K, Huang Y, Stark A, et al. Initial results of the phase 2 treatment naive cohort in a phase 1b/2 study of obinutuzumab, ibrutinib, and venetoclax in chronic lymphocytic leukemia. Blood 2017;130:431. abstract. [Google Scholar]
- 27.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018;378:1107–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.