Abstract
In the estimation of proportions by pooled testing, the MLE is biased, and several methods of correcting the bias have been presented in previous studies. We propose a new estimator based on the bias correction method introduced by Firth (Biometrika 80:27–38, 1993), which uses a modification of the score function, and we provide an easily computable, Newton–Raphson iterative formula for its computation. Our proposed estimator is almost unbiased across a range of problems, and superior to existing methods. We show that for equal pool sizes the new estimator is equivalent to the estimator proposed by Burrows (Phytopathology 77:363–365, 1987). The performance of our estimator is examined using pooled testing problems encountered in plant disease assessment and prevalence estimation of mosquito-borne viruses.
Keywords: Bias correction, Estimation of proportions, Group testing, Pooled testing, Virus prevalence
1. INTRODUCTION
Pooled testing (also known as group testing) occurs when individuals from a population are pooled together and tested as a group for the presence of an attribute, usually a pathogen. Since its introduction to the statistical literature by Dorfman (1943), pooled testing has been applied to a diverse array of fields, including seed health assays (Liu et al. 2011), HIV prevalence estimation (Hund and Pagano 2013), screening of mycobacteria in rodents (Durnez et al. 2008), and detection of genetically modified organisms (Yamamura and Hino 2007). Two common areas of application, which correspond to the current authors’ involvement, are plant disease assessment (e.g., Freeman et al. 2013) and prevalence of viruses in mosquitoes which transmit them (e.g., Aranda et al. 2009).
Research in pooled testing diverges into two areas—classification, in which the purpose is to identify the positive units, and estimation, where the aim is to estimate the proportion of positives (p) in a population. The statistical issues are quite different, and the literature generally focuses on one or the other. Our concern is with estimation, because of the subject areas which have led to our interest in pooled testing. In plant disease assessment, estimating disease levels in a population such as a field crop, a glasshouse, or a plant production process, has far higher priority than identifying infected plants. With mosquito-borne diseases, the aim is often to estimate the prevalence of virus infection in mosquitoes, which is used in a variety of ways in public health to aid control and prevention of human disease.
The maximum likelihood estimator (MLE) of p is (for a fixed sample size) positively biased, except in the trivial case of all pools consisting of one individual each. This bias, which can be severe, was recognized as an issue in early work on pooled testing (Thompson 1962), but point estimation has not generated as much interest as interval estimation. Some studies have simply encouraged better design of pooled testing procedures, with statements such as “the choice of experimental design is crucial to achieve negligible bias” (Schaarschmidt 2007). It is indeed the case that good planning can alleviate the problem to some extent. For example, Swallow (1985) produced tables showing the bias of the MLE for different numbers of pools and different pool sizes, and these have been used extensively by other studies, even recently (Ding and Xiong 2016). Hepworth and Watson (2009) showed that bias can be reduced by sequential testing with pools of decreasing size. But in many situations, a design (such as a testing plan with particular numbers of pools of a certain size) does not exist, or if it does, the process may not be able to be fully controlled. For example, the US Centers for Disease Control and Prevention may collect virus vector mosquitoes related to ongoing disease outbreaks or when conducting field-related experiments, and these samples are usually analyzed by trap location and collection date, so that pool sizes may not be predetermined, and pooling of individuals rarely results in equal-sized pools. An example of such a study is described by Godsey et al. (2005).
It is important that less biased alternatives to the MLE be available for the wide range of pooled testing scenarios that occur in practice. In this paper we describe estimators which are almost unbiased, and also have smaller mean squared error than the MLE. We first consider work that has already been done in this area, and then propose a new estimator based on the bias reduction method introduced by Firth (1993). We provide an easily computable, Newton–Raphson iterative formula for its computation. We show that Firth’s method is equivalent to that introduced by Burrows (1987) for pools of equal size. We then compare the new estimator with the bias-adjusted MLE resulting from the method described by Gart (1991), which has been previously shown to work well for pools of unequal size. These estimators are evaluated for a variety of pooled testing problems, chosen to reflect real situations encountered by the authors, in either plant disease assessment or mosquito-borne viruses.
2. BIAS CORRECTION OF THE MLE IN POOLED TESTING
Suppose that for i = 1,…, d, ni pools of size mi are tested, of which Xi = xi pools are positive. Assuming that the individuals in the pools follow i.i.d. Bernoulli distributions with parameter p, and that testing is conducted without error, the binomial parameters for the distribution of Xi are ni and 1 − (1 − p)mi, ni assumed fixed and known. The log-likelihood is therefore
| (1) |
where x = (x1,…, xd). The MLE of p is the solution to the score equation
| (2) |
which requires iteration except when all pools are of equal size m, in which case the MLE simplifies to . A convenient, iterative formula for this solution is given in Walter et al. (1980).
Burrows (1987) derived a bias correction for equal pool sizes by writing the MLE as , where y = n − x is the number of negative pools. Beginning with instead of the MLE, he found that the bias term of O(n−1) is eliminated when . This results in an estimator which can be expressed as either of the following:
| (3) |
This estimator could be reasonably described as “the MLE with about a half an additional negative pool.” In calculations across a range of n, p, and m, Burrows found the bias of to be less than about 5% of that of , and the mean squared error (MSE) to be uniformly less than that of .
Hepworth and Watson (2009) found the Burrows correction to be extremely effective in reducing bias for small p, and recommended as an estimator when pool sizes are equal, describing it as “essentially unbiased for values of p consistent with the design of the procedure.” Hund and Pagano (2013) concurred, stating that “the Burrows estimator has negligible bias, even for sample sizes as small as 100.” Colon et al. (2001) pointed out that the Burrows estimator belongs to a class of shrinkage estimators which shrink the MLE toward zero. They examined in some detail the expansions used to derive it, and compared its bias and MSE properties with the MLE, the jackknife, and other shrinkage estimators. For small sample sizes (n < 20) they found the Burrows estimator to have the least bias, and for larger n, it tied for best with the jackknife. Asymptotically, they found that the Burrows estimator tends to overestimate p and the jackknife to underestimate it. However, “for large enough sample sizes the bias of the Burrows estimator is essentially zero” (Mitchell and Pagano 2012).
For equal pool sizes, therefore, the Burrows correction produces an estimator which, for all practical purposes, is effectively unbiased. This perhaps explains the lack of proposed alternatives, though there have been a few. One is the jackknife, mentioned above; another is an empirical Bayes estimator proposed by Bilder and Tebbs (2005), which gave much smaller bias and MSE than , but not quite as small as . Gildow et al. (2008) applied this estimator to the transmission of Cucumber mosaic virus by aphids in snap bean.
The only serious competitor to the Burrows estimator is the general bias correction described by Gart (1991) for independent Xi and a single parameter, which matches the usual pooled testing framework we study here. Except for terms of , the bias of the MLE is
| (4) |
where I is the Fisher information. Using the fact that , it can be shown that
| (5) |
(Walter et al. 1980). The other terms in (4) were derived for pooled testing by Hepworth (2005), and lead to the following expression for the bias:
which provides an estimator
with the bias of removed.
For equal pool sizes, Hepworth and Watson (2009) found Gart’s bias correction to be effective in reducing bias for small p, though not quite as good as Burrows’ correction. A situation in which either correction could have been usefully employed was in the testing of blood samples for HIV described by Brookmeyer (1999). Seven hundred individual samples were grouped into 7 pools of 100, and 4 of the pools tested positive, resulting in an MLE of . At , the MLE has a (relative) bias of 243%, the Burrows estimator has a bias of 0.43%, and the Gart correction gives a bias of −1.81%.
The Gart correction has the disadvantage of not providing a result when due to I(p) being zero. For an extensive discussion of the problem of all positive pools in estimation of p, see Hepworth and Watson (2009).
For unequal pool sizes, the Gart correction was also effective. However, their main evaluation involved only one pooled testing procedure, comprising 8 pools of 20 and 8 pools of 5. The context for that particular evaluation was the estimation of virus prevalence in a carnation population, from which 200 plants were sampled, and tested in pools using ELISA. In the present study we evaluate a range of pooled testing scenarios, including larger ones typical of the monitoring of mosquito-borne viruses. Hepworth and Watson (2009) also assessed Gart’s correction with sequential pooled testing procedures, and found that it effectively accounted for the positive bias, but created a negative bias of similar magnitude. Sequential procedures have their own distinctive characteristics, and we do not consider them further here.
Because of the effectiveness of the Burrows correction for equal pool sizes, an extension of it to unequal pool sizes is desirable. No extension or generalization has yet been derived, however, as even obtaining for equal pool sizes, with a closed-form expression for the estimator, is not trivial, and the unequal pool case appears intractable.
Hepworth and Watson (2009) made an attempt at a generalization by defining yi = ni −xi as the number of negative pools for i = 1,…,d, replacing yi in (2) by yi + ai and ni by ni + bi, where , and iteratively solving (2). The result was an over-correction, with negative bias for all p, though it was less in absolute value than the positive bias of . They also pointed out that there is no inherent reason why ai must equal bi, or why either quantity has to equal , and so a direct generalization remains elusive.
3. FIRTH’S BIAS CORRECTION OF THE MLE
Firth (1993) proposed a general bias correction to the MLE, which involves a modification to the estimating function. Instead of solving the usual score equation S(p) = 0, a solution is found to
| (6) |
This removes bias of and has the feature of being “preventative” rather than “corrective,” as Gart’s correction and jackknife methods are. This is an advantage when an estimate can be infinite, as has been seen in logistic regression (Heinze and Schemper 2002). Firth’s method has been applied widely; for example, with a single binomial observation, is added to the number of positives and the number of negatives, which is not dissimilar from the Burrows correction for equal pool sizes. For pooled testing, we have derived expressions for S(p), I (p), and b(p), and so the numerical solutions to (6) are quite straightforward, though iterative. The resulting estimator, which is our proposed new estimator of p, therefore arises from applying Firth’s bias correction method to pooled testing.
To solve Eq. (6), the Newton–Raphson method provides a convenient iterative scheme for implementation. For each distinct pool size i = 1, …, d, write the unique pool size contribution to the information as
and set . Note that . Next compute
and
Using expressions given, the Newton–Raphson recursion may therefore be derived as
with equal to the number of individuals. A starting value at k = 0 should be predetermined, and we have found it convenient to use the MLE computed using the proportion of positives pools and average pool size, as follows:
Iteration proceeds until the change in successive iterates pk and pk+1 is less than some desired tolerance. For the case of all positive pools, the starting value for the iteration should be the so-called minimum infection rate (MIR), as the preceding one is 0 in this case, making the Newton–Raphson update undefined. As an alternative to this iterative scheme, commonly used statistical software often provides root-finding functionality.
For equal pool sizes, expressions S(p) and I (p) are (2) and (5), respectively, without the summations or subscripts, and the bias in Eq. (4) simplifies to
Piecing these together, Eq. (6) then becomes
which rearranges to
whose solution for p is equivalent to the Burrows estimator given in (3). This shows that the Firth bias correction is equivalent to the Burrows bias correction for equal pool sizes, and so may be considered a natural extension of the Burrows estimator for unequal pool sizes.
4. COMPARISON OF BIAS-CORRECTED ESTIMATORS
4.1. SMALL-SIZED POOLED TESTING EXAMPLE
We now compare Gart’s and Firth’s bias correction methods in some detail using the pooled testing example described above. There were 200 individuals grouped into 8 pools of 20 and 8 pools of 5, for which we adopt the notation N : mn = 200 : 58 208. It is useful to test the methods on a small example such as this, because we cannot rely on asymptotic properties to rescue them from poor performance. It is instructive first to examine the estimates themselves for a range of outcomes; these are presented in Table 1, with the outcomes selected to give a range of values of . It is evident that Firth’s method makes a slightly smaller correction than Gart’s method.
Table 1.
Estimates of p from the Gart and Firth bias correction methods, for selected outcomes of a procedure testing 8 pools of 20 and 8 pools of 5.
| Number of positive groups (x1, x2) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method | (1, 2) | (4, 0) | (2, 5) | (3, 7) | (6, 4) | (5, 7) | (7, 5) | (7, 8) | (8, 7) | (8, 8) |
| MLE | 0.016 | 0.025 | 0.042 | 0.067 | 0.085 | 0.099 | 0.128 | 0.205 | 0.341 | 1 |
| Gart | 0.015 | 0.024 | 0.039 | 0.062 | 0.079 | 0.091 | 0.116 | 0.180 | 0.291 | – |
| Firth | 0.015 | 0.024 | 0.040 | 0.064 | 0.080 | 0.093 | 0.118 | 0.187 | 0.296 | 0.455 |
For evaluation of the bias, there is not much to be gained by examining the entire range of p. A better approach, adopted by Hepworth and Watson (2009), is to concentrate on values of p consistent with the design of the procedure, i.e., values for which the probability of all positive groups is small, because this outcome is highly uninformative, and to be avoided if at all possible. We therefore let ψ be the value of p at which the probability of all positive groups is 0.05, and use ψ as an upper bound on p for purposes of evaluation. For this example, ψ = 0.211. Gart’s method does not produce an estimate for all positive groups, so it is necessary to allocate a value in an ad hoc way; we will use the Firth estimate. For small p the actual value chosen makes little difference, because the probability of all positives is negligible.
Table 2 gives the expected value of the estimators corrected by either Gart’s or Firth’s method, together with the percentage bias and root mean squared error (RMSE), for selected values of p. The corresponding figures for the MLE are also shown. Both corrected estimators are close to unbiased, with less than 1% absolute bias for p < ψ. The bias is slightly negative for the Gart estimator, and mostly slightly positive for the Firth estimator. The RMSE is virtually identical for the two estimators, and here it essentially equals the standard deviation, due to the extremely small bias. Both estimators have smaller RMSE than the MLE, especially for larger p.
Table 2.
Bias of estimators corrected by either Gart’s or Firth’s method, for testing 8 pools of 20 and 8 pools of 5.
| p | MLE |
Gart |
Firth |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % bias | RMSE | % bias | RMSE | % bias | RMSE | ||||
| 0.01 | 0.0105 | 4.6 | 0.0077 | 0.0100 | −0.06 | 0.0074 | 0.0100 | 0.13 | 0.0074 |
| 0.02 | 0.0210 | 5.0 | 0.0115 | 0.0200 | −0.07 | 0.0108 | 0.0200 | 0.15 | 0.0109 |
| 0.03 | 0.0317 | 5.5 | 0.0150 | 0.0300 | −0.08 | 0.0139 | 0.0301 | 0.17 | 0.0139 |
| 0.04 | 0.0424 | 6.0 | 0.0184 | 0.0400 | −0.09 | 0.0168 | 0.0401 | 0.19 | 0.0168 |
| 0.05 | 0.0533 | 6.6 | 0.0219 | 0.0499 | −0.10 | 0.0196 | 0.0501 | 0.22 | 0.0197 |
| 0.07 | 0.0755 | 7.8 | 0.0297 | 0.0699 | −0.14 | 0.0256 | 0.0702 | 0.25 | 0.0258 |
| 0.10 | 0.1099 | 9.9 | 0.0450 | 0.0998 | −0.22 | 0.0357 | 0.1003 | 0.25 | 0.0359 |
| 0.15 | 0.1715 | 14.3 | 0.0927 | 0.1494 | −0.41 | 0.0554 | 0.1501 | 0.09 | 0.0558 |
| 0.20 | 0.2448 | 22.4 | 0.1703 | 0.1983 | −0.85 | 0.0752 | 0.1994 | −0.30 | 0.0758 |
| 0.25 | 0.3363 | 34.5 | 0.2586 | 0.2459 | −1.64 | 0.0910 | 0.2474 | −1.05 | 0.0913 |
| 0.30 | 0.4447 | 48.2 | 0.3391 | 0.2910 | −3.00 | 0.1003 | 0.2927 | −2.42 | 0.1000 |
4.2. MEDIUM-SIZED POOLED TESTING EXAMPLE
We now consider a “medium-sized” example, representative of some procedures used by the CDC in assessing virus infection rates in mosquitoes. This example has 5 pools of 5, 5 pools of 10, 5 pools of 25, and 6 pools of 50, which we write N : mn = 500 : 55 105 255 506. Figure 1 shows the bias and RMSE of both estimators, for p < ψ = 0.183. For p less than about 0.05, the bias is extremely small for either, with the negative bias for the Gart estimator of about the same magnitude as the positive bias for the Firth estimator. For larger p, both estimators have negative bias, with Gart more negative. For this example, the Firth estimator is better overall, though the Gart estimator still has small bias, with the maximum absolute bias being 1.46% at p = ψ. The RMSE is virtually identical for the two methods, and less than the RMSE using the MLE (0.019 at p = 0.05, 0.193 at p = ψ).
Figure 1.
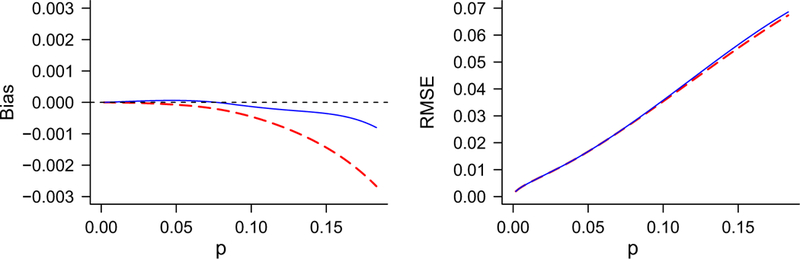
Bias and root mean squared error of estimators corrected by either Gart’s or Firth’s method, for pooled testing with N : mn = 500 : 55 105 255 506. Gart= broken line, Firth = unbroken line.
Another medium-sized example is the problem described by Brookmeyer (1999), which we introduced in section 2; this can be written N : mn = 700 : 1007. We do not provide details of the bias here, but overall Firth’s estimator performs better than Gart’s. At p = ψ = 0.0105, the bias is 0.17% for Firth and −2.39% for Gart.
4.3. LARGE-SIZED POOLED TESTING EXAMPLES
We now consider a range of larger examples, with N = 500, 1000 or 5000, and between 1 and 4 different pool sizes. Table 3 compares the Gart and Firth estimators for mean absolute percentage bias and RMSE, calculated over 100 equally spaced points in the interval [0.001,ψ ]. Also shown is the bias at p = ψ, where its maximum absolute value usually occurs over the range (0,ψ). Corresponding values for the MLE are not shown, as they always vastly exceed the values for the Gart and Firth estimators. Figure 2 plots the bias of both estimators for six of the procedures listed in Table 3, selected to show a range of N : mn and the resulting bias patterns. One of the plots arises from equal pool sizes, two of them from 2 pool sizes, two from 3 pool sizes, and one from 4.
Table 3.
Mean percentage bias, RMSE, and bias (×104) at p = ψ, for estimators corrected by either Gart’s or Firth’s method, for a range of pooled testing procedures.
| N | mn | ψ | Gart |
Firth |
||||
|---|---|---|---|---|---|---|---|---|
| Mean |% bias| |
Mean RMSE |
Bias at p = ψ |
Mean |% bias| |
Mean RMSE |
Bias at p = ψ |
|||
| 500 | 5100 | 0.506 | 0.020 | 0.0288 | −9.81 | 0.015 | 0.0289 | 2.12 |
| 500 | 1050 | 0.248 | 0.085 | 0.0220 | −13.04 | 0.032 | 0.0222 | 0.57 |
| 500 | 2025 | 0.103 | 0.224 | 0.0137 | −9.80 | 0.066 | 0.0139 | 0.13 |
| 500 | 5010 | 0.027 | 0.735 | 0.0063 | −4.81 | 0.200 | 0.0065 | 0.22 |
| 500 | 1005 | 0.008 | 2.069 | 0.0033 | −2.46 | 0.553 | 0.0034 | 0.27 |
| 1000 | 2050 | 0.133 | 0.122 | 0.0123 | −9.46 | 0.033 | 0.0125 | −0.10 |
| 1000 | 10010 | 0.013 | 0.791 | 0.0033 | −2.52 | 0.204 | 0.0034 | 0.10 |
| 1000 | 5200 | 0.569 | 0.012 | 0.0231 | −8.53 | 0.009 | 0.0232 | 1.75 |
| 5000 | 51000 | 0.687 | 0.004 | 0.0135 | −6.17 | 0.003 | 0.0135 | 1.21 |
| 1000 | 5100 5010 | 0.506 | 0.023 | 0.0286 | −9.81 | 0.019 | 0.0287 | 2.12 |
| 1000 | 2520 5010 | 0.078 | 0.251 | 0.0099 | −8.61 | 0.067 | 0.0101 | −0.20 |
| 5000 | 5500 5050 | 0.641 | 0.006 | 0.0170 | −7.09 | 0.005 | 0.0171 | 1.41 |
| 5000 | 25100 5050 | 0.132 | 0.076 | 0.0083 | −7.90 | 0.018 | 0.0084 | −0.33 |
| 1000 | 1020 258 5012 | 0.180 | 0.203 | 0.0219 | −15.18 | 0.024 | 0.0222 | −1.12 |
| 1000 | 1050 2512 504 | 0.248 | 0.085 | 0.0207 | −13.12 | 0.026 | 0.0209 | 0.23 |
| 1000 | 5100 1040 254 | 0.507 | 0.019 | 0.0262 | −9.74 | 0.014 | 0.0263 | 1.98 |
| 5000 | 10200 2560 5030 | 0.344 | 0.032 | 0.0155 | −11.33 | 0.010 | 0.0156 | 0.18 |
| 1000 | 510 1010 2510 5012 | 0.261 | 0.199 | 0.0335 | −19.35 | 0.037 | 0.0339 | −0.46 |
| 1000 | 520 1040 2512 504 | 0.350 | 0.065 | 0.0283 | −14.37 | 0.043 | 0.0286 | 3.49 |
| 5000 | 1050 2540 5030 10020 | 0.248 | 0.080 | 0.0187 | −13.17 | 0.019 | 0.0188 | −0.28 |
Figure 2.
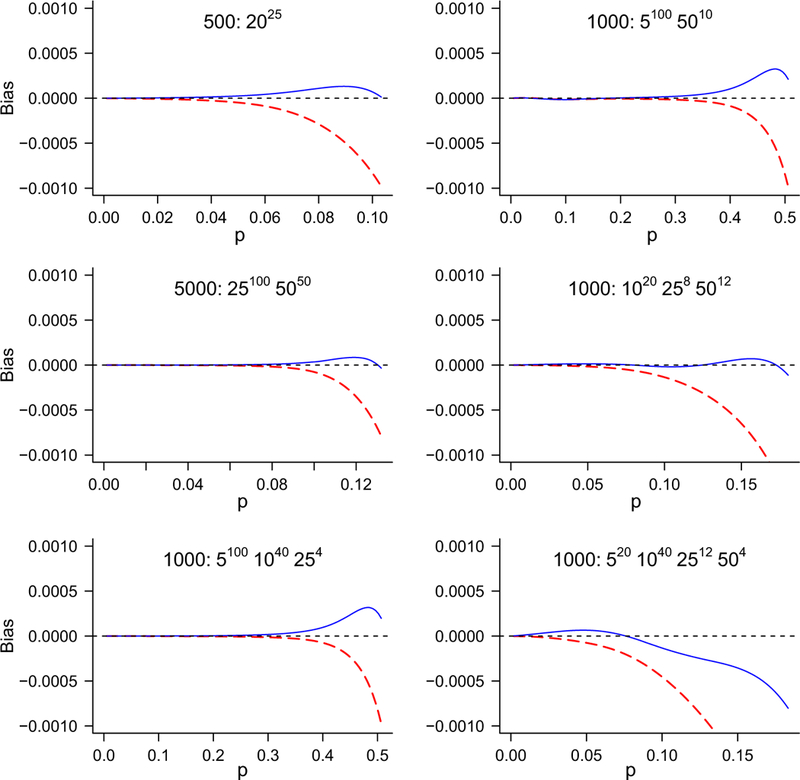
Bias of estimators over p <ψ corrected by either Gart’s or Firth’s method, for a range of pooled testing procedures. Gart = broken line, Firth = unbroken line.
Some trends emerge in these results. The most obvious and important is that, while the mean percentage (absolute) bias is small for both methods (generally < 1%), it is always smaller for Firth’s method than for Gart’s. The difference between the methods generally increases with pool size.
The “worst” bias (i.e., at the highest prevalence consistent with the testing procedure) is always much smaller for Firth’s method. It is always negative for Gart and usually positive for Firth. In percentage terms, the difference between the methods for worst bias decreases with increasing number of pools and increases with average pool size.
The average RMSE is always very slightly larger for Firth’s method, but the difference is of not practical consequence. For either method, it is generally worse (around 0.03) for procedures involving small pool sizes. However, this is still only about half the corresponding RMSE for the MLE.
5. DISCUSSION
We have considered bias correction in estimation of proportions by pooled testing, in which the MLE is clearly unacceptably biased for routine applications. We have proposed a new estimator based on the general bias reduction method applied to MLEs described by Firth (1993), provided an easily computable formula for its iterative computation, and shown that it is better overall than the method described by Gart (1991). Firstly and most importantly, it results in less absolute bias in a wide range of pooled testing situations. But in addition, being based on a modification of the score function, it is preventative rather than corrective, thus avoiding problems relating to undefined parameter estimates. Finally, we have shown that for pools of equal size, Firth’s method is equivalent to the method introduced by Burrows (1987), which is generally viewed as the best estimator available.
Firth’s method has been applied to a range of estimation problems. One study of interest in the current context is that of Mehrabi and Matthews (1995), who applied it to estimating the most probable number (the MLE) in dilution assays. Hepworth (1996) pointed out the strong parallel between pooled testing and dilution assays, especially when pools are of unequal size. Mehrabi and Matthews recommended Firth’s method, especially with the possibility of an infinite estimate. A close second was a “simple bias-corrected estimator,” which was an adjustment to the MLE based on Gart’s method.
Ding and Xiong (2016) proposed a new estimator for the case of equal pool sizes based on a weighted combination of order statistics. They showed by simulation that it was almost unbiased in most cases they considered, and claimed it to be at least as good as the Burrows estimate. This therefore provides a comparison of their proposed estimator with the Firth-adjusted estimator we propose, since for equal pool sizes Burrows and Firth agree.
If MSE or RMSE (which is composed largely of variance here) was used as the main criterion for choosing an estimator, we might place the Gart and Firth methods on an equal footing. As Firth (1993) states, “the merits of bias reduction in any particular problem will depend on a number of factors.” But given that the RMSE associated with the two methods is very similar, and much less than that of the MLE, the smaller bias of the Firth estimator gives it an advantage. This is likely to be particularly true for practitioners using pooled testing, whose disposition is often to prefer an unbiased estimator, even at the expense of a mild increase in variance.
We have assumed in this study that positive and negative bias are of equal detriment to an estimator. The fact that the corrected estimator has negative bias for Gart’s method (i.e., it is a slight over-correction) and generally positive bias for Firth’s method has therefore not been a consideration in recommending the Firth method.
Computation is not a major issue in deciding on an appropriate estimator. Estimation of p generally requires iteration when pools are of unequal size, even if there is no bias correction. In our computations, some of the bias calculations took considerable computing time when there were a large number of outcomes, e.g., N : mn = 1000 : 510 1010 2510 5012, for which there are 17303 outcomes. If the number of different group sizes was large, this was accentuated. However, the computation of the estimates themselves took very little time, and so provided a practitioner has access to statistical software, bias-corrected estimates can be found readily. R code to implement the methods in this paper is available from the authors, and for Firth’s estimator, R code implementing the Newton–Raphson iteration is provided in an online supplement accompanying the article at the journal’s website.
Contributor Information
Graham Hepworth, School of Mathematics and Statistics, The University of Melbourne, Victoria 3010, Australia, hepworth@unimelb.edu.au.
Brad J. Biggerstaff, Centers for Disease Control and Prevention, Fort Collins, CO 80521, USA.
REFERENCES
- Aranda C, Sanchez-Seco MP, Caceres F, Escosa R, Galvez JC, Masia M, Marques E, Ruz S, Alba A, Busquets N, Vazquez A, Castella J, and Tenorio A (2009). Detection and monitoring of mosquito flaviviruses in Spain between 2001 and 2005. (2009), Vector-borne and Zoonotic Diseases 9, 10.1089/vbz.2008.0073. [DOI] [PubMed] [Google Scholar]
- Bilder CR and Tebbs JM (2005). Empirical Bayes estimation of the disease transmission probability in multiple-vector-transfer designs. Biometrical Journal 47, 502–516. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R (1999). Analysis of multistage pooling studies of biological specimens for estimating disease incidence and prevalence. Biometrics 55, 608–612. [DOI] [PubMed] [Google Scholar]
- Burrows PM (1987). Improved estimation of pathogen transmission rates by group testing. Phytopathology 77 363–365. [Google Scholar]
- Colon S, Patil GP, and Taillie C (2001) Estimating prevalence using composites. Environmental and Ecological Statistics 8, 213–236. [Google Scholar]
- Ding J and Xiong W (2016). A new estimator for a population proportion using group testing. Communications in Statistics–Simulation and Computation 45, 10.1080/03610918.2013.854909. [DOI] [Google Scholar]
- Dorfman R (1943). The detection of defective members of large populations. Annals of Mathematical Statistics 14, 436–440. [Google Scholar]
- Durnez L, Eddyani M, Mgode GF, Katakweba A, Katholi CR, Machang’u RR, Kazwala RR, and Portaeis F and Leirs H (2008). First detection of mycobacteria in African rodents and insectivores, using stratified pool screening. Applied and Environmental Microbiology 74, 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth D (1993). Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38. [Google Scholar]
- Freeman AJ, Spackman ME, Aftab M, McQueen V, King S, van Leur JA, Loh MH, and Rodoni B (2013). Comparison of tissue blot immunoassay and reverse transcription polymerase chain reaction assay for virus-testing pulse crops from a South-Eastern Australia survey. Australasian Plant Pathology 42, 675–683. [Google Scholar]
- Gart JJ (1991). An application of score methodology: Confidence intervals and tests of fit for one-hit curves. In Handbook of Statistics, Rao CR, Chakraborty R (eds), 8, 395–406. Amsterdam: Elsevier. [Google Scholar]
- Gildow FE, Shah DA, Sackett WM, Butzler T, Nault BA, and Fleischer SJ (2008). Transmission efficiency of Cucumber mosaic virus by aphids associated with virus epidemics in snap bean. Phytopathology 98, 1233–1241. [DOI] [PubMed] [Google Scholar]
- Godsey MS, Nasci R, Savage HM, Aspen S, King R, Powers AM, Burkhalter K, Colton L, Charnetzky D, Lasater S, Taylor V, and Palmisano CT (2005). West Nile Virus-infected mosquitoes, Louisiana, 2002. Emerging Infectious Diseases 11, 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze G and Schemper M (2002). A solution to the problem of separation in logistic regression. Statistics in Medicine 21, 2409–2419. [DOI] [PubMed] [Google Scholar]
- Hepworth G (1996). Exact confidence intervals for proportions estimated by group testing. Biometrics 52, 1134–1146. [Google Scholar]
- Hepworth G (2005). Confidence intervals for proportions estimated by group testing with groups of unequal size. JABES 10, 478–497. [Google Scholar]
- Hepworth G and Watson R (2009). Debiased estimation of proportions in group testing. JRSS-C 58, 105–121. [Google Scholar]
- Hund L and Pagano M (2013). Estimating HIV prevalence from surveys with low individual consent rates: annealing individual and pooled samples. Emerging Themes in Epidemiology 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-C, Chiang K-S, Lin C-H, and Deng T-C (2011). Confidence interval procedures for proportions estimated by group testing with groups of unequal size adjusted for overdispersion. Journal of Applied Statistics 38, 1467–1482. [Google Scholar]
- Mehrabi Y and Matthews JN (1995). Likelihood-based methods for bias reduction in limiting dilution assays. Biometrics 51, 1543–1549. [Google Scholar]
- Mitchell S and Pagano M (2012). Pooled testing for effective estimation of the prevalence of Schistosoma mansoni. American Journal of Tropical Medicine and Hygiene 87, 850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaarschmidt F (2007). Experimental design for one-sided confidence intervals or hypothesis tests in binomial group testing. Communications in Biometry and Crop Science 2, 32–40. [Google Scholar]
- Swallow WH (1985). Group testing for estimating infection rates and probabilities of disease transmission. Phytopathology 75, 882–889. [Google Scholar]
- Thompson KH (1962). Estimation of the proportion of vectors in a natural population of insects. Biometrics 18, 568–578. [Google Scholar]
- Walter SD, Hildreth SW and Beaty BJ (1980). Estimation of infection rates in populations of organisms using pools of variable size. American Journal of Epidemiology 112, 124–128. [DOI] [PubMed] [Google Scholar]
- Yamamura K and Hino A (2007). Estimation of the proportion of defective units by using group testing under the existence of a threshold of detection. Communications in Statistics–Simulation and Computation 36, 949–957. [Google Scholar]


