Abstract
Folate metabolism makes a crucial contribution towards late-onset Alzheimer’s disease (LOAD). Moreover, methylenetetrahydrofolate reductase (MTHFR) constitutes the primary enzyme of the folate pathway. We hypothesize that there is an association of C677T polymorphism in the MTHFR gene with the susceptibility to LOAD. Previous published research has investigated the link between the MTHFR C677T polymorphisms and LOAD susceptibility; nevertheless, the findings have continued to be not only controversial, but also indecisive. Accordingly, we carried out the present meta-analysis for the assessment of the potential link that exists between the MTHFR C677T polymorphism and the susceptibility to LOAD. Furthermore, we carried out a literature search of the PubMed, EMBASE, Cochrane Library, and WanFang database up to August 10, 2018. The odds ratios (ORs) with the respective 95% confidence interval (95%CI) were put to use for the evaluation of the robustness of the link of the MTHFR C677T polymorphism with the vulnerability to LOAD. All statistical analyses were carried out using STATA 15.0. An aggregate of 14 case-control research works was retrieved, involving 2,467 LOAD patients as well as 2,877 controls. We found that a substantial link exists between C677T polymorphism and LOAD risk in a codominant framework (TC vs. CC: OR=1.22, 95%CI=1.00-1.49, P=0.049). In addition to the stratified analysis based on ethnicity, which suggested that C677T polymorphism was likely linked only to an augmented threat of LOAD in Asians, it did not exist among Caucasians. Furthermore, in the subgroup analysis carried out using APOE ɛ4 status, a substantial increase in the susceptibility to LOAD was detected in APOE ɛ4 carriers as well as non-APOE ɛ4 carriers. In sum, the current meta-analysis revealed that MTHFR C677T polymorphism was associated with susceptibility to LOAD. Further extensive case-control studies are required.
Keywords: Alzheimer’s disease, MTHFR, C677T, Polymorphism, Meta-analysis
1. Introduction
Alzheimer’s disease (AD) is among the most frequently found forms of neurodegenerative dementia in mature persons, constituting a crucial public health concern worldwide [1, 2]. In addition, 47.3 million people globally were suffering from AD in 2015, and the number of AD patients is expected to grow to 133 million by 2050, according to the International Alzheimer’s Disease Report [3, 4]. Furthermore, clinical characteristics of AD include memory impairment, in addition to behavioral as well as cognitive deficiencies [5]. AD comprises early-onset AD (EOAD) and late-onset AD (LOAD). LOAD, accounting for the most AD, is presumed to be a consequence of both ecological and genetic factors [3, 5].
The development of AD is assumed to result from an intricate relationship among several genetic, ecological and lifestyle factors [6]. There is increasing evidence suggesting that genetic factors substantially contribute toward the AD etiology through contact with the ecological components [7, 8]. In addition, the strongest known genetic risk factor with regard to LOAD is the ɛ4 allele of the apolipoprotein E (APOE ɛ4) gene [9, 10, 11]. Numerous investigations have reported several genetic variants, to be linked to LOAD along with the APOE genotype, including the ABCA7, EPHA1, CD33, MS4A6A, and MTHFR gene [12, 13, 14].
Methylene-tetrahydrofolate reductase (MTHFR) constitutes the primary enzyme of the folate metabolism pathway, impacting not only DNA synthesis, but also methylation as well as the repair mechanism [15]. It catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, in addition to being the methyl donor for the remethylation of homocysteine to methionine [16]. Numerous single nucleotide polymorphisms have been discovered in the MTHFR gene; among them, the C677T polymorphism is the most investigated as well as the most significant clinically. Lowered MTHFR activity gives rise to the dietary requirement for folic acid that is needed for the purpose of maintaining the normal homocysteine (Hcy) remethylation to methionine. Furthermore, folate deficiency, together with the consequent augmented levels of plasma homocysteine is associated with the poor cognitive performance, in addition to being linked to AD [17, 18].
Previous research has been carried out on the underlying link between MTHFR C677T polymorphism and the susceptibility to LOAD; nevertheless, conclusions of these studies are inconsistent.. Accordingly, we carried out the present meta-analysis of published investigations aimed at obtaining a more dependable conclusion of this link.
2. Methods and materials
2.1. Selection of literatures
Each of the possible studies was chosen through a search the databases of PubMed, EMBASE, Cochrane Library, and WanFang databases (the last search was updated on August 10, 2018), with the use of keywords as well as the subject terms “polymorphism*” or “variant*” or “mutation*”, “methylenetetrahydrofolate reductase” or “MTHFR”, and “Alzheimer’s disease”. There was no language limitation.
2.2. Inclusion and exclusion criteria
We selected those research works that met the following criteria; (i) the study evaluates the association between the C677T polymorphism of the MTHFR gene and the susceptibility to LOAD; (ii) the study provides sufficient data for the calculation of the odds ratios (ORs) with its respective 95% CI); (iii) exact diagnosis of LOAD was performed with the application of the generally accepted criteria; (iv) the study must be designed as a case-control and based on humans. The major exclusion criteria were as follows: (i) only a case study design; (ii) lacking available genotype frequency; (iii) an abstract of a meeting. In addition, the paper was commented upon and reviewed. When several studies reported the same data category, the largest or the most recent publication was selected.
2.3. Data extraction
Careful extraction of the data of competent research works was performed independently by two authors according to the inclusion criteria presented above. Furthermore, the data hereunder were recorded: (i) name of the first author, in addition to the date of publication, country as well as ethnicity of origin; (ii) genotyping methods, in addition to genotype distributions in both the cases and controls; criteria for LOAD diagnosis; and Hardy-Weinberg equilibrium (HWE) in controls. Resolution of disparities was performed with the assistance of discussion between authors.
2.4. Statistical analysis
All analyses were carried out using STATA 15.0 (Stata Corp, College Station, TX, USA). To evaluate the link of MTHFR C677T with the vulnerability to LOAD, the significance of the pooled odds ratios (ORs) was calculated by a Z-test. We also carried out a subgroup analysis based on ethnicity as well as APOE ɛ4 status. We accessed heterogeneity among included research works using a chi-square–based Q test, coupled with an I2 statistic. The fixed-effects model was put to use when the Q-test led to P-value > 0.1 or the I2 test of I2<50% [19]; alternatively, the random-effects model was adopted [20]. To evaluate the constancy of findings, we carried out a sensitivity analysis through the omission of a single investigation every time. Examination of the latent publication partiality was performed using Begg’s funnel plots as well as Egger’s test [21, 22]; and a P-value less than 0.05 suggested a substantial publication partiality.
3. Results
3.1. Study characteristics
As evident from Figure 1, an aggregate of 343 articles was recognized in the preliminary search in PubMed, EMBASE, the Cochrane Library, as well as WanFang databases. Subsequent to the deduplication and exclusion of the clearly irrelevant studies, an aggregate of 14 case-control research works was retrieved, which involved 2,467 LOAD patients with 2,877 controls [14, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35]. Key attributes of individual investigations are summarized in Table 1.
Figure 1.
Flow diagram of publication selection process.
Table 1.
Main characteristics of studies selected in the meta-analysis
| Author | Year | Country | Ethnicity | Diagnosis criteria | Case | Control | Genotyping methods | HWE |
|---|---|---|---|---|---|---|---|---|
| Belcavello | 2014 | Brazil | Mixed | NR | 82 | 161 | PCR-RFLP | 0.171 |
| Bi | 2009 | China | Asian | NINCDS–ADRDA/DSM-IV | 386 | 375 | PCR-RFLP | 0.125 |
| Coppede | 2012 | Italy | Caucasian | NINCDS–ADRDA/DSM-IV | 378 | 305 | PCR–RFLP | 0.44 |
| Elhawary | 2013 | Egypt | Mixed | NINCDS–ADRDA | 32 | 32 | PCR | 0.628 |
| Giedraitis | 2009 | Sweden | Caucasian | NINCDS-ADRDA/DSM-IV | 85 | 375 | IGA | 0.125 |
| Keikhaee | 2006 | Iran | Asian | NINCDS–ADRDA | 117 | 125 | PCR-RFLP | 0.07 |
| Kida | 2004 | Japan | Asian | NINCDS–ADRDA | 194 | 379 | PCR-RFLP | 0.056 |
| Pollak | 2000 | Israel | Caucasian | NINCDS–ADRDA | 92 | 82 | PCR | 0.501 |
| Prince | 2001 | Sweden | Caucasian | NINCDS–ADRDA | 204 | 172 | DASH | 0.232 |
| Ravaglia | 2004 | Italy | Caucasian | NINCDS-ADRDA/-AIREN | 48 | 122 | PCR | 0.412 |
| Seripa | 2003 | USA | Caucasian | NINCDS-ADRDA | 124 | 97 | PCR-SSCP | 0.781 |
| Stoccoro | 2017 | Italy | Caucasian | NINCDS-ADRDA/DSM-IV | 581 | 468 | PCR-RFLP | 0.139 |
| Wang | 2005 | China | Asian | NINCDS-ADRDA/DSM-III-R | 104 | 130 | PCR | 0.339 |
| Zuliani | 2001 | Italy | Caucasian | NINCDS-ADRDA | 40 | 54 | PCR | 0.627 |
Abbreviations: NR, not reported; ; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association Criteria; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; HWE, Hardy-Weinberg equilibrium in control population; PCR–RFLP, polymerase chain reaction-restriction fragment length polymorphism; PCR–SSCP, polymerase chain reaction-single strand conformation polymorphism; DASH, dynamic allele-specific hybridization; IGA, Illumina GoldenGate Assay.
3.2. MTHFR C677T polymorphism with the susceptibility to LOAD
In the current meta-analysis, we evaluated the association of MTHFR C677T polymorphism with the vulnerability to LOAD through five genetic model comparisons. The results indicated that the MTHFR C677T polymorphism was substantially associated with the LOAD risk in the co-dominant framework (TC vs. CC: OR=1.22, 95%CI=1.00-1.49, P=0.049, Figure 2) in populations in general. Performing the stratified analysis based on ethnicity, a substantially augmented threat of LOAD was observed in Asian populations subjected to the allelic (T vs. C: OR=1.43, 95%CI=1.09-1.87, P=0.009, Figure 3), in addition to recessive (TT vs. CC+TC: OR=1.82, 95%CI=1.05-3.15, P=0.032), dominant (TT+TC vs. CC: OR=1.43, 95%CI=1.10-1.85, P=0.007),
Figure 2.
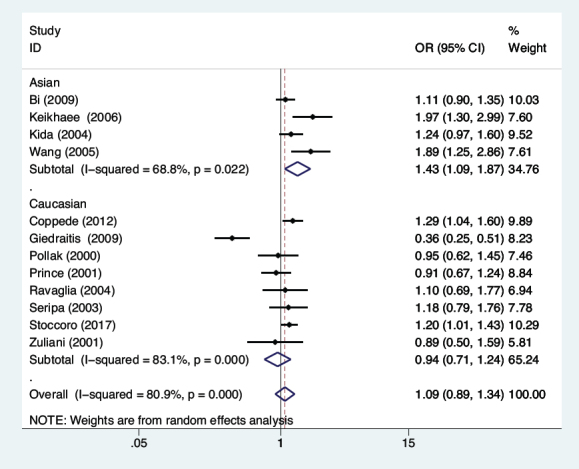
Meta-analysis for the association between MTHFR C677T polymorphism and LOAD susceptibility under the co-dominant model.
Figure 3.
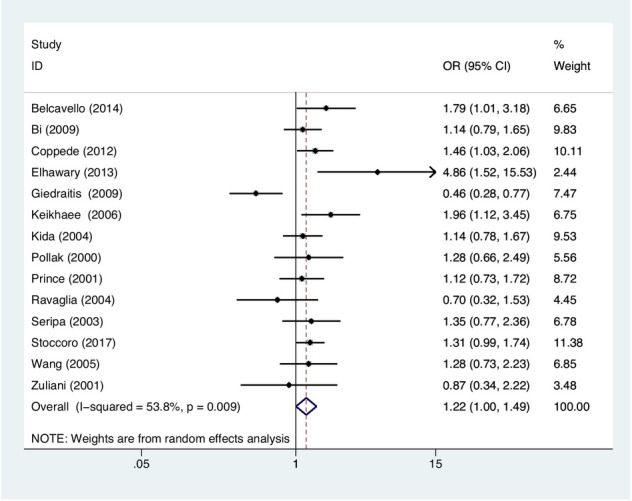
Meta-analysis for the association between MTHFR C677T polymorphism and LOAD susceptibility in Asian under the allelic model.
and co-dominant (TC vs. CC: OR=1.26, 95%CI=1.01-1.57, P=0.037; TT vs. CC: OR=2.09, 95%CI=1.16-3.77, P=0.014) models. The current meta-analysis showed an extensive between-study heterogeneity; therefore, the random-effect model was carried out to evaluate the association between MTHFR C677T polymorphism and the vulnerability to LOAD. Additionally, stratified analysis by APOE ɛ4 status suggested that a substantial rise in the susceptibility to LOAD was found in APOE ɛ4 carriers under the allelic (T vs. C: OR=1.47, 95%CI=1.14-1.91, P=0.003, Figure 4), together with dominant (TT+TC vs. CC: OR=1.67, 95%CI=1.16-2.42, P=0.006), and co-dominant (TC vs. CC: OR=1.54, 95%CI=1.04-2.28, P=0.030; TT vs. CC: OR=1.97, 95%CI=1.15-3.37, P=0.013) models. Additionally, similar results were found in non-APOE ɛ4 carriers. The fixed-effects framework was selected because there existed no substantial heterogeneity among between-study, per the main results in Table 2.
Figure 4.
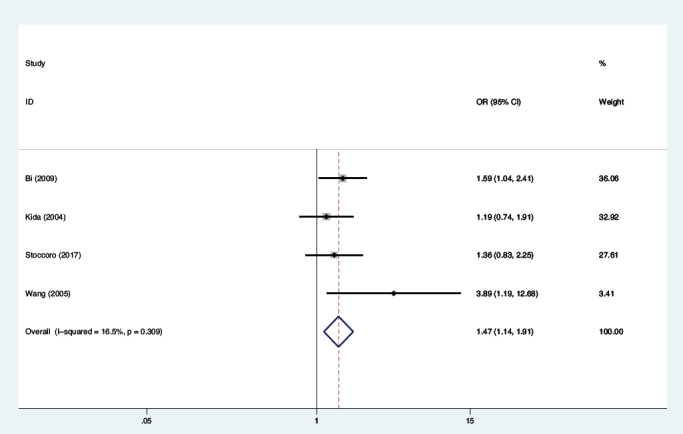
Meta-analysis for the association between MTHFR C677T polymorphism and LOAD susceptibility in APOE ɛ4 carriers under the allelic model.
Table 2.
Meta-analysis of the MTHFR C677T polymorphism and late-onset Alzheimer’s disease susceptibility
| Comparison | Subgroup | Studies | Test of heterogeneity | Test of association | Model | Publication bias | ||
|---|---|---|---|---|---|---|---|---|
| P Value | I2(%) | OR (95%CI) | P Value | Egger | ||||
| T vs. C | Overall | 14 | <0.001 | 80.6 | 1.16(0.95-1.41) | 0.147 | R | 0.839 |
| Asian | 4 | 0.022 | 68.8 | 1.43(1.09-1.87) | 0.009 | R | - | |
| Caucasian | 8 | <0.001 | 80.9 | 1.09(0.89-1.34) | 0.648 | R | - | |
| APOE ɛ4 carrier non-APOE ɛ4 | 4 | 0.309 | 16.5 | 1.47(1.14-1.91) | 0.003 | F | - | |
| carrier Overall | 4 | 0.241 | 28.5 | 1.31(1.12-1.54) | 0.001 | F | - | |
| TC vs. CC | Asian | 14 | 0.009 | 53.8 | 1.22(1.00-1.49) | 0.049 | R | 0.862 |
| Caucasian | 4 | 0.403 | 0 | 1.26(1.01-1.57) | 0.037 | R | - | |
| APOE ɛ4 carrier non-APOE ɛ4 | 8 | 0.016 | 59.4 | 1.05(0.80-1.39) | 0.706 | R | - | |
| carrier Overall | 4 | 0.212 | 33.4 | 1.54(1.04-2.28) | 0.030 | F | - | |
| Asian | 4 | 0.922 | 0 | 1.22(0.94-1.59) | 0.132 | F | - | |
| TT vs. CC | Caucasian | 14 | <0.001 | 75.4 | 1.23(0.84-1.81) | 0.286 | R | 0.915 |
| APOE ɛ4 carrier non-APOE ɛ4 | 4 | 0.027 | 67.2 | 2.09(1.16-3.77) | 0.014 | R | - | |
| carrier Overall | 8 | <0.001 | 78.7 | 0.84(0.49-1.42) | 0.507 | R | - | |
| Asian | 4 | 0.607 | 0 | 1.97(1.15-3.37) | 0.013 | F | - | |
| Caucasian | 4 | 0.094 | 53.0 | 2.06(1.23-3.47) | 0.006 | R | - | |
| TC+TT vs | APOE ɛ4 carrier non-APOE ɛ4 | 14 | <0.001 | 73.1 | 1.24(0.97-1.58) | 0.090 | R | 0.878 |
| CC | carrier Overall | 4 | 0.208 | 34.1 | 1.43(1.10-1.85) | 0.007 | R | - |
| Asian | 8 | <0.001 | 77.6 | 0.98(0.69-1.39) | 0.912 | R | - | |
| Caucasian | 4 | 0.347 | 9.3 | 1.67(1.16-2.42) | 0.006 | F | - | |
| APOE ɛ4 carrier non-APOE ɛ4 | 4 | 0.973 | 0 | 1.38(1.08-1.77) | 0.011 | F | - | |
| TT vs | carrier | 14 | <0.001 | 68.3 | 1.11(0.82-1.51) | 0.496 | R | 0.954 |
| TC+CC | 4 | 0.022 | 68.9 | 1.82(1.05-3.15) | 0.032 | R | - | |
| 8 | 0.002 | 69.9 | 0.84(0.56-1.26) | 0.409 | R | - | ||
| 4 | 0.262 | 25.0 | 1.58(0.98-2.56) | 0.061 | F | - | ||
| 4 | 0.011 | 73.0 | 1.83(1.02-3.30) | 0.044 | R | - | ||
Abbreviations: APOE, Apolipoprotein E; OR, odds ratio; CI, confidence interval; F, fixed-effects model; R, random-effects model; NA, not available.
3.3. Sensitivity analysis and publication bias
We performed a sensitivity analysis to assess the impact of the individual investigations, addressing the pooled OR by sequentially removing each eligible study. The evidence revealed the robustness and reliability of our results (Figure 5). Moreover, funnel plots and Egger’s test were performed to assess the latent publication partiality, and demonstrated that there was no obvious publication partiality (Table 2).
Figure 5.
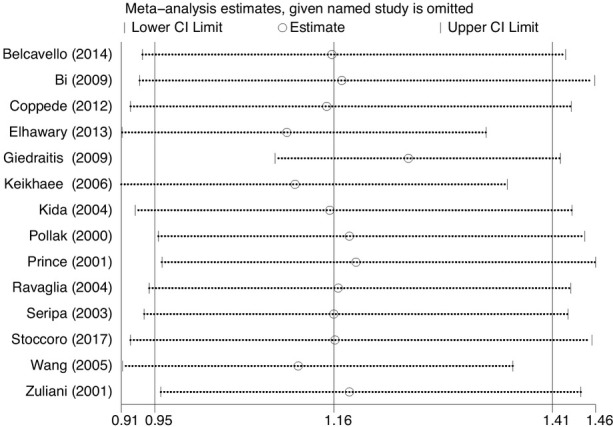
Sensitivity analysis result of the association between MTHFR C677T polymorphism and LOAD susceptibility under the allelic model.
4. Discussion
Alzheimer’s disease (AD) is a complex neurodegenerative dementia in aging persons. Memory impairment constitutes a typical clinical feature of AD. The pathogenesis of AD is regarded as the consequence of contact between ecological and genetic elements. The ɛ4 allele of Apolipoprotein E (APOE ɛ4) gene is hitherto the most robust known genetic risk in patients who have late-onset Alzheimer’s disease (LOAD). Identification of the genetic variants in addition to APOE genotype that contributed to the development of LOAD has the potential to reveal some effective interventions that could lower the incidence of the disease.
Several experimental and epidemiological study results have connected disorders of folate metabolism of homocysteine to vascular, neurodegenerative, neuropsychiatric, and neoplastic disease, including not only strokes, but also Parkinson’s disease, Alzheimer disease, and glioma [14, 36, 37, 38, 39, 40]. Moreover, methylenetetrahydrofolate reductase (MTHFR) is an important enzyme for in the folate metabolism of homocysteine. The human MTHFR gene is localized in the chromosome locus; the
protein it produces catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the methyl donor for the remethylation of homocysteine to methionine [16, 40]. Mutation in the MTHFR gene could result to reducing its methylene tetrahydrofolate reductase activity, putting homocysteine transferring to methionine in malfunctioning, thus giving rise to a higher plasma homocysteine concentration. Additionally, an augmented level of homocysteine and a lack of folate occur, increasing the risk of impairment to oligodendrocytes because of not only the amyloid precursor protein but also presenilin-1 [40]. Numerous single nucleotide polymorphisms in the MTHFR gene have been identified, whereof C677T polymorphism is the most studied and is the clinically important [41]. Nevertheless, the involvement of the MTHFR C677T with the vulnerability to LOAD is not consensual.
Since April 1998, associated research works addressing the link between the MTHFR genetic variants and the susceptibility to AD have been performed in study groups of differing ethnicities. Chapman et al. reported case-control investigations that suggested that MTHFR C677T polymorphism was not substantially linked to the susceptibility to Alzheimer’s dementia [42]. Concerning the risk for LOAD, the MTHFR C677T polymorphism had a slight link to the onset of senile dementia in men but not with LOAD by Nishiyama et al [43]. Stoccoro et al.’s study showed that the MTHFR C677T polymorphism constituted a risk factor for LOAD in non-Asian populations, either in APOE ɛ ɛ4 or in non-APOE ɛ4 carriers [14]. In the year 2010, a meta-analysis conducted by Zhang et al. demonstrated that the polymorphism of MTHFR C677T was substantially linked to the vulnerability to LOAD in the allelic and dominant model comparisons in East Asian populations [44]. Peng et al. performed a meta-analysis, which suggested that the MTHFR C677T polymorphism was linked to the augmented susceptibility to LOAD in Asians and APOE ɛ4 carriers, not in non-APOE ɛ4 carriers, early-onset Alzheimer’s disease (EOAD) as well as in Caucasians [45]. The present meta-analysis is the first comprehensive systematic evaluation of the potential link between the MTHFR C677T polymorphism and the vulnerability to LOAD: the polymorphism of MTHFR C677T was linked to an increased susceptibility to LOAD in the co-dominant model. Moreover, by means of the stratification analysis in accordance with ethnicity, a substantially augmented risk of LOAD was observed in Asians. IMportantly, we carried out the stratified analysis based on APOE ɛ4 status, wherein we have found a significant increase in the susceptibility to LOAD in APOE ɛ4 as well as in non-APOE ɛ4 carriers.
Interpreting findings through the meta-analysis, some constraints require mention. Firstly, the included research works were constrained to literature in English and Chinese only, which is likely to introduce a limitation for interpreting the findings. Secondly, substantially between-study heterogeneity was also discovered, which is likely to distort the meta-analysis. Thirdly, specimen size in some of the involved investigations was comparatively smaller for investigating the link existing between MTHFR C677T polymorphism and AD risk. Finally, lack of the genuine data for competent research works studies, we were not able to estimate the susceptibility to LOAD stratified analysis based on sex, life-style, and other risk factors.
5. Conclusion
The present meta-analysis revealed that the polymorphism of MTHFR C677T might contribute to individual susceptibility to LOAD in Asian populations, APOE ɛ4, and non-APOE ɛ4 carriers. Further research involving a large-scale, multi-center sample is required for the purpose of clarifying our findings.
Footnotes
Author Contributions: L.B.Y. designed this study and had full access to all of the data in the study; X.L., and Z.S.Q. extract data; Z.S.Q., Z.W.J., and X.L. analysis and interpretation of data; Y.J. wrote this paper. All authors reviewed the manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (81273989, 81202694); the Key Project of Natural Science Foundation of Hunan Province (2015JJ2105), and a project supported by Scientific Research Fund of Hunan Provincial Education Department(16B199).
Conflicts of interest: The authors have no conflicts of interest to declare.
Reference
- [1].El Kadmiri N, Said N, Slassi I, El Moutawakil B, Nadifi S. Biomarkers for Alzheimer Disease: Classical and Novel Candidates’ Review. Neuroscience. 2018;370:181–190. doi: 10.1016/j.neuroscience.2017.07.017. [DOI] [PubMed] [Google Scholar]
- [2].Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genetics in medicine : official journal of the American College of Medical Genetics. 2016;18(5):421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].2015 Alzheimer’s disease facts and figures. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2015;11(3):332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- [4].Vaudano E, Vannieuwenhuyse B, Van Der Geyten S. Boosting translational research on Alzheimer’s disease in Europe: The Innovative Medicine Initiative AD research platform. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2015;11(9):1121–1122. doi: 10.1016/j.jalz.2015.02.002. [DOI] [PubMed] [Google Scholar]
- [5].Bird TD. Adam MP, Ardinger HH, Pagon RA. GeneReviews((R)) Seattle (WA) GeneReviews is a registered trademark of the University of Washington, Seattle; 1993. Alzheimer Disease Overview. University of Washington, Seattle University of Washington, Seattle. All rights reserved. [Google Scholar]
- [6].Gatz M, Reynolds CA, Fratiglioni L. Role of genes and environments for explaining Alzheimer disease. Archives of general psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- [7].Scheltens P, Blennow K, Breteler MM. Alzheimer’s disease. Lancet (London, England) 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- [8].Roman GC. MTHFR Gene Mutations: A Potential Marker of Late-Onset Alzheimer’s Disease? J Alzheimers Dis. 2015;47(2):323–327. doi: 10.3233/JAD-150304. [DOI] [PubMed] [Google Scholar]
- [9].Saunders AM, Strittmatter WJ, Schmechel D. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- [10].Chouraki V, Seshadri S. Genetics of Alzheimer’s disease. Advances in genetics. 2014;87:245–294. doi: 10.1016/B978-0-12-800149-3.00005-6. [DOI] [PubMed] [Google Scholar]
- [11].Rosenberg RN, Lambracht-Washington D, Yu G, Xia W. Genomics of Alzheimer Disease: A Review. JAMA neurology. 2016;73(7):867–874. doi: 10.1001/jamaneurol.2016.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hollingworth P, Harold D, Sims R. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nature genetics. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Naj AC, Jun G, Beecham GW. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nature genetics. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stoccoro A, Tannorella P, Salluzzo MG. The Methylenetetrahydrofolate Reductase C677T Polymorphism and Risk for Late-Onset Alzheimer’s disease: Further Evidence in an Italian Multicenter Study. J Alzheimers Dis. 2017;56(4):1451–1457. doi: 10.3233/JAD-161081. [DOI] [PubMed] [Google Scholar]
- [15].Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Molecular genetics and metabolism. 2000;71(1-2):121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- [16].Mansouri L, Fekih-Mrissa N, Klai S, Mansour M, Gritli N, Mrissa R. Association of methylenetetrahydrofolate reductase polymorphisms with susceptibility to Alzheimer’s disease. Clin Neurol Neurosurg. 2013;115(9):1693–1696. doi: 10.1016/j.clineuro.2013.03.015. [DOI] [PubMed] [Google Scholar]
- [17].Ma F, Wu T, Zhao J. Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study. Nutrients. 2017;9(7) doi: 10.3390/nu9070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seshadri S, Beiser A, Selhub J. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. The New England journal of medicine. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- [19].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22(4):719–748. [PubMed] [Google Scholar]
- [20].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary clinical trials. 2015;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- [22].Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Belcavello L, Camporez D, Almeida LD, Morelato RL, Batitucci MC, de Paula F.. Association of MTHFR and PICALM polymorphisms with Alzheimer’s disease. Mol Biol Rep. 2015;42(3):611–616. doi: 10.1007/s11033-014-3806-1. [DOI] [PubMed] [Google Scholar]
- [24].Bi XH, Zhao HL, Zhang ZX, Zhang JW. Association of RFC1 A80G and MTHFR C677T polymorphisms with Alzheimer’s disease. Neurobiol Aging. 2009;30(10):1601–1607. doi: 10.1016/j.neurobiolaging.2007.12.010. [DOI] [PubMed] [Google Scholar]
- [25].Coppede F, Tannorella P, Pezzini I. Folate, homocysteine, vitamin B12, and polymorphisms of genes participating in one-carbon metabolism in late-onset Alzheimer’s disease patients and healthy controls. Antioxid Redox Signal. 2012;17(2):195–204. doi: 10.1089/ars.2011.4368. [DOI] [PubMed] [Google Scholar]
- [26].Elhawary NA, Hewedi D, Arab A. The MTHFR 677T allele may influence the severity and biochemical risk factors of Alzheimer’s disease in an Egyptian population. Dis Markers. 2013;35(5):439–446. doi: 10.1155/2013/524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giedraitis V, Kilander L, Degerman-Gunnarsson M. Genetic analysis of Alzheimer’s disease in the Uppsala Longitudinal Study of Adult Men. Dement Geriatr Cogn Disord. 2009;27(1):59–68. doi: 10.1159/000191203. [DOI] [PubMed] [Google Scholar]
- [28].Keikhaee MR, Hashemi SB, Najmabadi H, Noroozian M. C677T methylentetrahydrofulate reductase and angiotensin converting enzyme gene polymorphisms in patients with Alzheimer’s disease in Iranian population. Neurochem Res. 2006;31(8):1079–1083. doi: 10.1007/s11064-006-9119-6. [DOI] [PubMed] [Google Scholar]
- [29].Kida T, Kamino K, Yamamoto M. C677T polymorphism of methylenetetrahydrofolate reductase gene affects plasma homocysteine level and is a genetic factor of late‐onset Alzheimer’s disease. 2004;4(1):4–10. [Google Scholar]
- [30].Pollak RD, Pollak A, Idelson M, Bejarano-Achache I, Doron D, Blumenfeld A. The C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene and vascular dementia. Journal of the American Geriatrics Society. 2000;48(6):664–668. doi: 10.1111/j.1532-5415.2000.tb04725.x. [DOI] [PubMed] [Google Scholar]
- [31].Prince JA, Feuk L, Sawyer SL. Lack of replication of association findings in complex disease: an analysis of 15 polymorphisms in prior candidate genes for sporadic Alzheimer’s disease. European journal of human genetics : EJHG. 2001;9(6):437–444. doi: 10.1038/sj.ejhg.5200651. [DOI] [PubMed] [Google Scholar]
- [32].Ravaglia G, Forti P, Maioli F. Common polymorphisms in methylenetetrahydrofolate reductase (MTHFR): relationships with plasma homocysteine concentrations and cognitive status in elderly northern italian subjects. Arch Gerontol Geriatr Suppl. 2004;9:339–348. doi: 10.1016/j.archger.2004.04.044. [DOI] [PubMed] [Google Scholar]
- [33].Seripa D, Forno GD, Matera MG. Methylenetetrahydrofolate reductase and angiotensin converting enzyme gene polymorphisms in two genetically and diagnostically distinct cohort of Alzheimer patients. Neurobiology of Aging. 2003;24(7):933–939. doi: 10.1016/s0197-4580(03)00040-x. [DOI] [PubMed] [Google Scholar]
- [34].Wang B, Jin F, Kan R. Association of MTHFR gene polymorphism C677T with susceptibility to late-onset Alzheimer’s disease. Journal of molecular neuroscience : MN. 2005;27(1):23–27. doi: 10.1385/JMN:27:1:023. [DOI] [PubMed] [Google Scholar]
- [35].Zuliani G, Ble A, Zanca R. Genetic polymorphisms in older subjects with vascular or Alzheimer’s dementia. Acta neurologica Scandinavica. 2001;103(5):304–308. doi: 10.1034/j.1600-0404.2001.103005304.x. [DOI] [PubMed] [Google Scholar]
- [36].Kronenberg G, Colla M, Endres M.. Folic acid, neurodegenerative and neuropsychiatric disease. Current molecular medicine. 2009;9(3):315–323. doi: 10.2174/156652409787847146. [DOI] [PubMed] [Google Scholar]
- [37].Mao X, Han L. The Relationship of Methylenetetrahydrofolate Reductase Gene C677T Polymorphism and Ischemic Stroke in Chinese Han Population. Annals of clinical and laboratory science. 2018;48(2):242–247. [PubMed] [Google Scholar]
- [38].Yuan L, Song Z, Deng X, Xiong W, Yang Z, Deng H. Association of the MTHFR rs1801131 and rs1801133 variants in sporadic Parkinson’s disease patients. Neurosci Lett. 2016;616:26–31. doi: 10.1016/j.neulet.2016.01.031. [DOI] [PubMed] [Google Scholar]
- [39].Chen D, Dong J, Huang Y. Folate metabolism genetic polymorphisms and meningioma and glioma susceptibility in adults. Oncotarget. 2017;8(34):57265–57277. doi: 10.18632/oncotarget.18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. European journal of medical genetics. 2015;58(1):1–10. doi: 10.1016/j.ejmg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- [41].Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):14853–14858. doi: 10.1073/pnas.261469998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chapman J, Wang N, Treves TA, Korczyn AD, Bornstein NM. ACE, MTHFR, Factor V Leiden, and APOE Polymorphisms in Patients With Vascular and Alzheimer’s Dementia. Stroke. 1998;29(7):1401–1404. doi: 10.1161/01.str.29.7.1401. [DOI] [PubMed] [Google Scholar]
- [43].Nishiyama M, Kato Y, Hashimoto M, Yukawa S, Omori K. Apolipoprotein E, methylenetetrahydrofolate reductase (MTHFR) mutation and the risk of senile dementia--an epidemiological study using the polymerase chain reaction (PCR) method. Journal of epidemiology. 2000;10(3):163–172. doi: 10.2188/jea.10.163. [DOI] [PubMed] [Google Scholar]
- [44].Zhang MY, Miao L, Li YS, Hu GY. Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to Alzheimer’s disease. Neurosci Res. 2010;68(2):142–150. doi: 10.1016/j.neures.2010.06.011. [DOI] [PubMed] [Google Scholar]
- [45].Peng Q, Lao X, Huang X, Qin X, Li S, Zeng Z. The MTHFR C677T polymorphism contributes to increased risk of Alzheimer’s disease: evidence based on 40 case-control studies. Neurosci Lett. 2015;586:36–42. doi: 10.1016/j.neulet.2014.11.049. [DOI] [PubMed] [Google Scholar]


